Abstract
Second (2nd)-generation tyrosine kinase inhibitors (TKI) (dasatinib, nilotinib) are effective in patients with all phases of chronic myeloid leukaemia (CML). Dose reductions and treatment interruptions are frequently required due to toxicity, but their significance is unknown. We analysed the impact of dose reductions/interruptions and dose intensity of 2nd-generation TKI on response and survival. A total of 280 patients with CML (all phases) were analysed. Dose reductions were considered when the daily dose was below the standard dose. Dose intensity was determined based on the percentage of the ideal dose intensity. Overall, 176 patients (63%) required treatment interruptions and/or dose reduction at least once during therapy. Dose reductions/interruptions, analysed as a time-dependent covariate, were associated with worse failure-free survival only in patients with untreated CML. Dose intensity analysis did not reveal a worse response or survival in patients who received a lower dose intensity (<100%) during therapy or during the first 6 months. In conclusion, dose reductions and treatment interruptions of 2nd generation TKI in patients with CML have a minimal impact in the response rate and survival of these patients. Further studies are required to determine whether there might be a minimum adequate dose of these agents.
Keywords: Chronic myeloid leukaemia, dasatinib, nilotinib, dose reduction, treatment interruption, dose intensity
Introduction
Imatinib (Gleevec; Novartis, Basel, Switzerland) is standard therapy for patients with chronic myeloid leukaemia (CML) (Baccarani, et al 2006). Among patients with newly diagnosed chronic phase (CP) CML receiving therapy with standard dose imatinib the rate of major cytogenetic response (MCyR) is 89% with an overall survival (OS) of 86% at 7 years (O'Brien, et al 2008). Despite these excellent results 20–30% of patients discontinue therapy because of toxicity or inadequate response (O'Brien, et al 2008).
Second (2nd)-generation tyrosine kinase inhibitors (TKI) are effective therapy for patients who fail imatinib. Dasatinib (Sprycel; Bristol Myers Squibb, New York, NY) is an Abl/Src inhibitor that is 325-fold more potent than imatinib in vitro (O'Hare, et al 2005, Shah, et al 2004). Nilotinib (Tasigna; Novartis, Basel, Switzerland) is derived from imatinib, has improved binding affinity to Bcr-Abl, and is 20-fold more potent than imatinib (Golemovic, et al 2005, O'Hare, et al 2005, Weisberg, et al 2005). Both agents were shown to have clinical activity in CML patients with intolerance or resistance to imatinib (Cortes, et al 2008a, Guilhot, et al 2007, Hochhaus, et al 2008, Kantarjian, et al 2007, le Coutre, et al 2008).
Although generally well tolerated, adverse events are observed in some patients receiving 2nd-generation TKI, leading to transient treatment interruptions in 41–87% of patients receiving dasatinib and dose reductions in up to 73% (Cortes, et al 2008a, Guilhot, et al 2007, Hochhaus, et al 2008). Among patients receiving nilotinib, treatment interruptions occur in 15% of patients and dose reductions in up to 25% (Kantarjian, et al 2007, le Coutre, et al 2008). The impact of dose reductions and treatment interruptions on the clinical outcome of patients receiving therapy with 2nd generation TKI is not known. We thus conducted this analysis to determine whether treatment interruptions and dose reductions of 2nd-generation TKI affected the response to therapy with these agents and survival outcomes.
Patients and Methods
Patients
We reviewed the records of all adult patients with a diagnosis of CML or Philadelphia positive acute lymphoblastic leukaemia (Ph+-ALL) who received therapy with single-agent 2nd-generation TKI (dasatinib or nilotinib) in open label Phase I, II or III studies conducted at MD Anderson Cancer Center (MDACC) as part of multicentre trials. Studies were approved by the Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to study entry.
Among 343 patients treated between May 2004 and June 2008, 98 received therapy with 2nd generation TKI as initial therapy for early CP (ECP) CML, 238 received these agents after imatinib failure for CML in all phases, and 7 had Ph+ ALL (analysed together with blast phase [BP] patients). Criteria for accelerated phase (AP) and BP were as previously published (Baccarani, et al 2006). For this analysis, only patients who started treatment with the currently considered standard dose were included. Thus, 63 patients treated in phase I studies who initiated therapy with doses below or above the standard dose were excluded, and 280 patients remained for the analysis. We considered the currently considered standard dose (regardless of whether a twice daily or once daily schedule was used) as 100% of the dose. For nilotinib this was 800 mg/day (all phases), and for dasatinib it was 100 mg/day for patients in CP and 140 mg/day for AP/BP/Ph+-ALL. Thirty-seven patients in late CP (LCP; after failing imatinib) who received dasatinib at an initial starting dose of 140 mg/day were also included as this was initially considered the standard dose. These patients were considered to have had a dose reduction only if the dose was reduced to <100mg/day at any time and the starting dose was considered as 140% of the target dose.
To calculate the dose intensity of 2nd-generation TKI, we calculated the ideal dose intensity (i.e., 100%) by multiplying the number of days from the start of therapy to last follow-up by the standard dose based on the drug and the stage of the disease as mentioned above. We then calculated the actual dose received. The time that a patient was on treatment was divided into periods defined by dates of dose changes and treatment interruptions. Each period corresponded to a time the patient was receiving a particular dose. The number of days on each time period was multiplied by the dose the patient was receiving during that period (or by 0 if they were off therapy for treatment interruptions). The dose received during each of these periods was added up and divided by the ideal dose intensity. The result was the actual dose intensity the patient received.
Patient Monitoring and Response Criteria
Complete blood counts were performed weekly for the first 12 weeks, and every 3 months thereafter. Cytogenetic and haematological responses were assessed by bone marrow biopsies and aspirates every 12 weeks for the first year and at least every 6–12 months thereafter. The response criteria were as previously published (Baccarani, et al 2006). Briefly, a complete haematological response (CHR) required normalization, for at least 4 weeks, of the bone marrow (less than 5% blasts) and peripheral blood with a white blood cell (WBC) count <10×109/l, without blasts, promyelocytes, or myelocytes, a platelet count <450×109/l, and disappearance of all signs and symptoms of CML. Cytogenetic response was defined by the percentage of Ph+ metaphases in at least 20 metaphases from a bone marrow aspirate sample (fluorescent in situ hybridization [FISH] if insufficient metaphases) as follows: complete cytogenetic response (CCyR) – 0% Ph+ metaphases; partial cytogenetic response (PCyR) – 1–35% Ph+ metaphases; minor cytogenetic response (MiCyR) – 36–65% Ph+ metaphases; minimal cytogenetic response – 66–95% Ph+ metaphases; no response – 96–100% Ph+ metaphases. MCyR included CCyR and PCyR (<36% Ph+ metaphases).
Dasatinib and Nilotinib Dose Modification
Criteria for treatment interruption and dose reduction were as previously published (Cortes, et al 2008a, Guilhot, et al 2007, Hochhaus, et al 2008, Kantarjian, et al 2006, Kantarjian, et al 2007, le Coutre, et al 2008, Talpaz, et al 2006). In general, therapy could be interrupted or reduced in patients who had grade 3 haematological or non-haematological toxicity (in some instances also for persistent grade 2 toxicity). Treatment was then re-initiated at the previous dose, at a reduced dose, or discontinued altogether, depending on the severity and duration of the adverse event and on the number of times the same event had occurred. CML therapies other than the TKI were prohibited while on therapy with TKI except for the use of anagrelide and hydroxycarbamide for treatment of elevated platelet and WBC counts, respectively, with usage generally limited to 1–6 weeks. Administration of colony-stimulating factors for neutropenia and recombinant erythropoietin was also permitted at the discretion of the investigator in most studies, but not routinely used at MDACC.
Definition of Failure-Free Survival, Transformation-Free Survival and Overall Survival
Failure-free survival (FFS) was defined as the time from start of treatment to the occurrence of a failure, defined as death from any cause, transformation to AP/BP, loss of MCyR/CCyR, increase in WBC (>20×109/l), loss of CHR, or treatment discontinuation because of no response or toxicity. Patients who remained on drug therapy were censored at last follow-up. Overall survival (OS) was defined from the time treatment was started until death from any cause, and patients alive at last follow-up were censored.
Statistical analysis
Categorical and continuous variables between groups were compared using the Fischer exact test and Mann-Whitney U test, respectively. Survival curves and probabilities were estimated by the Kaplan-Meier method (Kaplan and Meier 1958). Time-dependent Cox regression analysis was used to evaluate the effect of dose reductions and treatment holidays on survival and rate of cytogenetic response (Cox 1972). Time to the first date of either treatment interruption or dose reduction (whichever occurred first) was used as a time-dependent covariate, and its effects on FFS and OS, and rates of CCyR, and MCyR were evaluated. A possible association of patient and disease characteristics with occurrence of dose reduction/delay was investigated using a multivariate logistic regression model. Covariates included in the multivariate model are presented in table 1. The multivariate logistic model was obtained by performing a backward elimination with P<0.05, then allowing treatment-covariate interactions or any variable previously deleted to reenter the final model if its P value was <0.05. All p-values were 2-sided. Calculations were done in Statistica, version 6.1 (StatSoft, Tulsa, OK).
Table 1.
Clinical Features of Patients Treated with 2nd Generation TKI.
| Clinical Feature | Median [range], or No. (%) | p | |
|---|---|---|---|
| No Dose Reduction (n = 104) |
Dose Reduction/Treatment Interruption (n = 176) |
||
| Age, years | 46 [15–82] | 55 [18–80] | <0.0001 |
| Male sex | 71 (68) | 77 (44) | <0.0001 |
| Weight, kg | 81.5 [45.5–174.3] | 75.8 [47.1–179.7] | 0.07 |
| Body Surface Area, m2 | 1.99 [0.91–2.98] | 1.89 [1.24–2.95] | 0.04 |
| WBC, ×109/l | 26.5 [1–400] | 16.4 [0.7–700] | 0.04 |
| Hb, g/l | 119 [71–158] | 112 [45–212] | 0.13 |
| Platelets, ×109/l | 232.5 [3–1103] | 266 [8–2427] | 0.29 |
| Peripheral blood blasts, % | 0 [0–97] | 0 [0–82] | 0.91 |
| Bone marrow blasts, % | 2.5 [0–97] | 3 [0–88] | 0.68 |
| Time on Imatinib, months* | 24 [2–77] | 33.5 [1–77] | 0.08 |
| Previous therapies* | 2 [1–7] | 2 [1–5] | 0.67 |
| • IFN | 22 (43) | 67 (53) | 0.14 |
| • SCT | 6 (12) | 9 (7) | 0.23 |
| • TKI other than imatinib | 11 (22) | 15 (12) | 0.08 |
| Ph+ at start of 2nd-generation TKI, % | 100 [0–100] | 100 [0–100] | 0.49 |
| Stage | 0.005 | ||
| • ECP | 48 (46) | 50 (28) | |
| • LCP | 17 (17) | 58 (33) | |
| • AP | 20 (19) | 39 (22) | |
| • BP/Ph+ ALL | 19 (18) | 29 (17) | |
| Time from diagnosis to 2nd-generation TKI therapy, years | 1 [0–19] | 4 [0–19] | 0.0002 |
| TKI received | <0.0001 | ||
| • Dasatinib | 38 (37) | 113 (64) | |
| • Nilotinib | 66 (63) | 63 (36) | |
Only for patients not in frontline therapy (N=177; no dose reduction/interruption = 51; dose reduction/interruption = 126)
TKI,; IFN, interferon; SCT, stem cell transplantation; ECP, early chronic phase; LCP, late chronic phase; AP, acute phase; BP/Ph+ ALL, blast phase/ Philadelphia positive acute lymphoblastic leukaemia
Results
Patient characteristics
A total of 280 patients were included: 276 with CML and 4 with Ph+-ALL. Among patients with CML, 98 (36%) were in ECP, 75 (27%) in LCP, 59 (21%) in AP, and 48 (16%) in BP (29 myeloid, 12 lymphoid, 4 Ph+-ALL and 3 extramedullary). Nilotinib was used in 129 (46%) patients (ECP=50, LCP=25, AP=28 and BP=26), while 151 (54%) patients received dasatinib (ECP=48, LCP=50, AP=31 and BP=22). Response rates, FFS and OS were similar for patients treated with either drug (data not shown), thus, for the remainder of the analysis, patients who received dasatinib and nilotinib were considered together, unless stated otherwise.
Overall, 176 patients (63%) required treatment interruptions and/or dose reduction at least once during therapy, and 115 of these patients (41%) required a dose reduction. Median time to first treatment interruption was 37 days (range 3–882 days) and median duration of treatment interruption was 35 days (range 2–321). Median time to first dose reduction was 93 days (range 0–972 days) and median duration of treatment with reduced dose was 306 days (range 8–1307 days). The characteristics of patients who had a dose reduction/interruption compared to those with no dose reduction/interruption are shown in Table 1. There was a trend for patients who had no dose reductions/interruption to be still on study drug therapy at the time of this analysis (54% vs 42%, p=0.06). By multivariate logistic regression analysis, the following factors were independently associated with dose reduction: older age (odds ratio [OR]=1.04, p<0.001), female sex (OR=2.34, p=0.002) and use of dasatinib (OR=3.2, p<0.001).
Dose reductions/interruptions occurred in 63 (49%) patients treated with nilotinib and 113 (75%) patients receiving dasatinib (p<0.0001), and the difference was more significant in patients in LCP (Table 2). When excluding the 37 patients in LCP that started with 140mg of dasatinib, there was still a similar difference (dasatinib – 79 patients (69%); nilotinib 63 patients (49%); p=0.001). Of the 113 patients receiving dasatinib who had a dose reduction/interruption, 61 (54%) were receiving 70mg b.i.d., 13 (11%) were receiving 140mg q.d., 14 (19%) 50mg b.i.d. and 13 (16%) 100mg q.d.. Dose reductions/interruptions were more common in patients receiving dasatinib at 140mg vs 100mg (92% vs 64%, p=0.01), b.i.d. vs q.d. schedule (80 vs 63%, p=0.02), and 70mg b.i.d. vs 100mg q.d. (84% vs 56% p=0.005). Among patients receiving dasatinib that had a dose reduction, the median lowest daily dose in patients in ECP/LCP was 60mg (range 20–80mg) and it was 80mg (range 20–100mg) in patients in AP/BP. Among patients receiving nilotinib who had a dose reduction, the median lowest daily dose was 400mg (range 200–600mg). The more common causes for dose reductions are summarized in Table 3.
Table 2.
Frequency of dose reduction/interruption by TKI
| Stage | Nilotinib | Dasatinib | |||
|---|---|---|---|---|---|
| No. Treated |
No. (%) Dose reduction/interruption |
No. Treated |
No. (%) Dose reduction/interruption |
p | |
| All | 129 | 63 (49) | 151 | 113 (75) | <0.0001 |
| ECP | 50 | 21 (42) | 48 | 29 (60) | 0.05 |
| LCP | 25 | 14 (56) | 50 | 44 (88) | 0.003 |
| AP | 28 | 16 (57) | 31 | 23 (74) | 0.18 |
| BP/Ph+ ALL | 26 | 12 (46) | 22 | 17 (77) | 0.03 |
ECP, early chronic phase; LCP, late chronic phase; AP, acute phase; BP/Ph+ ALL, blast phase/ Philadelphia positive acute lymphoblastic leukaemia
Table 3.
Most Common Reasons for Dose Reduction
| Cause of Dose Reduction | No. Episodes (%) |
|---|---|
| Nilotinib | 49 |
| Myelosuppression | |
| • Neutropenia | 7 (14) |
| • Thrombocytopenia | 15 (31) |
| Elevated bilirubin | 8 (16) |
| Rash | 5 (10) |
| Cardiac event* | 5 (10) |
| Elevated amylase/lipase | 4 (8) |
| Dasatinib | 137 |
| Pleural effusion | 54 (40) |
| Myelosuppression | |
| • Neutropenia | 20 (15) |
| • Thrombocytopenia | 32 (23) |
| Gastrointestinal bleeding | 8 (6) |
| Rash | 4 (3) |
| Dizziness | 4 (3) |
- Acute Myocardial Infarction, Atrial Fibrillation, Chest Pain, Right Bundle Branch Block and Tachycardia (1 each).
Response rate and survival outcomes by dose reduction/interruption
Response to therapy and survival for patients with or without dose reduction/interruption, stratified by disease stage, are presented in Table 4 and Figures 1–2. Median follow-up for all patients was 20 months (range 1–48 months). There was no statistically significant difference in the rates of MCyR and CCyR between patients who did and did not have a dose reduction and/or treatment interruption.
Table 4.
Response rate and survival (failure-free, and overall) according to the need for dose reduction and/or interruption
| Stage | Dose Reduction/Interruption |
N | % MCyR |
% CCyR |
2-year FFS % (median, months |
2-year OS % (median, months) |
|---|---|---|---|---|---|---|
| ECP | Yes | 50 | 94 | 92 | 72 (NR) | 100 (NR) |
| No | 48 | 88 | 88 | 83 (NR) | 100 (NR) | |
| p | 0.56 | 0.09 | 0.03 | 0.73 | ||
| LCP | Yes | 58 | 59 | 52 | 46 (20) | 93 (NR) |
| No | 17 | 53 | 53 | 33 (11) | 59 (29) | |
| p | 0.45 | 0.92 | 0.21 | 0.02 | ||
| AP | Yes | 39 | 49 | 28 | 19 (9) | 54 (29) |
| No | 20 | 20 | 20 | 17 (3) | 58 (27) | |
| p | 0.19 | 0.79 | 0.39 | 0.84 | ||
| BP/Ph+ ALL | Yes | 29 | 38 | 26 | 17 (4.5) | 26 (7) |
| No | 19 | 32 | 26 | 0 (3) | 26 (7) | |
| p | 0.71 | 0.87 | 0.35 | 0.7 |
MCyR, major cytogenetic response; CCyR, complete cytogenetic response; FFS, failure-free survival; OS, overall survival; ECP, early chronic phase; LCP, late chronic phase; AP, acute phase; BP/Ph+ ALL, blast phase/ Philadelphia positive acute lymphoblastic leukaemia; NR, not reached.
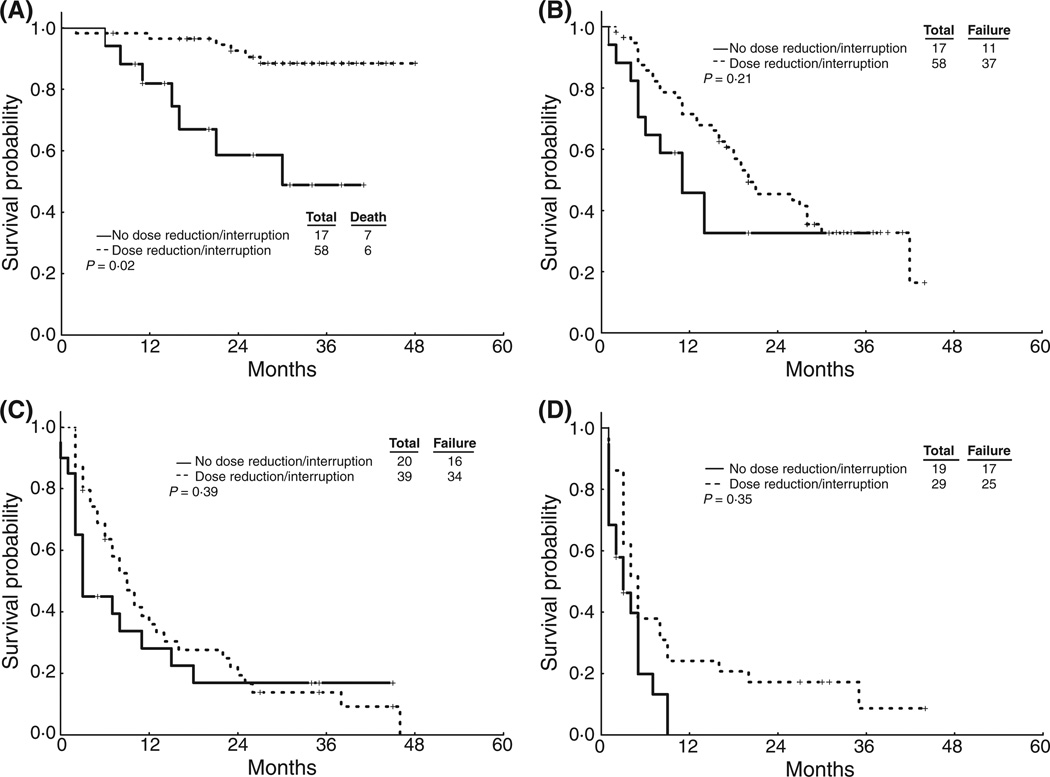
Figure 1. FFS by dose reduction/treatment interruption.
(a) ECP patients. (b) LCP Patients. (c) AP Patients. (d) BP Patients.
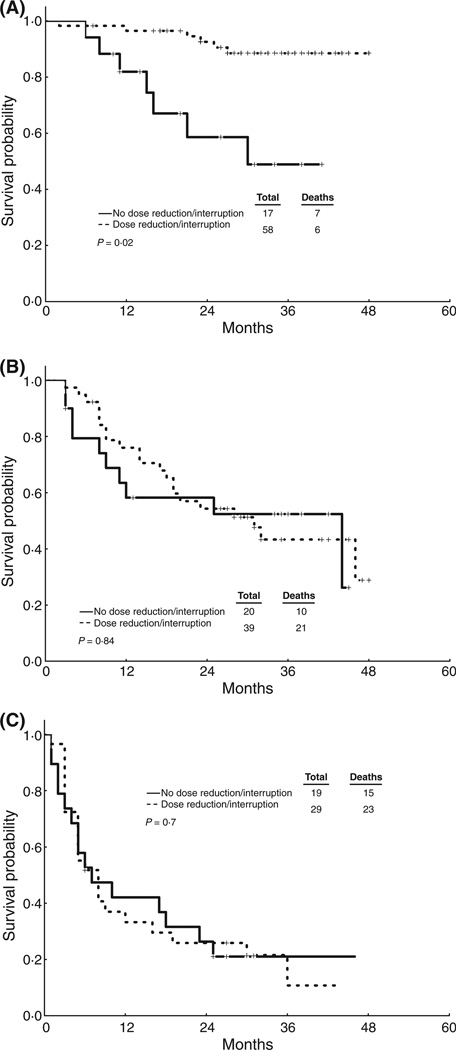
Figure 2. OS by dose reduction/treatment interruption.
(a) LCP Patients. (b) AP Patients. (c) BP Patients. 2-year OS for patients in ECP was 100% in both groups (data not shown).
Dose reductions/interruptions, analysed as a time-dependent covariate, were associated with worse FFS outcome in the overall patient population (P<0.001). When stratified by the disease stage, this effect was only significant in patients in ECP group (Table 4 and Figure 1). Dose reduction/interruption was not associated with worse OS in the entire group of patients (P=0.73) or in any stage group (Table 4 and Figure 2). In patients with LCP CML, patients with dose reductions/interruptions had an improved survival probability (2-year OS: 93% vs. 59%; p=0.02).
Survival outcomes by dose intensity
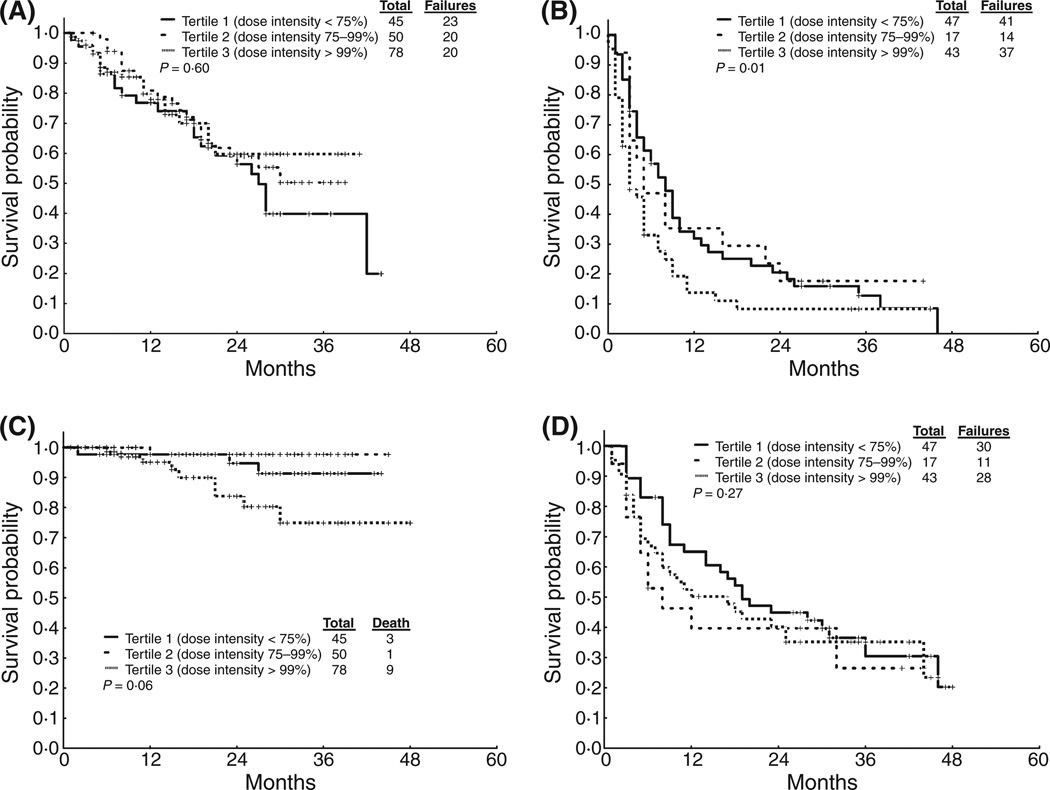
We next analysed the impact of dose intensity, which is a more accurate reflection of the intensity of treatment that was actually received by each patient. We divided dose intensity by tertiles: dose intensity below the 33th percentile (tertile 1, 75% dose intensity), between the 33th and 66th percentile (tertile 2, 75–99% dose intensity) and above the 66th percentile (tertile 3, ≥100% dose intensity). Patients were stratified into CP CML (ECP and LCP) and advanced phase CML (AP and BP). Results are shown in Table 5 and Figure 3. Patients (both CP and advanced stages) who received low- and intermediate-dose intensity (tertiles 1 and 2) had similar survival probabilities as those patients who received high-dose intensity (tertile 3). We also tested dose intensity at every 10% or 20% interval and failed to identify any dose intensity threshold that had a significant effect on FFS and OS (data not shown).
Table 5.
Response and survival by tertile of dose intensity
| Stage | Tertile Dose Intensity |
N | % MCyR | % CCyR | 2-year FFS % (median, months |
2-year OS % (median, months) |
|---|---|---|---|---|---|---|
| ECP/LCP | < 75% | 45 | 67 | 67 | 56 (27) | 95 (NR) |
| 75–99% | 50 | 86 | 76 | 59 (NR) | 98 (NR) | |
| ≥ 100% | 78 | 76 | 76 | 60 (NR) | 84 (NR) | |
| p | 0.08 | 0.49 | 0.60 | 0.06 | ||
| AP/BP/Ph+ ALL | < 75% | 47 | 45 | 26 | 20 (8) | 45 (19) |
| 75–99% | 17 | 47 | 47 | 18 (5) | 40 (7) | |
| ≥ 100% | 43 | 26 | 23 | 8 (3) | 40 (12) | |
| p | 0.11 | 0.15 | 0.01 | 0.27 |
MCyR, major cytogenetic response; CCyR, complete cytogenetic response; FFS, failure-free survival; OS, overall survival; ECP, early chronic phase; LCP, late chronic phase; AP, acute phase; BP/Ph+ ALL, blast phase/ Philadelphia positive acute lymphoblastic leukaemia; NR, not reached.
Figure 3. Survival by dose intensity tertile.
(a) FFS for CP patients. (b) FFS for AP+BP/Ph+ ALL patients. (c) OS for CP. (d) OS for AP+BP/Ph+ ALL.
Dose Intensity over the first 6 months
Recent reports suggest that maintaining high dose intensity during the first 6 months of therapy with imatinib for CML is an important factor for cytogenetic response and survival.(Hughes, et al 2008) We thus compared response rate and survival by dose intensity of 2nd-generation TKI during the first 6 months of therapy. Dose intensity was divided by tertiles as described above. Only patients who were alive and still taking the drug at 6 months (N = 202) were considered for this landmark analysis. Results are shown in Table 6. There were no statistically significant differences for response rate or survival for patients who received lower dose intensity compared with higher dose intensity. We also analysed response and survival by dose intensity before achievement of MCyR in 172 patients. No significant difference was identified (data not shown).
Table 6.
Response and survival by tertile of dose intensity during first 6 months of therapy
| Stage | Tertile Dose Intensity First 6 Months |
N | % MCyR | % CCyR | 2-year FFS % (median, months |
2-year OS % (median, months) |
|---|---|---|---|---|---|---|
| ECP/LCP | < 75% | 25 | 72 | 72 | 59 (27) | 100 (NR) |
| 75–99% | 35 | 86 | 83 | 65 (28) | 97 (NR) | |
| ≥ 100% | 87 | 88 | 85 | 62 (NR) | 92 (NR) | |
| p | 0.12 | 0.31 | 0.89 | 1.0 | ||
| AP/BP/Ph+ ALL | < 75% | 30 | 50 | 30 | 31 (9) | 57 (30) |
| 75–99% | 10 | 70 | 60 | 20 (14) | 77 (NR) | |
| ≥ 100% | 15 | 53 | 47 | 27 (11) | 65 (33) | |
| p | 0.54 | 0.20 | 0.59 | 0.67 |
MCyR, major cytogenetic response; CCyR, complete cytogenetic response; FFS, failure-free survival; OS, overall survival; ECP, early chronic phase; LCP, late chronic phase; AP, acute phase; BP/Ph+ ALL, blast phase/ Philadelphia positive acute lymphoblastic leukaemia; NR, not reached.
Discussion
Patients receiving TKI frequently require transient treatment interruptions and dose reductions because of toxicity (Cortes, et al 2008a, Guilhot, et al 2007, Hochhaus, et al 2008, Kantarjian, et al 2007, le Coutre, et al 2008). It is possible that decreased dose intensity may result in reduced efficacy of therapy. Such a correlation has been suggested when using imatinib for patients with CML. In one analysis, imatinib dose reductions mandated by associated myelosuppression in patients with CP CML correlated with a worse cytogenetic response rate (Sneed, et al 2004). The use of higher initial doses of TKI may lead to better response rates but also more toxicities and need for eventual dose reductions (Kantarjian, et al 2004). One study evaluated the clinical impact of dose reduction in a cohort of CP CML patients treated with high dose imatinib (800 mg/day) (Jain, et al 2007). There was no impact in event-free survival (EFS) in patients who had a dose reduction versus those who remained on high-dose therapy. However, patients who were dose reduced before 6 months of therapy (a time when nearly 90% of patients have achieved CCyR when treated with high-dose imatinib) had worse EFS than those who had a dose reduction after 6 months of therapy or those who had no dose reductions. Another report suggested that patients who were able to tolerate an average daily dose of imatinib of 600 mg/day for the first 6 months had a better response rate than patients who received an average daily dose of <600 mg/day (Hughes, et al 2008). A report from a randomized trial of 400 mg versus 800 mg as initial therapy for patients with ECP CML also suggested a significantly higher probability of response for patients maintaining higher dose intensity (Cortes, et al 2008b).
We thus analysed the frequency and significance of dose reductions and treatment interruptions among patients treated with 2nd generation TKI. Treatment holidays and dose reductions were frequently necessary, occurring in 63% of all patients treated. By multivariate analysis, older patients and women more frequently required dose reductions/interruptions. This could correlate with differences in metabolism of the drugs, possibly leading to increased plasma concentrations of the drugs, resulting in turn in more toxicity and/or reduced tolerance. Unfortunately, measurements of plasma concentrations of dasatinib and nilotinib are not widely available. However, plasma concentrations of imatinib have been found to have little correlation with adverse events (Guilhot, et al 2008, Larson, et al 2008). There was a higher incidence of dose reductions/interruptions in patients receiving dasatinib, especially in LCP. This was partly due to the use of higher doses (140 mg/day) and a twice-daily dosing schedule in some patients. A recent randomized, phase III trial reported that a 100 mg single daily dose of dasatinib achieves a similar response to higher doses (140 mg/day) and a twice-daily schedule, but with less toxicity and fewer dose interruptions (Shah, et al 2008). As expected, in our cohort, patients who received higher doses of dasatinib and a twice-daily schedule had a higher rate of dose interruption/reduction.
In the present analysis, dose reductions/interruptions of 2nd-generation TKI had no impact in the response rate, and were associated with worse FFS only in untreated patients (ECP). In most comparisons, response rates and survival were similar for patients regardless of the presence of dose reductions/interruptions. There was even an unexpected instance where the long-term benefit (improved OS for patients in LCP) was superior for the patients with dose reductions. However, because of the small sample size of the cohort in such subset analysis, these suggestions should be taken with extreme caution. We also analysed the impact of dose intensity on survival and response, and did not find any significant influence of dose intensity of 2nd-generation TKI on response rate and survival.
The lack of adverse impact of dose reductions/interruptions and dose intensity in response to therapy may be viewed as unexpected. One question that arises from our results is what would be the minimal effective dose of the 2nd-generation TKI. For imatinib there is data indicating that the standard dose of 400 mg/daily achieves a trough serum concentration of 1.0µM/l in most patients, which is the concentration that inhibits proliferation of Bcr-Abl positive cells in vitro (Druker, et al 1996, Peng, et al 2004). The use of higher dosages of imatinib leads to a more rapid achievement of cytogenetic and molecular responses, but the long-term impact on survival is still unknown (Baccarani, et al 2009, Cortes, et al 2008b). It has been suggested that the benefit of using high-dose imatinib may be limited to patients that have lower activity of the organic cationic transporter-1 (OCT-1), a transporter molecule responsible for the influx of imatinib into cells (White, et al 2007, White, et al 2008). In this regard, it is important to emphasize that neither dasatinib nor nilotinib depend on OCT-1 for cellular uptake (Hiwase, et al 2008, White, et al 2006).
Compared to imatinib, nilotinib and dasatinib are much more powerful at inhibiting proliferation of Bcr-Abl positive cells. Nilotinib inhibits cellular phosphorylation Bcr-Abl with a half maximal inhibitory concentration (IC50) of 20–57 nM/l (depending on cell line), compared to 250–280 nM/l for imatinib (Manley, et al 2005, O'Hare, et al 2005). Data from the nilotinib phase I study showed that a single daily dose of 400 mg/day, which is 50% of the standard dose, achieved a steady state trough concentration of 1.0µM/l, which is 50-fold the minimum concentration needed to inhibit Bcr-Abl and is also more than the IC50 for inhibition of most mutant forms of Bcr-Abl (19–709nM/l) (Manley, et al 2005, O'Hare, et al 2005, Talpaz, et al 2006). Dasatinib is even more potent, with an IC50 for inhibition of wild type Bcr-Abl of 0.6–3nM/l, and trough levels of 100nM are obtained with recommended doses (Sawyers, et al 2005).
Our analysis suggests that lower dasatinib and nilotinib doses may potentially have similar efficacy in therapy of CML as standard doses. However, these results need to be interpreted with caution as they represent a retrospective analysis of the data, even when all patients were treated on prospective studies with well defined guidelines for dose adjustments, and data on dosing was collected prospectively. In addition, all patients included in this analysis started with at least what is considered the standard dose for dasatinib and nilotinib. Thus, extrapolations cannot be made as to the potential use of lower starting doses of either of these agents. Still, exploring the use of lower doses of these agents might be warranted, either as initial dose or in a strategy that includes an early full-dose “induction” phase followed by a lower dose, pre-designed “maintenance” phase. Utilizing lower doses could decrease costs and diminish side effects. Only prospective randomized trials designed to evaluate the efficacy of lower doses could answer this question. Recent data supporting this approach comes from the recently presented ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials - newly diagnosed patients) trial (Saglio, et al 2009). In this study, untreated patients with CML were randomized between nilotinib 400 mg twice daily, 300 mg twice daily and imatinib 400 mg daily. Both nilotinib arms were superior to imatinib, while there was no difference among them in response and survival, thus suggesting that a lower dose of nilotinib (600 mg/daily) might have the same efficacy as the standard dose, at least in the setting of untreated patients.
In conclusion, dose reductions and treatment interruptions of 2nd generation TKI in patients with CML have a minimal impact in the response rate and survival of patients treated with these agents. Patients who need to stop treatment or to dose reduce due to toxicity should thus be managed accordingly. Further studies are required to determine whether there might be a minimum adequate dose of these agents.
Acknowledgments
Conflicts of interest disclosure: HK and JC have received research funding from Novartis and Bristol-Myers-Squibb. FR has received honoraria from Novartis.
Footnotes
Authorship Contributions: F.P.S.S. collected data, analysed data and wrote the manuscript. C.F collected data and reviewed the manuscript. J.S. collected and analysed the data. J.C. designed research, analysed data, wrote the manuscript and provided patient care. H.K., S.O., G.G.M., F.R., W.W. and D.T. provided patient care and reviewed the manuscript
References
- Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, Apperley J, Cervantes F, Cortes J, Deininger M, Gratwohl A, Guilhot F, Horowitz M, Hughes T, Kantarjian H, Larson R, Niederwieser D, Silver R, Hehlmann R. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- Baccarani M, Rosti G, Castagnetti F, Haznedaroglu I, Porkka K, Abruzzese E, Alimena G, Ehrencrona H, Hjorth-Hansen H, Kairisto V, Levato L, Martinelli G, Nagler A, Lanng Nielsen J, Ozbek U, Palandri F, Palmieri F, Pane F, Rege-Cambrin G, Russo D, Specchia G, Testoni N, Weiss-Bjerrum O, Saglio G, Simonsson B. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet Study. Blood. 2009;113:4497–4504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
- Cortes J, Kim DW, Raffoux E, Martinelli G, Ritchie E, Roy L, Coutre S, Corm S, Hamerschlak N, Tang JL, Hochhaus A, Khoury HJ, Brummendorf TH, Michallet M, Rege-Cambrin G, Gambacorti-Passerini C, Radich JP, Ernst T, Zhu C, Van Tornout JM, Talpaz M. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia. 2008a;22:2176–2183. doi: 10.1038/leu.2008.221. [DOI] [PubMed] [Google Scholar]
- Cortes J, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim D-W, Pane F, Rudoltz M, Yu R, Collins L, Krahnke T, Radich JP, Hughes TP. A Phase III, Randomized, Open-Label Study of 400 Mg Versus 800 Mg of Imatinib Mesylate (IM) in Patients (pts) with Newly Diagnosed, Previously Untreated Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Using Molecular Endpoints: 1-Year Results of TOPS (Tyrosine Kinase Inhibitor Optimization and Selectivity) Study. Blood. 2008b;112 Abstract 335. [Google Scholar]
- Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- Golemovic M, Verstovsek S, Giles F, Cortes J, Manshouri T, Manley PW, Mestan J, Dugan M, Alland L, Griffin JD, Arlinghaus RB, Sun T, Kantarjian H, Beran M. AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukemia. Clin Cancer Res. 2005;11:4941–4947. doi: 10.1158/1078-0432.CCR-04-2601. [DOI] [PubMed] [Google Scholar]
- Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, Amadori S, de Souza CA, Lipton JH, Hochhaus A, Heim D, Larson RA, Branford S, Muller MC, Agarwal P, Gollerkeri A, Talpaz M. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- Guilhot F, Hughes TP, Cortes J, Wang Y, Hayes M, Gichangi A, Druker BJ, Baccarani M. Imatinib (IM) Pharmacokinetic (PK) Exposure and Its Correlation with Clinical Outcome in Patients with Chronic-Phase Chronic Myeloid Leukemia (CML-CP) for 400 Mg and 800 Mg Daily Doses (Tyrosine Kinase Dose Optimization Study [TOPS]) [abstract] Blood. 2008;112 Abstract 447. [Google Scholar]
- Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P, Eadie L, To LB, Melo J, Kumar S, Hughes TP, White DL. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14:3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Baccarani M, Deininger M, Apperley JF, Lipton JH, Goldberg SL, Corm S, Shah NP, Cervantes F, Silver RT, Niederwieser D, Stone RM, Dombret H, Larson RA, Roy L, Hughes T, Muller MC, Ezzeddine R, Countouriotis AM, Kantarjian HM. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Branford S, White DL, Reynolds J, Koelmeyer R, Seymour JF, Taylor K, Arthur C, Schwarer A, Morton J, Cooney J, Leahy MF, Rowlings P, Catalano J, Hertzberg M, Filshie R, Mills AK, Fay K, Durrant S, Januszewicz H, Joske D, Underhill C, Dunkley S, Lynch K, Grigg A. Impact of early dose intensity on cytogenetic and molecular responses in chronic- phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112:3965–3973. doi: 10.1182/blood-2008-06-161737. [DOI] [PubMed] [Google Scholar]
- Jain N, Kantarjian HM, Fava C, Thomas D, Burger JA, Borthakur G, Pate O, Cortes J. Imatinib Dose Can Be Safely Reduced after Complete Cytogenetic Response (CCyR) in Patients (pts) with Chronic Myeloid Leukemia (CML) in Early Chronic Phase (CP) Treated with High-Dose Imatinib [abstract] Blood. 2007;110 Abstract 1043. [Google Scholar]
- Kantarjian H, Talpaz M, O'Brien S, Garcia-Manero G, Verstovsek S, Giles F, Rios MB, Shan J, Letvak L, Thomas D, Faderl S, Ferrajoli A, Cortes J. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103:2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F, Ossenkoppele GJ, Nicolini FE, O'Brien SG, Litzow M, Bhatia R, Cervantes F, Haque A, Shou Y, Resta DJ, Weitzman A, Hochhaus A, le Coutre P. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T, Gathmann I, Wang Y. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- le Coutre P, Ottmann OG, Giles F, Kim DW, Cortes J, Gattermann N, Apperley JF, Larson RA, Abruzzese E, O'Brien SG, Kuliczkowski K, Hochhaus A, Mahon FX, Saglio G, Gobbi M, Kwong YL, Baccarani M, Hughes T, Martinelli G, Radich JP, Zheng M, Shou Y, Kantarjian H. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111:1834–1839. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- Manley PW, Mestan J, Cowan-Jacob S, Wartmann M, Weisberg E, Fabbro D, Gilliland DG, Griffin JD. AMN107: Inhibitory profile against non-mutated and mutated forms of the Bcr-Abl tyrosine kinase [abstract] Proc Am Assoc Cancer Res. 2005;46 Abstract 5985. [Google Scholar]
- O'Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, Radich JP, Rudoltz M, Filian J, Gathmann I, Druker BJ, Larson RA. International Randomized Study of Interferon Versus STI571 (IRIS) 7-Year Follow-up: Sustained Survival, Low Rate of Transformation and Increased Rate of Major Molecular Response (MMR) in Patients (pts) with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CMLCP) Treated with Imatinib (IM) [abstract] Blood. 2008;112 Abstract 186. [Google Scholar]
- O'Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, Cowan-Jacob SW, Lee FY, Heinrich MC, Deininger MW, Druker BJ. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, Sawyers CL, Rosamilia M, Ford J, Lloyd P, Capdeville R. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22:935–942. doi: 10.1200/JCO.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Saglio G, Kim D-W, Issaragrisil S, le Coutre PD, Reiffers J, Lobo C, Pasquini R, Clark RE, Hughes TP, Hochhaus A, Gallagher NJ, Hoenekopp A, Dong M, Haque A, Kantarjian HM, Larson RA. Nilotinib Demonstrates Superior Efficacy Compared with Imatinib in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase: Results From the International Randomized Phase III ENESTnd Trial [abstract] Blood. 2009;114 Abstract LBA-1. [Google Scholar]
- Sawyers CL, Kantarjian H, Shah N, Cortes J, Paquette R, Donato N, Nicoll J, Bleickardt E, Chen TT, Talpaz M. Dasatinib (BMS-354825) in Patients with Chronic Myeloid Leukemia (CML) and Philadelphia-Chromosome Positive Acute Lymphoblastic Leukemia (Ph+ ALL) Who Are Resistant or Intolerant to Imatinib: Update of a Phase I Study [abstract] Blood. 2005;106 Abstract 38. [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, Vela-Ojeda J, Silver RT, Khoury HJ, Charbonnier A, Khoroshko N, Paquette RL, Deininger M, Collins RH, Otero I, Hughes T, Bleickardt E, Strauss L, Francis S, Hochhaus A. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- Sneed TB, Kantarjian HM, Talpaz M, O'Brien S, Rios MB, Bekele BN, Zhou X, Resta D, Wierda W, Faderl S, Giles F, Cortes JE. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100:116–121. doi: 10.1002/cncr.11863. [DOI] [PubMed] [Google Scholar]
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O'Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP, Sawyers CL. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daley GQ, Callahan L, Catley L, Cavazza C, Azam M, Neuberg D, Wright RD, Gilliland DG, Griffin JD. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC, Quinn SR, Manley PW, Hughes TP. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S, Zannettino A, Lynch K, Manley PW, Hughes T. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110:4064–4072. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- White DL, Saunders VA, Dang P, Frede A, Eadie L, Soverini S, Quarantelli F, Lin P, Thornquist M, Kim D-W, Pane F, Martinelli G, Radich J, Kalebic T, Saglio G, Hughes T. CML Patients with Low OCT-1 Activity Achieve Better Molecular Responses on High Dose Imatinib Than on Standard Dose. Those with High OCT-1 Activity Have Excellent Responses on Either Dose: A TOPS Correlative Study [abstract] Blood. 2008;112 Abstract 3187. [Google Scholar]