Abstract
Background
Physical exercise in early adulthood and mid-life improves cognitive function and enhances brain plasticity, but the effects of commencing exercise in late adulthood are not well-understood.
Method
We investigated the effects of voluntary exercise in the restoration of place recognition memory in aged rats and examined hippocampal changes of synaptic density and neurogenesis.
Results
We found a highly selective age-related deficit in place recognition memory that is stable across retest sessions and correlates strongly with loss of hippocampal synapses. Additionally, 12 weeks of voluntary running at 20 months of age removed the deficit in the hippocampally dependent place recognition memory. Voluntary running restored presynaptic density in the dentate gyrus and CA3 hippocampal subregions in aged rats to levels beyond those observed in younger animals, in which exercise had no functional or synaptic effects. By contrast, hippocampal neurogenesis, a possible memory-related mechanism, increased in both young and aged rats after physical exercise but was not linked with performance in the place recognition task. We used graph-based network analysis based on synaptic covariance patterns to characterize efficient intrahippocampal connectivity. This analysis revealed that voluntary running completely reverses the profound degradation of hippocampal network efficiency that accompanies sedentary aging. Furthermore, at an individual animal level, both overall hippocampal presynaptic density and subregional connectivity independently contribute to prediction of successful place recognition memory performance.
Conclusions
Our findings emphasize the unique synaptic effects of exercise on the aged brain and their specific relevance to a hippocampally based memory system for place recognition.
Keywords: Aging, exercise, memory, network, neurogenesis, pre-synaptic density
Western societies are rapidly aging, with attendant increases in the prevalence of dementia (1). Interest has therefore focused on physical exercise as an effective, inexpensive, and low-risk strategy for maximizing brain health in later life (2). For example, prospective studies have shown that physical activity acts as a protective factor in the incidence of dementia (3), and randomized clinical trials have found that regular aerobic exercise can reduce rates of cognitive decline in older adults (4). Whether such exercise can arrest cognitive decline associated with aging when begun later in life, however, is mostly unknown.
The neural substrates underlying the protective effects of exercise on cognitive performance in elderly people are not well-understood (5). In rodents, voluntary running at 2–3 months of age leads to several changes in the hippocampus, including increased neurogenesis (6), neurotrophin gene expression (7), dendritic spine density (8), synaptogenesis (9), synaptic plasticity (10), and enhanced survival of neural progenitor cells (11). Consistent with these changes, exercise leads to im provements in various hippocampal-dependent tasks such as spatial reference memory (6,10,12) and context fear conditioning (13). Initiating exercise in older rodents might also lead to changes in the hippocampus and improve performance on hippocampal-dependent tasks. For instance, van Praag et al. (14) provided aged mice (18-month-old) with access to running wheels for 45 days and found increased neurogenesis in the dentate gyrus, as well as improved acquisition and retention of spatial reference memory in the Morris water maze (MWM).
We used a combination of place and object recognition memory paradigms as well as a spatial learning task to determine whether exercise begun when rats are old can arrest or even reverse age-related cognitive decline and whether these effects are independent of age-related changes in noncognitive factors (e.g., motor ability). Short-term place recognition memory is dependent on hippocampal integrity (15), whereas object recognition involves the perirhinal cortex and is largely independent of the hippocampus (16,17). In our study, aging was associated with deficits in place but not object recognition performance, and this deficit was correlated with loss of presynaptic density. Twelve weeks of voluntary running restored place recognition performance in aged rats to that of younger adults but did not produce any detectable differences in the place recognition performances of the younger adults. Running also led to increased neurogenesis in both younger and older rats, but these effects did not predict place recognition performance. Running also led to a marked increase in presynaptic density in older rats, surpassing levels seen in younger animals, and was linked to changes in intrahippocampal synaptic connectivity and predicted place recognition performance. Our findings show that exercise begun in old age reverses some forms of cognitive decline and increases presynaptic density as well as connectivity, raising the possibility that the former is mediated by changes in the latter.
Methods and Materials
Subjects
Aged subjects were 28 experimentally naïve 20-month-old female ex-breeder Fischer 344 rats, whereas younger subjects were 30 experimentally naïve 7-week-old female virgin Fischer 344 rats, both obtained from a commercial supplier (Australian Research Centre, Perth, Australia). Experimental procedures were consistent with the ethical guidelines established by the American Psychology Association and were approved by the Animal Care and Ethics Committee of the University of New South Wales. Further details are provided in Supplement 1.
Apparatus
The water maze consisted of a circular pool constructed from fiberglass. A circular escape platform was submerged 1.5 cm below water level. Black curtains enclosed the pool, with three external cues within the curtains to reduce the amount of visuospatial information available to the animals. Object and place recognition memory tests were conducted in an open field arena constructed from black polyvinyl chloride plastic. Running wheels were made of plastic with a solid back running surface (Wodent Wheels, Salem, Oregon). There were two wheels/cage. Body weight and number of wheel revolutions/cage were measured weekly. Further details and behavioral procedures are described in Supplement 1.
Immunohistochemistry
At the completion of the behavioral tests, rats were anesthetized with sodium pentobarbital (100 mg/kg, intraperitoneal) and per-fused transcardially. Brains were postfixed for 1 hour and placed in 20% sucrose solution overnight. The entire hippocampus was cut in 40-μm coronal sections in alignment with the atlas of Paxinos and Watson (18) with a cryostat (Microm HM560; Microm International, Walldorf, Germany). Six serially adjacent sets of sections were obtained from each brain and stored in .1% sodium azide in .1 mol/L phosphate-buffered saline, pH 7.2. Further immunohistochemical details can be found in Supplement 1.
Statistical Analysis
Data were analyzed by Student t test or a one-way analysis of variance for multiple comparisons in the recognition paradigms and neural measures. A repeated measures analysis of variance was used for testing outcomes from multiple learning trials in the MWM, and analysis of covariance was used when controlling for covariates. Differences were considered significant when p <.05. A matrix to matrix test was computed on the basis of all possible pairwise cross-correlational presynaptic density differences between groups (19). Computational details for graph network analyses are available in Supplement 1.
Results
Aged Rats Are Selectively Impaired in Hippocampally Dependent Short-Term Place Recognition Memory
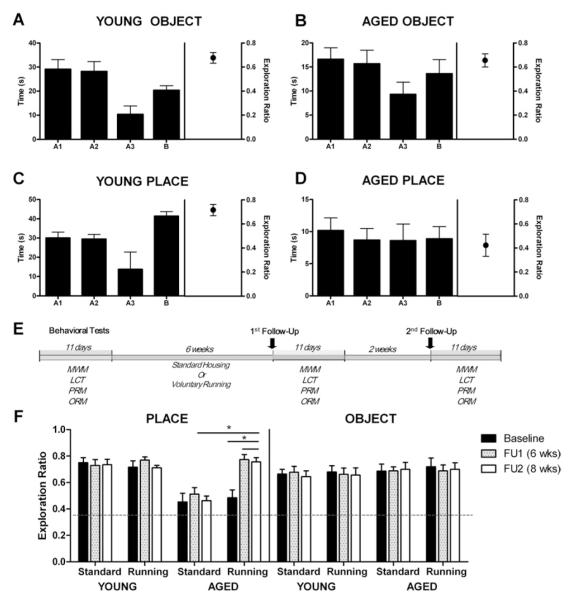
We used object and place recognition tasks to evaluate whether there is a loss of short-term memory associated with advanced age (20). Place recognition was significantly impaired in aged rats compared with young rats [F(1,18) = 16.497, p <.001] (Figure 1C, D). In contrast, there were no significant age differences on the hippocampally independent object recognition memory task [F(1,18) = .025, p > .87] (Figure 1A, B). Among the aged rats only, place recognition performance was significantly worse than object recognition performance [F(1,9) = 7.826, p <.02], confirmed by a significant age × task interaction [F(1,18) = 9.697, p <.006] (Figure 1B, D). Furthermore, there were no significant differences between the place and the object recognition tasks in younger rats [F(1,9) = 3.920, p > .08], suggesting that the tasks were of equivalent difficulty in these rats but not in older animals (Figures 1A and 2C).
Figure 1.
Age-related impairment in hippocampal-dependent place recognition memory (PRM) in older rats is rescued by exercise. Young (n = 10) and aged (n = 10) animals were assessed on the object recognition memory (ORM) (A, B) and PRM (C, D) behavioral paradigms. These involved exposing the rat to two identical objects (A1, A2) and then either replacing one of them with a new object (B in A and B) or to moving one of the original objects to a new location (B in C and D). Exploration Ratio (chance performance indicative of recognition memory failure = .5) was computed as the amount of time spent exploring the novel object or place over the entire time spent exploring. Compared with young rats, aged rats were impaired in PRM but not ORM. (E) Behavioral timeline of exercise experiment. Animals were initially tested on a battery of behavioral tests before being allocated to running or standard housing conditions. Animals remained in these conditions for the remaining duration of the study. First follow-up (FU) testing occurred at 6 weeks post running/standard housing, and second FU testing started 2 weeks later. (F) Voluntary running increased PRM performance in aged rats from impaired levels to that of young animals. There was no effect of running on ORM in older rats or on either object or PRM in young rats. Error bars in all bar graphs depict SEM. LCT, localized cue task; MWM, Morris water maze.
Figure 2.
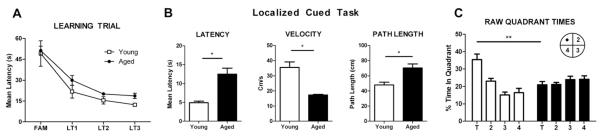
Performance in the hippocampal-dependent spatial version of the Morris water maze task. (A) Latencies of young (n = 10) and aged (n = 10) animals to locate a hidden platform over 3 training days averaged across trials. Performance was identical on the first day of training but was impaired in aged rats across subsequent training. (B) Significant age differences on a control paradigm, the localized cue task, were observed on all three outcome measures: latency to reach the visually signed platform, swim speed, and path length. (C) Average percentage of time within target (T) and nontarget quadrants (quadrants 2–4) on the delayed probe test in the absence of a platform. Aged animals spent a lower proportion of time in the quadrant where the platform used to be compared with young animals (raw means, left). *p <.05; **p <.001.
We next used the MWM, a classic hippocampally dependent spatial learning and memory task. Young and aged rats learned the location of the submerged platform, evidenced by a decrease in time taken to locate the hidden platform across trials [F(1,18) = 6.123, p <.05] (Figure 2A). Younger rats were faster at locating the platform during training and better at recalling this location on the delayed probe trial as evidenced by more time spent in the target quadrant than the older rats (p values <.05) (Figure 2A, C). To determine whether these age-associated differences were due to changes in spatial cognition rather than to other factors (e.g., motoric abilities), we used the localized cue task in which the submerged platform is clearly signposted. Younger rats reached the signposted platform more quickly than the aged rats, swam faster, and had shorter path lengths (p values <.05) (Figure 2B). After controlling for individual differences in performance in an analysis of covariance, there no longer remained any significant age differences on the probe trial in the spatial learning task (p > .05) (Figure S1 in Supplement 1). Our water maze data therefore could not unambiguously isolate age-associated effects on learning and spatial reference memory from other factors, such as sensory acuity, motor ability, or motivation. By contrast, place recognition memory was selectively sensitive to advanced age, an effect not seen in the object recognition paradigm, and therefore not a function of general age-related changes in other factors, including changes in exploratory behavior.
Voluntary Running Reverses Place Recognition Memory Deficits in Aged Animals
We next evaluated whether exercise improves recognition memory. Young and aged rats were randomly assigned to a 12-week voluntary running or standard housing condition, tested at baseline and after a first (6 weeks post baseline) and second (8 weeks post baseline) follow-up period. Our main observations with regard to age-specific effects on place recognition memory but not object recognition memory were replicated (Figure 1F, Figures S2 and S3 in Supplement 1). Additionally, both groups ran consistently throughout experimentation. Both young and aged rats ran in the wheels, with younger rats covering significantly more distance than aged rats when averaged across time [F(1,8) = 79.557, p = .0001] (Figure S4A in Supplement 1). However, at the end of exercise, there were no significant differences between the two groups (Figure S4B in Supplement 1).
Running improved place recognition memory performance at the first follow-up [F(1,16) = 15.466, p <.001] but had no effect on object recognition performance [F(1,16) = .145, p > .70] (Figure 1F). Accordingly, there was a significant age × exercise × task interaction [F(1,24) = 7.972, p <.009]. Furthermore, recovery of place recognition memory in older runners was stable, remaining elevated at the second follow-up test [F(1,16) = 1.210, p > .28]. Because object recognition was unaffected, non-cognitive effects on motivation, motor, and sensory functions were unlikely to have contributed to these results. Running therefore produced a strong and stable corrective effect on age-related place recognition memory dysfunction, independent of noncognitive factors. In contrast to our place recognition memory results, spatial learning and reference memory was not affected by exercise in aged animals (Figure S5 in Supplement 1).
Voluntary Running Rescues Hippocampal Presynaptic Density in Aged Animals
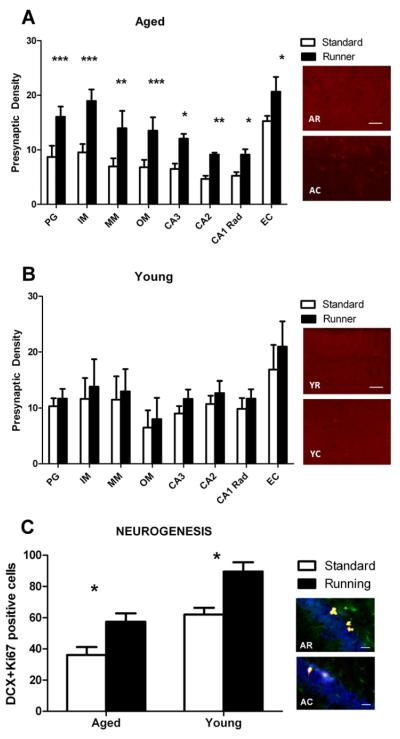
Synaptic loss is regarded as one of the primary bases for cognitive dysfunction in both normal age-related cognitive decline and Alzheimer’s disease (21). Accordingly, one mechanism that might account for improved hippocampal function is restoration of age-dependent loss of synapses. We therefore examined presynaptic density with synaptophysin immunocytochemistry (22–24). Aged nonrunners had lower synaptophysin density in comparison with younger nonrunners across most hippocampal subregions, including the dentate gyrus polymorph layer and inner molecular layer, CA3, CA2, and CA1 radiatum (p values <.05) (Figure 3A, B). By contrast, there were no significant age differences in nonrunners in the middle and outer molecular layers of the dentate gyrus (p values > .05). Running effectively counteracted this pattern of age-related presynaptic loss, increasing synaptophysin density in aged rats across all subfields of the hippocampus (p values <.05). Moreover, this was greatest in the dentate gyrus, where increases were to levels beyond those seen in younger animals (Supplement 1). In contrast, running did not alter presynaptic density estimates in any hippocampal subregion in younger rats (p values > .05).
Figure 3.
Running increases presynaptic density to supranormal levels in older rats and neurogenesis in both younger and older rats. Under standard housing conditions, densities were lower in aged rats (A) than younger animals (B) across most hippocampal subregions. Running increased synaptic density in aged rats only across many subregions of the hippocampus as well as the entorhinal cortex (EC). There was a significant linear trend for stronger effects nearest to the dentate gyrus, where levels were significantly higher than those in younger animals. Inserts show synaptophysin immunohistochemistry staining intensity in dentate gyrus polymorph layer in exemplar control subjects and runners. Scale = 50 μm. (C) Positive cells counted by co-expression of proliferation marker (Ki67) and doublecortin (DCX) antibody staining in the subgranular zone. Younger rats had higher baseline levels than older animals, and running increased levels of neurogenesis in both groups by similar relative amounts. Inserts show exemplar images. Scale = 20 μm. AC, aged control; AR, aged runner; IM, inner molecular layer; MM, middle molecular layer; OM, outer molecular layer; PG, dentate gyrus polymorph layer; YC, young control; YR, young runner. *p <.05; **p <.01; ***p <.005.
We also investigated the effect of age and exercise on presynaptic density in the entorhinal cortex, given that subparts of the molecular layer of the dentate gyrus receive and project to distinct areas of the brain, including the polymorphic layer and the medial and lateral entorhinal cortices. Under standard conditions, there were no differences between younger and older control rats (p <.716). By contrast, running significantly increased presynaptic density in the entorhinal cortex of older rats (p <.011) but not in younger rats (p <.076).
Voluntary Running Enhances Neurogenesis in Both Younger and Older Rats
Neurogenesis is another mechanism that might account for improved hippocampal function (25). To assess neurogenesis in the subgranular zone, we identified the number of triple-labeled Ki67 (proliferation marker), doublecortin (DCX, migrating neuroblast marker), and nuclear dapi-positive cells, an approach that closely matches bromodeoxyuridine (BrDU)-based results (26,27). Aged nonrunners had significantly less neurogenesis than younger non-runners (p <.004) (Figure 3C). Running increased neurogenesis in both younger and older rats (p values <.001). Importantly, the magnitude of the increase in young and older rats was similar, with neurogenesis in older runners restored to levels of younger non-running rats. Hippocampal neurogenesis did not, however, correlate with place or object recognition memory performance.
Voluntary Running Leads to Profound Changes in Hippocampal Presynaptic Connectivity
Next, we explored the impact of voluntary running on hippocampal mechanisms with graph-based network theory. We adapted a technique widely used to characterize dependencies between distributed human brain properties on magnetic resonance imaging (MRI), including regional white matter diffusivity (28) and localized functional MRI timeseries (29), where strong intercorrelations suggest a high level of structural or functional connectivity, respectively. This information can be summarized in the form of a correlational matrix that after thresholding can be expressed as a graph with discrete network properties (30). Presynaptic network characteristics were therefore assessed on the basis of synaptophysin covariance between hippocampal subregions.
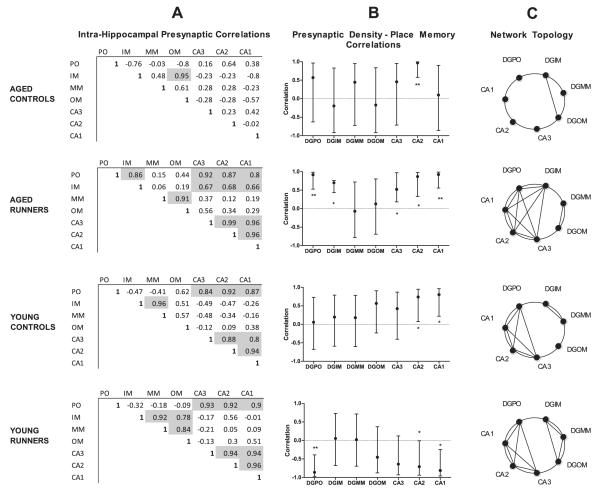
In the absence of running, subregional presynaptic densities were not strongly cross-correlated in old rats and moderately cross-correlated in younger rats (Figure 4A). Brain-behavior relationships were also examined: in old nonrunners, presynaptic density in CA2 was strongly correlated with place recognition memory performance (r2 = .932); and in younger nonrunners, both CA1 and CA2 (r2 = .832) were strongly correlated with that performance (Figure 4B). Twelve weeks of voluntary running profoundly altered this connectivity pattern. A test of synaptophysin correlational matrices in runners versus nonrunners found these to be more dissimilar than would be expected by chance (p <.05) (19).
Figure 4.
Running induces complex age-dependent synaptic connectivity changes in the hippocampus. (A) Correlation coefficients of synaptophysin intensity between seven intrahippocampal subregions in young and older animals, under standard or running conditions (n = 10 each). Shaded boxes indicate significant correlation after correction for multiple comparisons (p <.005). (B) Correlation coefficients (±95% confidence interval) between place recognition memory performance and presynaptic density in hippocampal subregions. Running increases the number of significant brain-behavior correlations in both age groups but in a negative direction in younger rats and in a positive direction in older rats, suggesting the role of different exercise-dependent mechanisms in older animals. (C) Synaptic topology is now graphically displayed as a nonweighted five-node network, where an edge connects nodes if a significant correlation exists between two hippocampal subregions. A cluster coefficient appears for each node, ranging from 0 to 1, where 1 indicates a maximal number of edges between that node and its neighbors. A global efficiency estimate is provided for each group, ranging from 0 to 1, where 1 suggests a theoretical maximal efficiency of information transfer between nodes. Running restored efficiency in both age groups. DGIM, dentate gyrus inner molecular layer; DGMM, dentate gyrus middle molecular layer; DGOM, dentate gyrus outer molecular layer; IM, inner molecular layer; MM, middle molecular layer; OM, outer molecular layer; PO, polymorph layer; DGPO, dentate gyrus polymorph layer. *p <.05; **p <.01.
Age modulated the impact of exercise on presynaptic connectivity. In younger rats, running increased intrahippocampal presynaptic connectivity from 7 to 9 significant cross-correlations (of a possible 21) (Figure 4A). By contrast, 11 cross-correlation pairs were significant among older runners, versus only 1 correlation pair in sedentary older animals. Interestingly, place recognition memory was positively correlated with density estimates across most hippocampal subregions among old runners only, in comparison with one–two regions in the aged and young nonrunners (Figure 4B).
Graphical analyses captured some of these complex exercise-dependent network changes (Figure 4C). Running decreased network path length while increasing clustering coefficients of each node, resulting in greater global efficiency in both young and older runners. This was most prominent in the older rats, where global efficiency increased from theoretical minimum (zero) among the nonrunners to intermediate (.34) after 12 weeks of voluntary running. In older humans, global efficiency based on structural connectivity measures across the whole brain declines with age, and individual differences on this metric are linked to specific cognitive abilities such as working memory and speed of information processing (28). For the first time in rodents, graphical network analysis found that aging was accompanied by a similar degradation of hippocampal network efficiency and that this network property can be restored by voluntary running.
Synaptic Predictors of Place Recognition Memory
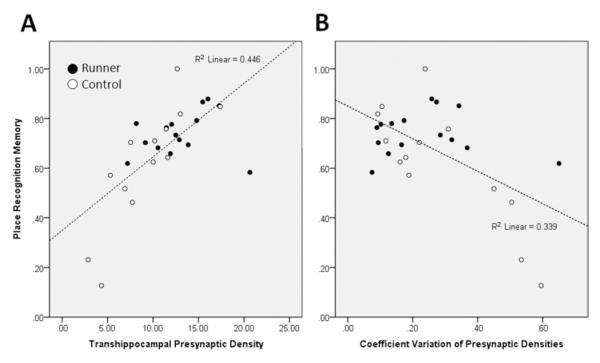
We pooled all data and used multiple regression analyses to test the relative contributions of presynaptic density, regional variation in presynaptic density, and neurogenesis to place recognition memory performance at an individual animal level. Within-animal regional variability was assessed by the coefficient of variation of hippocampal synaptophysin across subregions, and the overall average was used to indicate transhippocampal presynaptic density. A backward elimination procedure retained both transhippocampal presynaptic density (standardized β = .501) (Figure 5A) and presynaptic coefficient of variation (standardized β = −.32) (Figure 5B) in a model that accounted for a majority of place recognition memory variance (R2 = .52, p <.001). Overall, our voluntary running experiments suggest that the combined effect of boosting presynaptic densities across the hippocampus and fostering greater connectivity between these subregions might be critical to the reversal of place recognition memory deficits in aged animals.
Figure 5.
Overall hippocampal presynaptic density and regional uniformity predict place recognition memory. Scatterplots showing correlation between place recognition memory and (A) average hippocampal presynaptic density (p <.001) and (B) coefficient of variation of synaptic densities across five subregions (p = .001). Both features were found to independently predict place memory in a multiple regression analysis. Runners had higher overall hippocampal presynaptic density, less sub-regional variability, and superior memory performance.
Discussion
Aging produced a selective impairment in short-term place recognition memory, a deficit that was stable across repeated testing and highly correlated with loss of hippocampal presynaptic proteins. Moreover, 12 weeks of voluntary exercise commenced in old age reversed these behavioral and synaptic defects, in the latter case to beyond young adult levels. Exercise-dependent upregulation of neurogenesis was also found in both younger (6) and older animals (14) but was unrelated to recognition memory. We applied graphical network analyses to patterns of synaptic covariance in the rodent hippocampus and found that running fully restores impoverished intrahippocampal connectivity that is associated with old age. In fact, the combined impact of heightened presynaptic density throughout the hippocampus and minimal interregional variation after running was critical to predicting intact place recognition memory. Physical exercise therefore produces different effects on old and young brains.
In healthy elderly people, aerobic exercise over a 1-year period increases hippocampal volume and is accompanied by enhanced short-term spatial working memory (31). Precise cognitive outcomes are therefore critical to translating exercise studies between animals and humans. In control navigation tests, old rats took longer to reach a visually signaled platform, swam slower, and traversed a longer, more indirect route than younger ones. We therefore could not clearly separate age-related changes in spatial learning and memory in the MWM (32) from noncognitive age-related effects. Additionally, because our young female rats were at different stages of the ovarian hormonal cycle and cycle-related effects have been documented in the water maze (33), this might have contributed to behavioral variance in the young group and so decreased the likelihood of detecting age-related effects on this paradigm. In this context, it is difficult to interpret the lack of any definitive exercise-induced improvements in our old rats on this paradigm in comparison with previous reports (10,14).
By contrast, the short-term place recognition memory task was sensitive and specific to age-related change, was stable over repeated administrations, and we are not aware of any estrus-cycle-related effects. Place recognition performance not only declined with age but also exhibited an age-related increase in variability, replicating two cardinal findings from cognitive neuroscience studies of human aging (34,35). Age-related decline in place recognition www.sobp.org/journal memory also dissociated from age-invariant performance on object recognition. When lengthy delay intervals are used between the sample and test phases of the object recognition memory task (>20 min), aged animals show impaired performance (36,37) that might reflect hippocampal dysfunction (38). However, we used a short 5-min delay and, as previously reported (39), found no age differences on object recognition memory. Hence, at this short inter-stimulus period, place and object recognition memory dissociate with respect to aging and hippocampal dependency.
Twelve weeks of voluntary running rescued short-term place recognition memory deficits in an independent group of older animals and had no observable behavioral effect in younger animals or on object recognition memory. These improvements were reliable, being maintained at the second follow-up test. Given that we were able to discount the role of noncognitive factors, as object recognition was unaffected, we found that voluntary exercise reverses age-related deficits on a hippocampally dependent recognition memory task. These improvements occurred in the older rats but not younger animals, despite older rats running less overall. These results suggest that exercise exerts a disproportionate effect on older animals. It remains to be determined at an individual animal level how much running is required to rescue age-related place recognition memory deficits.
Neurobiological mechanisms potentially mediating these cognitive changes were investigated by focusing on neurogenesis and synaptogenesis. In the absence of exercise, we found that aging was associated with a significant reduction in neurogenesis, in accord with previous studies (14). Effects of advanced age on synaptic proteins are less clear. In 28-month-old male Fischer rats, electron microscopy found that the total number of axospinous synaptic contacts per granule cell is significantly diminished in the middle and inner molecular layer (40). Additionally, in 24-month-old male Wistar rats, electron microscopy found that the total number of axospinous, perforated, and nonperforated synapses decreased in the CA1 stratum radiatum (41). By contrast, no significant age differences were found with 24–28-month-old male Long-Evans rats with confocal microscopy in the inner, middle, or outer layers of the dentate gyrus. In our study of 20-month-old female Fisher rats, aging was associated with a reduction in presynaptic density across several hippocampal subregions, including the polymorph layer, CA1 (24,41,42), CA2, and CA3 (23), but was unchanged in the middle and outer molecular layers of the dentate gyrus (23). These competing findings might reflect differences in the precise age of the animals, gender, breed, hormonal cycle, and the nature of the synaptic assay (43).
Voluntary running counteracted both types of age-related neurobiological deficits. Neurogenesis levels were increased by 59% in aged runners, effectively restored back to levels of younger control animals. Such a large relative increase is greater than that observed previously and might be due to our extended running plus behavioral testing period (12 weeks) compared with prior studies (14). Because running also produced similar proportional increases in young rats (44%), neurogenesis in aged runners remained well below those of young runners, as seen previously (14). Yet the functional significance of exercise-induced neurogenesis in aged animals remains unclear. In one study, number of BrDU-labeled cells in the hippocampus correlated modestly with spatial separation performance in younger mice but was uncorrelated in older mice, possibly due to the inability of the extremely old mice (22 months) to complete the task or undergo exercise-induced neurogenesis (44). Another study in aged rats found that latency to a hidden platform was positively correlated with subgranule zone cell proliferation (45), whereas another study reported a negative correlation (46). We observed exercise-induced neurogenesis in both young and older animals but were unable to detect any correlations between neurogenesis and performance in the place or object recognition tasks. Further research using BrDU-based protocols is required to differentiate between potential functional differences between neurogenic cell proliferation, survival, and network integration.
Voluntary running also resulted in striking increases in presynaptic density in older animals, by up to 102%. By contrast, presynaptic density did not change in response to running in younger animals and so was higher in older runners than either young control subjects or young runners. Upregulation of presynaptic proteins also seemed to follow a topographic pattern. The greatest increase was in the polymorphic dentate gyrus, followed by a drop-off in response magnitude along the outgoing mossy fiber pathway. Interestingly, cortical input into the dentate gyrus originates mainly from the entorhinal cortex, where we also found evidence of presynaptic increases after running, as well as the middle and outer layers of the dentate gyrus molecular layer, one of the main targets of entorhinal projections.
Voluntary running therefore seemed to produce similar positive changes to both presynaptic density and place recognition memory, effects that might be linked through a strengthening of hippocampal connectivity. In network theory, enhanced global efficiency implies a better ability to integrate information and has been proposed to mediate cognitive function in humans (28,30). Interest has also recently turned to the impact of exercise on human brain connectivity, including hippocampal networks (47). Here, we translated this approach to hippocampal synaptic networks in rodents. In older control animals, hippocampal network efficiency fell to zero by virtue of only a single suprathreshold connection between the inner and outer molecular layer. Running overturned this age-related network degeneration, rescuing seven different hippocampal subregions in a coordinated manner. Global efficiency as well as place recognition memory was thereby fully restored by voluntary running in aged animals to that of younger animals. In fact, with a regression model we found that overall hippocampal synaptic densities as well as a more uniform distribution of densities between hippocampal subregions are dual and independent predictors of successful place recognition memory. Synaptic networks in the hippocampus might therefore have an important role in the mediation of age-related memory dysfunction as well as the therapeutic impact of physical exercise on the aged brain.
Electrophysiological in vivo studies of running provide a possible mechanistic link between exercise and hippocampal network function. The temporal and spatial dynamics of individual CA3 neuron firing patterns during running resemble those during place navigation but only when the two activities are repetitively interleaved (48). These firing patterns are more intense during running and can predict subsequent navigational choices, suggesting that working memory processes are active during an otherwise stimulus-free running task. In the absence of a place navigation task, running animals do not show this complex neuronal activity pattern. Animals in our experiment were between these two extremes, undertaking the place recognition memory paradigm, exercising, and then repeating the tests. During the intertest period, runners might have continued to process their newly learned spatial representation, accompanied by coordinated neural firing dynamics within CA3 as well as other hippocampal subfields, ultimately leading to the presynaptic changes that we observed. A major challenge for future work is to connect the established molecular machinery of exercise-dependent neuroplasticity—including neurotrophic cascades (49), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor expression (50), and induction of somatic factors (51,52)—to these complex systems-level phenomena.
In summary, our study found an age-related dissociation of short-term recognition memory, whereby place recognition memory was age-sensitive and linked to hippocampal presynaptic loss. Voluntary running rescues this specific memory defect in older animals. Although exercise stimulated neurogenesis irrespective of age, exercise-dependent increases in hippocampal presynaptic density, synaptic connectivity, and brain-behavior dependencies were isolated to older animals. Exercise might therefore have specialized effects on synaptic plasticity in the older brain.
Supplementary Material
Acknowledgments
This work was funded by the Australian New Zealand Mason Foundation, The Rebecca Cooper Medical Research Fund, the National Health and Medical Research Council of Australia Program Grant (#568969), and the National Institute of Aging Grant (AG00538). MJV is a National Health and Medical Research Council of Australia Career Development Fellow. JS is a recipient of an Australian Postgraduate Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Henriette van Praag and Margaret Morris for their helpful comments on an earlier version of this manuscript.
Footnotes
The authors declare no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Brayne C. The elephant in the room—healthy brains in later life, epidemiology and public health. Nat Rev Neurosci. 2007;8:233–239. doi: 10.1038/nrn2091. [DOI] [PubMed] [Google Scholar]
- 2.Larson EB. Prospects for delaying the rising tide of worldwide, late-life dementias. Int Psychogeriatr. 2010;22:1196–1202. doi: 10.1017/S1041610210001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarsland D, Sardahaee FS, Anderssen S, Ballard C. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health. 2010;14:386–395. doi: 10.1080/13607860903586136. [DOI] [PubMed] [Google Scholar]
- 4.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 5.Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: Learning from animal models. Alzheimers Dement. 2007;3:S30–S37. doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 8.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synapto-genesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci. 2008;28:10766–10771. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 11.Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, et al. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- 12.Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121:324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann H, Glenn MJ, Mumby DG. Consolidation of object-discrimination memory is independent of the hippocampus in rats. Exp Brain Res. 2007;180:755–764. doi: 10.1007/s00221-007-0895-2. [DOI] [PubMed] [Google Scholar]
- 17.Winters BD, Bussey TJ. Removal of cholinergic input to perirhinal cortex disrupts object recognition but not spatial working memory in the rat. Eur J Neurosci. 2005;21:2263–2270. doi: 10.1111/j.1460-9568.2005.04055.x. [DOI] [PubMed] [Google Scholar]
- 18.Paxions G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed Academic; San Diego: 1997. [Google Scholar]
- 19.Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O. Modeling the impact of lesions in the human brain. PLoS Comput Biol. 2009;5:e1000408. doi: 10.1371/journal.pcbi.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavoy A, Delacour J. Spatial but not object recognition is impaired by aging in rats. Physiol Behav. 1993;53:527–530. doi: 10.1016/0031-9384(93)90148-9. [DOI] [PubMed] [Google Scholar]
- 21.Scheff SW, Price DA. Synaptic pathology in Alzheimer’s disease: A review of ultrastructural studies. Neurobiol Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Yamasaki TR, Blurton-Jones M, Morrissette DA, Kitazawa M, Oddo S, LaFerla FM. Neural stem cells improve memory in an inducible mouse model of neuronal loss. J Neurosci. 2007;27:11925–11933. doi: 10.1523/JNEUROSCI.1627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb Cortex. 2005;15:571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- 27.Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 28.Wen W, Zhu W, He Y, Kochan NA, Reppermund S, Slavin MJ, et al. Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J Neurosci. 2011;31:1204–1212. doi: 10.1523/JNEUROSCI.4085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 31.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: A common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Hedden T, Gabrieli JD. Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 35.Morse CK. Does variability increase with age? An archival study of cognitive measures. Psychol Aging. 1993;8:156–164. doi: 10.1037//0882-7974.8.2.156. [DOI] [PubMed] [Google Scholar]
- 36.Pitsikas N, Rigamonti AE, Cella SG, Sakellaridis N, Muller EE. The nitric oxide donor molsidomine antagonizes age-related memory deficits in the rat. Neurobiol Aging. 2005;26:259–264. doi: 10.1016/j.neurobiolaging.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Vannucchi MG, Scali C, Kopf SR, Pepeu G, Casamenti F. Selective muscarinic antagonists differentially affect in vivo acetylcholine release and memory performances of young and aged rats. Neuroscience. 1997;79:837–846. doi: 10.1016/s0306-4522(97)00091-2. [DOI] [PubMed] [Google Scholar]
- 38.Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15:347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- 39.Winters BD, Bartko SJ, Saksida LM, Bussey TJ. Scopolamine infused into perirhinal cortex improves object recognition memory by blocking the acquisition of interfering object information. Learn Mem. 2007;14:590–596. doi: 10.1101/lm.634607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 41.Long LH, Liu RL, Wang F, Liu J, Hu ZL, Xie N, et al. Age-related synaptic changes in the CA1 stratum radiatum and spatial learning impairment in rats. Clin Exp Pharmacol Physiol. 2009;36:675–681. doi: 10.1111/j.1440-1681.2008.05132.x. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rummel J, Epp JR, Galea LA. Estradiol does not influence strategy choice but place strategy choice is associated with increased cell proliferation in the hippocampus of female rats. Horm Behav. 2010;58:582–590. doi: 10.1016/j.yhbeh.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 47.Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, et al. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Rao VR, Finkbeiner S. NMDA and AMPA receptors: Old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.