Abstract
Lentiviral vectors are widely used for the stable expression of genes and shRNA-mediated knockdown and are currently under development for clinical use in gene therapy. Pseudotyping of the vectors with VSV-G allows them to infect a wide range of cell-types. However, myeloid cells, such as dendritic cells and macrophages are relatively refractory to lentiviral vector transduction as a result of the myeloid-specific restriction factor, SAMHD1. SIVmac/HIV-2 and related viruses relieve the SAMHD1-mediated restriction by encoding Vpx, a virion-packaged accessory protein that induces the degradation of SAMHD1 upon infection. HIV-1 does not encode Vpx and cannot package the protein. We report the development of an HIV-1-based lentiviral vector in which the Vpx packaging motif has been placed in the p6 region of the Gag/Pol expression vector that is used to generate the lentiviral vector virions. The virions package Vpx in high copy number and infect myeloid cells with a two-log increase in titer. Transduction of dentritic cells with an shRNA against transportin-3 resulted in >90% knock-down of the encoding mRNA. The system can be applied to any HIV-based lentiviral vector and is useful for laboratory and clinical applications where the efficient transduction of myeloid cells is required.
Keywords: HIV, Vpx, dendritic cells, macrophages, lentiviral vectors, SAMHD1, host restriction
Introduction
Lentiviral vectors are widely used for the expression of proteins in mammalian cells in culture and in transgenic animals1. The vectors are currently being developed clinically to achieve stable long-term expression in vivo for the treatment of conditions including HIV-1 infection2, 3, human beta-thalassaemia4, X-linked adrenoleukodystrophy (ALD)5, 6, and B-cell malignancies7. Lentiviral vectors provide stable expression of transduced genes; they do not express any viral proteins; and, unlike murine leukemia virus-based gamma retroviral vectors, they can infect non-dividing cells. The vectors are resistant to silencing by Krüppel-associated box (KRAB) proteins8 and, as a result, are expressed in stem cells and have been useful in the generation of transgenic mice9. Lentiviral vector stocks are typically produced by cotransfection of 293T cells with a Gag/Pol expression vector and a lentiviral genome that contains both a packaging site and cDNA that encodes a protein of interest or an shRNA10. Inclusion of VSV-G expression vector in the transfection results in pseudotypes with titers >1 × 106 that can transduce a broad range of cultured and primary cell types11, 12.
Unlike the majority of cell-types, myeloid lineage cells, such as monocyte-derived macrophages (MDM) and monocyte-derived dendritic cells (DC), are relatively refractory to infection by VSV-G pseudotyped HIV-1, making transduction of these cells with HIV-1-based lentiviral vectors of limited use. The failure to efficiently infect these cells is caused by the myeloid-specific restriction factor, SAMHD1, that blocks infection at early reverse transcription 13, 14. SAMHD1 belongs to a family of related HD domain-containing DNA hydrolases and nucleases. The protein is a GTP-activated triphospho-deoxynucleoside hydrolase that dephosphorylates cellular dNTPs, decreasing their level to below that required to support reverse transcription15–17.
HIV-2 and viruses of the SIVsm lineage encode Vpx, an accessory protein that counteracts SAMHD113, 14, 18–21. Vpx is packaged into the virions as they assemble via an interaction with an amino acid motif in the p6 domain of Pr55gag22, 23. In the newly infected cell, virion-packaged Vpx binds to SAMHD1, inducing its degradation13, 14. The dNTP pool is then restored, and the block to reverse transcription is removed17. HIV-1 and HIV-1-based lentiviral vectors do not encode Vpx, but have Vpr, an accessory protein that is related in sequence to Vpx. However, Vpr does not have activity against SAMHD1 and only has a modest effect on myeloid cell infection24.
Despite the absence of Vpx in HIV-1, the virus is sensitive to SAMHD1-mediated restriction. If Vpx is provided to DC by pretreatment of the cells with virus-like particles (VLP) that contain Vpx, subsequent infection with HIV-1 or lentiviral vectors is markedly increased 19–21, 25. We previously reported on the production of chimeric HIV-1 virions that package Vpx. These were generated by transferring the p6 Vpx packaging motif (amino acids 17–26) of SIVmac239 to the corresponding position in HIV-1 Gag p6. Production of virions in cells cotransfected with Vpx expression vector and the modified Gag/Pol provirus resulted in the production of virions that contained Vpx in high copy numbers. The resulting virus was nearly 100-fold more infectious on DC than control virions that lacked Vpx 22.
Here, we used the chimeric p6 principle to generate Vpx-containing HIV-1 based lentiviral vector virions. We introduced the Vpx packaging motif of SIVmac239 p6 at amino acids 17–26 into an HIV-1 Gag/Pol packaging vector and used the modified plasmid to produce lentiviral vector virus stocks. The resulting virus was significantly more infectious on DC compared to the controls and induced stable production of vector-encoded protein. Additionally, the Vpx-containing virus allowed for efficient knockdown of an shRNA-targeted mRNA. The system can be used for any HIV-1 based lentiviral vector and could be applied to other lentiviruses to produce Vpx-containing virions, as diagrammed in Figure 1A.
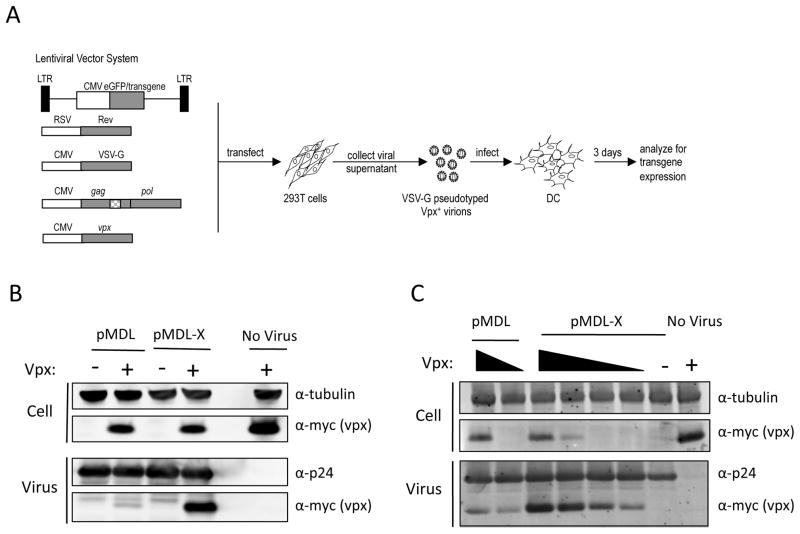
Fig. 1. Modified HIV-1 Gag/Pol expression vector generates virions that package SIV Vpx.
(A) To generate Vpx-cotaining lentiviral vector stock, 293T cells are transfected with a lentiviral vector plasmid, pMDL-X chimeric p6 HIV-1 Gag/pol expression vector and expression vectors for Vpx, VSV-G and HIV-1 Rev. The virus is normalized by infectious titer on 293T cells and used to infect DC differentiated with GM-CSF and IL-4. Approximate location of the inserted Vpx packaging motif is indicated in the Gag/Pol vector as cross-hatched (B) 293T cells were cotransfected with pMDL or pMDL-X containing wild-type (WT) or chimeric p6SIV(17–26), respectively and pcVpx.myc (+) or pcDNA (−). Two days later, cell lysates and virions were analyzed by immunoblotting. The immunoblot was probed with an antibody to myc-tagged Vpx, HIV-1 p24 CA, or tubulin. Transfection with pcVpx-myc.His alone was included in order to rule out nonspecific release of Vpx. (C) Cotransfection of 293T cells with pMDL or pMDL-X and decreasing amounts of pcVpx.myc. The amount of packaging plasmid harboring p6 remained constant, whereas the amount of pcVpx.myc decreased.
Results
A lentiviral vector system harboring chimeric Gag p6 efficiently packages Vpx into virions
We previously reported that transfer of the DNA sequence encoding Gag p6 amino acids 17–26 from SIVmac239 into the corresponding site in HIV-1 allowed for the production of virions that packaged SIV Vpx expressed in trans in transfected 293T cells22. The resulting virions counteract SAMHD1-mediated restriction and are able to efficiently transduce MDM and DC. Location of the Vpx packaging motif was chosen such that it did not disturb the neighboring PTAPP late domain motif and ALIX binding site and was not within the overlapping Gag open reading frame. Insertion of the Vpx packaging motif did not adversely affect Gag processing, virion production, or virus titer22. To establish an HIV-1 based lentiviral vector system that allows for the efficient transduction of myeloid cells, we introduced the Vpx packaging motif into the Gag p6 in the lentiviral packaging vector, pMDL26. The resulting plasmid, pMDL-X, encodes an NL4-3 derived Gag/Pol protein identical to the one we previously reported22.
To characterize the virions produced by the modified vector, we cotransfected 293T cells with either the wild-type or chimeric p6 Gag/Pol expression vector and the lentiviral vector plasmid pPGK-GFP that contains a GFP gene driven by the PGK promoter. Also included in the transfection were the myc-tagged SIVmac239 Vpx expression vector pcVpx.myc, VSV-G expression vector, and HIV-1 Rev expression vector. Rev was required for Rev-mediated enhancement of the gag/pol mRNA expression. After 48 h, cell culture supernatants were harvested and the virions were pelleted by ultracentrifugation. The cell and virion lysates were then analyzed on an immunoblot (Fig. 1B). Analysis of the cell lysates showed that Vpx was expressed at similar levels in each of the transfections. Analysis of the amount of capsid (CA) present in the virion lysates showed that the chimeric and wild-type Gag/Pol viruses were produced at comparable levels. As expected, the chimeric virus efficiently packaged Vpx while only a small amount of Vpx was present in lysate of the wild-type virus. Vpx was not detected in the supernatant of cells transfected with pcVpx.Myc lacking pMDL-X, demonstrating that Vpx detected in the virus-containing supernatant was virion-associated.
To determine the efficiency with which Vpx was packaged by the chimeric virions, we cotransfected 293T cells with decreasing amounts of pcVpx.myc and a constant amount of wild-type or chimeric Gag/Pol expression vector. Immunoblot analysis of the virions showed that wild-type virus packaged only a small amount of Vpx and that this did not increase much with the amount of transfected pcVpx.myc (Fig. 1C). In contrast, the chimeric virions packaged Vpx in an amount that correlated with the amount of pcVpx.myc transfected. Vpx packaging appeared to be efficient as cells that expressed Vpx at a level that could not be detected on the immunoblot produced virions that contained a readily detectable amount of Vpx. The amount of CA detected was similar for all of the viruses, demonstrating that Vpx did not affect virus production.
Vpx-containing lentiviral vectors efficiently infect DC
To test the ability of the Vpx-containing lentiviral vectors to transduce DC, we prepared VSV-G pseudotyped chimeric and wild-type lentiviral vector virions in which the lentiviral vector genome expressed a CMV-driven EGFP. To determine the effect of the amount of packaged Vpx on infectivity, virions were prepared from 293T cells transfected with a constant amount of pMDL-X and a decreasing amount of pcVpx.myc at a Gag/Pol:Vpx ratio of 1:1, 1:0.33, 1:0.11 and 1:0.037. We normalized the infectious titer of the viruses on 293T cells by determining the number of GFP transducing units per milliliter and then infected donor-derived DC at an MOI of 2. Three days later, the number of GFP+ cells was determined by flow cytometry (Fig. 2A). The results showed that wild-type viruses were poorly infectious on the DC, regardless of whether they had been produced in cells transfected with a large amount of pcVpx.myc. In contrast, the chimeric viruses that contained Vpx were significantly more infectious on the DC. Viruses that contained a low or intermediate amount of Vpx were the most infectious, having a titer about 30-fold higher than virus lacking Vpx. A further increase in the Vpx content reduced the titer by about 50%. Infection of DC derived from two additional donors further corroborated these findings (Fig. 2B).
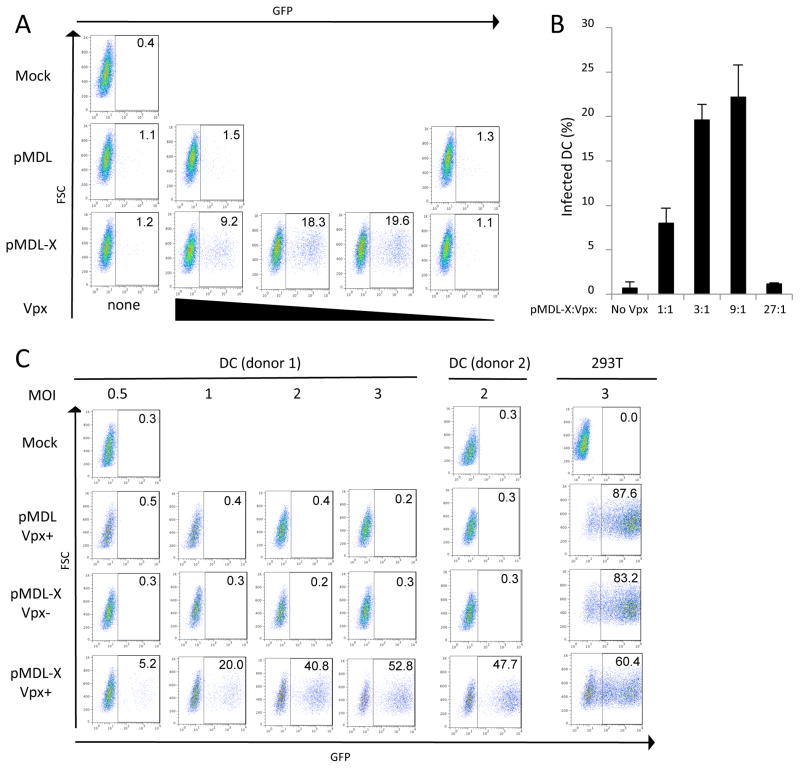
Fig. 2. Effect of Vpx packaging on infection of DC by pLenti-CMV-GFP.
(A) DC were infected at an MOI of 2 with the GFP reporter virus produced with pMDL or pMDL-X and decreasing amounts of pcVpx.myc. Whereas the amount of the packaging plasmid containing p6 remained constant, the amount of pcVpx.myc was decreased stepwise 3-fold (1/3, 1/9, 1/27). Three days post-infection, cells were collected and analyzed for GFP expression by FACS. (B) The graph represents the average result of two independent infections of DC derived from PBMCs of two different donors. (C) DC were infected at MOIs of 0.5, 1, 2 or 3 with a GFP reporter virus produced with pMDL-X and either pcVpx.myc or pcDNA. Three days post-infection, cells were collected and analyzed for GFP expression by FACS. The data shown are representative of four independent experiments with different donor DC. The panel on the right depicts 293T cells infected an MOI of 3 to assess the general infectivity of the viruses.
We next determined the extent to which the DC population could be infected with Vpx-containing lentiviral vector by varying the amount of viral inoculum. For this, we infected DC at an MOI of 0.5, 1, 2 and 3 with the wild-type or chimeric lentiviruses that contained the optimal amount of Vpx (produced at Gag/Pol:Vpx ratio of 1:0.3). Wild-type lentivirus cotransfected with Vpx did not detectably infect the cells (Fig. 2C). In contrast, chimeric lentivirus that contained Vpx infected the cells to an extent proportional to the MOI. Similar results were obtained with DC from a second donor (Fig. 2C). Parallel infection of 293T cells resulted in infections of 65%–84% transduction efficiency at MOI of 3. Infection of the DC by the Vpx-containing lentivirus nearly reached the infectivity on 293T, a cell-line that is highly permissive to VSV-G pseudotyped HIV-1. Of note, the chimeric virus containing Vpx was reproducibly less infectious on 293T cells than virus without Vpx, suggesting that in the absence of host restriction, Vpx interferes slightly with virus infectivity. This finding is similar to what we previously found for HIV-1 Vif, where in the absence of APOBEC3G, wild-type virus was slightly less infectious than Δvif virus 27.
Packaged Vpx is as effective as Vpx provided in VLP
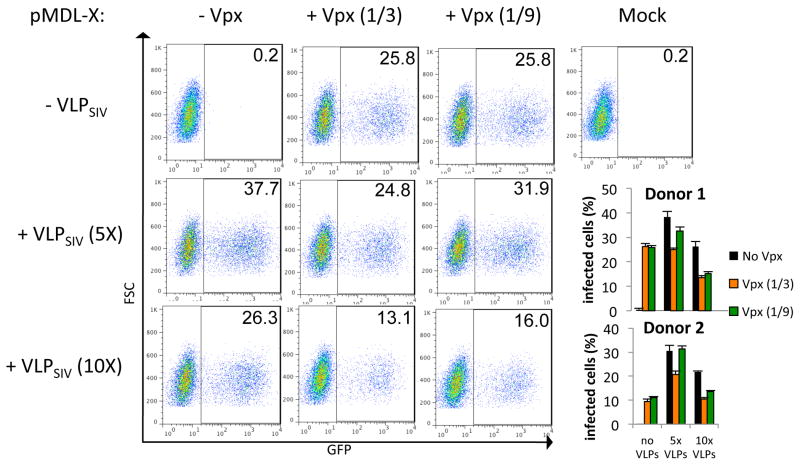
The ability of Vpx to enhance lentiviral transduction of DC was first shown in cells pretreated with Vpx-containing VLP and then infected with a lentiviral vector19–21, 25. This delivery differs from that of the chimeric lentiviral vector in which Vpx is introduced into the target cells directly through the Vpx-containing lentivirus. To determine whether delivery of Vpx by the virion itself was as effective as delivery by VLP, we compared the infection of DC by these two strategies. To do this, we pretreated DC with Vpx-containing VLP and, after two hours, infected the cells with Vpx-containing chimeric lentivirus. The amount of VLP corresponded to 5- or 10-fold more CA than that of the lentiviral vector. The results showed that pretreatment of the DC with 5X and 10X VLP increased the number of infected cells 188- and 131-fold, respectively, while transduction with Vpx-containing chimeric virus increased the number of infected cells 129-fold (Fig. 3). Introduction of Vpx into the cell by both VLP treatment and infection with Vpx-containing chimeric lentiviral virions did not further increase the number of infected cells. Instead, this combination caused a small reduction in the number of cells infected. Analysis of DC from two additional donors showed similar results (Fig. 3, lower right). Overall, these findings demonstrated that the chimeric lentivirus was comparable to VLP in providing enhanced DC infection. Furthermore, taking into account the fact that the VLP were used in large excess, the Vpx in the lentiviral vector virions was quite effective at promoting infection as it resulted in maximal infection with many fewer virions.
Fig. 3. Lentiviral vector that packages Vpx bypasses the need for VLP pre-treatment.
DC were infected at an MOI of 2 with the GFP reporter virus produced with pMDL-X and pcVpx.myc or pcDNA at a plasmid mass ratio of pMDL:pcVpx.myc 1:0.33 (1/3) and 1:01 (1/9), respectively. Where indicated, DC were treated with VLP for 2 h prior to infection with the virus at 5X or 10X p27 to p24, respectively. Three days post-infection, cells were collected and analyzed for GFP expression by FACS. The experiment was done in triplicate and the graphs depict the results of two experiments conducted with DC obtained from two different donors.
Efficient shRNA knock-down in DC by transduction with Vpx-containing lentiviral vector
mRNA knock-down in DC and MDM is technically challenging because of the low transfection efficiency of siRNA and low infectability by retrovirus and lentiviral vectors-expressing shRNA. Because of their ability to overcome the restriction to infection of myeloid cells, Vpx-containing chimeric lentiviral vectors might provide a means to achieve effective knock-down in these cells. As a proof-of-principle, we prepared a Vpx-containing lentiviral vector that expressed an shRNA targeting the mRNA that encodes transportin 3 (TNPO3). TNPO3 shRNA and a control scrambled shRNA in the pLKO.1-pgk-GFP lentiviral vector were packaged in Vpx-containing virions and then normalized for infectivity on 293T cells. We then infected DC from two donors with each virus. Analysis of the number of GFP+ cells by flow cytometry three days post-infection showed that these cells had been efficiently infected (Fig. 4 left). RNA was prepared from duplicate cultures, and the TNPO3 mRNA was quantified by quantitative real-time RT-PCR. The analysis showed that the TNPO3 mRNA levels were reduced to 25.4 % and 38.2 % when DC were transduced with the specific shRNA (Fig. 4, right) Considering that the lentiviral vectors had infected about 80% of the cells, the efficiency of knock-down per infected cell was 95% and 82%, respectively.
Fig. 4. Use of chimeric p6 lentiviral vector system for shRNA mediated knock-down of transportin 3 in DC.
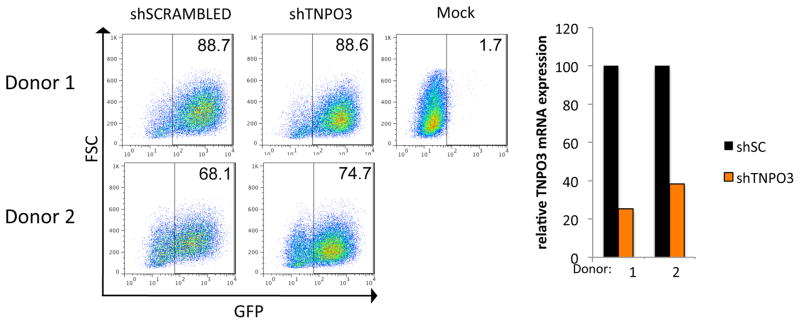
DC were infected at an MOI of 5 with pLKO.1-pGK-GFP, harboring an shRNA specific for TNPO3 or a nonspecific, scrambled shRNA control, as well as pMDL-X and pcVpx.myc. Three days post-infection, cells were collected and either analyzed for GFP expression by FACS (A) or for mRNA expression levels of TNPO3 (B). For the latter, RNA was prepared and the reverse transcripts were quantified by qRT-PCR using primers specific for TNPO3. The results were normalized to G6PDH and compared to DC infected with the control shRNA.
Discussion
We report here on a modified lentiviral packaging vector that packages the SIV Vpx accessory protein, thereby allowing for improved transduction of myeloid cells. This strategy is similar to that developed by Goujon et al., who used Vpx-containing VLP to treat DC prior to infection with lentiviral vector19–21, 25, 28. In our approach, Vpx is packaged in the lentiviral vector virions themselves, obviating the need for pretreatment with Vpx-containing VLP. This approach would allow the use of Vpx-boosted lentiviral vectors in vivo where VLP would not be feasible or desirable. Comparison of the two strategies showed that they achieved a comparable efficiency of transduction. The current system requires transfection of 293T cells with five different plasmids. However, it may be possible to streamline the system by establishing a packaging cell line that stably expresses some of the components.
The infectivity of the virions on myeloid cells was dependent on the amount of Vpx packaged. Virions that contained a relatively small amount of Vpx were the most infectious on DC, demonstrating the effectiveness with which Vpx counteracts SAMHD1-mediated restriction in myeloid cells. Moreover, the effect is likely boosted by the fact that when a cell becomes productively infected, it has been exposed to many virions, each of which can deliver its content of Vpx into the cytoplasm to contribute to the depletion of SAMHD1. It might have been expected that VLP treatment would be more effective than direct packaging of Vpx because the VLP are added prior to the virus. This would cause the effective depletion of SAMHD1 and an increase in the dNTP level upon infection. However, providing Vpx to the cell by pretreatment with Vpx-containing VLP did not further augment infection of the Vpx-containing virus. This finding suggests that the packaged Vpx was sufficient to fully relieve the SAMHD1-mediated block.
The Vpx-containing lentiviral vectors used in this report were VSV-G pseudotypes. As such, they infect DC and MDM cells but do not specifically target them. The vectors could be made more specific by pseudotyping with CCR5-tropic HIV-1 envelope glycoprotein, focusing them on CD4/CCR5+ cells. In addition, the Vpx-packaging lentiviral vectors reported here are based on HIV-1 but the approach could be applied to vectors based on other lentiviruses by introducing the Vpx packaging motif into their respective Gag protein.
Vpx-containing lentiviral vectors have potential applications for research and clinical use. In the laboratory, they offer the ability to efficiently knock-down a gene of interest or to stably transduce the expression of an exogenous protein in myeloid cells. In addition, the vectors offer the possibility of genome-wide shRNA screening of primary myeloid cells or cell-lines for viral dependency genes. Large-scale screens have not been feasible in these cells because of the difficulty of introducing shRNA or siRNA into them. The high transduction efficiency of Vpx-containing shRNA lentiviruses may be sufficient to make such screening possible. Clinically, Vpx-containing lentiviral vectors could be useful for vaccine strategies that require antigen expression in myeloid cells. DC are professional antigen presenting cells that play a central role in triggering adaptive and innate immune responses. They are relatively long-lived, offering the potential for sustained expression of transduced antigenic genes. A recent report in the vesicular stomatitis mouse model suggested that targeting of a subset of MDM is crucial for inducing a protective response to the infection 29. DC-based vaccines that are currently being evaluated in clinical trials are based on ex vivo pulsing of DC with tumor antigen peptide 30–32. Stable transduction of the cells using Vpx-containing lentivirus vectors might provide improved immunogenicity by stimulating a class I-restricted T cell response to the encoded antigen.
Materials and Methods
Cells and cell culture
293T cells were cultured in Dulbecco’s modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. DC were cultured in RPMI 1640/5% human AB serum and antibiotics. Peripheral blood mononuclear cells (PBMC) were purified from normal human donor blood by Ficoll density gradient centrifugation. Monocytes were purified from healthy donor PBMC by positive selection on anti-CD14-coated magnetic beads (Miltenyi Biotec Inc.) according to the manufacturer’s instruction. Bead purified monocytes were typically >98% CD14+. Monocytes were differentiated to DC by culturing for 5–6 days with 50 ng/ml GM-CSF (Invitrogen Inc.) and 100 ng/ml IL-4 (R&D Systems).
Plasmids
The codon-optimized SIVmac239 Vpx expression vector pcVpx.myc has been described previously22. The plasmid SIV3+ was used to produce SIV virus-like particles (VLP) and was described previously 21. The Gag/Pol p6 chimeric plasmid (pMDL-X) was generated by cloning the p6 region of the 17–26 HIV-1 p6 chimera we reported on previously into the AgeI and MfeI sites of pMDL22. pLenti (pLenti CMV GFP Puro33), pRSV-Rev, pMDL (wild-type p6 Gag/pol), and pVSV-G (pMD2.G) have been previously described34. The lentiviral vector pPGK.GFP (pCCL.PPT.hPGK.IRES.eGFP.Wpre) has been described previously 35. The pLKO.1-puro shRNA lentiviral vector that targets nucleotides 1956 to 1977 of TNPO336 was modified to replace the puror EGFP coding sequence taken from pEGFP-N1 (Clontech). To construct this plasmid, a DNA fragment containing the EGFP coding sequence was isolated by cleaving pEGFP-N1 with NotI, blunting with T4 DNA polymerase and then cleaving with BamHI. The EGFP fragment was then ligated to pLKO.1-shRNA DNA that had been cleaved with KpnI, blunted with T4 DNA polymerase and cleaved with BamHI to excise the puror.
Virus preparation and infection
Vpx-containing lentiviral vector stocks were produced by trans-complementation. Briefly, 5 × 106 293T cells in a 10 cm plate were cotransfected by the calcium phosphate method with 14 μg pLenti, 2.5 μg pRSV-Rev, 3.5 μg pVSV-G and 5 μg pMDL or pMDL-X. PcVpx.myc or pcDNA were added at a mass ratio of 3:1 to the Gag/Pol packing plasmid or as indicated. To generate VSV-G-pseudotyped shRNA knock-down virus, 293T cells were transfected with pRSV-Rev, pLKO.1 harboring the transportin 3 (TNPO3) specific shRNA or a scrambled shRNA, and pMDL-X and pcVpx.myc. To produce VLP, 293T cells were cotransfected with pSIV3+ and pVSV-G at a mass ratio of 2:1. The viral supernatants were harvested 48 h post-transfection, passed through a 0.45 μm pore-size filter, aliquoted, and frozen at −80°C. To determine the infectious titer, 1 × 105 293T cells were infected with 5 μl, 10 μl, or 20 μl of virus-containing supernatant. Two days later, the number of GFP+ cells was determined by flow cytometry. Virus titers were typically 3 × 106/ml with a p24 concentration of >300 ng/ml. VLP-containing supernatants were normalized for p27.
To infect the DC, the cells were seeded at 1.25 × 105 cells per well in a 96-well plate in 200 μL, in duplicate. The cells were infected the next day with virus normalized for infectious titer on 293T cells. After 20 h, the virus was removed. Two days later, the cells were removed from the plate using phosphate-buffered saline (PBS) containing 5 mM EDTA and fixed in 1% paraformaldehyde. The number of infected cells was quantified by flow cytometry.
Quantitative Real-time PCR (qRT-PCR)
To determine the efficiency of shRNA knock-down, 2.5 × 106 DC were infected with the shRNA vector at an MOI of 5. The cells were harvested 72 h post-infection and total RNA was prepared using TRIzol (Invitrogen Inc.). The RNA (250 ng) was reverse transcribed using the Roche high fidelity reverse transcription system and poly-dA primer. TNPO3 cDNA was quantified by qRT-PCR with an ABI Prism 7300 using SYBR green detection (Applied Biosystems) and TNPO3 forward (5′ CTGCTGCAGCCAAAGCCATTCATA) and TNPO3 reverse (5′ AGCAGCTTCTGGAGACAACAGGAA) primers. TNPO3 mRNA levels were compared in DC infected with the TNPO3-specific or scrambled shRNA vector. Glucose-6-phosphate dehydrogenase (G6PDH) was used as a reference gene.
Immunoblot analysis
293T cells were cotransfected with PGK.GFP, pRSV-Rev, pMDL or pMDL-X, and pcVpx.myc or pcDNA using lipofectamine 2000. Two days post-transfection, culture supernatants were filtered, pelleted through 20% sucrose at 100,000 × G for 20 min. at 4° C, and lysed in buffer containing 0.5% NP40. Cellular and viral proteins were detected by immunoblot analysis as previously described 24. The filters were probed with anti-myc MAb 9E10 (Covance), anti-p24 MAb #183-H12-SC (AIDS Research and Reference Reagent Program, NIH), and anti-α-tubulin (Sigma). The filters were hybridized with biotinylated goat anti-mouse immunoglobulin and Streptavidin DyLight 800 conjugate (Pierce) and imaged on an Odyssey Infrared Imaging System (LiCOR) at 800 nm.
Acknowledgments
We thank Antonia Follenzi for the pPGK.GFP plasmid and Kayleigh Taylor for technical assistance. The studies were funded by the National Institutes of Health (A1067059 and 5T32 A1007180). NRL is an Elizabeth Glazer Scientist of the Pediatric AIDS Foundation.
Footnotes
Conflict of Interest. The authors declare that they have no conflict of interest.
Literature Cited
- 1.Manilla P, Rebello T, Afable C, Lu X, Slepushkin V, Humeau LM, et al. Regulatory considerations for novel gene therapy products: a review of the process leading to the first clinical lentiviral vector. Human gene therapy. 2005;16(1):17–25. doi: 10.1089/hum.2005.16.17. [DOI] [PubMed] [Google Scholar]
- 2.Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 3.Porter DL, Kalos M, Zheng Z, Levine B, June C. Chimeric Antigen Receptor Therapy for B-cell Malignancies. J Cancer. 2011;2:331–2. doi: 10.7150/jca.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467(7313):318–22. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 6.Cartier N, Hacein-Bey-Abina S, Von Kalle C, Bougneres P, Fischer A, Cavazzana-Calvo M, et al. Gene therapy of x-linked adrenoleukodystrophy using hematopoietic stem cells and a lentiviral vector. Bull Acad Natl Med. 2010;194(2):255–64. discussion 264–8. [PubMed] [Google Scholar]
- 7.Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17372–7. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herchenröder O, Hahne JC, Meyer WK, Thiesen HJ, Schneider J. Repression of the human immunodeficiency virus type 1 promoter by the human KRAB domain results in inhibition of virus production. Biochim Biophys Acta. 1999;1445(2):216–23. doi: 10.1016/s0167-4781(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 9.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–72. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 10.Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol. 1998;9(5):457–63. doi: 10.1016/s0958-1669(98)80029-3. [DOI] [PubMed] [Google Scholar]
- 11.Spector DH, Wade E, Wright DA, Koval V, Clark C, Jaquish D, et al. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol. 1990;64(5):2298–308. doi: 10.1128/jvi.64.5.2298-2308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu ZH, Chen SS, Huang AS. Phenotypic mixing between human immunodeficiency virus and vesicular stomatitis virus or herpes simplex virus. J Acquir Immune Defic Syndr. 1990;3(3):215–9. [PubMed] [Google Scholar]
- 13.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474(7353):658–61. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474(7353):654–7. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011 doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 16.Powell RD, Holland PJ, Hollis T, Perrino FW. The Aicardi-Goutieres syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J Biol Chem. 2011 doi: 10.1074/jbc.C111.317628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nature immunology. 2012;13(6):621. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laguette N, Benkirane M. How SAMHD1 changes our view of viral restriction. Trends Immunol. 2012;33(1):26–33. doi: 10.1016/j.it.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goujon C, Riviere L, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, et al. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology. 2007;4:2. doi: 10.1186/1742-4690-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goujon C, Arfi V, Pertel T, Luban J, Lienard J, Rigal D, et al. Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J Virol. 2008;82(24):12335–45. doi: 10.1128/JVI.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC) Gene Ther. 2006;13(12):991–4. doi: 10.1038/sj.gt.3302753. [DOI] [PubMed] [Google Scholar]
- 22.Sunseri N, O’Brien M, Bhardwaj N, Landau NR. Human immunodeficiency virus type 1 modified to package Simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells. J Virol. 2011;85(13):6263–74. doi: 10.1128/JVI.00346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Conway JA, Kim J, Kappes JC. Localization of the Vpx packaging signal within the C terminus of the human immunodeficiency virus type 2 Gag precursor protein. J Virol. 1994;68(10):6161–9. doi: 10.1128/jvi.68.10.6161-6169.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gramberg T, Sunseri N, Landau NR. Evidence for an activation domain at the amino terminus of simian immunodeficiency virus Vpx. J Virol. 2010;84(3):1387–96. doi: 10.1128/JVI.01437-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger G, Durand S, Goujon C, Nguyen XN, Cordeil S, Darlix JL, et al. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat Protoc. 2011;6(6):806–16. doi: 10.1038/nprot.2011.327. [DOI] [PubMed] [Google Scholar]
- 26.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–7. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 27.Schrofelbauer B, Chen D, Landau NR. A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif) Proceedings of the National Academy of Sciences of the United States of America. 2004;101(11):3927–32. doi: 10.1073/pnas.0307132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. Heterologous human immunodeficiency virus type 1 lentiviral vectors packaging a simian immunodeficiency virus-derived genome display a specific postentry transduction defect in dendritic cells. J Virol. 2003;77(17):9295–304. doi: 10.1128/JVI.77.17.9295-9304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M, et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nature immunology. 2012;13(1):51–7. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Koldovsky U, Xu S, Mick R, Roses R, Fitzpatrick E, et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer. 2012 doi: 10.1002/cncr.26734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler CJ, Black KL. DCVax-Brain and DC vaccines in the treatment of GBM. Expert opinion on investigational drugs. 2009;18(4):509–19. doi: 10.1517/13543780902841951. [DOI] [PubMed] [Google Scholar]
- 32.Trepiakas R, Berntsen A, Hadrup SR, Bjorn J, Geertsen PF, Straten PT, et al. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. 2010;12(6):721–34. doi: 10.3109/14653241003774045. [DOI] [PubMed] [Google Scholar]
- 33.Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS ONE. 2009;4(8):e6529. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72(11):8463–71. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nature genetics. 2000;25(2):217–22. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 36.Logue EC, Taylor KT, Goff PH, Landau NR. The cargo-binding domain of transportin 3 is required for lentivirus nuclear import. J Virol. 2011;85(24):12950–61. doi: 10.1128/JVI.05384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]