Abstract
SUMMARY
Inflammation due to Shigella flexneri can cause damage to the colonic mucosa and cell death by necrosis and apoptosis. This bacteria can reach the bloodstream in this way, and the liver through portal veins. Hypoxia is a condition present in many human diseases, and it may induce bacterial translocation from intestinal lumen. We studied the ability of S. flexneri to invade rat hepatocytes and Caco-2 cells both in normoxic and hypoxic microenvironments, as well as morphological and physiological alterations in these cells after infection under hypoxia. We used the primary culture of rat hepatocytes as a model of study. We analyzed the following parameters in normoxic and hypoxic conditions: morphology, cell viability, bacterial recovery and lactate dehydrogenase (LDH) released. The results showed that there were fewer bacteria within the Caco-2 cells than in hepatocytes in normoxic and hypoxic conditions. We observed that the higher the multiplicity of infection (MOI) the greater the bacterial recovery in hepatocytes. The hypoxic condition decreased the bacterial recovery in hepatocytes. The cytotoxicity evaluated by LDH released by cells was significantly higher in cells submitted to hypoxia than normoxia. Caco-2 cells in normoxia released 63% more LDH than hepatocytes. LDH increased 164% when hepatocytes were submitted to hypoxia and just 21% when Caco-2 cells were in the same condition. The apoptosis evaluated by Tunel was significantly higher in cells submitted to hypoxia than normoxia. When comparing hypoxic cells, we obtained more apoptotic hepatocytes than apoptotic Caco-2 cells. Concluding our results contribute to a better knowledge of interactions between studied cells and Shigella flexneri. These data may be useful in the future to define strategies to combat this virulent pathogen.
Keywords: Shigella flexneri, Hepatocytes, Caco-2 cells, Hypoxia, Apoptosis
Abstract
RESUMO
A inflamação causada por Shigella flexneri pode causar danos à mucosa do cólon e morte celular por necrose e apoptose. Esta bactéria pode atingir a corrente sanguínea por esta via e o fígado através da veia porta. A hipóxia é uma condição presente em muitas doenças humanas, podendo induzir a translocação bacteriana a partir do lúmen intestinal. Nós estudamos a capacidade de S. flexneri invadir hepatócitos de rato e células Caco-2 nos microambientes de normóxia e hipóxia, bem como as alterações morfológicas e fisiológicas dessas células após a infecção sob hipóxia. Utilizamos a cultura primária de hepatócitos de ratos como modelo de estudo. Nós analisamos os seguintes parâmetros em condições de normóxia e hipóxia: morfologia, viabilidade celular, recuperação bacteriana e liberação de lactato desidrogenase (LDH). Os resultados mostraram menor quantidade de bactérias dentro das células Caco-2 do que em hepatócitos em condições de normóxia e hipóxia. Nós observamos que quanto maior foi a multiplicidade de infecção (MOI), maior também foi a recuperação bacteriana em hepatócitos. A condição hipóxica foi capaz de diminuir a recuperação de bactérias dos hepatócitos. A citotoxicidade avaliada pela liberação de LDH foi significativamente maior em células submetidas à hipóxia do que normóxia. As células Caco-2 em normóxia produziram 63% mais LDH do que os hepatócitos. O LDH aumentou 164% quando os hepatócitos foram submetidos à hipoxia e apenas 21% quando as células Caco-2 estavam na mesma condição. A apoptose avaliada por TUNEL foi significativamente maior em células submetidas à hipóxia que normóxia. Quando comparamos células hipóxicas houve mais apoptose entre hepatócitos do que nas células Caco-2. Concluindo, nossos resultados contribuem para um melhor conhecimento das interações entre as células estudadas e S. flexneri. Estes dados podem ser úteis no futuro, para definir estratégias de combate a este patógeno virulento.
INTRODUCTION
Shigella flexneri (S. flexneri) is a gram-negative enterobacteria responsible for bacillary dysentery in humans. After oral ingestion, these bacteria reach the colon and may invade the intestinal barrier, mainly via translocation across M-cells of the Peyer's patches25. Then, bacterial translocation occurs through mesenteric lymph nodes or portal veins allowing bacterial access to liver16 24.
Many study models have been used for S. flexneri research as intragastric infection in guinea pigs and i.v. rabbit infection. These studies contributed to knowledge of Shigella sp. dissemination mechanisms as well as the recovery rates of these bacteria from the liver2 7.
Inflammatory processes present in atherosclerotic disease, dermal wounds, cardiovascular disorders, and microbial infections are implicated in the formation of tissue hypoxic areas3. Several processes can result in a hypoxic microenvironment, such as increased metabolic demand for leukocytes, microcirculation impairment and microorganism proliferation15 20.
Intestinal hypoxia and reperfusion increase bacterial and endotoxine translocation and diminish their hepatic clearance14.
Furthermore, hypoxia can also be present in many liver diseases such as tumors, chronic hepatitis and cirrhosis, leading to morphological and functional alterations of the hepatocytes4 21 22. Hepatitis after Shigella spp. infection has also been reported in humans, although we cannot exclude other causes of hepatitis in this study30.
Most of the research of S. flexneri enterocyte invasiveness in vitro was made in intestinal epithelial cells lineages like Caco-2 or T848 34. The Caco-2 cell line is a continuous line of heterogeneous human epithelial colorectal adenocarcinoma cells that can be invaded by intestinal bacteria, among them S. flexneri 9 19. S. flexneri has the powerful type III secretion system used to penetrate nonphagocitic cell types as enterocytes and hepatocytes5 10 19.
There is no animal model that fully mimics the pathogenesis of shigellosis in humans. Therefore, alternative systems have been applied to study shigellosis, like the murine pulmonary model after intranasal administration of bacteria and the intestinal infection of the newborn mice18. The only model that mimics natural shigellosis in humans is the intragastric infection of Macacus rhesus. However, the scarcity of non-human primates and their cost creates difficulties in shigellosis studying18. For this reason, other study models were needed to advance the research in shigellosis. Our research group has then developed a experimental model of bacterial infection of primary rat hepatocytes cultured26.
This study has addressed the effect of hypoxia on hepatocytes and Caco-2 cells susceptibility to S. flexneri infection. The results showed that our model of cellular infection was effective to study the mechanisms underlying Shigella's infection.
MATERIAL AND METHODS
1. Primary culture of rat hepatocytes: Newborn female Wistar rats were obtained from the Central Bioterium of the Faculty of Medicine - University of São Paulo (USP), in a number of 15 rats per assay. The animals were anesthetized with CO2 before surgical procedures. The livers were excised using an aseptic technique under biological safety cabinet as described previously26. The material was then transferred to 24-well plates (Corning) previously covered with 50 µg/mL laminin (Sigma). Cells were incubated with Williams' E medium (Sigma) containing 2% of fetal calf serum (Cultilab) enriched with several supplements as described previously26. Cells were placed in an incubator at 37 °C in a humidified atmosphere of 5% CO2 in air, and used after 7-8 days of culture. The study design was approved by the Research Ethics Committee of the institution.
2. Culture of Caco-2 cells line: Caco-2 cell line was kindly provided by Dr. André Zonetti de Arruda Leite from Gastroenterology Laboratory (LIM-07) of the Faculty of Medicine - University of São Paulo (USP). Cells were incubated with DMEM medium (Sigma) containing 20% of fetal bovine serum (Cultilab). After this, Caco-2 cells were placed in an incubator at 37 °C in a humidified atmosphere of 5% CO2 in air and used after 7-8 days of culture.
3. Hypoxic conditions: Both hepatocytes and Caco-2 cells were cultured for 7-8 days. The experimental groups are referred to as hypoxia (H) and normoxia (N). Another two experimental groups were formed: “normoxia + infection” with cells cultured for 7-8 days and then infected with S. flexneri in multiplicity of infection (MOI) 5, 50 and 500, and “hypoxia + infection” with cells cultured for 7-8 days submitted to hypoxia for 24h and then infected with S. flexneri in MOIs 5, 50 and 500. The normoxia group was placed in an incubator at 37 °C. The hypoxia groups were placed into a gas-tight modular chamber (Billups-Rothenberg, Del Mar, CA) for 24h. The chamber was gassed for 30 min at a flow rate of 10 liters/min using certified gases containing N2 (White Martins - São Paulo, Brazil) and placed in an incubator at 37 °C. The oxygen concentration in cell cultures under hypoxic was < 1%, measured at a gas meter apparatus (Radiometer ABL800 Flex, Denmark).
4. Bacterial invasion and recovery assay: Shigella flexneri 2a strain (NCTC 9729) was grown in Brain Heart Infusion medium (Difco) for 16h at 37 °C. Cells cultured in 24-well plates (Corning) at 1x105 cells/mL were infected with S. flexneri at MOIs 5, 50 and 500, one h for hepatocytes and three h for Caco-2 cells, at 37 °C with previous centrifugation of cells/bacteria at 1000 rpm for 10 min. Cells were incubated with 50 µg/mL gentamicin (Sigma) for one h at 37 °C, followed by PBS washing and later lyses with Triton X-100 (Sigma) 1% during five min for intracellular bacteria recovery. The number of viable intracellular bacteria was determined by plating serial dilutions of the cells lysates on blood agar and CFU counting. The number obtained was divided by the well's area of the cell culture.
5. Hematoxylin-eosin staining: Cells monolayers grown on glass coverslips were washed three times with PBS, fixed with methanol (Merck) for two min and then hydrated in a graded series of alcohol (Merck) for five min. Subsequently, the coverslips were stained with hematoxylin (Sigma) for 20 min and then washed with tap water. After this they were stained with eosin (Sigma) for 20 min, washed with distilled water and dehydrated in a graded series of alcohol, immersed in xylenol (Merck) and mounted in Canada balsam (Vetec). Images were analyzed in an optical microscope with digital image capture system (Leica)26.
6. Viability assay: Hepatocytes and Caco-2 cells cultures in normoxic and hypoxic conditions were washed three times with PBS, and detached from wells using Trypsin-EDTA solution. The cells' suspensions were centrifuged at 1500 rpm for five minutes and the supernatants were removed. The cell pellets were resuspended in 1 mL PBS. After this the cells were stained with Trypan blue and counted in a Neubauer chamber.
7. Detection of the LDH released: Detection of LDH (normoxic and hypoxic cells) released was made by specific colorimetric kit (Sigma). The assays were performed in two steps: 1) without cell lysis to measure the LDH released; 2) with cell lysis to measure the total LDH (released + intracellular). The steps were carried out in the following sequence:
Step 1: The cell cultures in 24-well plates (Corning) were centrifuged at 1100 rpm for four minutes and then an aliquot of 50 µL from each well was transferred to 96-well plates.
Step 2: We added 1/10 lysis buffer in each well of 24-well plates (kit), followed by incubation at 37 °C for 45 minutes. After this, the plate was centrifuged at 1100 rpm for four minutes and then an aliquot of 50 µL from each well was transferred in 96-well plates.
The enzyme dosage was performed to both types of samples (LDH released and LDH total). We added 100 µL of solution in each well containing equal volume of substrate, cofactor and dye. The plate was then incubated for 30 minutes at room temperature protected from light. The reaction was blocked by adding 15 µL of HCl 1M to each well, then readings were taken with a spectrophotometer at the wavelengths of 490 nm and 690 nm. The results were obtained by subtracting the result of the wavelength of 490 nm wavelength from that of the wavelength of 690 nm; this was carried out using specific software (Soft max Pro).
Data analysis were performed by calculating the percentage of cytotoxicity according to the formula:
 |
LDH released: absorbance result of non-lysed cells. LDH total: absorbance result of cell lysates.
8. Apoptosis detection: Detection of fragmented DNA (TUNEL technique) was assayed using Fragment End Labeling Kit (FragEL kit; Calbiochem, Oncogene Research Products, Cambridge, MA, USA), in accordance with the manufacturer's instructions. Hepatocytes and Caco-2 cells were cultured in glass cover slips and a total of 10 successive fields were counted in fluorescence microscope (Leica).
9. Statistical analysis: All the experiments were repeated at least ten times, and the Kruskal-Wallis test along with the Student Newman Keuls post-hoc test were used for statistical analysis. The alpha risk was considered statistically significant when p ≤ 0.05.
RESULTS
1. Cell viability: The cellular viability data after invasion by S. flexneri can be seen in Figure 1. Caco-2 cells submitted to hypoxia presented significant difference when compared to Caco-2 cells in normoxia only at MOI 500. In all other experimental conditions no significant differences were observed.
Fig. 1. Evaluation of Hepatocytes (HEP) and Caco-2 cells (CACO) viability under hypoxia (H) or normoxia (N) submitted to infection by S. flexneri in MOIs 5, 50 and 500. (*= p ≤ 0.001).
2. Oxygen consumption: We found that Caco-2 cells consumed oxygen in a quantity significantly greater than hepatocytes in normoxic conditions (Fig. 2). In contrast, hepatocytes consumed more oxygen than Caco-2 cells under hypoxia. Apparently Caco-2 cells were able to drastically reduce their intake of oxygen at nearly undetectable levels under hypoxia.
Fig. 2. Oxygen consumption of hepatocytes and Caco-2 under normoxia and hypoxia conditions (*= p ≤ 0.001).
3. Bacterial recovery: The minimum time required for bacterial invasion was one hour for hepatocytes and three hours for Caco-2 cells. The results showed that bacterial invasion was lower in Caco-2 cells than in hepatocytes in both normoxic and hypoxic conditions (Fig. 3).
Fig. 3. Bacterial recovery from Caco-2 cells (CACO) and hepatocytes (HEP) under normoxia (N) or hypoxia (H) submitted to infection by S. flexneri in MOIs 5, 50 and 500 (*= p ≤ 0.001) (CACO-N5/50/500 versus HEP-N5/50/500, CACO-H500 versus HEP-H500; HEP-H5 versus H500, H5 versus N5, H50 versus H500, H50 versus N50, N5 versus N500, N5 versus N50, N50 versus N500, H500 versus N500).
We observed that the higher the MOI the greater the bacterial recovery's rate in the hepatocytes. The hypoxic condition reduced the bacterial recovery in comparison to normoxic condition in these cells. We also found that there was no significant difference among Caco-2 groups (normoxia and hypoxia).
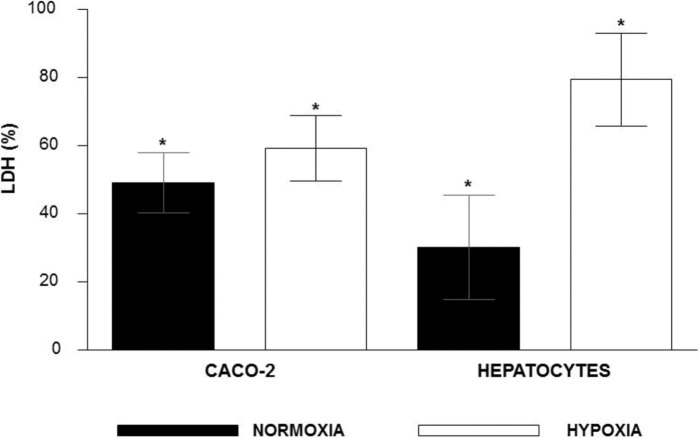
4. Evaluation of cytotoxicity: Percentage of cytotoxicity can be seen in Figure 4. The rates of LDH released in normoxic condition were higher in Caco-2 cells, while in hypoxic condition the hepatocytes produced the highest rates of LDH. In both cell types studied there was higher LDH released in hypoxia condition than in normoxia. There were significant differences in all comparisons.
Fig. 4. Percentage of hepatocytes and Caco-2 cells cytotoxicity under hypoxic and normoxic atmosphere conditions according the release of lactate dehydrogenase from cells (* = p ≤ 0.001).
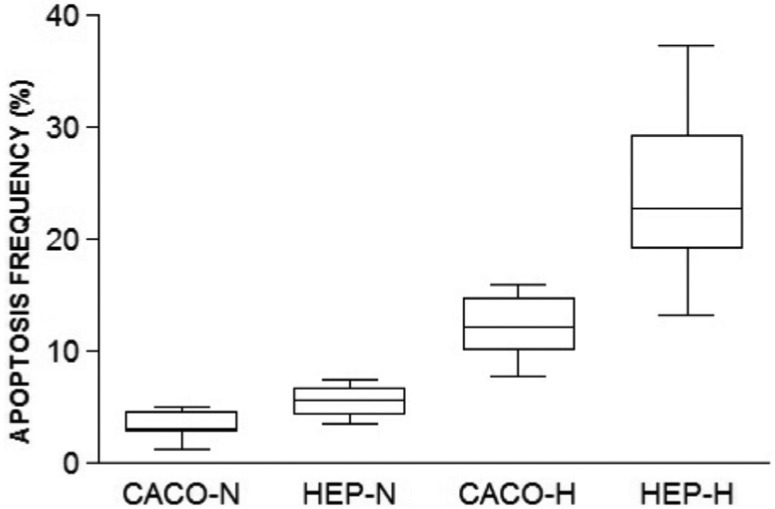
5. Apoptosis quantification: Hypoxic hepatocytes and Caco-2 cells groups had higher apoptosis rates when compared to normoxic control groups. There was a significant difference in apoptosis rate among normoxic and hypoxic groups and Caco-2 cells and hepatocytes both hypoxic (Fig. 6).
Fig. 6. Percentage of apoptotic cells cultured under normoxic or hypoxic conditions. (p < 0.005, all comparisons except Caco-N × Hep-N).
6. Morphological analysis: Normoxic hepatocyte cultures presented cells arranged in monolayer with wide cell junctions, while cells submitted to 24h hypoxia showed piknotic nuclei as well as monolayer ruptures (Fig. 5). Caco-2 cells cultured in normoxic condition presented a confluent monolayer. These cells were larger than hepatocytes and they had close contact with each other. When submitted to hypoxia, Caco-2 monolayer showed ruptures as well as piknotic nuclei.
Fig. 5. Caco-2 cells in Normoxia (A) and Hypoxia (B); Hepatocytes in Normoxia (C) and Hypoxia (D). The arrows indicate piknotic nuclei and monolayer disruption (X400).

DISCUSSION
Hepatocytes and Caco-2 cells were able to maintain their viability (ranging between 74-87%) in order to allow the experimental assessment. Although hypoxia reduces cell proliferation and increases cell damage33, we observed that hypoxia alone doesn't decrease cell viability significantly. Only in the Caco-2 cells group infected at MOI 500 there was a significant decrease in the Caco-2's viability in hypoxia condition compared to normoxia. There was no difference between hepatocytes and Caco-2 viabilities in all other groups. These results suggest that the presence of bacteria in different concentrations did not bring significant changes in hepatocytes and Caco-2 viability.
O2 homeostasis has evolved into a complex system to regulate O2 delivery27. In our study we observed that Caco-2 cells consumed more oxygen than hepatocytes in normoxic condition (Fig. 3). In contrast hepatocytes consumed more oxygen than Caco-2 cells under hypoxia. These results suggest that Caco-2 cells are more adapted to hypoxic environments. We speculate that their tumoral origin is the explanation for this phenomenon. The selection of hypoxia-resistant cells usually occurs in tumor tissues28.
S. flexneri required less time to invade hepatocytes (one hour) than Caco-2 cells (three hours). This period was lower when compared to other intestinal tumoral lineages with similar bacterial recovery rate18. We also observed that S. flexneri were less able to invade hepatocytes under hypoxic conditions. This result is similar to that obtained by other authors with other pathogens and cells types6 23 29. Hypoxia can modify gene expression, intra and extracellular pH as well as membrane receptors1 12 32. These changes may disturb the bacterial invasion both in hepatocytes and Caco-2.
The results about bacterial recovery from cells studied showed that there were fewer bacteria within the Caco-2 cells than in hepatocytes on normoxia and hypoxia conditions (Fig. 4). There were significant differences in bacterial recovery rate among hepatocytes groups, as well as among hepatocytes and Caco-2 groups. We also observed that the higher the MOI the greater the bacterial recovery from hepatocytes. The hypoxic condition decreased the bacterial recovery in hepatocytes. Structural alterations of the cells under hypoxia probably obstruct the entry of bacteria. There is evidence that Shigella sp undergoes adjustments against hypoxia. MARTEYN et al. 17 showed that S. flexneri have the ability to detect oxygen varying tensions, which allows T3SS activation enhancing invasion and virulence of this bacteria. This bacterial adaptation could not overcome the structural alteration of the cells.
Cytotoxicity evaluated by LDH released was significantly higher in cells submitted to hypoxia than normoxia (Fig. 4). These results are in agreement with other studies with human hepatocytes, cardiomyocytes and mesenchymal cells31. We also observed that Caco-2 cells in normoxia produced 63% more LDH than hepatocytes. On the other hand hepatocytes produced 34% more LDH than Caco-2 cells under hypoxia. LDH increased 164% when hepatocytes were submitted to hypoxia and just 21% when Caco-2 cells were in the same condition. The percentage of LDH released is a cytotoxic index widely used in studies of hepatocyte injury, primarily reflecting membrane integrity13. In agreement with the results of LDH released, the morphological analysis also showed greater injury to hepatocytes under hypoxia compared to Caco-2 cells (Fig. 5). These results agree to evaluation of apoptosis by Tunel, where there are more hypoxic apoptotic hepatocytes than hypoxic apoptotic Caco-2 cells. We speculated that hypoxia produced more changes in the cell membrane of epithelial origin (hepatocytes) than those of tumoral origin (Caco-2). Besides this, hypoxia is a common condition when a tumor expands and it may induce the expression of hypoxia-inducible factor (HIF), a transcription factor that initiates a range of pro-survival mechanisms11. It is possible that HIF was more expressed in Caco-2 cells than in the hepatocytes due to their tumoral origin. This phenomenon would contribute to lower injury Caco-2 under hypoxia. Hepatocytes submitted to hypoxia had increased apoptosis rates compared to normoxic cells (Fig. 4).
In brief, our results contribute to a better knowledge of interactions between studied cells and S. flexneri. Hypoxia appears to influence significantly the bacterial cell invasiveness. The differences observed between these two cellular model systems could be due to us having compared primary cells (rat hepatocytes) and tumoral cell lines (Caco-2). Furthermore, our results suggest that tumoral cells are more resistant to infection and hypoxia than primary cells. More details on this complex interaction await further studies. This knowledge will be important to improve strategies to combat this virulent pathogen in the future.
Acknowledgments
This work was supported by the “Fundação de Amparo à Pesquisa do Estado de São Paulo” (FAPESP, Foundation for the Support of Research in the state of São Paulo).
REFERENCES
- 1.Berger ML, Reynolds RC, Hagler HK, Bellotto D, Parsons D, Mulligan KJ, et al. Anoxic hepatocyte injury: role of reversible changes in elemental content and distribution. Hepatology. 1989;9:219–28. doi: 10.1002/hep.1840090210. [DOI] [PubMed] [Google Scholar]
- 2.Bernardini ML, Arondel J, Martini I, Aidara A, Sansonetti PJ. Parameters underlying successful protection with live attenuated mutants in experimental shigellosis. Infect Immun. 2001;69:1072–83. doi: 10.1128/IAI.69.2.1072-1083.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosseto MC, Palma PV, Covas DT, Giorgio S. Hypoxia modulates phenotype, inflammatory response, and leishmanial infection of human dendritic cells. APMIS. 2010;118:108–14. doi: 10.1111/j.1600-0463.2009.02568.x. [DOI] [PubMed] [Google Scholar]
- 4.Bottaro DP, Liotta LA. Cancer: out of air is not out of action. Nature. 2003;423:593–5. doi: 10.1038/423593a. [DOI] [PubMed] [Google Scholar]
- 5.Bukholm G, Modalsli K, Degré M. Effect of measles-virus infection and interferon treatment on invasiveness of Shigella flexneri in HEp2-cell cultures. J Med Microbiol. 1986;22:335–41. doi: 10.1099/00222615-22-4-335. [DOI] [PubMed] [Google Scholar]
- 6.Colhone MC, Arrais-Silva WW, Picoli C, Giorgio S. Effect of hypoxia on macrophage infection by Leishmania amazonensis . J Parasitol. 2004;90:510–5. doi: 10.1645/GE-3286. [DOI] [PubMed] [Google Scholar]
- 7.Etheridge ME, Hoque AT, Sack DA. Pathologic study of a rabbit model for shigellosis. Lab Anim Sci. 1996;46:61–6. [PubMed] [Google Scholar]
- 8.Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahé MM, et al. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2011;60:473–84. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- 9.Fogh J, Trempe G. New human tumor cell lines. In: Fogh J, editor. Human tumor cells in vitro. New York: Plenum; 1975. pp. 115–41. [Google Scholar]
- 10.Galan JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–8. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 11.Greer SN, Metcalf JL, Wang Y, Ohh M. Review. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–60. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman B, Gores GJ, Nieminen AL, Kawanishi T, Harman A, Lemasters JJ. Calcium and pH in anoxic and toxic injury. Crit Rev Toxicol. 1990;21:127–48. doi: 10.3109/10408449009089876. [DOI] [PubMed] [Google Scholar]
- 13.Jauregui HO, Hayner NT, Driscoll JL, Williams-Holland R, Lipsky MH, Galletti PM. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes - freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro. 1981;17:1100–10. doi: 10.1007/BF02618612. [DOI] [PubMed] [Google Scholar]
- 14.Kondo S, Wang D, Mayumi T, Klein AS, Bulkley GB. Effect of hemorrhagic shock and resuscitation upon hepatic phagocytic clearance and killing of circulating microorganisms. Shock. 1996;5:106–11. doi: 10.1097/00024382-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 16.Macutkiewicz C, Carlson G, Clark E, Dobrindt U, Roberts I, Warhurst G. Characterisation of Escherichia coli strains involved in transcytosis across gut epithelial cells exposed to metabolic and inflammatory stress. Microbes Infect. 2008;10:424–31. doi: 10.1016/j.micinf.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Marteyn B, West NP, Browning DF, Cole JA, Shaw JG, Palm F, et al. Modulation of Shigella virulence in response to available oxygen in vivo . Nature. 2010;465:355–8. doi: 10.1038/nature08970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martino MC, Rossi G, Tattoli I, Martini I, Chiavolini D, Cortese G, et al. Intravenous infection of virulent shigellae causes fulminant hepatitis in mice. Cell Microbiol. 2005;7:115–27. doi: 10.1111/j.1462-5822.2004.00441.x. [DOI] [PubMed] [Google Scholar]
- 19.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti PJ. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–48. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–63. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 21.Raurich JM, Llompart-Pou JA, Ferreruela M, Colomar A, Molina M, Royo C, et al. Hypoxic hepatitis in critically ill patients: incidence, etiology and risk factors for mortality. J Anesth. 2011;25:50–6. doi: 10.1007/s00540-010-1058-3. [DOI] [PubMed] [Google Scholar]
- 22.Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, et al. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol. 1999;155:1065–73. doi: 10.1016/S0002-9440(10)65209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth A, König P, van Zandbergenc G, Klinger M, Hellwig-Bürgel T, Däubener W, et al. Hypoxia abrogates antichlamydial properties of IFN-gamma in human fallopian tube cells in vitro and ex vivo . Proc Natl Acad Sci USA. 2010;107:19502–7. doi: 10.1073/pnas.1008178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–30. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sansonetti PJ. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. III. Shigellosis: from symptoms to molecular pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;280:319–23. doi: 10.1152/ajpgi.2001.280.3.G319. [DOI] [PubMed] [Google Scholar]
- 26.Santos SA dos, Andrade DR de, Júnior, Andrade DR de. TNF-alpha production and apoptosis in hepatocytes after Listeria monocytogenes and Salmonella Typhimurium invasion. Rev Inst Med Trop Sao Paulo. 2011;53:107–12. doi: 10.1590/s0036-46652011000200009. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda LA, Polak J. Hypoxia. 4. Hypoxia and ion channel function. Am J Physiol Cell Physiol. 2011;300:C951–C67. doi: 10.1152/ajpcell.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shipp C, Derhovanessian E, Pawelec G. Effect of culture at low oxygen tension on the expression of heat shock proteins in a panel of melanoma cell lines. PLoS One. 2012;7: doi: 10.1371/journal.pone.0037475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spear W, Chan D, Coppens I, Johnson RS, Giaccia A, Blader IJ. The host cell transcription factor hypoxia-inducible factor 1 is required forToxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–52. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 30.Stern MS, Gitnick GL. Shigella hepatitis. JAMA. 1976;235: [PubMed] [Google Scholar]
- 31.Tüzüner E, Liu L, Shimada M, Yilmaz E, Glanemann M, Settmacher U, et al. Heme oxygenase-1 protects human hepatocytes in vitro against warm and cold hypoxia. J Hepatol. 2004;41:764–72. doi: 10.1016/j.jhep.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–62. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- 33.Zitta K, Meybohm P, Bein B, Huang Y, Heinrich C, Scholz J, et al. Salicylic acid induces apoptosis in colon carcinoma cells grown in-vitro: influence of oxygen and salicylic acid concentration. Exp Cell Res. 2012;318:828–34. doi: 10.1016/j.yexcr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Zurawski DV, Mumy KL, Faherty CS, McCormick BA, Maurelli AT. Shigella flexneri type III secretion system effectors OspB and OspF target the nucleus to downregulate the host inflammatory response via interactions with retinoblastoma protein. Mol Microbiol. 2009;71:350–68. doi: 10.1111/j.1365-2958.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]