Abstract
OBJECTIVE
Repeated exposure to cannabis in nonpsychotic adolescents is associated with impairments in executive control of attention, similar to those observed in young adults with first-episode schizophrenia. To assess the impact of recurrent exposure to cannabis on cognitive function, this study characterized attention performance in both nonpsychotic adolescents and adolescents with early-onset schizophrenia (EOS).
METHOD
The Attention Network Test, a standard procedure that estimates the functional state of neural networks controlling the efficiency of three different attentional behaviors (i.e., alerting, orienting and executive attention) was administered to four groups of participants: (1) adolescents with EOS and comorbid cannabis use disorder (EOS+CUD; n = 18), (2) “Pure” schizophrenia (EOS; n = 34), (3) “Pure” cannabis use disorder (CUD; n = 29), and (4) Healthy controls (HC; n = 53). Task performance was examined with a 2 × 2 design (EOS+ versus EOS− and CUD+ versus CUD−) using multivariate analysis of covariance. Correlative analyses were conducted between executive attention performance and measures of surface area in the right anterior cingulate cortex.
RESULTS
A significant EOS × CUD interaction was observed. In the executive attention network, adolescents with EOS+CUD showed reduced efficiency relative to adolescents with pure EOS, whereas no group differences were found between adolescents with pure CUD and HC. Less efficient executive attention was significantly associated with smaller surface area of the right caudal anterior cingulate cortex in EOS+CUD.
CONCLUSIONS
These preliminary data suggest that the presence of CUD has a moderating effect on attentional performance in adolescents with schizophrenia compared to nonpsychotic adolescents. These deficits could have a role in difficulties in self-regulation and predisposition to substance misuse in this patient group. The anatomic substrate of this cognitive deficit may be related to surface area in the right caudal anterior cingulate cortex.
Keywords: Schizophrenia, Cannabis, Attention Network Test, Adolescent, Anterior Cingulate
1. Introduction
Attention refers to both the preparedness for and selection of certain aspects of our physical environment or some ideas in our mind that are stored in our memory (Raz and Buhle, 2006). Current models show that attention is not a unitary function but the result of three different attention networks (i.e., alerting, orienting and executive attention), which can be independently evaluated using the Attention Network Test (ANT) (Fan et al., 2002). Alerting is manifested by achieving and maintaining the alert state; orienting by the ability to direct attention to sensory events; and executive attention by efficient control of the attentional mechanism itself (i.e., shifting, disengaging, and alternating attention). In infancy and toddlerhood, when external cues overwhelmingly guide attention, alerting and orienting are the predominant systems used. From around age 4, an executive attention network gradually takes over the alerting and orienting systems and becomes the dominant factor in cognitive control (Rothbart et al., 2011) as children develop the ability to use rules, strategies, and plans to guide their behavior (Berger et al., 2007). In parallel, improvement in cognitive control begins at age 4 with a steep developmental trajectory that gradually decreases in slope and plateaus at around age 14 to 15 years. In general, self-regulation and executive attention continue to develop throughout childhood and well into adolescence (Fjell et al., 2012).
Cannabis use disorder (CUD) is highly prevalent among adolescents with early-onset schizophrenia (EOS) (Kumra et al., 2012), but the basis of this comorbidity remains unclear. It is possible that attentional impairment is a common risk factor that predisposes adolescents to both disorders. To understand the basis of the comorbidity between CUD and EOS, this study characterized attention performance in both nonpsychotic adolescents and adolescents with EOS using the ANT (Fan et al., 2002). Applying the ANT to patients with schizophrenia, Wang and colleagues found a marked deficit in the executive control network and a less pronounced deficit in the orienting network. Using the ANT, impairments in executive attention have been described in both nonpsychotic adolescents with CUD (Abdullaev et al., 2010) and in adults with schizophrenia alone (Breton et al., 2011; Orellana et al., 2012; Wang et al., 2005). To our knowledge, attention networks in patients suffering from the schizophrenia-cannabis comorbidity have not been evaluated with the ANT. Based on these previous reports (Abdullaev et al., 2010; Orellana et al., 2012; Wang et al., 2005), we hypothesized that the presence of CUD might moderate the association between impairments in executive attention and EOS. Examining executive attention deficits in both healthy adolescents and adolescents with EOS is an important topic of inquiry because these impairments could lead to difficulties in exerting control over thoughts, feelings, and actions, and predispose these individuals to cannabis misuse.
As an exploratory aim, we examined whether performance on the executive attention component of the ANT was associated with surface area in the anterior cingulate cortex, the central structure of the executive attention network (Fair et al., 2009; Posner, 2012). Recently, a large multicenter study showed that surface area in the right anterior cingulate cortex was strongly correlated with a measure of effortful self-control in healthy children and adolescents (Fjell et al., 2012). We attempted to replicate this finding in healthy adolescents and in patient groups.
2. Method
2.1 Participants
The details of the clinical protocol have been described elsewhere (Kumra et al., 2012). In brief, a total of 141 participants ranging in age from 10 to 23 years were recruited from clinical programs at the University of Minnesota under an approved Institutional Review Board protocol. Of the 141 participants (55 HC; 31 CUD; 34 EOS; 21 EOS+CUD) who completed structural imaging scans, 134 (53 HC; 29 CUD; 34 EOS; 18 EOS+CUD) completed the ANT; the characteristics of this subgroup are described in Table 1.
TABLE 1.
Demographic Features and Clinical Characteristics for Participants
| HC (n=53) |
CUD (n=29) |
EOS (n=34) |
EOS+CUD (n=18) |
χ2 or F | |
|---|---|---|---|---|---|
| Demographic Features | |||||
| Sex, male | 26 | 20 | 15 | 14 | 8.43* |
| Mean age, years (SD) | 16.4 (2.6) | 17.4 (2.3) | 16.5 (1.9) | 17.7 (1.2) | 2.63 |
| Handedness, right | 45 | 28 | 28 | 16 | 3.31 |
| Clinical Characteristics, Means | |||||
| Psychosis age of onset, years (SD) | - | - | 12.5 (3.4) | 14.9 (2.6) | 6.50* |
| BPRSc total score (SD) | - | - | 22.7 (12) | 21.9 (16) | .04 |
| SANSd total score (SD) | - | - | 9.3 (4.9) | 7.2 (5.0) | 1.94 |
| Cannon-Spoor premorbid adjustment | - | - | 9.5 (3.8) | 7.9 (3.5) | 2.09 |
| Currente CPZf antipsychotic dose, 100 CPZ equivalents (SD) | - | 1.4 (.78) | 2.7 (1.8) | 2.8 (2.0) | 1.15 |
| Lifetimeg CPZf exposure, dose-years (SD) | 0 (0) | .06 (.21) | 2.24 (2.7)a,b | 2.74 (2.9)a,b | 21.00*** |
| Age of onset, cannabis use (SD) | - | 12.7 (2.5) | - | 12.9 (1.9) | .074 |
| Lifetime cannabis episodes (SD) | - | 934 (483) | - | 953 (456) | .02 |
| Median days since last use (SD) | - | 57 | - | 80 | |
| Urine analysis for cannabis, positive | 0 | 11 | 0 | 7 | 38.50*** |
| Cigarette smoking, yes | 1 | 14 | 1 | 6 | 37.86*** |
| Alcohol Drinking, yes | 8 | 19 | 3 | 8 | 32.98*** |
| Cognitive measures | |||||
| WRATh Reading decoding scores (SD) | 111 (16) | 104 (17) | 103 (19)a | 95 (13)a | 4.73** |
| WASIi full-scale IQ | 116 (9) | 100 (14)a | 90 (17)a,b | 90 (16)a,b | 29.33*** |
| ANTj mean reaction time, ms (SD) | 603 (70) | 636 (108) | 702 (86)a,b | 652 (128) | 8.05*** |
| ANTj mean accuracy, % (SD) | 96 (3.4) | 94 (8.8) | 88 (13.3)a,b | 93 (7.7) | 6.17** |
Note: HC=healthy controls; CUD=cannabis use disorder; EOS=early-onset schizophrenia; EOS+CUD=EOS with comorbid CUD;
Significant difference from HC, p<.05;
Significant difference from CUD;
BPRS=Brief Psychiatric Rating Scale;
SANS=Scale for Assessment of Negative Symptoms;
Current dose based on participants taking antipsychotic medications at time of scan (5 CUD, 27 EOS, 18 EOS+CUD);
CPZ=chlorpromazine equivalents;
Estimates of lifetime CPZ exposure includes all members of patient groups;
WRAT=Wide Range Achievement Test;
WASI=Weschler Abbreviated Scale of Intelligence;
ANT=Attention Network Test.
p<.05
p<.01
p<.001
All EOS participants met criteria for schizophrenia (n = 41), schizoaffective (n = 4), or schizophreniform disorder (n = 7), and reported an onset of psychotic symptoms prior to age 18 years. Thirty-four “pure” EOS patients out of 52 total EOS patients had no past or current DSM-IV diagnosis for substance or alcohol-use disorders. Eighteen EOS+CUD patients out of 52 EOS met lifetime criteria for a co-occurring CUD of abuse or dependence. In EOS, participants with co-occurring CUD were included if a history of psychotic symptoms was present when there was no evidence of substance misuse or withdrawal. Thirty out of 34 pure EOS patients were taking antipsychotic medication at the time of scanning, which included quetiapine (n=5), aripriprazole (n=8); risperidone (n=10); clozapine (n=3); olanzapine (n=2); and ziprasidone (n=2). Fourteen out of 18 EOS+CUD patients were taking antipsychotic medication at the time of scanning, which included quetiapine (n=5), aripriprazole (n=3); risperidone (n=4); olanzapine (n=1); and paliperidone (n=1). Chlorpromazine equivalent (CPZ) dose and lifetime antipsychotic exposure was calculated using a standardized method (Andreasen et al., 2010).
Nonpsychotic adolescents with CUD (n=29) were recruited from treatment settings for chemical dependency. Adolescents were selected who reported cannabis as their drug of choice with significant cannabis exposure by age 17 years (> 50 exposures to cannabis), and who did not meet lifetime criteria for abuse of or dependence on other illicit drugs with the exception of alcohol abuse or nicotine dependence. Exclusion criteria for the CUD group included a lifetime diagnosis of bipolar disorder or schizophrenia-spectrum disorder. Fourteen of the 31 CUD participants were taking psychotropic medication at the time of scanning, which included stimulants (n=1), antidepressants (n=12), mood stabilizers (n=1), and second-generation antipsychotic agents (n=5).
A total of 53 HC were recruited from the same geographic area in response to flyers and by word of mouth to match the EOS group on age, sex, and handedness. Control participants were excluded if they had any current or past DSM-IV diagnosis, prior or current treatment with psychotropic medications, history of psychological counseling, reported history of more than five lifetime exposures to any illicit drug, and/or history of schizophrenia or psychosis in a first-degree relative.
General exclusion criteria for all participants included any contraindication to magnetic resonance imaging (MRI), positive pregnancy test, history of a DSM-IV diagnosis of mental retardation, a neurological disorder, head injury with loss of consciousness for more than 30 seconds, or active medical illness that could potentially affect brain structure.
2.2 Clinical Measures
Diagnoses of Axis I disorders including schizophrenia and of substance use disorders were made using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (<18 years of age) or the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (>=18 years of age) using multiple data sources. The Wide Range Achievement Test-3rd Edition Reading Subtest was administered to estimate premorbid intellectual ability (Wilkinson, 1993).
2.3 Attention network test (ANT)
One hundred thirty-four participants completed the ANT (53 HC, 29 CUD, 34 EOS, 18 EOS+CUD). The stimuli and procedures for the ANT followed the experimental procedures that have been previously described by Fan and colleagues (2002). In brief, participants viewed a computer screen from a distance of 65 cm and responses were collected via two input keys on a keyboard that rested on their laps. Stimuli consisted of a row of five visually-presented horizontal black lines with arrowheads pointing left or right against a gray background. The central target, a left- or right-pointing arrowhead, was flanked by two arrows on each side; these four flanker arrows all pointed either in the same direction as the central target (congruent condition) or in the opposite direction as the central target (incongruent condition). For the neutral condition, four horizontal lines instead of four arrows flanked the central target. Participants responded to the direction of the centrally-presented target by pressing one key for the left direction and a different key for the right direction. Preceding the presentation of the target, one of four cue conditions was provided: no cue; center cue; double cue; and spatial cue. These four conditions interact with the flanker task to influence reaction times (RT). The center cue and double cue conditions provide alerting without spatial information, while the spatial cue conditions direct attention toward (or away from) the location where the target will appear.
2.4 MRI Protocol
MRI acquisition parameters and protocol have been described in detail previously (Kumra et al., 2012).
Image Acquisition: MRI scans were obtained using a Siemens 3T Tim Trio MR System (Erlangen, Germany) at the Center for Magnetic Resonance Research, University of Minnesota. After a localizer sequence for orientation, a T1-weighted image (1mm isotropic MPRAGE sequence: TR=2530ms, TE=3.65ms, TI=1100ms, Flip angle=7deg, FOV=256mm × 176mm, Number of slices=224, IPAT acceleration factor 2, in-plane resolution 1 × 1 mm, Slice thickness=1 mm) was collected for each patient.
Anatomical images were then processed using the FreeSurfer (Version 4.5) image analysis suite. We measured surface area in the right rostral and caudal anterior cingulate cortex because this structural characteristic has been shown to predict effortful self-control in healthy children and adolescents (Fjell et al., 2012). Correlative analyses between these surface area measurements and executive attention scores were performed within each group for exploratory purposes.
3. Statistical Analysis
Statistical analyses were performed with SPSS version 18 (SPSS Inc, Chicago, Illinois).
An alerting score was calculated by subtracting the mean RT of the double-cue condition from the mean RT of the no-cue condition. Orienting was calculated by subtracting the mean RT of the spatial cue condition from the mean RT of the center cue condition. Executive attention was calculated by subtracting the mean RT of all congruent flanking conditions, summed across cue types, from the mean RT of incongruent flanking conditions, summed across cue types.
To test the hypothesis that a CUD moderated the relationship between EOS and attention network scores, group differences in ANT performance for all three networks were analyzed using an omnibus multivariate analysis of covariance (MANCOVA) test with the ANT network scores as the dependent variables and two factors, Psychosis (EOS+ versus EOS−) and Cannabis use disorder (CUD+ versus CUD−), as the independent variables. Potential covariates included age, sex, WRAT reading score, mean RT on the ANT, positive urine analysis for cannabis, and lifetime antipsychotic exposure in chlorpromazine equivalents. Of these possible covariates, only age and mean RT were significant and retained in the final model. Post-hoc pairwise group comparisons using analysis of covariance (ANCOVA) were conducted with age and mean RT as covariates to clarify any significant main effects or interactions.
4. Results
4.1 Demographics and Clinical Characteristics
Demographic and clinical characteristics are summarized in Table 1. There were no significant differences in age or handedness between the four groups, although there was a significant difference in gender distribution, with CUD and EOS+CUD having significantly more males (χ2 =9.43, p=.04).
All patient groups (CUD, EOS, EOS+CUD) were observed to have lower WRAT reading scores compared to HC, and EOS+CUD had significantly lower scores than pure EOS (F(3,135)=5.32, p=.002). In addition, EOS+CUD had a significantly later age of onset of psychotic symptoms compared with EOS (F(1,50)=6.50, p=.01).
Urine toxicology tests were performed on the day of the MRI scan using the K012B 12 Panel Drug Screen Test (THC sensitivity 50 ng/ml) from Drug Test Systems (Dover, NH). We imaged 18 participants who tested positive for cannabis on the day of MRI scanning (11 CUD, 7 EOS+CUD).
4.2 Effects of Age
We examined the relationship between ANT network scores with age separately in each group. In HC, age was found to be significantly correlated with measures of alerting (r= −.39, n=53, p=.004), orienting (r=−.36, n=53, p<.001), and executive attention (r=−.31, n=53, p=.02), as well as overall mean RT (r=−.53, n=53, p<.001) and mean accuracy (r = .39, p =.004). With increasing age, reaction times decrease across all neural networks in HC, indicating greater efficiency and better performance. This robust age-performance correlation did not appear in patient groups, with the exception of alerting scores in pure EOS. In pure EOS, reaction times decreased with increasing age in the alerting network only (r=−.51, n=34, p=.002). Based on these data, age was included as a covariate in the subsequent group comparison of ANT performance.
4.3 ANT Behavioral Performance
The general performance on the ANT was measured by two components: mean RT and accuracy (percent correct). These data, by subject groups, are shown in Table 1. Adolescents with pure EOS had significantly longer reaction times (F(3,130)=8.05, p<.001) than HC and CUD, indicating worse performance. Also, pure EOS participants had lower accuracy scores than HC and CUD (F(3,130)=6.17, p=.01). We included RT as a covariate in the subsequent group comparison of ANT performance.
4.4 Efficiencies of the Three Networks
The means and standard deviations of specific attentional networks (alerting, orienting, and executive attention) are shown in Table 2. The overall MANCOVA showed a significant main effect of EOS (F(3,126)=2.86, p=.04), a significant main effect of CUD (F(3,126)=5.30, p=.002), and a significant EOS × CUD interaction (F(3,126)=2.80, p=.04). Covariates of no interest that were significant (and therefore retained in the final model) were: mean RT on the ANT (F(3,126)=35.7, p=.001) and age (F(3,126)=3.8, p=.01). Covariates that were not significant and dropped from the model included: WRAT score, urine toxicology, cumulative antipsychotic exposure, and sex.
TABLE 2.
Neuropsychological Tests: Means, Standard Deviations, and Main Effects of Schizophrenia and Cannabis
| CUD (−) (n=87) Mean (SD) |
CUD (+) (n=47) Mean (SD) |
Main Effect: EOS |
Main Effect: CUD |
EOS-by-CUD Interaction |
||
|---|---|---|---|---|---|---|
| ANT subtest | ||||||
| Alerting (ms) | F1,128=2.6, p=.11 | F1,128=5.3, p=.02 | F1,128=.25, p=.62 | |||
| EOS (−) (n=82) | 53.5 (31.6) | 38.6 (52.1) | ||||
| EOS (+) (n=52) | 74.3 (39.8) | 46.5 (29.5) | ||||
| Orienting (ms) | F1,128=5.7, p=.02 | F1,128=3.8, p=.05 | F1,128=.44, p=.51 | |||
| EOS (−) (n=82) | 52.5 (22.2) | 42.1 (44.6) | ||||
| EOS (+) (n=52) | 73.1 (48.8) | 52.5 (25.4) | ||||
| Conflict (ms) | F1,128=1.8, p=.18 | F1,128=10.5, p=.002 | F1,128=8.3, p=.005 | |||
| EOS (−) (n=82) | 96.4 (34.6) | 120.2 (66.8) | ||||
| EOS (+) (n=52) | 104.5 (75.7) | 144.5 (114.4) |
Note: Multivariate tests (Wilk’s lambda): Age: F=3.8, p=.01; Mean Reaction Time: F=35.7, p<.001. EOS=early-onset schizophrenia; CUD=cannabis use disorder.
A summary of between-subjects effects is presented in Table 2. For the alerting network, there was a significant main effect of cannabis status, but no main effect of EOS, and no EOS × CUD interaction.
For the orienting network, there was a significant main effect of EOS, but no main effect of CUD, and no EOS × CUD interaction.
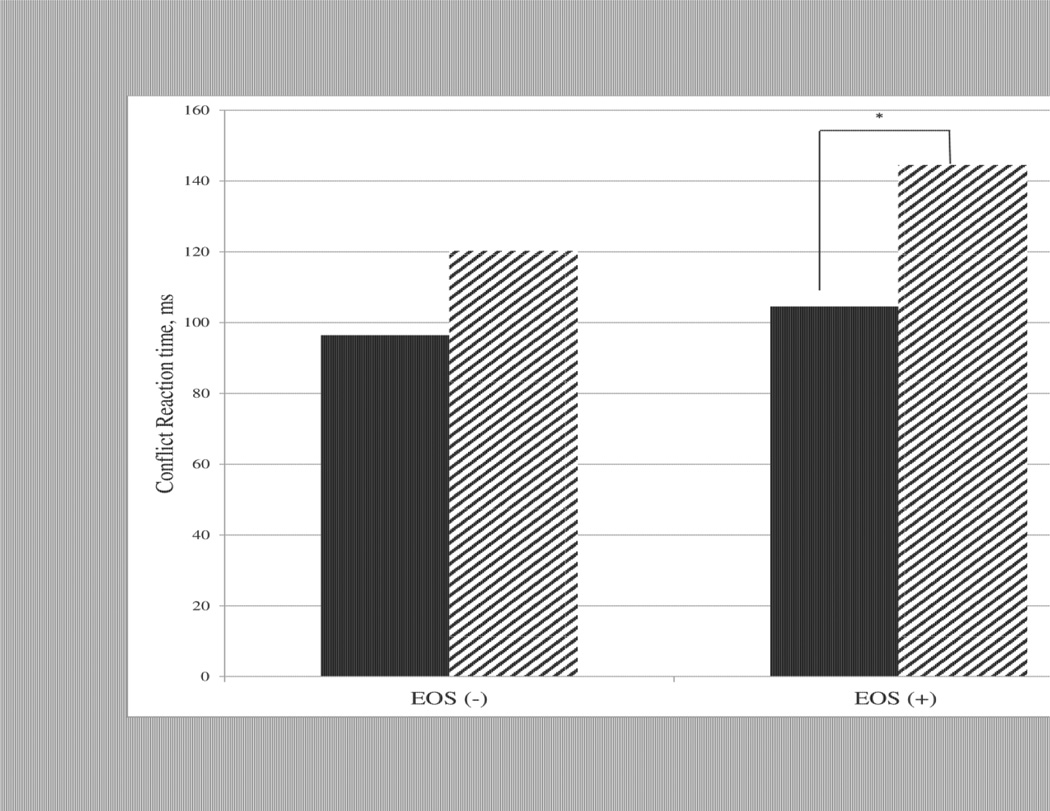
For the executive attention (conflict) network, there was no main effect of EOS, a significant main effect of CUD, and a significant EOS × CUD interaction. As shown in Figure 1, post-hoc testing indicated that there was reduced efficiency of the executive attention network in comorbid patients relative to adolescents with pure EOS (F3,48 = 7.17, p=.01). In contrast, although the efficiency of the executive attention network was worse in patients with CUD compared to HC, this difference did not reach statistical significance (F(3,78)=1.16, p=.28).
Figure 1.
Conflict scores are significantly different between adolescents with schizophrenia (EOS +) with (CUD +) and without a comorbid cannabis use disorder (CUD −) (F(3,48)=7.05, p=.01). Nonpsychotic adolescents (EOS −) have no significant difference in conflict scores based on cannabis status (F(3,78)=1.17, p=.28).
To assess the effect of accuracy on executive attention network efficiency, the above analyses were repeated excluding participants with accuracy less than 80% (0 HC, 3 CUD, 8 EOS, 2 EOS+CUD), since these participants may not have been sufficiently engaged and/or understood the task. A significant main effect of CUD (F(1,116)=8.87, p=.004), and a significant EOS × CUD interaction (F(1,116) = 6.05, p = .02) was still observed for the executive attention network.
4.5 Analysis of Brain Volumes
An ANCOVA was used to examine surface area in the right rostral and right caudal anterior cingulate cortex, correcting for age. For descriptive purposes, the estimated marginal means for the regions of interest in which a significant main effect of diagnostic group was identified are shown in Table 3.
TABLE 3.
Brain measures: estimated marginal means and standard errors for all participants
| Means (SE) | |||||
|---|---|---|---|---|---|
| HC (n=53) | CUD (n=29) | EOS (n=34) | EOS+CUD (n=18) | F(df = 3, 129) | |
| Surface area, mm2 | |||||
| R. rostral anterior cingulate | 628.3 (18) | 576.5 (25) | 552.0 (23)a | 535.8 (32)a | 3.37* |
| R. caudal anterior cingulate | 832.1 (21) | 839.5 (29) | 806.7 (26) | 779.7 (36) | .77 |
Note: Note: Univariate tests corrected for Age.
HC=healthy controls; CUD=cannabis use disorder; EOS=early-onset schizophrenia; EOS+CUD=EOS with comorbid CUD.
p<.05
4.6 Brain Structure-function Relationships
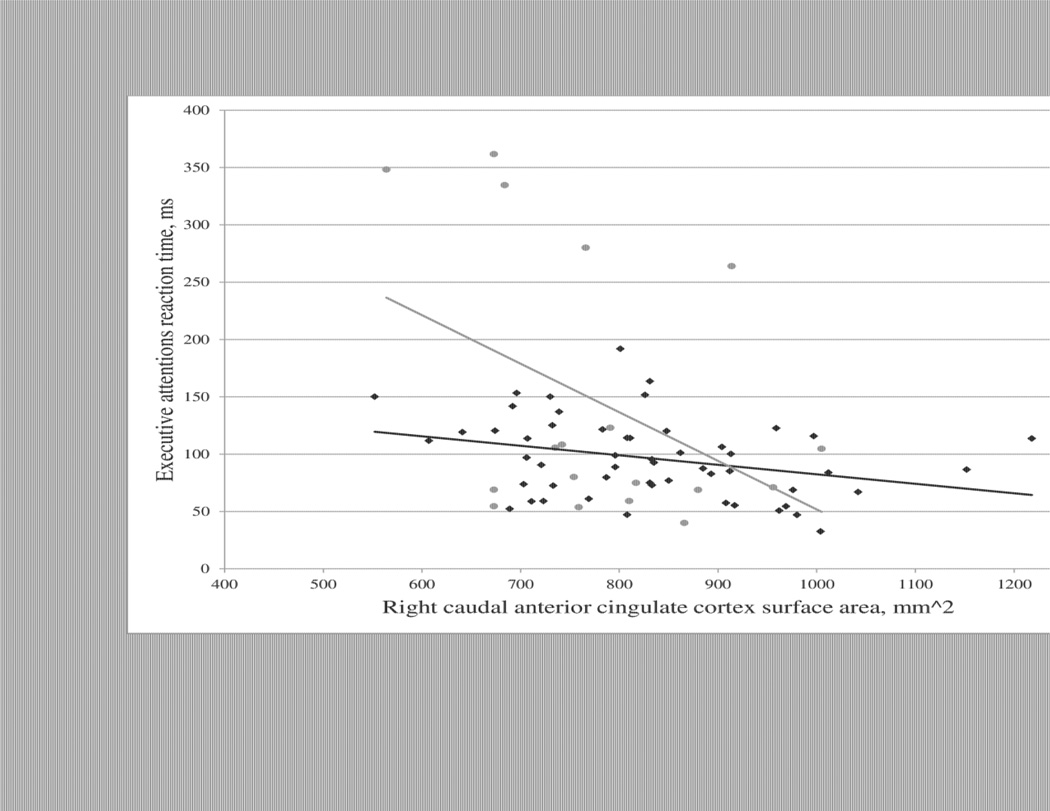
Pearson correlations were used to test the hypothesis that measures of surface area in the right rostral or caudal anterior cingulate cortex were correlated with executive attention scores on the ANT. In HC, surface area in the right caudal anterior cingulate was significantly correlated with executive attention (r=−.316, n=53, p=.02); Greater surface area was associated with a shorter reaction time and better performance. In EOS+CUD, there was a trend toward a correlation between surface area in the right caudal anterior cingulate and executive attention (r=−.416, n=18, p=.09). These relationships are depicted in Figure 2. There were no significant correlations between surface area in the right rostral anterior cingulate and executive attention scores in any group.
Figure 2.
In healthy controls, surface area of the right caudal anterior cingulate cortex predicts better performance (shorter reaction time) on the executive attention task of the Attention Network Task (ANT) (r=−.32, n=53, p=.02). In adolescents with early onset schizophrenia and a comorbid cannabis use disorder (EOS+CUD), the correlation between increased surface area of the right caudal anterior cingulate cortex and improved executive attention is significant at a trend level (r=−.42, n=18, p=.09).
Since age and mean RT were found to be significant covariates of ANT scores, we used partial correlations to control for these variables. After controlling for age and reaction time, we found that surface area of the right caudal anterior cingulate cortex was significantly correlated with performance on the executive attention task of the ANT in the EOS+CUD group (r=−.66, n = 14, p = .006). This correlation remained significant at a trend level in HC (r = −.27, n= 49, p = .06). Partial correlations for each group are described in Table 4.
TABLE 4.
Partial Correlations between surface area in right anterior cingulate and conflict scores
| Surface area, mm2 | ||||
|---|---|---|---|---|
| Group | HC (n=49) | CUD (n=25) | EOS (n=30) | EOS+CUD (n=14) |
| Brain region | ||||
| R. rostral anterior cingulate | .049 | .09 | −.22 | −.49 |
| R. caudal anterior cingulate | −.27 | .11 | −.13 | −.66** |
Note: Partial correlations corrected for Age and Mean Reaction Time on Attention Network Test. HC=Healthy Controls; CUD=cannabis use disorder; EOS=early onset schizophrenia; EOS+CUD=EOS with comorbid CUD.
p<.01
There were no significant relationships found between parameters of antipsychotic medication dosage at time of MRI scan (as indexed by chlorpromazine equivalent dose at time of testing or cumulative lifetime antipsychotic exposure), or clinical symptoms (as measured by BPRS and SANS scores) and ANT performance measures in either EOS group.
To test for the possibility of a dose-response effect of cannabis exposure on conflict scores, we used partial correlations, correcting for age and mean RT, to test for a relationship between lifetime cannabis exposure and conflict scores. There was no significant correlation between these two measures in either CUD (r = −.26, n=25, p=.19) or in EOS+CUD (r =.24, n=14, p=.38).
4. Discussion
To our knowledge, this study is the first to comprehensively examine alterations in attention network efficiency associated with recurrent exposure to cannabis in both healthy adolescents and adolescents with EOS using the ANT. In HC, we found that with increasing age, reaction time decreased across all networks, indicating improved performance in healthy subjects. These data, along with the existing literature (Fjell et al., 2012; Jepsen et al., 2010; Konrad et al., 2005), suggest a protracted developmental trajectory for attentional networks during adolescence.
Consistent with our hypothesis, there was a significant CUD × EOS interaction across all attention networks indicating that the effect of CUD on task performance was dependent on diagnostic grouping. Subsequent analyses suggested that only the executive attention network, related to resolution of conflict, differed between comorbid adolescents and adolescents with pure EOS. Although executive attention performance was numerically worse in pure CUD compared to healthy controls, this did not reach statistical significance. These data suggest that the presence of CUD moderates the association between EOS and executive attention.
In this study, we also found a significant relationship between surface area of the right caudal anterior cingulate cortex and executive attention network scores in EOS+CUD and a trend-level relationship in healthy participants, after correcting for age and mean RT. These findings are similar to a recent report from a large normative study of young participants aged 4–21 years (Fjell et al., 2012). Fjell and colleagues found a stronger correlation between surface area and performance in younger healthy subjects, suggesting that with age, improved efficiency of neural circuits may decrease reliance on this brain structure for task performance. Based on these data, we would speculate that the EOS+CUD patients may use a less efficient or more immature strategy to perform this task well into adolescence.
Several findings in the present study suggest that performance deficits in executive attention in EOS+CUD may indicate a pre-existing vulnerability rather than a potential “neurotoxic” effect of exposure to cannabis. First, there was no evidence of a dose-dependent deleterious effect of cannabis on executive attention performance. Second, we found a relationship between anterior cingulate surface area and executive attention performance. Cortical surface area is determined prenatally, by proliferation of radial unit progenitors, and decreased surface area may reflect a reduced number of cortical columns (Pontious et al., 2008; Rakic, 1995). By contrast, cortical thickness changes dynamically across the lifespan, influenced by development and disease (Frye et al., 2010). Third, deficits in executive attention have been associated with increased risk for several neuropsychiatric disorders including schizophrenia, such as 22q11 deletion syndrome (which confers a genetic high risk for schizophrenia) (Takarae et al., 2009), post-traumatic stress disorder (Aupperle et al., 2012), and attention-deficit disorder (Mullane et al., 2011). In contrast, a previous study comparing ANT performance in heavy cannabis users, heavy cannabis + inhalant users, and healthy controls found no differences in alerting, orienting, or executive attention across the three groups (Vilar-Lopez et al., 2013). This result was unexpected given the well-described neurotoxicity of inhalants (Rosenberg et al., 2002; Yucel et al., 2008). However, heavy cannabis + inhalant users had significantly more errors across all conditions, suggesting that accuracy, not efficiency, may be a more sensitive measure of drug neurotoxicity in adolescents.
5. Strengths and Limitations
Limitations to this study should be noted. The sample size was small and there was a significant group difference in sex ratios (see Table 1) driven by the higher proportion of males in EOS+CUD. Although we did not find sex to be a significant predictor of performance on the ANT, a previous study of patients with schizophrenia showed that patients had significantly worse conflict ratio scores on the ANT than healthy controls, but that these differences were driven by worse performances in females with schizophrenia than in males (Urbanek et al., 2009). This gender effect was in the opposite direction of our current findings, where the male-heavy EOS+CUD group had the worst performance. A previous study in healthy participants showed a significant sex difference in orienting, but not alerting or conflict scores (Liu et al., 2013).
Another potential limitation to this study is the test-retest reliability of the ANT, which has been questioned in both healthy and clinical populations (Hahn et al., 2011). A longitudinal study found that patients with schizophrenia had moderate test-retest correlations (across a 7.4 month interval) for mean RT, orienting, and conflict scores. The analysis of accuracy in patients with schizophrenia revealed very low test-retest correlations. In general, healthy participants have stronger test-retest correlations than patients, and conflict scores in HC and patients have consistently stronger test-retest correlations than alerting or orienting scores (Fan et al., 2002; Fan et al., 2001; Greene et al., 2008; Hahn et al., 2011). Thus, these findings do not appear to seriously undermine the results of the present study, since conflict scores and mean RT were found to best discriminate adolescents with EOS versus EOS+CUD, and these measures have been shown to have acceptable test-retest correlations across a 7.4 month interval in patients and controls (Hahn et al., 2011). A final limitation of the present study is its cross-sectional design, which makes it impossible to draw causal inferences about the effect of cannabis exposure on executive attention performance.
Despite these limitations, the present findings suggest that executive attention may be an important prognostic factor in adolescents with EOS as well as adolescents at clinical high risk for developing schizophrenia. Studies of children and adolescents have shown that executive attention performance in the ANT is related to a temperamental measure of effortful control from parent reports of child behavior (Rueda et al., 2005). Failures in effortful control have in turn been related to increased risk-taking behavior in children and adolescents (Honomichl, 2012; Steinberg, 2008). One reason adolescents with psychosis may use cannabis is that the perceived benefits such as reduction in anxiety and increased sociability outweigh any perceived harmful consequences. It is possible that youth with attentional deficits are prone to use cannabis when the opportunity arises, and to become dependent on cannabis over time compared to adolescents that have developed higher levels of self-regulation. Executive attention and cognitive control have been increasingly studied as important predictors of outcome and achievement in healthy children (Diamond et al., 2007) and adolescents (Duckworth and Seligman, 2005). Future prospective studies of adolescents with prodromal features of schizophrenia are needed to examine whether executive attention and cognitive control are predictors of risk for cannabis misuse or for transition to psychosis.
Acknowledgement
We thank Dr. Kathryn Cullen and Dr. Bonnie Klimes-Dougan for their comments on an earlier draft of the manuscript, and Dr. Susanne Lee, who served as our statistical expert.
Role of the Funding Source
Funded by National Institute of Mental Health Grant MH073150-05 (Cannabis and Schizophrenia; to S.K.). The funding source played no role in study design, in the collection, analysis, or interpretation of the data, in the writing of this manuscript, or in the decision to submit it for publication.
Dr. Kumra has received research support from the National Alliance for Research on Schizophrenia and Depression and Otsuka Pharmaceutical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Kumra designed the study and wrote the protocol. Dr. Kumra and Ms. Epstein managed the literature searches, conducted statistical analyses, and wrote the manuscript together. Both authors contributed to and have approved the final manuscript.
Conflict of Interest
Ms. Epstein has no financial disclosures or potential conflicts of interest to report.
REFERENCES
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav. Brain Res. 2010;215(1):45–57. doi: 10.1016/j.bbr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Kofman O, Livneh U, Henik A. Multidisciplinary perspectives on attention and the development of self-regulation. Prog. Neurobiol. 2007;82(5):256–286. doi: 10.1016/j.pneurobio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Breton F, Plante A, Legauffre C, Morel N, Ades J, Gorwood P, Ramoz N, Dubertret C. The executive control of attention differentiates patients with schizophrenia, their first-degree relatives and healthy controls. Neuropsychologia. 2011;49(2):203–208. doi: 10.1016/j.neuropsychologia.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Seligman ME. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychological science. 2005;16(12):939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a"local to distributed" organization. PLoS computational biology. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC neuroscience. 2001;2:14. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Jr, Venkatraman V, Roddey JC, Erhart M, McCabe C, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Darst BF, Schork NJ, Casey BJ, Chang L, Ernst TM, Gruen JR, Kaufmann WE, Kenet T, Frazier J, Murray SS, Sowell ER, van Zijl P, Mostofsky S, Jernigan TL, Dale AM, Pediatric Imaging N, Genetics S. Multimodal imaging of the self-regulating developing brain. Proc. Natl. Acad. Sci. U. S. A. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Liederman J, Malmberg B, McLean J, Strickland D, Beauchamp MS. Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cereb. Cortex. 2010;20(11):2625–2635. doi: 10.1093/cercor/bhq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Barnea A, Herzberg K, Rassis A, Neta M, Raz A, Zaidel E. Measuring attention in the hemispheres: the lateralized attention network test (LANT) Brain Cogn. 2008;66(1):21–31. doi: 10.1016/j.bandc.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E, Ta TM, Hahn C, Kuehl LK, Ruehl C, Neuhaus AH, Dettling M. Test-retest reliability of Attention Network Test measures in schizophrenia. Schizophr. Res. 2011;133(1–3):218–222. doi: 10.1016/j.schres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Honomichl RDD, M B. Dimensions of Temperament in Preschoolers Predict RIsk Taking and Externalizing Behaviors in Adolescents. Social Psychology and Personality Science. 2012;3(1):14–22. [Google Scholar]
- Jepsen JR, Fagerlund B, Pagsberg AK, Christensen AM, Nordentoft M, Mortensen EL. Deficient maturation of aspects of attention and executive functions in early onset schizophrenia. Eur. Child Adolesc. Psychiatry. 2010;19(10):773–786. doi: 10.1007/s00787-010-0126-4. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz-Dahlmann B, Fink GR. Development of attentional networks: an fMRI study with children and adults. Neuroimage. 2005;28(2):429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kumra S, Robinson P, Tambyraja R, Jensen D, Schimunek C, Houri A, Reis T, Lim K. Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(2):171–180. doi: 10.1016/j.jaac.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Liu G, Hu PP, Fan J, Wang K. Gender differences associated with orienting attentional networks in healthy subjects. Chin. Med. J. (Engl) 2013;126(12):2308–2312. [PubMed] [Google Scholar]
- Mullane JC, Corkum PV, Klein RM, McLaughlin EN, Lawrence MA. Alerting, orienting, and executive attention in children with ADHD. Journal of attention disorders. 2011;15(4):310–320. doi: 10.1177/1087054710366384. [DOI] [PubMed] [Google Scholar]
- Orellana G, Slachevsky A, Pena M. Executive attention impairment in first-episode schizophrenia. BMC psychiatry. 2012;12:154. doi: 10.1186/1471-244X-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev. Neurosci. 2008;30(1–3):24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Posner MI. Imaging attention networks. Neuroimage. 2012;61(2):450–456. doi: 10.1016/j.neuroimage.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18(9):383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nature reviews. Neuroscience. 2006;7(5):367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Rosenberg NL, Grigsby J, Dreisbach J, Busenbark D, Grigsby P. Neuropsychologic impairment and MRI abnormalities associated with chronic solvent abuse. J. Toxicol. Clin. Toxicol. 2002;40(1):21–34. doi: 10.1081/clt-120002883. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing Mechanisms of Self-Regulation in Early Life. Emotion review : journal of the International Society for Research on Emotion. 2011;3(2):207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proc. Natl. Acad. Sci. U. S. A. 2005;102(41):14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Schmidt L, Tassone F, Simon TJ. Catechol-O-methyltransferase polymorphism modulates cognitive control in children with chromosome 22q11.2 deletion syndrome. Cognitive, affective & behavioral neuroscience. 2009;9(1):83–90. doi: 10.3758/CABN.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek C, Neuhaus AH, Opgen-Rhein C, Strathmann S, Wieseke N, Schaub R, Hahn E, Dettling M. Attention network test (ANT) reveals gender-specific alterations of executive function in schizophrenia. Psychiatry Res. 2009;168(2):102–109. doi: 10.1016/j.psychres.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Vilar-Lopez R, Takagi M, Lubman DI, Cotton SM, Bora E, Verdejo-Garcia A, Yucel M. The effects of inhalant misuse on attentional networks. Developmental neuropsychology. 2013;38(2):126–136. doi: 10.1080/87565641.2012.745547. [DOI] [PubMed] [Google Scholar]
- Wang K, Fan J, Dong Y, Wang CQ, Lee TM, Posner MI. Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr. Res. 2005;78(2–3):235–241. doi: 10.1016/j.schres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Achievement Test, 3rd Edition (WRAT-3) Manual. Wilmington, DE: Wide Range, Inc.; 1993. [Google Scholar]
- Yucel M, Takagi M, Walterfang M, Lubman DI. Toluene misuse and long-term harms: a systematic review of the neuropsychological and neuroimaging literature. Neurosci. Biobehav. Rev. 2008;32(5):910–926. doi: 10.1016/j.neubiorev.2008.01.006. [DOI] [PubMed] [Google Scholar]