Abstract
Epidemiological studies have demonstrated that heavy drinking and alcohol abuse and dependence peak during the transition between late adolescence and early adulthood. Studies in animal models have demonstrated that alcohol exposure during adolescence can cause a modification in some aspects of behavioral development, causing the “adolescent phenotype” to be retained into adulthood. However, the “adolescent phenotype” has not been studied for a number of behavioral tests. The objective of the present study was to investigate the ontogeny of behaviors over adolescence/young adulthood in the light/dark box, open field conflict and forced swim test in male Wistar rats. These data were compared to previously published data from rats that received intermittent alcohol vapor exposure during adolescence (AIE) to test whether they retained the “adolescent phenotype” in these behavioral tests. Three age groups of rats were tested (post-natal day (PD) 34–42; PD55-63; PD69-77). In the light/dark box test, younger rats escaped the light box faster than older adults, whereas AIE rats returned to the light box faster and exhibited more rears in the light than controls. In the open field conflict test, both younger and AIE rats had shorter times to first enter the center, spent more time in the center of the field, were closer to the food, and consumed more food than controls. In the forced swim test no clear developmental pattern emerged. The results of the light/dark box and the forced swim test do not support the hypothesis that adolescent ethanol vapor exposure can “lock-in” all adolescent phenotypes. However, data from the open field conflict test suggest that the adolescent and the AIE rats both engaged in more “disinhibited” and food motivated behaviors. These data suggest that, in some behavioral tests, AIE may result in a similar form of behavioral disinhibition to what is seen in adolescence.
Keywords: Adolescence, Anxiety, Depression, Disinhibition, Ethanol Vapor
1. Introduction
Adolescence is a period between childhood and adulthood that is defined both behaviorally and biologically, and has been suggested to encompass almost the entire second decade of life (10–20 yrs) (Spear, 2000). It has been suggested that during this time individuals learn to function more independently, and fluency in emotional and social functioning is acquired (Dahl and Spear, 2004). While adolescence is usually defined by a series of endocrine events resulting in puberty, it actually involves changes in a number of organ systems including the brain that may occur in a separate time frame from endocrine events (Spear and Varlinskaya, 2010). During this developmental period there are changes in neurobiological organization and behavior that appear to be generally conserved during evolution with similarities seen across a number of mammalian species. In the rat it has been suggested, as a conservative estimate, that the adolescent period may span post-natal days 28–42 (Ojeda and Skinner, 2006; Spear and Brake, 1983; Varlinskaya et al., 1999). However depending on gender and the measures used to define adolescence, peri-adolescence may be seen as early as post-natal day (PD) 22 in females, and it may last until PD55 in males (Spear, 2000).
Adolescence is also a critical stage of brain development when humans initiate a range of exploratory behaviors that can result in exposure to ethanol and other drugs of abuse (Clark et al., 2008; Squeglia et al., 2009a). Since the brain continues to develop throughout the adolescent period into early adulthood (Markus and Petit, 1987; Sowell et al., 1999a,b), ethanol exposure during this time period may have unique and deleterious consequences including changes in disinhibitory, cognitive, and affectively driven behaviors. Several studies in humans have demonstrated that early alcohol exposure is associated with neurobehavioral deficits as measured by magnetic resonance imaging (MRI) scans and psychological testing (Hanson et al., 2011; McQueeny et al., 2009; Schweinsburg et al., 2011; Squeglia et al., 2009b). However, it is still not entirely clear whether these deficits are caused by alcohol exposure or are a result of pre-existing conditions (Nagel et al., 2005). The use of animal models of adolescent alcohol exposure allows for the testing of the direct effects of alcohol on the developing brain under more controlled conditions.
Exposure to chronic intermittent ethanol (CIE) during adolescence in rats has been demonstrated to cause a number of changes in brain and behavior that persist into adulthood. It has been demonstrated that chronic intermittent exposure to ethanol vapor during adolescence (14 hours exposure/10 hours no exposure, daily, over a 5-week period) can cause: increases in voluntary ethanol drinking, reductions in the size of the hippocampus as imaged by diffusion tensor imaging, reductions in measures of hippocampal neurogenesis, increases in the latency of the P300 component of the event-related potential, signs of behavioral disinhibition in the light/dark box and open field conflict test, immobility in the forced swim test, and reductions in cholinergic tone in the basal forebrain (see Criado and Ehlers, 2013; Ehlers et al., 2011, 2013a,b, 2014).
Studies in animal models have also shown that adolescent rats are less sensitive than adult rats to the effects of acute alcohol administration on hypothermia, motor in-coordination, sedation, as well as electrophysiological effects (Pian et al., 2008a; Silveri and Spear, 1998, 2000). Adolescent rats display greater tolerance to the sedative effects of acute ethanol administration than adult rats, and regain consciousness at higher blood alcohol levels (Pian et al., 2008b; Silveri and Spear, 1998). For adult rats exposed to CIE during adolescence, the adolescent phenotype of reduced sensitivity to ethanol has been shown to be “retained” in behavioral measures (Slawecki, 2002; White et al., 2000 a,b), electrophysiological measures in the hippocampus (Fleming et al., 2012, 2013; Slawecki, 2002), and in the cortex (Ehlers et al., 2013c, 2014; Slawecki, 2002). Some authors have suggested that adolescent ethanol exposure may “lock-in” adolescent sensitivity to ethanol and then sustain it into adulthood (Fleming et al., 2012). However, whether adolescent alcohol exposure “locks-in” the adolescent phenotype of other behaviors is not as well understood.
The present study investigated whether adolescent intermittent ethanol (AIE) could “lock-in” the adolescent phenotype in behaviors using three different test apparatus: the open field conflict, the light/dark box and the forced swim test. Data on the normal ontogenic development of behaviors in these tests over the course of adolescence and adulthood is sparse. Therefore we examined whether adolescent Wistar rats at different ages would display more or less “anxiety-like” and “depressive-like” behaviors in these tests as compared to adult rats. We examined three age groups of rats ranging from middle adolescence to early adulthood to determine the normal ontogenic responses in adolescence (group 1: PD34-42; group 2: PD55-63; group 3: PD69-77). We compared these groups to previously published data from control adult rats and adult rats that underwent AIE as adolescents and were tested in the behavioral apparatuses between PD70 to PD126. It was our hypothesis that adult rodents with ethanol exposure during adolescence may exhibit a behavioral profile that is more reminiscent of an adolescent animal rather than an adult.
2. Methods
2.1. Animals
The animals used in this study were male Wistar rats that were received and weaned at post-natal day (PD) 21 (n = 144 juveniles, Charles River, USA). On PD22 the adolescent animals were housed three per cage in standard plastic cages until PD63 to PD70, and then they were pair-housed for the rest of the experiment. Animals were kept in a light/dark (12 hrs light/12 hrs dark, lights on at 6:00am or 8:00am) and temperature-controlled environment. Food and water were available ad libitum throughout the experiment, except where noted. All experimental protocols were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
Three age groups of rats (group 1: PD34-42 (n = 15); group 2: PD55-63 (n = 15); group 3: PD69-77 (n = 18)) were run in three different behavioral paradigms (light/dark box, open field conflict and forced swim test) as described below. These data were then compared to previously published behavioral data (Ehlers et al., 2011, 2013a,b) on adult rats (PD70-107) that were exposed to 5 weeks of ethanol vapor or control (air) conditions during adolescence.
2.2. Light/Dark Box
The methodology used to test animals in the light/dark box has been described previously (see Ehlers et al., 2013a; Slawecki, 2005). Rats were tested at three different ages (group 1: PD34 (weight = 127.8 ± 2.2g); group 2: PD55 (weight = 303.8 ± 5.4g) group 3: PD69 (weight = 386.9 ± 7.3g)) and were compared to AIE rats (weight = 506.0 ± 10.8g) and their controls (542.1 ± 10.3g) at PD106-107. The apparatus consisted of two 34cm × 24cm × 24cm chambers joined together length-wise. Between the two chambers was an 8cm × 8cm square hole that the rat could use to move between chambers. One chamber was black and covered with a black lid (i.e. the dark box), while the other chamber was white, left uncovered, and had a 60 lux light shining on it (i.e. the light box). The light was located approximately 120cm above the apparatus and there was no appreciable illumination within the dark box (i.e. < 2 lux). During the test, an individual rat was placed in the center of the light box, facing 180 degrees away from the square hole connecting the two chambers, and was allowed to freely explore both chambers for a period of five minutes. Behaviors on the light side were recorded for later analysis. Both chambers were cleaned between sessions with a 3% acetic acid (vinegar) solution and then with water. Behaviors being analyzed include the latency time for entering the dark box, the number of rears in the light box, and the latency time for re-entering the light box. The comparative results of AIE on behavior in the light/dark box have been previously published (Ehlers et al., 2011, 2013a).
2.3. Open Field Conflict Test
Assessment of anxiety-like behavior/disinhibition was accomplished in the modified open field for three adolescent/early adult groups (group 1: PD38 (weight = 131.8 ± 2.3g); group 2: PD59 (weight = 291.3 ± 5.2g); group 3: PD73 (weight = 369.3 ± 7.2g)) and were compared to AIE rats (weight = 450.0 ± 11.0g) and their controls (weight = 496.8 ± 9.5g) at PD105-106. This procedure has been demonstrated previously in our lab to be highly sensitive to periadolescent drug exposure (Ehlers et al., 2012; Slawecki et al., 2003). The description of the test apparatus as well as the comparative results of vapor exposure on their behavior has been previously published (Ehlers et al., 2011, 2013b). Rats were placed for five minutes in a modified open field with a 3×3 grid pattern in a 122cm × 122cm × 46cm box. The center grid square was lighted (100 lux) and a single 5 gram food pellet was placed in a plastic holder in the center. This test provided an assessment of anxiety (approaching or retreating from the light) and disinhibition (overall movement towards the middle center) in the context of a motivator (the food pellet in the light), thus producing a behavioral conflict situation.
To start each test, the rat was positioned away from the light and food, head facing the left bottom corner. The light was located above the center of the apparatus. To ensure that the food pellet was a strong motivator, the animals were food deprived for 24 hours prior and the pellet was coated with peanut butter (Jif brand, creamy). To prevent the possibility that the food would be considered a novelty during the test, the animals were exposed to food pellets coated in peanut butter one week prior to testing. Each food pellet with peanut butter was weighed before and after the experiment to assess how much the rat had eaten. Between subjects the apparatus was cleaned with a 3% acetic acid (vinegar) solution then water, and a new food pellet was placed in the holder. Animals were videotaped on a low light sensitive camera with a 3-watt infrared light and the videos were later analyzed with ANY-maze Video Tracking System (version 4.84, Wood Date, IL: Stoelting Co.). The latency to first entry into the middle center square, total time in the middle center, mean distance from food pellet, and total food eaten were assessed.
2.4. Forced Swim Test
Immobility in the forced swim test has been demonstrated to be enhanced in animals that experience periadolescent alcohol vapor exposure (Ehlers et al., 2011; Slawecki et al., 2004) as well as adults exposed to ethanol vapors (Walker et al., 2010). Three adolescent/early adulthood groups were assessed (group 1: PD42 (weight = 183.7 ± 3.3g), group 2: PD63 (weight = 339.9 ± 6.1g), and group 3: PD77 (weight = 410.3 ± 8.1g) and were compared to AIE rats (weight = 353.5 ± 6.8g) and their controls (weight = 370.5 ± 8.5g) at PD70. The apparatus used for this test was a white plastic tub (diameter = 34cm, height = 66cm) filled to a height of 48cm of water at a temperature of 24–26 degrees Celsius. A light was placed above the apparatus and the water surface registered a light reading of 60 lux. A video camera was set up above to observe the entire surface of water and the edges of the apparatus. Two swim sessions were conducted on two consecutive days. On the first day, sessions were ten minutes and no behaviors were recorded. On the second day, sessions were five minutes and the behaviors were videotaped. Two researchers later analyzed the video independently and there was less than a 10% deviation between scorers on all parameters that were evaluated. The behaviors that were measured during the five minute test session consisted of number of sinks, total immobile time, time to first immobile episode, and wall climbing time.
2.5. Ethanol Vapor Exposure
Data from previously published studies evaluating the effect of adolescent ethanol exposure on behavior for the three different tests (light/dark box, open field conflict, and forced swim test) were compared to the data collected in the present study at the three different time points over adolescence/young adulthood. The ethanol vapor inhalation procedure used in those studies has been previously described (Ehlers et al., 2011). In brief, 96 adolescent rats were randomly assigned to a control group (n = 38) or vapor exposed group (n = 58). The vapor group was housed in sealed chambers and received vaporized 95% ethanol in air for five weeks. Ethanol vapor infusion began at the start of the rodent’s dark cycle (6 or 8pm) and ended 14 hours later (8 or 10am). For the remaining 10 hours, ethanol vapor was not infused into the chambers. Control rats were in similar cages but were exposed to ambient air, and were handled identically to ethanol-exposed rats. All rats started the exposure when they were 22 days old and continued it until they were 57 days old. The chambers were calibrated to produce high to moderate blood alcohol levels (BALs) between 150–225 mg/dL (BAL grand average = 157.5 ± 4.2 mg/dL). Tail tip bleeds were administered at least once a week. BALs were determined using the Analox micro-statGM7 (Analox Instr. Ltd., Lunenberg, MA). The control rats also had blood removed from their tails at the same time points. Following the 5 week exposure, the animals were transferred to standard vivarium cages for the duration of the study. Body weights and blood ethanol concentrations before, during, and after ethanol exposure have been previously published (Ehlers et al., 2011). Ethanol and control exposed rats were run in: (1) the light/dark box (22 AIE, 16 controls) on PD106-107, 49–50 days post ethanol/air exposure, (2) the open field conflict (12 AIE, 10 controls) on PD105-106, 48–49 days post ethanol/air exposure, and (3) the forced swim test (24 AIE, 12 controls) on PD70, 13 days post ethanol/air exposure.
2.6. Statistical Analysis
The first aim of the study was to determine if the three ontogenic groups and adult controls differed on their responses to the behavioral tests. The second aim was to test whether AIE exposure produced responses suggestive of a “retention” of the adolescent phenotype as compared to controls. To test these aims, analysis of variance (ANOVA) was carried out using the statistical program SPSS (version 15.0, Chicago, IL: SPSS Inc.). If the ANOVA was significant (p < 0.05), it was followed by Dunnett’s pairwise 2-tailed t-test, a planned comparison that served as a post hoc against a reference group. For the post hocs, the adult controls for the alcohol vapor group were used as the reference group against the three ontogenic groups. The weights between the AIE and control rats differed in the open field conflict test, but not in the other two behavioral tests. The previously published AIE and control rat data controlled for body weight (univariate ANOVA, co-varied for body weight) in the open field conflict test (Ehlers et al., 2013b). All means are reported as mean ± standard error.
3. Results
3.1. Light/Dark Box
Behavior in the light/dark box was assessed in three groups of animals: group 1: post-natal day (PD) 34; group 2: PD55; group 3: PD69 and the results are presented in Table 1. Two different data analyses were conducted to determine if there was: (1) an ontogenic effect between young animals and older adults and (2) an effect due to AIE exposure.
Table 1.
Effects of AIE (adolescent intermittent ethanol vapor) and ontogenic development (adolescence to adulthood) on the behaviors measured in the light/dark box.
| Latency to Dark (s) |
Latency Back to Light (s) |
Rears in Light |
|
|---|---|---|---|
| PD34 | 27.60 ± 8.3* | 59.27 ± 14.5 | 8.07 ± 2.9 |
| PD55 | 57.00 ± 15.2 | 50.10 ± 13.1 | 13.47 ± 2.1 |
| PD69 | 86.00 ± 13.6 | 61.20 ± 11.8 | 13.67 ± 1.6 |
| PD106-7 AIR | 74.87 ± 12.1 | 48.15 ± 5.6 | 13.31 ± 1.5 |
| PD106-7 AIE | 57.77 ± 6.6 | 34.67 ± 3.7* | 19.36 ± 1.6* |
AIE and Adult ethanol vapor air controls = postnatal day (PD) 106-7, Group 1 = PD34, Group 2 = PD55, Group 3 = PD69. Ethanol vapor and air control data have been previously published and included for reference to current study. Data reported as mean ± SE.
p < 0.05: statistically significant difference from the adult controls, PD106-7 AIR (ANOVA, Dunnett’s test)
When comparing the three ontogenic adolescent/young adult groups directly with the older adults, younger animals differed from older animals in the amount of time they spent initially in the light box before entering the dark box. An age effect was observed (F = 4.24, df = 3,55, p < 0.0092). Post hoc analyses of the data revealed that the youngest adolescent rats at post-natal age 34 (group 1, mean = 27.6 ± 8.3s) had significantly shorter latencies for entering the dark box than older adults at post-natal age 106–107 (mean = 74.9 ± 12.1s, p < 0.025). No significances were detected for the ontogenic groups with the latency back to the light box or the rears displayed in the light box.
A comparison of the behavior of AIE exposed rats to controls in the light/dark box has been reported previously (Ehlers et al., 2013a). AIE exposed rats spent a significantly shorter time in the dark box before returning to the light box and they exhibited more rears while in the light box. However, there was no significant effect of ethanol vapor for the latency to enter the dark box.
3.2. Open Field Conflict Test
Behavior in the modified open field conflict test was assessed for the ontogenic groups (group 1: PD38; group 2: PD59; group 3: PD73), and for the control and AIE exposed group at PD105-106. The data was co-varied by body weight and the reported means were unadjusted.
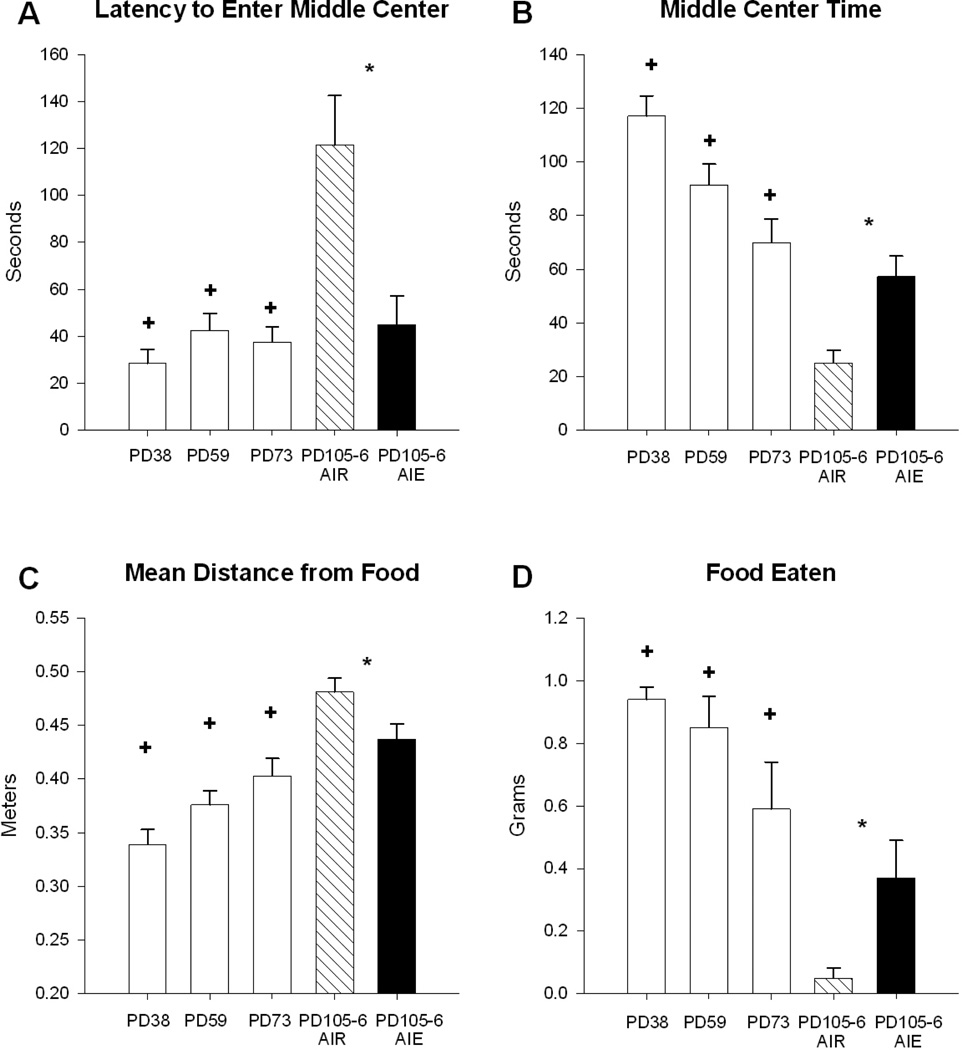
A comparison of the three younger ontogenic groups and the older adults was conducted in order to determine if behaviors in this apparatus change over adolescent/young adult development. Shorter times to the first entry into the center square were observed for all the younger groups (means = 28.44 – 42.47s) as compared to the older adults (mean = 121.47 ± 21.1s) (F = 15.89, df = 3,53, p < 0.001; post hocs: all p’s < 0.001; Fig.1A). All three of the younger animal groups (means = 69.95 – 117.07s) spent more time in the middle center square than older adults (mean = 25.13 ± 4.7s) (F = 18.18, df = 3,53, p < 0.001; post hocs: all p’s < 0.002; Fig.1B). Younger animals also exhibited more motivated behaviors towards the food pellet than the older adults. All younger animals (means = 0.33 – 0.40m) stayed, on average, closer to the food than older adults (mean = 0.48 ± 0.01m) (F = 12.77, df = 3,53, p < 0.001; post hocs: all p’s < 0.003; Fig.1C). The food eaten during the experiment was much higher for all three younger groups (means = 0.59 – 0.94g) as compared to the older adults (mean = 0.05 ± 0.03g) (F = 10.55, df = 3,54, p < 0.001; post hocs: all p’s < 0.005; Fig.1D).
Figure 1.
(A-D): Behavioral measures in the open field conflict test. Group 1: post-natal day (PD) 38; group 2: PD59; group 3: PD73 (open bars) and were compared to ethanol vapor controls (lined bars) at PD105-106. Ethanol vapor control animals (AIR) (lined bars) were also compared to adolescent intermittent ethanol vapor (AIE) rats (black bars) of same age (data previously published and included for reference to current study). (A) All ontogenic groups and AIE treated rats took significantly less time to enter the lit middle center of the open field and spent more time there (B) compared to older controls. (C) Controls were on average further from the food pellet than AIE treated and all ontogenic groups. (D) Controls ate less food than all ontogenic groups and AIE treated animals. All means are unadjusted. * p<0.01 AIE effect and co-varied by body weight, + p<0.01 ontogenic age effect (ANOVA, Dunnett’s test).
A comparison of the behavior of AIE exposed rats to controls in the open field conflict, co-varied for body weight, has been reported previously (Ehlers et al., 2013b). AIE exposed rats: first entered the center square faster, spent more time in the center square, ate more food, and were a shorter mean distance from the food square than the control group. This suggests that ethanol exposure resulted in more disinhibition-type and food-motivated behaviors in the open field conflict test not unlike what was observed for adolescents.
3.3. Forced Swim Test
The forced swim test was conducted in the ontogenic groups (group 1: PD42; group 2: PD63; group 3: PD77) and for the AIE exposed and control adult groups at PD70. These data are presented in Table 2.
Table 2.
Effects of AIE (adolescent intermittent ethanol vapor) and ontogenic development (adolescence to adulthood) on the behaviors measured in the forced swim test.
| Time to Immobile (s) |
Total Immobile Time (s) |
Number of Sinks |
Climb Time (s) |
|
|---|---|---|---|---|
| PD42 | 86.47 ± 14.0* | 152.83 ± 13.3 | 0.00 ± 0.0* | 52.43 ± 7.0 |
| PD63 | 56.93 ± 9.6* | 162.97 ± 16.2* | 0.53 ± 0.4* | 53.20 ± 5.3 |
| PD77 | 82.06 ± 14.6* | 114.75 ± 18.4 | 0.72 ± 0.4* | 62.39 ± 9.3 |
| PD70 AIR | 145.42 ± 20.6 | 101.92 ± 18.1 | 3.42 ± 1.4 | 81.42 ± 8.9 |
| PD70 AIE | 175.25 ± 17.1 | 69.75 ± 12.7 | 9.00 ± 1.9 | 110.46 ± 10.7 |
AIE and Adult ethanol vapor air controls = postnatal day (PD) 70, Group 1 = PD42, Group 2 = PD63, Group 3 = PD77. Ethanol vapor and air control data have been previously published and included for reference to current study. Data reported as mean ± SE.
p < 0.05: statistically significant difference from the adult controls, PD90 AIR (ANOVA, Dunnett’s test)
No clear developmental pattern could be determined in the observed behaviors in the forced swim test. Significant differences between the ontogenic groups and the adult controls did occur for the two immobility measures and for the number of sinks but they were not associated with the age of the animal. Time to first instance of immobility was significantly faster for all three ontogenic groups (means = 56.93 – 86.47s) than the adult controls (mean = 145.42 ± 20.6s) (F = 5.62, df = 3,56, p < 0.002; post hoc: all p’s < 0.025). Controls rats at PD70 also had a higher number of sinks (mean = 3.42 ± 1.4) than the three ontogenic groups (means = 0.00 – 0.72) (F = 5.04, df = 3,56, p < 0.004; post hoc: p < 0.015). A weak significance was observed for total immobile time (F = 2.87, df = 3,56, p < 0.05). The post hoc analyses revealed that group 2 rats at PD63 (mean = 162.97 ± 16.2s) spent more time being immobile than the adult controls at PD70 (mean = 101.92 ± 18.1s) (post hoc: p < 0.05). No significances were observed in the amount of time spent climbing the walls.
Immobility measures (time to immobility and total immobility), number of sinks, and wall climbing time were also examined in the AIE exposed adults and their controls and these comparative results have been published in a previous paper (Ehlers et al., 2011). Those analyses showed that the AIE exposed animals had more episodes of immobility and/or number of sinkings than controls, and were significantly more likely to defecate during the test session.
4. Discussion
This study evaluated whether there were ontogenic differences in behavior in three behavioral testing apparatuses the light/dark, the open field conflict test, and the forced swim test. These data were then compared to previous data collected from adult rats with a history of ethanol exposure during adolescence, and their controls, in order to determine whether alcohol exposure caused retention of the “adolescent phenotype”. In behavioral circumstances involving disinhibition or food motivated behavior, the AIE exposed rats displayed some behaviors that resembled their adolescent counterparts.
The light/dark box is based on the innate aversion that rodents have of brightly illuminated areas and on the spontaneous exploratory behavior of rodents in response to mild stressors (Bourin and Hascoet, 2003; Ramos, 2008). Adolescent rats were found to move more quickly out of the light box and into the dark box. This decrease in latency to enter the dark may represent an overall increase in locomotion in the face of novelty or it could be interpreted as a measure consistent with increased caution or “anxiety-like” behaviors (Bourin and Hascoet, 2003; Ramos, 2008). If the decreased latency to enter the dark box was a result of overall hyperactivity then other measures of locomotion would be predicted to also be increased in this test. However, when compared to the older adults, no differences for the adolescent groups emerged in the latency to return to the light box or rears in the light. The finding in the present study that adolescent rats may be more “anxious” or “stressed” in the light is supported by a previous study that also examined anxiety-like behaviors across different ontogenic time points of adolescence. That study also found that anxiety-like behavior, in traditional behavioral tests, appears to decrease from adolescence into adulthood in rats (Lynn and Brown, 2010).
The AIE exposed adult rats demonstrated a different profile of behavior in the light/dark box. Ethanol rats returned to the light box much quicker and reared more often in the light than controls, indicating that the ethanol rats were not as inhibited in their exploratory movements by the light as the controls were. Heightened arousal and impulsivity may account for this increase of movement and exploration in the light. Increased entries to the light side of the light/dark box have been reported in adolescent rats that were exposed to alcohol during late adolescence (Hughes, 2011), but not in mice exposed to alcohol during early adulthood (Pang et al., 2013). This suggests that ethanol exposure during adolescence (as opposed to adulthood) may underlie some of the disinhibitory behaviors exhibited in the light/dark box by the ethanol exposed rats in our study. In the light/dark box, adolescents seemed to act with greater caution and fear of the anxiety-provoking stimuli (i.e. the bright light) while early ethanol exposure in the vapor rats appeared to produce a less cautious profile.
In the light/dark box, different strategies appear to have been employed by the adolescents and the AIE rats in response to the test conditions. In contrast, the open field conflict test seems to support our hypothesis that ethanol vapor exposure can “lock-in” certain adolescent phenotypes for disinhibitory and food motivated behaviors under certain testing conditions. Adolescent animals and AIE exposed adult rats employed similar behavioral strategies for a number of measurements in the open field conflict test. Both of the younger ontogenic groups and the adolescent ethanol vapor exposed rats engaged in more “disinhibited”, behaviors by initially entering more quickly and spending more time overall in the lighted center than control adults. The convergence of these behaviors between groups suggests that at least, in certain behavioral tests, AIE exposure may result in a superficially similar form of behavioral disinhibition to what is seen in adolescents.
When comparing across the light/dark box (where no reward was presented) and the open field conflict test (where a motivational reward was present), the ethanol rats did not change their strategies like their adolescent counterparts, in that they acted in a more “disinhibited” manner in both tests as compared to normal adults. The open field conflict test presented a condition of motivational conflict, where there were incentives to both inhibit and approach the stimuli (i.e. food in the bright light). Fillmore and Vogel-Sprott (2000) tested the effect of motivational conflict on a response inhibition test in male human adults 30 to 130 minutes after a moderate dose of alcohol. The addition of motivational conflict to the situation, where there were incentives to both inhibit and respond to the stimuli, did not intensify the degree to which alcohol impaired inhibitory control; the impairment was no greater than that observed under a conflicting condition where no reinforcement was present. These findings support our results of increased disinhibition in both behavioral tests, irrespective of whether there was a motivational component or not, in the AIE exposed adult rats. It further suggests that alcohol exposure may have the ability to weaken inhibitory control in a variety of contexts.
In the open field conflict test adolescents more readily approached the anxiety-provoking lighted area compared to the older adults. One potential interpretation of these behaviors is that the adolescent groups may “overcome” their reticence to approach the light because they are strongly motivated by the food in the field. The younger animals ate more food during the experiment and spent more time eating the food than the older adults. Relative to other age groups, adolescents exhibit a disproportionate amount of novelty-seeking, sensation seeking, and risk-taking behaviors (Spear, 2000; Steinberg, 2008). Steinberg (2008) has theorized that the substantial increase in sensation-seeking and risk-taking seen during adolescence is likely due to changes in reward salience and reward sensitivity. The fact that the adolescent rats in our study only approached the light in the behavioral test where the rat could obtain a highly desired reward appears to support this theory.
The forced swim test yielded more complicated results than the light/dark box and the open field conflict test. Younger animals did not exhibit a clear developmental difference from older adults. Significances were exhibited between the ontogenic group and the adult controls for the two measures of immobility (time to first instance of immobility and total immobility time) and the number of sinks, but no clear pattern related to age could be discerned from these findings. The comparative results of the AIE exposed adult group however did display more depressive-like or stress-like behaviors than the air-exposed controls (Ehlers et al., 2011). Previous studies with the forced swim test have shown increased immobility time in ethanol exposed rodents (Pang et al., 2013; Ribeiro-Carvalho et al., 2011; Slawecki et al., 2004; Walker et al., 2010). These data suggest that alcohol exposure and withdrawal can lead to more depressive-like or stress-like behavior; however, those responses do not appear to mimic any aspect of adolescent development.
In summary, our study revealed that the behavioral changes associated with AIE exposure in rats could at times coincide with behaviors specific to adolescents for particular circumstances. AIE exposure resulted in global disinhibition across the two tests associated with anxiety-like behaviors (the light/dark box and the open field conflict test) where the younger animals displayed disinhibition on a conditional basis, that is, in the presence of a reward in the open field conflict test. In the forced swim test no clear developmental pattern emerged. AIE exposed animals had more episodes of immobility and/or number of sinkings than controls, and were significantly more likely to defecate during the test session as compared to controls. The results of the light/dark box and the forced swim test do not support the hypothesis that AIE can “lock-in” adolescent phenotypes on those behavioral tests. However, data from the open field conflict test suggest that adolescent animals and adolescent ethanol vapor exposed adult rats may employ similar behavioral strategies in the open field conflict test. The convergence of these behaviors between groups suggests that at least, in certain behavioral tests, alcohol vapor exposure during adolescence may result in a similar form of behavioral disinhibition and/or food motivation to that which is seen in adolescents.
Highlights.
Binge drinking peaks between late adolescence and early adulthood
Anxiety, disinhibition and depressive behaviors were measured during adolescence
Adolescent animals and alcohol exposed animals showed disinhibition
Acknowledgements
This study was supported in part by the National Institutes of Health (NIH), National Institute on Alcoholism and Alcohol Abuse grants, AA006059 and AA019969 awarded to CLE. The authors thank Shirley Sanchez for assistance in editing the manuscript.
Abbreviations
- AIE
adolescent intermittent ethanol
- ANOVA
analysis of variance
- BALs
blood alcohol levels
- CIE
chronic intermittent ethanol
- PD
post-natal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bourin M, Hascoet M. The mouse light/dark box test. Eur. J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin. Exp. Res. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent onset voluntary drinking followed by ethanol vapor exposure on subsequent ethanol consumption during protracted withdrawal in adult Wistar rats. Pharmacol. Biochem. Behav. 2013;103:622–630. doi: 10.1016/j.pbb.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. New York, N.Y: The New York Academy of Sciences. 2004 doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Havstad J. Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res. 2012;1450:67–79. doi: 10.1016/j.brainres.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Oguz I, Budin F, Wills DN, Crews FT. Peri-adolescent ethanol vapor exposure produces reductions in hippocampal volume that are correlated with deficits in prepulse inhibition of the startle. Alcohol Clin. Exp. Res. 2013a;37:1466–1475. doi: 10.1111/acer.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013b;244C:1–15. doi: 10.1016/j.neuroscience.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Desikan A, Wills DN. Developmental differences in EEG and sleep responses to acute ethanol administration and its withdrawal (hangover) in adolescent and adult Wistar rats. Alcohol. 2013c;47:601–610. doi: 10.1016/j.alcohol.2013.09.040. Eub 2013 Oct 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Desikan A, Wills DN. Event-related potential responses to the acute and chronic effects of alcohol in adolescent and adult Wistar rats. Alcohol Clin. Exp. Res. 2014 Jan 31; doi: 10.1111/acer.12299. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Response inhibition under alcohol: effects of cognitive and motivational conflict. J Stud Alcohol. 2000;61:239–246. doi: 10.15288/jsa.2000.61.239. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcohol Clin. Exp. Res. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Li Q, Risher ML, Sexton HG, Moore SD, Wilson WA, et al. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABA(A) receptor-mediated tonic inhibition in dentate granule cells. Alcohol Clin. Exp. Res. 2013;37:1154–1160. doi: 10.1111/acer.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of adolescent alcohol and drug use on neuropsychological functioning in young adulthood: 10-year outcomes. J Adolesc Subst Abuse. 2011;20:135–154. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Adult anxiety-related behavior of rats following consumption during late adolescence of alcohol alone and in combination with caffeine. Alcohol. 2011;45:365–372. doi: 10.1016/j.alcohol.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Lynn DA, Brown GR. The ontogeny of anxiety-like behavior in rats from adolescence to adulthood. Dev. Psychobiol. 2010;52:731–739. doi: 10.1002/dev.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus EJ, Petit TL. Neocortical synaptogenesis, aging, and behavior: lifespan development in the motor-sensory system of the rat. Exp. Neurol. 1987;96:262–278. doi: 10.1016/0014-4886(87)90045-8. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin. Exp. Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Skinner MK. Puberty in the rat. In: Knobil E, Neill JD, editors. Knobil and Neill's physiology of reproduction. Amsterdam: Elsevier; 2006. pp. 2061–2126. [Google Scholar]
- Pang TY, Renoir T, Du X, Lawrence AJ, Hannan AJ. Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur. J Neurosci. 2013;37:1803–1810. doi: 10.1111/ejn.12195. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008a;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Ehlers CL. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcohol Clin. Exp. Res. 2008b;32:2062–2073. doi: 10.1111/j.1530-0277.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol. Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A, Lima CS, Nunes-Freitas AL, Filgueiras CC, Manhaes AC, Abreu-Villaca Y. Exposure to nicotine and ethanol in adolescent mice: effects on depressive-like behavior during exposure and withdrawal. Behav. Brain Res. 2011;221:282–289. doi: 10.1016/j.bbr.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin. Exp. Res. 2002;26:246–254. [PubMed] [Google Scholar]
- Slawecki CJ. Comparison of anxiety-like behavior in adolescent and adult Sprague-Dawley rats. Behav. Neurosci. 2005;119:1477–1483. doi: 10.1037/0735-7044.119.6.1477. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol. Biochem. Behav. 2003;75:355–361. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann. N. Y. Acad. Sci. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999a;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999b;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev. Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev. Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin. EEG. Neurosci. 2009a;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav. 2009b;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000a;24:1251–1256. [PubMed] [Google Scholar]
- White AM, Matthews DB, Best PJ. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus. 2000b;10:88–93. doi: 10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]