Oncolytic virus (OV) therapy with T-VEC for patients with advanced melanoma in a successful phase III clinical trial has showcased the potential of this novel modality of cancer treatment. OVs kill cancer cells and associated stromal cells via multimodal mechanisms, killing directly by infection and oncolysis, indirectly by vasculature-targeting, bystander effect and elicited antitumor immunity. For most OVs, the antitumor immunity elicited by the OV contributes to, or even plays essential roles in the overall efficacy of the oncolytic virotherapy (OVT).1–3
We and others believe that OVT will be much more efficacious when combined with other modalities of cancer treatments (Figure 1).4 In this short communication, we would like to expand and update this idea of multimodal therapy using OVs as a fruitful platform. Generally speaking, multimodal therapy is more effective than single-agent for cancer treatment. In 1998, Chiocca and colleagues pioneered such an approach by showing that prodrug-enzyme therapy can be integrated into OVT. They had designed a herpes simplex viruses type 1 (HSV-1) with an inactivated gene for viral ribonucleotide reductase (ICP6) to target tumor cells with upregulated mammalian ribonucleotide reductase (mRR), an enzyme whose expression is regulated by the p16/pRB tumor suppressor pathway. A recombinant HSV-1 was generated with ICP6 gene-deletion and expressing rat CYP2B1 transgene, whose product can activate the prodrugs cyclophosphamide and ifosfamide. The recombinant HSV replicated selectively in mRR-expressing cancer cells. Addition of cyclophosphamide potentiated oncolytic effects against cultured tumor cells and subcutaneous tumor xenografts in athymic mice.5 This and many other studies later on have demonstrated the potentials of such combinatorial strategies in both preclinical studies and clinical trials.
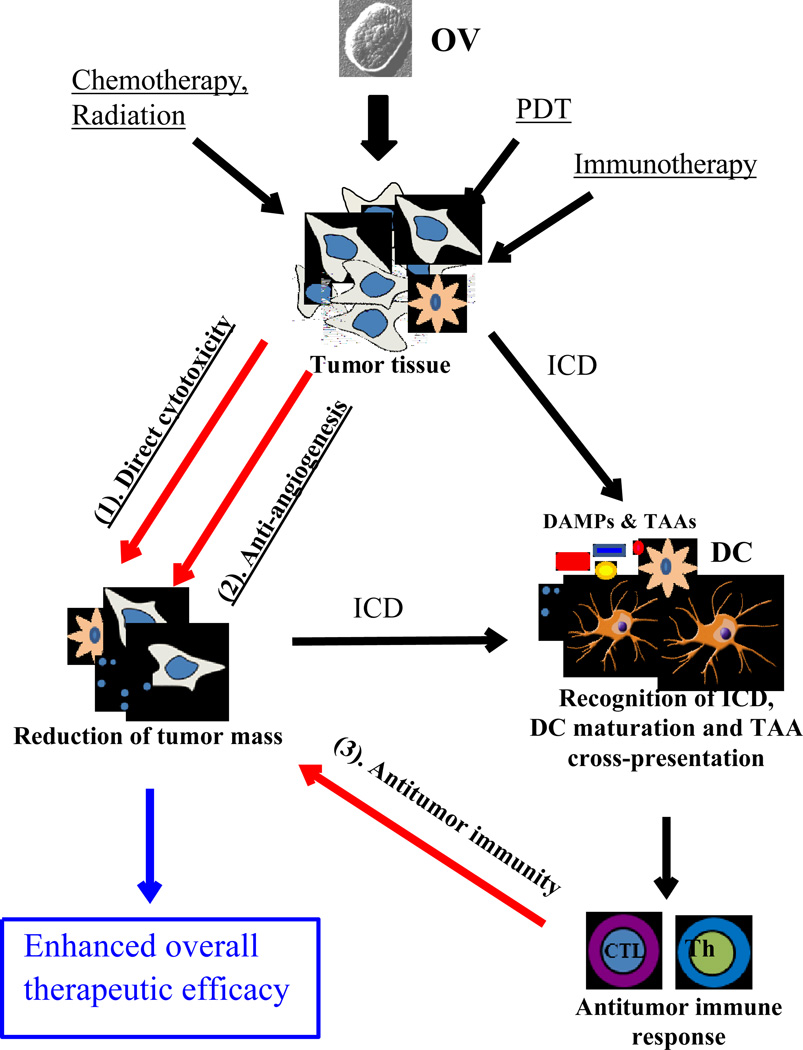
Figure 1.
Combination therapy with OV may enhance the overall therapeutic efficacy through multimodal modality mechanisms. Some chemotherapeutic drugs, radiation, photodynamic therapy (PDT) lead to direct cytotoxicity, and induce immunogenic cancer cell death (ICD), leading to danger signals (DAMPs) and TAAs to dendritic cells and thus further potentiates the antitumor immunity co-initiated by OV-mediated therapy. In addition, a number of therapeutics as well as OVT have been shown to be anti-angiogenic, which further induces massive cancer cell death. As a result, combinatorial therapies with OVs lead to enhanced efficacy.
Combination with chemotherapy or radiation therapy
We and many others have explored combining OVs with chemotherapy. The first approved oncolytic virus for clinical use in the world is to be used in conjunction with chemotherapy (Fluorouracil and cisplatin) for head and neck cancer. Two recent studies represent the latest developments in the field. OVs such as HSV can mediate DNA damage responses, and the combination with a chemotherapeutic agent (temozolomide) acts synergistically to kill glioma stem cells through the HSV-mediated manipulation of DNA damage responses. This strategy is highly efficacious in preclinical models and warrants further clinical study.6 The other study is about the immune consequence of cancer cell death induced by a particular OV and a specific chemotherapeutic drug and their potential impact on therapeutic outcome. It is thought that the cure of particularly aggressive malignancies may require induction of immunogenic cancer cell death (ICD), which leads to oncolysis and presentation of tumor-associated antigens (TAAs) coupling with exposure/release of danger signal molecules, such as cell surface exposure of calreticulin, and release of ATP, and high-mobility group box protein B1 (HMGB1) from dying tumor cells, eliciting a potent antitumor immunity. Angelova and colleagues have shown complementary induction of ICD by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer cells.7 These results warrant further studies of such combination therapy in animal tumor models.
The potential synergy between OVs and radiation may depend on the specific virus, specific genetic mutations of the cancer and type/dose of radiation. A recent study has found that synergistic cytotoxicity of radiation and oncolytic Lister strain vaccinia virus to BRAF mutant melanoma depends on JNK and TNF-α signaling.8 The virus GLV-1h68 combined with radiotherapy significantly increased cytotoxicity and apoptosis relative to either single agent in V600DBRAF/V600EBRAF mutant melanoma cells in vitro and in vivo. The mechanism of enhanced cytotoxicity from the combination was independent of viral replication but due to attenuation of JNK, p38 and ERK MAPK phosphorylation specifically in BRAF mutant cells.
Combination with vasculature targeting or photodynamic therapy
Many studies have shown that the OV alone or OV armed with angiogenesis inhibitors can work to inhibit tumor growth and metastasis.9; 10 Photodynamic therapy (PDT) is a clinically approved regimen for cancer treatment. PDT induced vascular disruption and resulted in higher viral titers in the tumor compared with the untreated tumor. Combination of PDT with oncolytic VV resulted in growth inhibition of primary and metastatic tumors compared with either monotherapy.11 PDT enhances viral replication in the tumor mass and the overall therapeutic efficacy. Intriguingly, Garg et al. have demonstrated that hypericin-based PDT (Hyp-PDT) is a bona fide ICD inducer. Hyp-PDT triggered a signaling pathway that leads to calreticulin (CRT) exposure and ATP release by cancer cells, a type of ICD, thus potentially eliciting antitumor immune responses.12 Therefore, multi-mechanistic pathways, including oncolysis, anti-angiogenesis and antitumor immunity, all contribute to therapeutic efficacy for oncolytic-PDT therapy.
Combination with targeted therapy on specific signaling pathways
Cancer cells often display alteration/mutations in specific signaling pathways, which have been utilized for targeting and selective replication of OVs.13 It is possible that inhibition/promotion of specific signaling pathways may promote OV viral replication and oncolysis, or simply promote cancer cell death, thus leading to improved therapeutic efficacy. As an example, MG18L, an oncolytic HSV with US3 deletion, replicated well in glioblastoma stem cells (GSCs), and had anti-glioblastoma activity in mice. PI3K/Akt inhibitors displayed significant but variable antiproliferative activities in GSCs. The combination of the two synergized in killing GSCs and glioma cells, but not human astrocytes, through enhanced induction of apoptosis. Somehow, synergy was independent of inhibitor sensitivity. In vivo, the combination significantly prolonged survival of mice. This study establishes a novel therapeutic strategy using the ability of an oncolytic HSV to manipulate critical oncogenic pathways to sensitize cancer cells to a molecularly targeted drug.14 OVT of gallbladder cancer with myxoma virus can be enhanced by hyaluronan (HA).15 The recent study has shown that HA interacts with CD44 to activate the Akt signaling pathway, which increases rates of oncolysis. HA also enhanced the MMP-9 secretion, which contribute to collagen IV degradation, thus the viral diffusion and spread.
Cancer cells often overexpress anti-apoptotic proteins and thus are resistant to apoptosis-inducing agents including a number of OVs. Smac mimetic compounds (SMC) are a class of drugs that sensitize cancer cells to apoptosis by counteracting the activity of inhibitor of apoptosis (IAP) proteins. Inflammatory cytokines can induce cancer cell death when combined with SMC. Korneluk and colleagues reasoned that SMCs would synergize with agents that stimulate a potent yet safe “cytokine storm”. They demonstrated that OVs and adjuvants such as poly (I:C) and CpG induce bystander death of cancer cells treated with SMCs. This is mediated by cytokines interferon beta (IFN-β), tumor necrosis factor alpha (TNF-α) and/or TNF-related apoptosis-inducing ligand (TRAIL). This combinatorial treatment resulted in tumor regression and extended survival in two murine tumor models.16
Combination with immunotherapy
As we have reviewed recently, OVT itself is a novel form of immunotherapy.1; 2 Many studies have focused on combination of OVs with cancer vaccines, adoptive immune cell transfer, immunostimulatory cytokines, Toll-like receptor ligands, and have otained promising results. We would like to discuss a few recent significant studies. We have utilized Th1-Tc1-attacting chemokine-armed oncolytic virus for combined oncolytic immunotherapy in a model of colorectal cancer.17 In the late stage of cancer, OV alone is insufficient to break immune tolerance in the highly immunosuppressive tumor microenvironment. It might be possible to induce an immune response if OV is combined with an ICD-inducing chemotherapeutic drug.18 The combination of a HSV-1 OV with mitoxantrone provided significant survival benefit to BALB/c mice bearing Her2/neu TUBO-derived tumors. This protection was mediated by increased infiltration of neutrophils and tumor antigen-specific CD8+ T cells into tumor. This study suggests that OVs in combination with an ICD-inducing drug can enhance cancer immunogenicity, breaking immunologic tolerance established toward these tumor antigens.18 Song and coworkers have made seminal discovery using an oncolytic VV that encodes a secretory bispecific T-cell engager consisting of two single-chain variable fragments specific for CD3 and the tumor cell surface antigen EphA2 [termed EphA2-T-cell engager-armed VV].19 The function of this virus is two-fold: first acting as an oncolytic virus and second as a virally secreted molecule to activate T cells. The OV plus human T cells had potent antitumor activity in a lung cancer xenograft model in comparison to either monotherapy. Thus, this armed oncolytic VV may represent a promising new approach to improve oncolytic virotherapy. The fourth study is combination of OV with immune checkpoint blockade. Recent investigations led to two important concepts that, first, preexisting lymphocytic infiltration of tumors is associated with superior prognostic outcomes in a variety of cancers; second, immune checkpoint blockade could work together with strategies that induce tumor inflammation. Built on these notions, Allison, Zamarin and coworkers have just shown that localized oncolytic virotherapy using an oncolytic Newcastle disease virus can overcome systemic tumor resistance to immune checkpoint blockade immunotherapy in the immunosuppressive B16 melanoma model.20 More specifically, localized intratumoral injection of NDV induces inflammatory responses, leading to lymphocytic infiltrates and antitumor effect in distant tumors without distant virus spread. Localized NDV injection and systemic CTLA-4 blockade led to rejection of pre-established distant tumors and protection from tumor rechallenge in poorly immunogenic tumor models, irrespective of tumor cell line sensitivity to NDV-mediated lysis. This study provides a strong rationale for clinical trials on such combination therapies in human cancer patients.20
Perspectives
The rationales of combination therapy are multifacious. First, specific OVs induce direct oncolysis of the cancer cells, usually via specific cell death pathways with one as a predominant pathway. Cancer cells have evolved multiple mechanisms to evade cell death, inhibiting one or more specific pathways of cell death. This provides a rationale for combining another therapeutic regimen to kill cancer cells through another death pathway, thus functionally additively or synergistically. Second, cancer cell death has immunological consequences. OVs often induce ICD, releasing/presenting danger signals, and present tumor-associate antigens (TAAs), which are critical initial steps in activating DCs and eliciting adaptive antitumor immunity. Thus it is logical to combine OV with some other form of immunotherapy to enhance the overall efficacy. Third, metastases are the main reason for cancer fatality. Many standard therapeutic regimens cannot target micrometastases and cancer stem cells effectively, resulting in cancer relapse. Immunotherapy is very effective in inhibiting distant metastases. A variety of anticancer cytotoxic treatments, such as a variety of modes of chemotherapy, radiation, photodynamic therapy, all lead to multimodal ICD as a consequence (Figure 1). Thus it is only rational to integrate immunotherapy into most therapeutic regimens in order to maximize the therapeutic efficacy, and prolong the disease progression-free survival. Some of these strategies have generated exciting and promising results in tumor models, and it is high time to test them in clinical trials.
Contributor Information
Z. Sheng Guo, Email: guozs@upmc.edu.
David L. Bartlett, Email: bartlettdl@upmc.edu.
REFERENCES
- 1.Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2:295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chase M, Chung RY, Chiocca EA. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998;16:444–448. doi: 10.1038/nbt0598-444. [DOI] [PubMed] [Google Scholar]
- 6.Kanai R, Rabkin SD, Yip S, Sgubin D, Zaupa CM, Hirose Y, et al. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst. 2012;104:42–55. doi: 10.1093/jnci/djr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelova AL, Grekova SP, Heller A, Kuhlmann O, Soyka E, Giese T, et al. Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer. J Virol. 2014;88:5263–5276. doi: 10.1128/JVI.03688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyula JN, Khan AA, Mansfield D, Karapanagiotou EM, McLaughlin M, Roulstone V, et al. Synergistic cytotoxicity of radiation and oncolytic Lister strain vaccinia in (V600D/E)BRAF mutant melanoma depends on JNK and TNF-alpha signaling. Oncogene. 2014;33:1700–1712. doi: 10.1038/onc.2013.112. [DOI] [PubMed] [Google Scholar]
- 9.Angarita FA, Acuna SA, Ottolino-Perry K, Zerhouni S, McCart JA. Mounting a strategic offense: fighting tumor vasculature with oncolytic viruses. Trends Mol Med. 2013;19:378–392. doi: 10.1016/j.molmed.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Gil M, Seshadri M, Komorowski MP, Abrams SI, Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110:E1291–E1300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gil M, Bieniasz M, Seshadri M, Fisher D, Ciesielski MJ, Chen Y, et al. Photodynamic therapy augments the efficacy of oncolytic vaccinia virus against primary and metastatic tumours in mice. Br J Cancer. 2011;105:1512–1521. doi: 10.1038/bjc.2011.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai R, Wakimoto H, Martuza RL, Rabkin SD. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin Cancer Res. 2011;17:3686–3696. doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng M, Gong W, Ma M, Chu B, Qin Y, Zhang M, et al. Targeting gallbladder cancer: oncolytic virotherapy with myxoma virus is enhanced by rapamycin in vitro and further improved by hyaluronan in vivo. Mol Cancer. 2014;13:82. doi: 10.1186/1476-4598-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beug ST, Tang VA, LaCasse EC, Cheung HH, Beauregard CE, Brun J, et al. Smac mimetics and innate immune stimuli synergize to promote tumor death. Nat Biotechnol. 2014;32:182–190. doi: 10.1038/nbt.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, O'Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, et al. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther. 2011;19:650–657. doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Workenhe ST, Pol JG, Lichty BD, Cummings DT, Mossman KL. Combining oncolytic HSV-1 with immunogenic cell death-inducing drug mitoxantrone breaks cancer immune tolerance and improves therapeutic efficacy. Cancer Immunol Res. 2013;1:309–319. doi: 10.1158/2326-6066.CIR-13-0059-T. [DOI] [PubMed] [Google Scholar]
- 19.Yu F, Wang X, Guo ZS, Bartlett DL, Gottschalk SM, Song XT. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol Ther. 2014;22:102–111. doi: 10.1038/mt.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]