Abstract
Objective
To assess the relationship between estimated residential maternal exposure to atrazine during pregnancy and risk for choanal atresia or stenosis in offspring.
Study Design
Data for 280 nonsyndromic cases and randomly selected, population-based controls delivered during 1999 to 2008 were obtained from the Texas Birth Defects Registry. County-level estimates of atrazine levels obtained from the United States Geological Survey were assigned to cases and controls based on maternal county of residence at delivery. Unconditional logistic regression was used to assess the relationship between maternal residential atrazine exposure and risk for choanal atresia or stenosis in offspring.
Results
Compared to offspring of mothers with low levels of estimated residential atrazine exposure, those with high levels had nearly a two-fold increase in risk for choanal atresia or stenosis (adjusted odds ratio: 1.79, 95% confidence interval: 1.17–2.74). A significant linear trend was also observed with increasing levels of atrazine exposure (adjusted P = 0.002).
Conclusions
A link between maternal exposure to endocrine disruptors, such as atrazine, and choanal atresia risk is plausible based on previous findings. Our results further support this hypothesis.
Keywords: Texas, epidemiology, maternal exposure, congenital malformations
INTRODUCTION
Choanal atresia and stenosis are characterized by a complete blockage or narrowing, respectively, of the opening between the posterior nasal cavity and the nasopharynx on the left side, right side, or both.1 Choanal atresia and stenosis are the most common craniofacial abnormalities of the nose and often require multiple corrective surgeries or can lead to life-threatening airway obstruction.2, 3 In spite of its clinical significance, the etiology of choanal atresia or stenosis in the absence of a chromosome abnormality or malformation syndrome or sequence is not well understood in humans.4
Although there has been limited research evaluating the etiology of nonsyndromic choanal atresia or stenosis, a few risk factors involved in endocrine function are suspected. For example, multiple studies have demonstrated that in utero exposure to hyperthyroid medications (i.e., methimazole and carbimazole, a carboxy derivative of methimazole) may increase risk for choanal atresia.5–11 The exact mechanism of this suspected teratogenic effect is unclear and a teratogenic role of the underlying hyperthyroidism has not been ruled out. Other mechanisms suspected to increase choanal atresia risk include suppression of retinoic acid and activation of fibroblast growth factor signaling pathways, both of which regulate endocrine function.12, 13
Atrazine, the most widely used herbicide in the U.S., has been suggested to have teratogenic effects for other birth defects and is considered to be a potent endocrine disruptor (i.e., a chemical that interferes with the normal function of the endocrine system).14–18 However, to our knowledge, atrazine exposure has not been evaluated with regards to choanal atresia or stenosis specifically. Given the plausibility of a possible teratogenic effect of atrazine via endocrine disruption, we evaluated the relationship between residential maternal exposure to atrazine during pregnancy and risk for choanal atresia or stenosis in offspring in Texas during 1999 to 2008.
METHODS
STUDY SUBJECTS
Analyses were conducted using data from the Texas Birth Defects Registry, which is an ongoing, population-based registry maintained by the Texas Department of State Health Services Birth Defects Epidemiology and Surveillance Branch (BDESB). The registry uses state-wide active case surveillance at hospitals, birthing centers, and midwife facilities to identify cases with birth defects, including live births, still births, and induced pregnancy terminations. Potential cases are included in the registry when a structural birth defect or chromosome abnormality is present and the mother resided in Texas at the time of delivery. Medical records data for each case are abstracted and case diagnoses are reviewed and classified using a six-digit code defined by the Centers for Disease Control and Prevention, which is based on the British Pediatric Association Classification of Diseases and the International Classification of Diseases, 9th Revision, Clinical Modification system (BPA code).19 Registry data is also linked to reproductive and sociodemographic data (e.g., maternal address at delivery) from birth and fetal death certificates obtained from the Vital Statistics Unit of the Texas Department of State Health Services. For the present analyses, corresponding vital records data were also obtained for potential controls, sampled from all live births in Texas during the study period. These data included infant sex, delivery date, and maternal race/ethnicity, birthplace, age, education, smoking (yes versus no), and history of live births. The protocol for this study was approved by the Institutional Review Board for the University of Texas Health Science Center at Houston.
Data for cases with documented postnatal diagnoses of choanal atresia or stenosis (BPA code 748.000) delivered between January 1, 1999 and December 31, 2008 were included in these analyses. Cases were not included when there was a choanal atresia or stenosis BPA code but written comments indicating that the case did not have choanal atresia or stenosis (N=64). To reduce the potential for etiologic heterogeneity among cases, all analyses were restricted to nonsyndromic cases (i.e., those without possible diagnoses of chromosome abnormalities or malformation syndromes or sequences). Further, analyses were repeated among the subset of isolated cases (i.e., nonsyndromic cases without additional major malformations). We also selected a random sample of controls without major malformations delivered during the study period, using a ratio of ten controls to one case.
EXPOSURE ASSESSMENT
Annual estimates of atrazine levels were obtained for all counties in Texas from the United States Geological Survey (USGS) for the period 1999–2007.20 Atrazine estimates from the USGS model are based on agricultural crops (crop acreage and reported crops) that were likely to have applications of atrazine. The method for developing these estimates has been described elsewhere.20 Briefly, the USGS method uses AgroTrak survey data on pesticide use and Census of Agriculture and National Agriculture Statistics Service data on harvested crop acreages for all counties in the contiguous Unites States. To ensure that atrazine use was accounted for in all geographic areas, atrazine crop application rates for Crop Reporting Districts (CRDs) not surveyed were derived from adjacent CRDs that had been surveyed. CRDs consist of multiple adjacent counties, which were grouped to represent similar geography, climate, and cropping practices. For this assessment, data on the counties in Texas were linked to maternal county of residence at delivery and year of delivery for cases and controls. Because data for 2008 were not available, 2007 atrazine data were used for 2008 deliveries. For main analyses, atrazine exposure was categorized as low, medium, medium-high, or high, defined by the distribution of atrazine levels in controls (i.e., based on cutoffs below the 25th percentile, above the 25th and less than the 75th percentile, above the 75th and less than the 90th percentile, and above the 90th percentile, respectively, as used by Reynolds et al.21).
STATISTICAL ANALYSIS
The distribution of infant and maternal sociodemographic and reproductive characteristics among cases and controls were by tabulated using counts and proportions and were compared using a chi-square test. The distribution of overall atrazine levels in Texas was also described by year. Further, for each county, mean atrazine levels across 1999–2007 were plotted.
Frequencies of cases and controls were determined for each atrazine category (i.e., those based on the 25th, 75th, and 90th percentile cutoffs in controls). In the main analyses, unconditional logistic regression was used to assess the relationship between categories of atrazine exposure and choanal atresia or stenosis. Using the category of low atrazine exposure as a referent, crude odds ratios (ORs) were estimated for each atrazine exposure level category. We also estimated ORs adjusted for the following potential confounders (based on previous literature): season of conception, infant sex, birth year, and maternal race/ethnicity, education, age, and smoking. Furthermore, adjusted p-values were estimated to assess the potential for a linear trend across increasing categories of atrazine exposure. We also assessed a linear trend using a continuous measurement of atrazine levels, based on 10,000 pound increments. To further limit potential heterogeneity, main analyses were repeated among isolated cases. Analyses were conducted using SAS (version 9.2 copyright 2002–2008, SAS, Inc., Cary, NC) and STATA version 12 (StatCorp LP, College Station, TX).
RESULTS
From 1999 to 2008, there were 372 cases in Texas with postnatal diagnoses of choanal atresia or stenosis. Controls were randomly selected among all live births in Texas during the study period at a ten to one control to case ratio (N=3720 controls). There were 28 cases that had a chromosome abnormality and 64 cases that had a malformation syndrome or sequence. The main analyses focused on the remaining 280 nonsyndromic cases. The proportion of subjects missing maternal county of residence at delivery, and therefore missing atrazine exposure level, was similar between cases and controls (0.4% and 0.5%, respectively).
There were no significant differences in the distribution of characteristics (i.e., infant sex, delivery year, or maternal race/ethnicity, birthplace, age at delivery, education, history of previous live births, smoking, or season of conception) between cases and controls (Table I). Further characteristics of a subset of these cases have been previously described in a descriptive epidemiology study.4 The overall distribution of atrazine levels in Texas between 1999 and 2007 are presented (Table II). The annual mean levels of atrazine application in Texas ranged from a high of 19.5 pounds per square mile in 2000 to a low of 9.2 pounds per square mile in 2006. There was no obvious trend in estimated levels over time.
Table I.
Characteristics of controls and nonsyndromic cases with choanal atresia in Texas, 1999–2008
| Characteristic | Cases (N=280) | Controls (N=3720) | P |
|---|---|---|---|
| Infant Sex | |||
| Female | 138 (49.5%) | 1813 (48.7%) | 0.68 |
| Male | 141 (50.5%) | 1907 (51.3%) | |
| Delivery year | |||
| 1999 | 28 (10.0%) | 363 (9.8 %) | 0.65 |
| 2000 | 29 (10.4%) | 364 (9.8 %) | |
| 2001 | 32 (11.4%) | 353 (9.5%) | |
| 2002 | 25 (8.9%) | 387 (10.4%) | |
| 2003 | 25 (8.9%) | 354 (9.5%) | |
| 2004 | 23 (8.2%) | 406 (10.9%) | |
| 2005 | 29 (10.4%) | 365 (9.8%) | |
| 2006 | 36 (12.9%) | 364 (9.8%) | |
| 2007 | 22 (7.9%) | 368 (9.9%) | |
| 2008 | 31 (11.1%) | 396 (10.7%) | |
| Maternal race/ethnicity | |||
| Non-Hispanic white | 110 (39.4%) | 1310 (35.2%) | 0.41 |
| Non-Hispanic black | 30 (10.8%) | 447 (12.0%) | |
| Hispanic | 133 (47.7%) | 1831 (49.3%) | |
| Other | 6 (2.2%) | 130 (3.5%) | |
| Maternal birthplace | |||
| United States | 207 (73.9%) | 2638 (70.9%) | 0.21 |
| Outside United States | 73 (26.1%) | 1082 (29.1%) | |
| Maternal age | |||
| <20 | 33 (11.8%) | 562 (15.1%) | 0.48 |
| 20–24 | 77 (27.5%) | 989 (26.6%) | |
| 25–29 | 79 (28.2%) | 1034 (27.8%) | |
| 30–34 | 56 (20.0%) | 755 (20.3%) | |
| 35–39 | 28 (10.0%) | 320 (8.6%) | |
| ≥40 | 7 (2.5%) | 60 (1.6%) | |
| Maternal education | |||
| < High school | 92 (33.5%) | 1167 (31.8%) | 0.90 |
| High school | 79 (28.7%) | 1069 (29.1%) | |
| > High school | 104 (37.8%) | 1439 (37.2%) | |
| Previous live births | |||
| No | 102 (37.5%) | 1388 (38.4%) | 0.98 |
| Yes | 170 (62.5%) | 2231 (61.7%) | |
| Maternal smoking | |||
| No | 259 (92.8%) | 3499 (94.4%) | 0.25 |
| Yes | 20 (7.2%) | 209 (5.6%) | |
| Season of conception | |||
| Spring | 73 (26.2%) | 920 (24.8%) | 0.26 |
| Summer | 74 (26.5%) | 858 (23.1%) | |
| Fall | 72 (25.8%) | 939 (25.3%) | |
| Winter | 60 (21.5%) | 992 (26.8%) | |
Table II.
Distribution of atrazine levels (pounds per square mile) in Texas by year, 1999–2007
| Year | Mean | Median | Minimum | Maximum |
|---|---|---|---|---|
| 1999 | 14.71 | 3.83 | 0.00009 | 194.6 |
| 2000 | 19.53 | 4.83 | 0.00009 | 223.8 |
| 2001 | 15.18 | 3.04 | 0.0002 | 211.3 |
| 2002 | 14.80 | 2.16 | 0.0002 | 235.5 |
| 2003 | 15.73 | 3.58 | 0.001 | 161.1 |
| 2004 | 13.82 | 2.28 | 0.0004 | 177.6 |
| 2005 | 11.89 | 2.47 | 0.0001 | 211.4 |
| 2006 | 9.24 | 2.27 | 0.0004 | 115.9 |
| 2007 | 15.68 | 3.03 | 0.0006 | 352.5 |
Atrazine exposure was categorized based on the cutoffs in controls at the 25th, 75th, and 90th percentiles: 0–<1.40 pounds/square mile (low), 1.40–<15.03 pounds/square mile (medium low), 15.03–<47.63 pounds/square mile (medium), and ≥47.63 pounds/square mile (high). A map plotting atrazine exposure categories for each Texas county, based on mean atrazine application over 1997–2007, is presented in Figure I. Based on these estimates; levels of atrazine appeared to be both high and variable throughout the state.
Figure I.
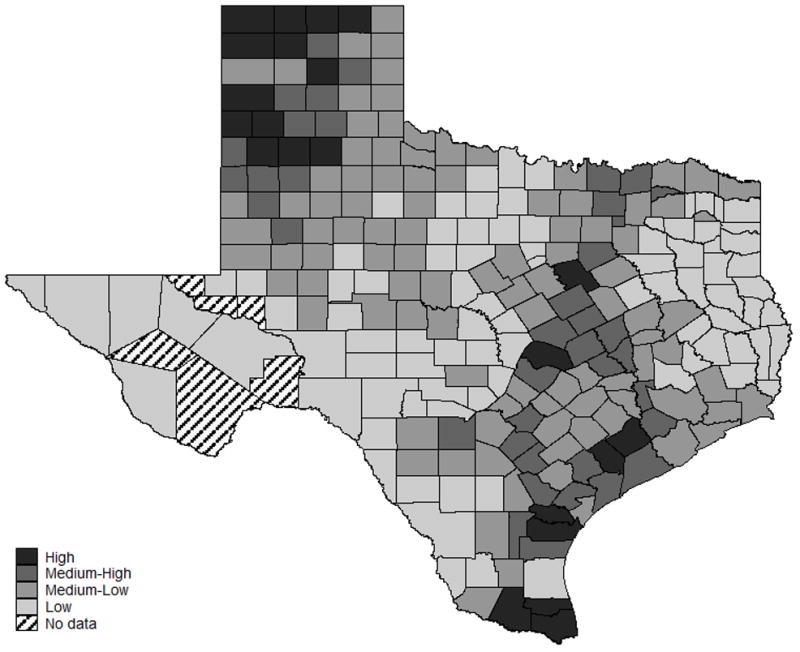
Distribution of mean atrazine levels in Texas by county over 1999–2007
Offspring of mothers with high levels of estimated residential atrazine exposure had a significantly increased risk of choanal atresia or stenosis compared to those with low exposure levels (OR: 1.65, 95% CI: 1.10–2.48) (Table III). A similar association was observed after adjustment for season of conception, infant sex, birth year, and maternal race/ethnicity, education, age, and smoking (adjusted OR: 1.79, 95% CI: 1.17–2.74). The unadjusted and adjusted magnitudes of association across the categories of exposure were consistent with a dose-response over increasing levels of atrazine exposure. A significant linear trend was also observed with increasing category of atrazine exposure (adjusted P=0.002). Main analyses were also repeated using a continuous measure of atrazine exposure (i.e., based on 100 pound per square mile increments). A significant linear increase was observed (OR: 1.40, 95% CI: 1.04–1.89; adjusted OR: 1.49, 95% CI: 1.09–2.04).
Table III.
Association between atrazine and choanal atresia in Texas, 1999–2008
| Atrazine levels a | Pounds/square mile | Casesb (%)(N=289) | Controls (%)(N=3720) | OR | 95% CI | aORc | 95% CI |
|---|---|---|---|---|---|---|---|
| Low (reference) | 0–<1.40 | 64 (22.9) | 922 (24.9) | 1.00 | 1.00 | ||
| Medium-low | 1.40–<15.03 | 120 (43.0) | 1856 (50.1) | 0.93 | 0.68–1.27 | 0.93 | 0.68–1.29 |
| Medium | 15.03–<47.63 | 53 (19.0) | 557 (15.1) | 1.37 | 0.94–2.00 | 1.35 | 0.90–2.01 |
| High | ≥47.63 | 42 (15.1) | 367 (9.9) | 1.65 | 1.10–2.48 | 1.79 | 1.17–2.74 |
| Pd | 0.002 |
Atrazine categories based on 25th, 75th, and 90th percentiles in controls
Nonsyndromic cases
Odds ratio adjusted for season of conception, infant sex, birth year, and maternal race/ethnicity, education, age, and smoking
P-value for linear trend across the four increasing categories of atrazine exposure
To further limit heterogeneity, analyses were repeated among isolated cases (N=147). The results from these analyses were similar to the main results (data not shown).
DISCUSSION
We found a significant association between estimated maternal residential exposure to atrazine and risk for nonsyndromic or isolated choanal atresia or stenosis in offspring. Specifically, the prevalence of choanal atresia or stenosis was highest among offspring of women who lived in counties with the highest estimated levels of atrazine use. Our results suggest a monotonic dose-response relationship, whereby living in counties with increasing atrazine application is associated with increasing risk of having a child with choanal atresia or stenosis.
As there are no large-scale population-based measurements of personal atrazine exposure, our exposure assessment strategy was based on county-level estimates of atrazine application provided by the USGS, and therefore, our results should be interpreted cautiously. However, living in areas with high levels of atrazine application (e.g., proximity to agricultural fields) appears to correlate with personal exposure.14, 15 For instance, families living in farm households have higher levels of urine atrazine metabolites compared to families living in non-farm households.22
Based on research in animal models, the primary target of atrazine toxicity is the female reproductive system; therefore, there is growing interest in the association between atrazine exposure in humans and birth defects.15 Although the relationship between maternal atrazine exposure and risk for choanal atresia or stenosis has not been previously evaluated, our findings are consistent with previous reports of associations between atrazine exposure and adverse pregnancy outcomes and other birth defects, such as gastroschisis, spina bifida, cleft lip, congenital heart defects, limb reduction defects and urogenital defects.15–18
Additionally, as atrazine is a suspected endocrine disruptor in humans (including disruption of thyroid hormone), and there is evidence that choanal atresia risk is associated with in utero exposure to hyperthyroid medications, it is plausible that choanal atresia may be an atrazine-susceptible phenotype.5–11 While the pathways involved in atrazine-induced tertatogenesis have not been elucidated (particularly for choanal atresia), there are three potential mechanisms that warrant further exploration. First, it is possible that atrazine may interfere with maternal thyroid hormone levels during pregnancy, thereby increasing the risk of choanal atresia. Second, atrazine exposure (and exposure to other endocrine disruptors) may actually lead to maternal hyperthyroidism prior to pregnancy, which may lead to this effect. Third, previous studies have shown that risk for choanal atresia or stenosis increases in mice with suppression of retinoic acid synthesis (i.e., vitamin A deficiency) or activation of fibroblast growth factor signaling pathways.12, 13 Although the relationships between the thyroid hormone, retinoic acid, and fibroblast growth factor signaling pathways during development are not fully understood, it has been suggested that retinoic acid regulates thyroid function and that thyroid hormones regulate fibroblast growth factor receptor signaling.23, 24 While it is unclear if atrazine might be involved in retinoic acid synthesis or fibroblast growth factor signaling, it is possible that precursors or derivatives of atrazine might be involved.
This study must be considered in the light of certain limitations. Given that the critical period for exposure for choanal atresia is early in pregnancy,25 residential atrazine exposure based on maternal residence at delivery may not have accurately represented residential atrazine exposure during the critical period for all subjects. However, we have previously shown that residential mobility from one county to another between conception and delivery is infrequent in Texas (i.e., ≤6%) and is expected to occur at similar proportions between cases and controls.26 As stated, we used a county-based estimate of atrazine exposure in the present study, which may not adequately account for variability of exposure within counties or within years; however, there are no large-scale population-based measures of personal exposure, which could be used in order to evaluate this relatively rare phenotype. Thus, the present study is an important first step in determining what associations may be present. Although we adjusted for several potential confounders, we cannot rule out the possibility of confounding by unmeasured variables. Future research is needed to confirm our findings, using other exposure assessment methodologies, including biomarkers of exposure. We were also limited in our case sample size, due to the rarity of choanal atresia or stenosis.
In spite of these limitations, the present study had several strengths, including use of a sample that utilized active surveillance to identify cases. The sample also included cases that were still births and elective pregnancy terminations, which reduced the potential for selection bias. Although choanal atresia or stenosis is a rare condition, the Texas Birth Defects Registry represents one of largest population-based birth defects registries in the world, and our sample contained more cases with nonsyndromic choanal atresia or stenosis than most previous studies. Additionally, to reduce heterogeneity, we restricted our case definition to nonsyndromic cases and also considered isolated cases separately.
In summary, we report the first identified association between maternal atrazine exposure and risk for choanal atresia or stenosis. Moreover, our findings, in conjunction with previous studies, suggest an important role of maternal endocrine disruption in risk for choanal atresia or stenosis. If confirmed, future research evaluating the many other factors that influence endocrine function may shed further light on the etiology of choanal atresia or stenosis.
Acknowledgments
Support: This project was supported in part by the Centers for Disease Control and Prevention (CDC)-funded Texas Center for Birth Defects Research and Prevention (#5U01DD000494) through a cooperative agreement with Texas Department of State Health Services (DSHS), as well as the Title V Office of Texas DSHS.
We thank M.A. Canfield and the staff of the Birth Defects Epidemiology and Surveillance Branch, Texas Department of State Health Services, for help with collecting data for the Texas Birth Defects Registry. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of the CDC.
Abbreviations and acronyms
- OR
odds ratio
- CI
confidence interval
- BDESB
Birth Defects Epidemiology and Surveillance Branch
- BPA code
birth defect code based on the British Pediatric Association Classification of Diseases and the International Classification of Diseases, 9th Revision, Clinical Modification system
- USGS
United States Geological Survey
Footnotes
Conflict of interest declarations: None
First draft statement: The first draft of the manuscript was written by A.J. Agopian and P.J. Lupo. No honorarium, grant, or other form of payment was given to a co-author to produce the manuscript.
References
- 1.Ramsden JD, Campisi P, Forte V. Choanal atresia and choanal stenosis. Otolaryngol Clin North Am. 2009;42:339–52. x. doi: 10.1016/j.otc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Cedin AC, Atallah AN, Andriolo RB, Cruz OL, Pignatari SN. Surgery for congenital choanal atresia. Cochrane Database Syst Rev. 2012;2:CD008993. doi: 10.1002/14651858.CD008993.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrow TA, Saal HM, de Alarcon A, Martin LJ, Cotton RT, Hopkin RJ. Characterization of congenital anomalies in individuals with choanal atresia. Arch Otolaryngol Head Neck Surg. 2009;135:543–7. doi: 10.1001/archoto.2009.53. [DOI] [PubMed] [Google Scholar]
- 4.Case AP, Mitchell LE. Prevalence and patterns of choanal atresia and choanal stenosis among pregnancies in Texas, 1999–2004. Am J Med Genet A. 2011;155A:786–91. doi: 10.1002/ajmg.a.33882. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg F. Choanal atresia and athelia: methimazole teratogenicity or a new syndrome? Am J Med Genet. 1987;28:931–4. doi: 10.1002/ajmg.1320280419. [DOI] [PubMed] [Google Scholar]
- 6.Barbero P, Ricagni C, Mercado G, Bronberg R, Torrado M. Choanal atresia associated with prenatal methimazole exposure: three new patients. Am J Med Genet A. 2004;129A:83–6. doi: 10.1002/ajmg.a.20668. [DOI] [PubMed] [Google Scholar]
- 7.Barbero P, Valdez R, Rodriguez H, Tiscornia C, Mansilla E, Allons A, et al. Choanal atresia associated with maternal hyperthyroidism treated with methimazole: a case-control study. Am J Med Genet A. 2008;146A:2390–5. doi: 10.1002/ajmg.a.32497. [DOI] [PubMed] [Google Scholar]
- 8.Kannan L, Mishra S, Agarwal R, Kartikeyan V, Gupta N, Kabra M. Carbimazole embryopathy-bilateral choanal atresia and patent vitello-intestinal duct: a case report and review of literature. Birth Defects Res A Clin Mol Teratol. 2008;82:649–51. doi: 10.1002/bdra.20483. [DOI] [PubMed] [Google Scholar]
- 9.Wolf D, Foulds N, Daya H. Antenatal carbimazole and choanal atresia: a new embryopathy. Arch Otolaryngol Head Neck Surg. 2006;132:1009–11. doi: 10.1001/archotol.132.9.1009. [DOI] [PubMed] [Google Scholar]
- 10.Bowman P, Vaidya B. Suspected Spontaneous Reports of Birth Defects in the UK Associated with the Use of Carbimazole and Propylthiouracil in Pregnancy. J Thyroid Res. 2011;2011:235130. doi: 10.4061/2011/235130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clementi M, Di Gianantonio E, Cassina M, Leoncini E, Botto LD, Mastroiacovo P. Treatment of hyperthyroidism in pregnancy and birth defects. J Clin Endocrinol Metab. 2010;95:E337–41. doi: 10.1210/jc.2010-0652. [DOI] [PubMed] [Google Scholar]
- 12.Hehr U, Muenke M. Craniosynostosis syndromes: from genes to premature fusion of skull bones. Mol Genet Metab. 1999;68:139–51. doi: 10.1006/mgme.1999.2915. [DOI] [PubMed] [Google Scholar]
- 13.Dupe V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci U S A. 2003;100:14036–41. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes TB, Stuart AA, Mendoza M, Collins A, Noriega N, Vonk A, et al. Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17beta-estradiol): Support for the demasculinization/feminization hypothesis. Environ Health Perspect. 2006;114 (Suppl 1):134–41. doi: 10.1289/ehp.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Atrazine. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; 2003. p. 222. [Google Scholar]
- 16.Munger R, Hanson J, Isacson P. Birth defects and pesticide-contaminated water supplies in Iowa. Am J Epidemiol. 1992;136:959. [Google Scholar]
- 17.Waller SA, Paul K, Peterson SE, Hitti JE. Agricultural-related chemical exposures, season of conception, and risk of gastroschisis in Washington State. Am J Obstet Gynecol. 2010;202:241, e1–6. doi: 10.1016/j.ajog.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Winchester PD, Huskins J, Ying J. Agrichemicals in surface water and birth defects in the United States. Acta Paediatr. 2009;98:664–9. doi: 10.1111/j.1651-2227.2008.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center on Birth Defects and Developmental Disabilities C. Appendix A: ICD-9 and CDC/BPA codes. Teratology. 2002;66 (Suppl 1):S218–9. doi: 10.1002/tera.90015. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Geological Survey. Method for Estimating Annual Atrazine Use for Counties in the Conterminous United States, 1992–2007. 2010 [Google Scholar]
- 21.Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Smith DF. Childhood cancer incidence rates and hazardous air pollutants in California: an exploratory analysis. Environ Health Perspect. 2003;111:663–8. doi: 10.1289/ehp.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, et al. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in iowa. Ann Occup Hyg. 2007;51:53–65. doi: 10.1093/annhyg/mel062. [DOI] [PubMed] [Google Scholar]
- 23.Barnard JC, Williams AJ, Rabier B, Chassande O, Samarut J, Cheng SY, et al. Thyroid hormones regulate fibroblast growth factor receptor signaling during chondrogenesis. Endocrinology. 2005;146:5568–80. doi: 10.1210/en.2005-0762. [DOI] [PubMed] [Google Scholar]
- 24.Silva AC, Marassi MP, Muhlbauer M, Lourenco AL, Carvalho DP, Ferreira AC. Retinoic acid effects on thyroid function of female rats. Life Sci. 2009;84:673–7. doi: 10.1016/j.lfs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Hengerer AS, Brickman TM, Jeyakumar A. Choanal atresia: embryologic analysis and evolution of treatment, a 30-year experience. Laryngoscope. 2008;118:862–6. doi: 10.1097/MLG.0b013e3181639b91. [DOI] [PubMed] [Google Scholar]
- 26.Lupo PJ, Symanski E, Chan W, Mitchell LE, Waller DK, Canfield MA, et al. Differences in exposure assignment between conception and delivery: the impact of maternal mobility. Paediatr Perinat Epidemiol. 2010;24:200–8. doi: 10.1111/j.1365-3016.2010.01096.x. [DOI] [PubMed] [Google Scholar]


