Anemia is a pervasive and difficult-to-treat consequence of a severe burn injury. The most effective method of correcting anemia is the transfusion of packed red blood cells, but this therapy is not without complications. Surgical techniques including tourniquets and epinephrine tumescence have reduced blood loss and stricter thresholds have limited transfusions, but for severe burns, transfusion requirements are still massive. In this review of the current literature of anemia of thermal injury, we will provide a new framework for addressing this anemia and will show how this approach may help to develop better interventions and further reduce transfusion rates.
CLASSIFYING ANEMIA
The terms burn anemia,1 anemia of thermal injury,2–9 anemia of thermal burns,10 and anemia in burns11 have all been used to describe anemia in burn patients. These terms encompass anemia occurring throughout the entire duration of burn care. They describe not only acute onset decreases in hemoglobin concentration immediately following the burn injury and before and after operative intervention but also, later, during wound healing and resolution of the critical injury. The causes of anemia at each stage of treatment differ; not distinguishing between them may limit patient care and research into the specific causes of anemia. Clearly defining the type of anemia will better coordinate research efforts to identify the mechanisms responsible for anemia and develop methods to reduce transfusion rates. As a result, we recommend the terms, acute blood loss anemia and anemia of critical illness, to describe the two types of anemia present in burn patients.
Acute blood loss is well established as a major contributor to anemia in burn patients. Little argument is needed to prove that acute blood loss anemia occurs in burn patients and that this inevitable blood loss requires correction with transfusion. Acute blood loss anemia occurs during the first 1 to 2 weeks after a burn injury. Blood is lost directly from the thermal injury and from the surgical management of the wounds. Other sources of blood loss may be from concurrent traumatic injury, red blood cell (RBC) sequestration,12 and direct erythrocyte damage.13
On the other hand, anemia of critical illness is an evolving concept with increasing support in the critical care literature. Anemia of critical illness is responsible for decreased hemoglobin concentrations between operative events, during wound healing, and throughout resolution of the acute phase of injury. Studies on critical care patients have found that 77% of intensive care unit (ICU) patients are anemic at the time of hospital discharge.14 Following up patients further from their ICU stay, 13 weeks after discharge, 63% of former ICU patients remain anemic.15 At 26 weeks, anemia persisted in 53%. In pediatric burn patients, Birdsell and Birch11 found that transfusion requirements commonly continued up to 10 weeks but as far as 20 weeks postburn. In a recent, large, multicenter retrospective review of transfusion characteristics in 666 burn patients, on average, the last transfusion was administered 28.8 days after admission, clearly after all excision and grafting. In addition, 13.7 ± 1.1 units of blood were transfused per patient during their hospital stay. Of these, 4.3 ± 0.3 units were transfused in the operating room (OR). This leaves almost 70% of blood not given in the OR and not related to surgical, acute blood loss. Anemia of critical illness is responsible for these additional (70%) transfusions.
Although the presence of anemia of critical illness has been confirmed in multiple epidemiologic studies, little is known about the mechanisms for this anemia. Most likely, this phenomenon is multifactorial and results from an imbalance between production (blunted erythropoiesis) and destruction (increased sequestration) of RBCs.
The distinction between acute blood loss anemia and anemia of critical illness is not just verbiage but rather an essential framework to continue the momentum toward lower incidence of anemia and reduced transfusions in burn patients. Anemia is not strictly the result of acute blood loss or the critical illness as there is some overlap (Figure 1). However, transfusion-reducing techniques such as erythropoietin (EPO) administration or lower transfusion thresholds may have an even greater impact if studied within the context of either acute blood loss anemia or anemia of critical illness rather than being lumped together as anemia of thermal injury. Examining the causes of both acute blood loss anemia and anemia of critical illness should bring to light the importance of this distinction. Throughout this review, advantages of using this distinction to improve patient care and research are highlighted.
Figure 1.
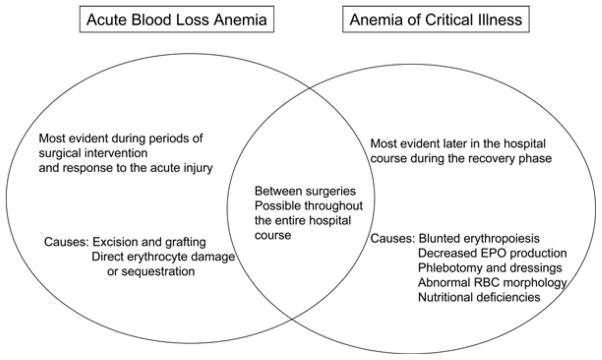
The relationship between acute blood loss anemia and anemia of critical illness in the spectrum of burn care.
CAUSES OF ANEMIA
Acute Blood Loss Anemia
Acute blood loss occurs mainly from the surgical management of the burn wound. Because of the extensive blood loss, much effort has been made to develop methods to reliably quantify and predict operative blood loss. Additional mechanisms for acute blood loss anemia include direct erythrocyte destruction and increased RBC sequestration, but conclusions from these studies are unclear and do not provide evidence for significant RBC loss.
A significant amount of blood is lost during surgical management of a burn wound. Although the burn eschar is devoid of viable blood vessels, proper tangential excision of a burn wound requires debridement to viable tissue evidenced by bleeding. Harvesting of donor sites for grafting leaves an oozing wound bed, which adds to an extensive blood loss. Multiple studies have estimated blood loss (EBL) during wound excision and skin grafting to compare techniques at blood loss reduction and to help in estimating operative transfusion rates. These studies vary significantly in their methodology for determining blood loss. Therefore, these studies should be viewed in light of the surgical techniques used and transfusion thresholds in relation to current standard practice and matched to individual practice as best possible. Methods used to estimate surgical blood loss include combining differences in preoperative and postoperative hemoglobin concentrations with volume of transfusion,16,17 simple estimates of blood loss by surgical and anesthesia teams,18 measuring areas of grafting and debridement and dividing or multiplying by predetermined constants,19–21 complex formulas based on patient age, weight, and area of excision,22 weighing of laparotomy pads pre- and postoperatively,23 and measurements from gowns and drapes using a swab washing machine.21 Interestingly, Budny et al18 compared blood loss using a calculation based on hemoglobin concentrations and transfusion volume with surgical and anesthesia team estimates and found that the surgical and anesthesia estimates were very close to calculated blood loss and may be just as reliable. Risk factors for increased blood loss include surface area of wound excised, percentage third degree burn, and longer time to first wound excision. The longer time to first excision may lead to both Gram-negative and Gram-positive colonization of the wound, which may increase blood loss and impair hemostasis.24
Estimating blood loss preoperatively is important as unnecessary crossmatching is wasteful, costly, and time consuming to the entire health care team. In a 5-year retrospective review of blood bank utilization by a burn unit, 3393 red cell units were prepared after type and crossmatch, but only 1691 were actually transfused, yielding a cross-match/transfused ratio of 2.01. The cross-match/transfused ratio was highest, 2.69, for patients with 0 to 10% TBSA burn as 988 units were cross-matched but only 367 were transfused questioning the need to obtain a type and crossmatch for patients with burns of small size.25 When estimating preoperative transfusion requirements, 1.78 units of blood per 1000 cm2 of burn wound excised is a reliable measure of transfusion needs.26
RBCs in burn patients may be destroyed or sequestered at a higher rate than normal. Kimber and Lander found that the morphology of RBCs change with longer duration of thermal insult and that RBC half-life decreased with increasing duration of thermal injury. However, to determine this, whole blood was removed from healthy volunteers, radiolabeled, heated ex vivo to 50°C, histologically examined, and then transfused back into the same patient. Patient radioactivity was then used to assess RBC destruction and sequestration. Although an interesting method, the ex vivo direct heating of RBCs to 50°C does not provide a realistic model for the heat applied to RBCs during a burn injury.13 Loebl et al transfused both healthy and burn patients with either burn or healthy radiolabeled sera. The half-life of burn RBCs transfused into the healthy patient was similar to that of healthy RBCs transfused into a healthy patient. However, when either burn or healthy RBCs were transfused into burn patients, the half-life of the RBCs was significantly reduced. These findings indicate that there may be a mechanism in the burn patient that favors early or more active sequestration of RBCs rather than just direct destruction of RBCs from the thermal injury.12
If at all present, direct erythrocyte injury and sequestration do not have a profound effect on overall hemoglobin concentrations as packed RBCs (pRBCs) are rarely transfused within the first few postburn days. On average, burn patients are transfused 5.3 ± 0.3 days after admission.27 If the thermal injury alone had such a profound impact on RBC survival, transfusion of pRBCs would likely have been necessary before postburn day 5. In contrast, studies on transfusion in the critically ill show a trend toward earlier transfusion. In the CRIT study, the average time to first transfusion was only 2.3 ±3.7 days.28 In the Anemia and Blood Transfusion in Critically Ill Patients (ABC) study, 70% of all transfused patients received their first transfusion within the first 2 ICU days.29 von Ahsen et al30 found that, in their patient population, ~50% of all transfusions were administered in the first 5 days of ICU stay. This earlier first transfusion day may reflect the acute, surgical blood loss occurring before or at times of ICU admission in trauma and surgical patients. For burn patients, the first operative day often occurs several days following the injury or ICU admission, as evidenced by their later days of first transfusion.
Extensive blood loss from excision and grafting is a major factor leading to acute blood loss anemia. Efforts to prevent and reduce surgical blood loss have resulted in decreased operative transfusion rates (see Operative Strategies to Reduce Transfusions). Other suggested mechanisms for acute blood loss in burn patients do not contribute significantly to this anemia. In addition, techniques to prevent their occurrence are unlikely to be effective as they are the direct result of the thermal injury.
Anemia of Critical Illness
Anemia of critical illness describes the persistent anemia that plagues critically ill patients after the resolution of their initial acute event. Although acute blood loss anemia is due to removal of RBCs, anemia of critical illness is the inability to produce enough RBCs to meet demand. It has been described as an acute form of anemia of chronic disease.31 It is a multifactorial entity related to a variety of factors including wound care, phlebotomy, impaired nutrition and metabolism, blunted EPO production and/or response, and reprioritization of bone marrow cell production.32
Iatrogenic factors such as blood loss from dressing changes and laboratory draws may play a small role in the anemia of critical illness. Blood loss can be as high as 41 ml/d in ICU patients29 and may contribute 17% of total blood loss during the ICU stay.30 The amount of blood drawn per laboratory test and the need for each test should be carefully scrutinized to avoid unnecessary blood loss.33 In fact, methods of reducing blood draws have been implemented and successful in reducing total blood loss including the use of pediatric blood collection tubes34,35 and blood-conserving arterial line systems.36,37 These iatrogenic causes of anemia, although necessary for patient care, may be a modifiable factor in the reduction of anemia of critical illness.
Nutritional deficiencies may play a role in the anemia of critical illness. By studying iron, B12, and folate levels in long-term ICU patients, Rodriguez et al38 found that 13% of patients had correctable abnormalities of these nutrients. Some studies have suggested that decreased nutrition levels in burn patients leads to an abnormal erythrocyte morphology, which leads to a decreased half-life/earlier sequestration of these cells and a decrease in RBCs.3
Bone marrow dysfunction may contribute to anemia of critical illness. Wallner and Warren examined the bone marrow at autopsy of patients who died from burns, acute myocardial infarction, or sepsis and compared the overall bone marrow cellularity and bone marrow cellular components. The overall cellularity of the bone marrow was increased in sepsis and burn patients. The percentage of granulocytes was increased and the percentage of erythroblasts was decreased in the burn patients.39 Similar to this autopsy study, Wallner et al found that, in burned mice, erythroid colony formation was severely blunted in the bone marrow. This decreased erythroid colony formation was not a transient event, but rather, persisted for up to 40 days after burn injury. Decreased erythroid colony formation led to the persistent decrease in peripheral RBCs and the prolonged anemia of critical illness.40 When sera of burn and healthy patients were added to mouse bone marrow cells, the erythroid colony forming production of bone marrow exposed to burn sera was reduced even with increasing doses of EPO.9 As a result, Wallner et al8 postulated that there was an erythroid inhibitory substance present in the sera of burn patients that stalled erythroid production. Further identification of this substance and investigations into the blunted erythropoietic response in the bone marrow following burn injury has not yet been undertaken.
The anemia of critical illness has been likened to an acute form of anemia of chronic disease.31 The anemia of chronic disease is an anemia of inflammation, because the chronic disease processes that lead to anemia (chronic kidney disease, rheumatoid arthritis, lupus, malignancy, chronic transplant rejection, acute and chronic infections, etc) cause an increase in the baseline inflammatory state.41 Burn patients, as a result of the overwhelming response to severe thermal injury, have increased levels of proinflammatory cytokines.42,43 Proinflammatory cytokines, including tumor necrosis factor,44,45 β-interferon,45 interferon-γ,46,47 and interleukin-6,48 have all been found to inhibit erythroid cell formation in the bone marrow. Although these studies suggest a role for proinflammatory cytokines in anemia of critical illness, no clear connection between these studies in the bone marrow compartment and the end peripheral RBC exists.
The anemia of critical illness in burn patients is most likely multifactorial, making it difficult to foresee one intervention that reduce transfusion rates. Rather, a collective effort integrating multiple techniques or therapies may lead to small reductions in anemia, which can add up to a major impact on transfusion rates. However, incorporating multiple techniques is difficult in clinical practice. In addition, the mechanism behind blunted erythropoiesis, a major factor in anemia of critical illness, is unclear and leaves a hole in the effort of both understanding and treating anemia of critical illness in burn patients.
TRANSFUSION RATES
Given the acute blood loss from excision and grafting of large wounds and the prolonged critical illness, transfusion of pRBCs is common and can be quite substantial in burn patients. Several studies have defined the transfusion trends in burn patients, and most have focused on the relationship between %TBSA burn and transfusion needs. Graves et al49 found that patients with >10% TBSA burn received an average of 19.7 units of blood with a range of 0 to 201 units. For Vasko et al, their patients with >10% TBSA burn required an average of 8.94 units per patient, with one patient with >90% TBSA burn receiving 117 units. For patients with >30% TBSA, the mean transfusion requirement was 17 units.7 Palmieri et al found that, on average, 13.7 ± 1.1 units of pRBCs were transfused per patient with ≥20% TBSA. For burns of ≥50% TBSA, >30 units of blood were transfused per patient.27 In a single-centered retrospective review from 1999 to 2004, patients with <10% TBSA had 4 ± 0.6 units of pRBCs transfused; for 11 to 19% TBSA, 8 ± 1 units; for 20 to 40% TBSA, 12 ± 3 units; and for >40% TBSA, 20 ± 4 units. As expected, as burn size increased, so did the chance of requiring a transfusion. Approximately 5.7% of patients with <10% TBSA burn required a transfusion, 21% with 11 to 20% TBSA burn, 39% with 21 to 30% TBSA burn, and 62% of patients with >30% TBSA burn25 (Table 1). In 109 pediatric patients, Birdsell and Birch found that 100% of children with >30% TBSA burn required a blood transfusion, but no transfusions were required for TBSA of <5%. They did not report the number of transfusions needed per patient.11
Table 1.
Transfusion rates in the burn population
| Author | Yr of Publication | pRBC Transfusions per Patient Based on %TBSA |
|---|---|---|
| Graves et al49 | 1989 | >10% TBSA = 19.7 units |
| Vasko et al7 | 1991 | >10% TBSA = 8.94 units >30% TBSA = 17.0 units |
| Palmieri et al27 | 2006 | ≥20% TBSA = 13.7 ± 1.1 units ≥50% TBSA = >30 units |
| Yogore et al25 | 2006 | <10% TBSA = 4 ± 0.6 units 11–19% TBSA = 8 ± 1 units 20–40% TBSA = 12 ± 3 units >40% TBSA = 20 ± 4 units |
pRBC, packed red blood cell.
These data show that massive transfusions occur following a severe burn injury. With increased burn size, there are increased blood transfusion requirements. This may be attributed to increased surgical blood loss from more extensive excision and grafting (acute blood loss anemia) and also from worsened illness severity leading to impaired erythropoiesis (anemia of critical illness). Little is known about other patient characteristics predisposing to transfusion. In a survey of transfusion trends among burn surgeons, Palmieri and Greenhalgh50 found that aside from %TBSA burn, other factors increasing transfusion rates are cardiac disease, acute respiratory distress syndrome (ARDS), and age. Identifying additional clinical and demographic characteristics of patients who require transfusions is key to promoting techniques that reduce transfusion rates. Dividing anemia of burns into acute blood loss anemia and anemia of critical illness may help to identify additional risk factors for transfusion.
CONSEQUENCES OF TRANSFUSION
Transfusion of blood products are an immediate and effective treatment for anemia as hemoglobin concentration is restored with donor RBCs. Increased RBCs improve oxygen carrying capacity, augment cardiac function, and prevent cellular damage from hypoxia. As in much of medicine though, such a simple treatment is not without risk. It is well known that the transfusion of pRBCs can lead to the direct transmission of infectious diseases. Although better screening methods have reduced transmission, HIV, hepatitis B, and hepatitis C can still be acquired from a pRBC transfusion.51 A recent review cites transmission rates of HIV as 1 in 2 million transfusions, hepatitis B transmission in 1 in every 250,000 transfusions, and hepatitis C in 1 in every 1.5 to 2 million transfusions.52 However, the immunomodulatory effect of pRBC transfusion may actually be more detrimental to patient morbidity and mortality, especially in the critically ill patients. The correlation between transfusion and infection has been established for many years in the burn,27,49,52,53 trauma/surgical,54–58 and critical care populations.28,29,59–61 In a mouse model of burn injury, Gianotti et al53 found that burn injury combined with blood transfusion increased microbial translocation from the gut and bacterial survival on dissemination. Graves et al found that %TBSA burn, age, inhalation injury, and number of transfusions were associated with increased infection risk. Logistic regression analysis showed that there was a relationship between the number of units transfused and infectious morbidity regardless of patient age, inhalation injury, or burn size.49 Palmieri et al27 showed similar findings in that there was a 13% increase in developing an infection per unit of blood transfused to burn patients.
A more recently characterized consequence is transfusion-related acute lung injury (TRALI). In fact, TRALI is the most frequent cause of transfusion-related mortality (51%)62 and may occur in a staggering 1 in every 5000 blood transfusions.52 TRALI is diagnosed clinically and radiographically and is defined as “a new episode of acute lung injury (ALI) that occurs during or within 6 hours of a completed transfusion, which is not temporally related to a competing ALI.”63 Symptoms include respiratory distress, hypoxemia, and possibly hypotension and fever.64 TRALI appears as bilateral patchy infiltrates on chest x-ray.63,64 The pathophysiology of TRALI is unclear, but it is speculated that TRALI is caused by neutrophil-mediated endothelial cell toxicity leading to capillary leak and severe local inflammation in the lungs.62,64,65 TRALI has not been well documented in burn patients although there is no reason to suggest it does not to occur. Higgins et al66 attempted to estimate the prevalence of TRALI in burn patients but making an actual diagnosis of TRALI was limited by preexisting ALI and ARDS, pathologies that exclude the diagnosis of TRALI. Because ALI from massive resuscitation or inhalation injury are already present in many burn patients, diagnosing TRALI in this population may require unique clarifiers similar to other disease processes in burns.67
The most morbid consequence of transfusion is ABO incompatibility from clerical error. When ABO incompatible blood is transfused, a severe, possibly fatal, hemolytic transfusion reaction occurs. In 2008, nine deaths transpired in the United States from errors in recipient identification, blood bank clerical error, and sample being collected from the incorrect patient.62 The transfusion error rate may be as high as 1 in 14,000 units, and fatal ABO incompatibility occurs with every 1 in 1,800,000 units, a rate higher than HIV transmission.68
Although a necessity to augment arterial oxygen content and organ perfusion, pRBC transfusion is associated with the direct transmission of infectious disease, immunomodulation leading to increased rates of nosocomial infections, and even death from ABO incompatibility. Methods to limit transfusion have already been shown to improve patient outcome. The following section addresses these methods specifically in burn patients and, when appropriate, in the critical care population.
REDUCING TRANSFUSION: EPO, SURGICAL TECHNIQUES, AND TRANSFUSION THRESHOLDS
Anemia does not impair wound healing17 and only affects hemodynamics and oxygen delivery when quite low.69 Therefore, techniques that limit transfusion should decrease infectious complications and improve morbidity and mortality in burn patients without detriment to wound healing or organ perfusion. The techniques described here (EPO, tourniquets, epinephrine tumescence, and transfusion restriction) are the most well-described and researched methods of reducing transfusions. The effectiveness of each technique varies, and addressing each technique in light of either acute blood loss anemia or anemia of critical illness will bring to light the true efficacy of the intervention.
Erythropoietin
EPO is secreted by the peritubular cells of the kidney in response to low oxygen content in the hemoglobin of RBCs.70 EPO binds to the EPO receptor on erythroid progenitor cells stimulating cell division, differentiation via the initiation of erythroid specific genes, and prevention of cellular apoptosis.71 Therefore, burn patients who are anemic should have elevated EPO level, which should then stimulate RBC production in the bone marrow. The administration of exogenous, recombinant human EPO (rhEPO) should augment the effects of endogenous EPO and increase RBC production as well. In patients with chronic kidney disease, in which EPO production is deficient, the administration of rhEPO can correct anemia and decrease transfusion.72,73 However, these assumptions do not hold true for burn patients. Studies on endogenous EPO production in burn patients are inconclusive, and in cases in which EPO is increased, it has not augmented RBC production. In burn patients, exogenous EPO does not stimulate RBC production to the point of reducing transfusions. The following section will explore these studies and similar work in ICU patients.
Endogenous EPO
Despite multiple studies assessing endogenous EPO levels after a burn injury and correlating these EPO levels to measures of erythropoiesis and, thus, effectiveness in RBC production, there is no clear picture on endogenous EPO levels after burn injury. Much of the confusion stems from the use of different assays. An analysis of these studies is supplied in Table 2.
Table 2.
Endogenous EPO
| Study | Sample Size | Method of EPO Measurement | Measure of Erythropoiesis | Main Findings |
|---|---|---|---|---|
| Robinson et al4 | 19 | Urine bioassay | None | Normal to subnormal EPO in patients with burn index >30 Normal to elevated EPO in patients with burn index <30 |
| Andes et al2 | 5 | Urine bioassay | Hemoglobin, reticulocyte counts, bone marrow morphology, and transfusion requirement | Increased EPO production in 4/5 patients but no increase in hemoglobin, reticulocyte counts, and bone marrow erythroid cells. No decrease in transfusion requirement |
| Sanders et al5 | 7 | Serum bioassay and radioimmunoassay | Hemoglobin | EPO via bioassay increased immediately and then undetectable by postburn day 7. EPO via radioimmunoassay increased immediately postburn and then gradually decreased during recovery |
| Sheldon et al6 | 7 | Serum bioassay and radioimmunoassay | Hemoglobin, reticulocyte count | Bioassay and radioimmunoassay results did not correlate |
| Vasko et al7 | 27 | Serum radioimmunoassay | Hemoglobin, reticulocyte count | Increased EPO production with decreased hemoglobin concentrations. No increase in reticulocytes |
| Deitch and Sittig1 | 24 | Serum radioimmunoassay | Hemoglobin, iron, total iron binding capacity, ferritin, transferrin saturation, and reticulocyte count | Increased EPO and reticulocyte counts with decreased hemoglobin levels |
EPO, erythropoietin.
Urine bioassay of EPO was used by both Robinson et al and Andes et al. Robinson et al4 studied 19 patients at different time points during their hospitalization and, as a whole, found a deficiency in EPO production when compared with healthy patients. Andes et al studied only five patients but also measured hemoglobin, reticulocyte counts, bone marrow morphology, and transfusion requirements. Even though EPO production was increased in comparison with normal, healthy volunteers, there was no corresponding increase in reticulocyte numbers and the bone marrow had decreased erythroid components, highlighting the dampened erythropoietic response to elevated EPO levels.2
Direct comparison of urine bioassay and serum radioimmunoassay was made by Sanders et al and Sheldon et al. Sanders et al found that both the urine bioassay and serum radioimmunoassay levels increased initially following burn. Seven days postburn, the urine bioassay was unable to detect EPO, whereas the radioimmunoassay detected a gradual decline in EPO levels.5 Sheldon et al found no correlation in EPO levels with bioassay and radioimmunoassay. With both methods, EPO concentrations were elevated with anemia. EPO concentration peaked within the first few postburn days using the bioassay but not until approximately postburn day 10 for the radioimmunoassay. The sensitivity or reliability of either test was not discernible.6 In both of these articles, the presumption that the radioimmunoassay is the more sensitive or superior test is suggested, because the radioimmunoassay results match the hypothesis that EPO levels should be increased following the burn injury. However, as mentioned in the discussion by Sheldon et al, the two assays may actually measure different proteins or moieties of EPO. Regardless, since these studies in the 1970s, serum radioimmunoassay has become the standard measure of EPO.74
Correlations between EPO radioimmunoassay levels and hemoglobin have been performed twice. For Vasko et al, in 27 burn patients, EPO levels were appropriately increased as hemoglobin concentration decreased. However, a reticulocytopenic anemia persisted despite this increase in EPO.7 Deitch and Sittig measured EPO, serum iron, total iron binding capacity, ferritin, transferrin saturation, hemoglobin, and reticulocyte counts in 24 burn patients and stratified them based on burn size. There was an inverse correlation between hemoglobin and EPO, indicating an appropriate response to anemia. Patients with supranormal levels of EPO developed a reticulocytosis. However, the reticulocytosis is less than what would be predicted in a normal patient, and thus these patients remained anemic.1
In the critical care literature, much of the data support inappropriately low EPO levels and reticulocyte counts for the degree of anemia.30 When ICU patients with severe sepsis were compared with those with iron-deficiency anemia, Rogiers et al75 found no correlation between EPO and hematocrit levels in the septic patients, but an inverse correlation for the controls. In pediatric critically ill patients, Krafte-Jacobs et al76 found that EPO levels in anemic critically ill patients did not differ from those of critically ill patients without anemia. In anemic, severe trauma patients, EPO levels are not increased as would be expected for the given hemoglobin concentration.77
As a whole, these studies do not provide a clear picture of the EPO response to anemia in both burn and critically ill patients. Regardless of whether EPO is increased or decreased, there does not seem to be an appropriate response to endogenous EPO in these patients.
Exogenous EPO
The administration of exogenous, rhEPO is indicated for patients with end-stage kidney disease,72,73 HIV patients taking zidovudine,78 anemia from the chemotherapy for nonmyeloid malignancies, and before elective, noncardiac, nonvascular surgery to reduce the need for transfusion.79 The use of exogenous EPO has not been found to be beneficial to burn or critically ill patients. Two small trials evaluated exogenous EPO administration on RBC indices and transfusion rates in burn patients. Fleming et al compared EPO administration on unburned, healthy volunteers with burned patients. Exogenous EPO enhanced reticulocyte counts in both groups. However, there was no increase in hematocrit for either group.80 Still et al performed a prospective randomized trial on the effects of exogenous EPO on RBC indices (hemoglobin, hematocrit, and reticulocyte count) and transfusion requirements in 40 burn patients with 25 to 65% TBSA burns. Despite high doses of EPO (300 U/kg within 72 hours of admission and daily for 7 days and then 150 U/kg every other day for 23 days), there was no statistical difference in RBC indices between groups except for an increase in reticulocytes for a subpopulation of patients (25–35% TBSA burns). There was no difference in transfusion rates.81
The effectiveness of exogenous EPO in reducing transfusion rates in burn patients has not been established in any large, prospective trials. However, a recent large prospective, randomized controlled trial82 and an extensive meta-analysis83 found no benefit in exogenous EPO administration in the critically ill. Corwin et al administered exogenous EPO or placebo weekly, for 3 weeks, to medical, surgical, and trauma patients. At 29 days, the mean hemoglobin was increased in the EPO group (16 vs 13 g/dl). Unfortunately, there was neither a decrease in the number of patients who received a transfusion nor a decrease in the mean number of transfusions in the EPO group. EPO may have effects on processes other than erythropoiesis, because the EPO group had a statistically significant increase in thrombotic events but decreased mortality for a subset of trauma patients.82 Zarychanski et al83 performed a meta-analysis of nine studies investigating exogenous EPO use and found only a 0.41-unit per patient decrease in transfusions with EPO use. A small randomized trial in burn patients,81 larger, randomized trials in the critically ill,82 and a meta-analysis of studies in critically ill patients,83 all point toward exogenous EPO administration having no effect on transfusion rates.
Regardless of whether endogenous levels of EPO are increased or decreased following a burn injury, both endogenous EPO and administration of exogenous EPO do not augment erythropoiesis to the point of decreasing transfusion rates. In fact, Deitch and Sittig noted one patient in their series with >40% TBSA burn who required multiple transfusions. Despite the highest endogenous EPO levels of any patient in their study, this patient could not manifest a reticulocytosis.1 This “EPO resistance” parallels findings of bone marrow dysfunction associated with anemia of critical illness.9 Whether an increase in endogenous EPO or supplemental exogenous EPO, the bone marrow does not seem to respond to EPO following a burn injury in the manner that it would under normal conditions or in cases of chronic disease. This is an important point, as EPO resistance may reflect a dysfunctional bone marrow. Looking at later time points postinjury, gradual decreases in hemoglobin or in the context of anemia of critical illness may help to better determine the actual mechanism behind the EPO resistance and bone marrow dysfunction and provide an additional avenue for transfusion intervention and/or reduction.
Although not indicated, the use of EPO in burn patients should not be completely ignored, as the small increase in erythropoiesis with EPO administration may have a greater impact on transfusion rates now with the practice of transfusion restriction. Therefore, EPO use in light of changes in transfusion threshold may warrant further investigation. Also, EPO may benefit burn patients unrelated to erythrogenesis as EPO has been found to have cellular protective effects84–88 and outcome benefit in trauma patients.82
Operative Strategies to Reduce Transfusion
Burn wound excision and skin grafting lead, inevitably, to acute blood loss anemia. Tangential excision, although leading to better cosmetic and functional grafts, is associated with increased blood loss. This acute blood loss anemia leads to pRBC transfusion, which may then lead to infectious complications. A significant amount of research has been performed to establish surgical techniques to reduce surgical blood loss and, in turn, decrease transfusion. These techniques include placement of tourniquets, topical epinephrine soaked pads, and tumescence with vasoconstricting agents. Many studies have detailed the benefits of these techniques. Details of the studies describing the effectiveness of these techniques are supplied in Table 3. Highlights of the most recent, reliable, or effective studies are described below.
Table 3.
Summary of surgical techniques to decrease transfusion
| Study | Technique | Outcome | Limitations |
|---|---|---|---|
| Tourniquets | |||
| Warden et al89 | Two-staged technique with tourniquet placed in the first stage during wound excision, followed by thrombin-epinephrine soaked pads | Decreased blood loss from 1.26 to 0.72 ml/cm2 excised | No mention of graft success |
| Rosenberg and Zawacki23 | Tourniquet placement from excision until grafting and compression dressing placement | Decreased blood loss from 172 to 29 ml/%TBSA 91% graft success |
Compared blood loss with other study outcomes rather than a control |
| Smoot91 | Partial exsanguination, intermittent release and rapid reinflation, epinephrine soaked pad; graft placement without tourniquet | No transfusions in patients with <12% TBSA of the extremities | High graft loss (14% of patients with average of 120 cm2) |
| O’Mara et al92 | Tourniquet placement from excision until grafting; thrombin and epinephrine soaked pads for hemostasis after excision; no exsanguination before tourniquet placement | Decreased blood loss from 0.58 ml/cm2 in controls to 0.19 ml/cm2 for tourniquet 98% graft success vs 96% in controls |
Could not compare transfusion rates as each patient was their own control |
| Epinephrine tumescence | |||
| Kahalley et al94 | Subdermal injection of 0.5 mg/L epinephrine or 50 mg/L phenylephrine at the excision and donor sites | Decreased intraoperative blood transfusion from 2.73 to 0.47–0.88 units per case | Use of a historical control for comparison No mention of graft success or hemodynamic effects. |
| Janezic et al22 | Subdermal injection of 1 mg/501 mL epinephrine at the excision site | Decreased blood loss to 0.97% of blood volume/% TBSA excised and grafted | No matched control group or mention of graft success or hemodynamic effects |
| Sheridan and Szyfelbein95 (pediatric) | Subdermal injection of 0.5 μg/ml epinephrine at both excision and donor sites | Decreased blood loss from 3.5–5% to 0.98% of total blood volume/% of body excised and grafted Graft take of 98% No cardiovascular compromise |
No matched control group |
| Robertson et al93 | Subdermal injection of 1 mg/L epinephrine at both excision and donor sites, followed by thrombin and warm saline soaked pads; Control group only received thrombin and saline soaked pads | Decreased blood loss from 1.15 to 0.37 ml/cm2 and units transfused from 1.91 to 1.08; all statistically significant | No discussion of graft success or impact on transfusion rate |
| Combined Techniques | |||
| Cartotto et al17 | Historical control: epinephrine and thrombin soaked pads Conservative strategy: epinephrine tumescence, tourniquets and epinephrine soaked pads |
Decreased EBL from 211 to 123 ml/% TBSA excised and grafted Decreased intraoperative transfusion from 3.3 to 0.1 units per case 96% graft take with conservative strategy |
Use of historical control |
| Sheridan and Szyfelbein100 | Two 3-year periods separated by a decade were compared; new techniques used in the later period include tourniquets with exsanguination, fascial excision with electrocautery, excision as early as possible following burn, and epinephrine tumescence | 63–89% reduction in pRBC transfusion based on burn size | No report of graft success |
| Gomez et al101 | Traditional surgical technique: epinephrine and thrombin soaked pads Modified tumescent technique: epinephrine tumescence, tourniquets, and epinephrine and thrombin soaked pads |
Decreased total units of blood transfused from 15.7 to 7.9 units per patient Decreased intraoperative units of blood transfused from 8.9 to 4.7 units per patient |
Use of historical control No report of graft success |
| Djurickovic et al108 | Epinephrine tumescence along the trunk or proximal limb vs tourniquet use along the distal limb | Blood loss for tourniquet use was 2.07% of circulating blood volume/%BSA excised vs 3.42 for epinephrine tumescence | Impossible to compare groups when performed in different anatomic locations; tourniquet technique can not possibly be performed on the trunk |
pRBC, packed red blood cell.
Tourniquets
When operating on a burned extremity, tourniquet placement with and without prior limb exsanguination has been found to decrease blood loss. Studies on tourniquet use differ in technique, effect on blood loss, and also graft success. Therefore, each study must be evaluated in light of their relative differences. Any technique that reduces blood loss at the expense of graft failure must be scrutinized for the overall benefit to patient care.
In a description of their two-staged technique, Warden et al89 found a significant decrease in blood loss (1.26 to 0.72 ml/cm2 excised) with tourniquet use during excision, followed by placement of thrombin-epinephrine soaked pads in comparison with a group in which no tourniquet was used. Rosenberg and Zawacki describe a technique in which a tourniquet was placed before excision and remained in place until after grafting. They identified a >80% reduction in blood loss by comparing their blood loss with a recently published study.23 Smoot studied 52 upper extremities using a modified tourniquet technique originally described by Marano.90 For patients with the upper extremity burn compromising <12% TBSA, no transfusions were necessary. Disappointingly, graft viability was low.91 A more thorough and controlled examination of the effect of tourniquet placement on blood loss was performed by O’Mara et al. They randomized the bilateral upper extremities of burn patients into two groups: no tourniquet and tourniquet placement without exsanguination. For the tourniquet technique, a tourniquet was placed for both excision and grafting, but the limb was not exsanguinated. After excision, thrombin and epinephrine soaked pads were used to achieve hemostasis in both groups. Blood loss (259 vs 100 ml) and blood loss per area excised and grafted (0.32 vs 0.10 ml/%TBSA) were significantly decreased in the tourniquet group. Graft take was 98% in the tourniquet group and 96% in the control group, demonstrating decreased blood loss while maintaining graft success.92
Taken together, these studies point favorably toward the use of tourniquets during excision and/or grafting of an extremity to reduce blood loss. When deciding on which technique to use, optimal graft take should be taken into account.
Epinephrine Tumescence
Intradermal clysis or tumescence with a vasoconstricting agent (epinephrine and phenylephrine) has been validated in multiple studies as a safe and effective method of decreasing both surgical blood loss and operative transfusion rates. Because epinephrine is the most commonly used vasoconstricting agent and the term tumescence commonly is used to describe this technique, for ease of association throughout the rest of this review, the phrase “epinephrine tumescence” will be used to describe this technique. Similar to tourniquet use, these studies must be evaluated with respect to both blood loss measures and graft success. A summary of these studies is provided in Table 3.
Robertson et al compared a control group (10 patients) that received thrombin spray and warm saline soaked laparotomy pads on the excised and grafted wounds with a tumescent group that received epinephrine tumescence at the excision and donor sites in addition to thrombin spray and warm saline soaked laparotomy pads (10 patients). The tumescent technique decreased blood loss from 1.15 ml/cm2 excised to 0.37 ml/cm2. Perioperative blood transfusions were decreased from 1.91 to 1.08 units per case.93 Kahalley et al used tumescence with epinephrine or phenylephrine (nine patients with known arrhythmias). Intraoperative transfusion rates decreased from 2.73 units in a historical control group with similar %TBSA debrided and grafted to 0.47 to 0.88 units of blood per patient.94 Sheridan and Szyfelbein reported the safety, efficacy, and transfusion benefit of epinephrine tumescence in pediatric burns. Intraoperative blood loss decreased to 0.98% of the total blood volume per percentage of the body excised and grafted in comparison with the reported literature estimate of 3.5 to 5% at the time. Only 28% of patients required a perioperative blood transfusion. Graft take was 98%.95 The hemodynamic safety of epinephrine tumescence has been well established in several studies.96–98
Only one study showed no improvement with epinephrine tumescence. This study, however, was limited by evaluating this technique at one anatomic location. Barrett et al99 found that topical epinephrine and thrombin and epinephrine tumescence at the scalp compared with thrombin spray alone did not decrease operative blood loss. Given the dense capillary network, epinephrine tumescence may be of less value on the scalp. The results of this one study should not detract from data present on the benefits of epinephrine tumescence.
These studies, along with near universal use, support the notion that epinephrine tumescence can significantly reduce both intraoperative blood loss and transfusions and that the technique is hemo-dynamically safe. Epinephrine tumescence may have a greater reduction in blood loss in comparison with tourniquets because this technique can be used in most areas of excision and can be used at the donor site.
Combined Surgical Techniques
Tourniquets and epinephrine tumescence can be used simultaneously and with other techniques (Table 3). Sheridan and Szyfelbein reviewed pRBC use in children during two 3-year periods separated by a decade. During that decade, several operative measures were undertaken to reduce surgical blood loss including tourniquets, fascial excision with electrocautery, excision as early as possible after the injury, and epinephrine tumescence. When comparing groups based on burn size, there was a 63 to 89% reduction in pRBC transfusion with the use of these surgical techniques.100 Cartotto et al retrospectively compared a conservation strategy of surgical debridement against a historical control group. The conservation strategy used donor site and burn wound epinephrine tumescence, donor site and excised wound topical epinephrine, and limb tourniquets, whereas the historical control group used only topical epinephrine and thrombin at the donor and excision sites. They compared EBL, intraoperative transfusion requirements, and wound outcome for the two groups. The conservation group had an EBL of only 123 ml/%BSA excised and grafted as opposed to 211 ml for the historical control group. The intraoperative transfusion requirement was reduced from 3.3 to 0.1 units per case. Twenty-eight percent of all conservation strategy cases required an intraoperative transfusion or a transfusion within the first 24 hours postoperatively, while 83% of the historical controls required a transfusion.17 Gomez et al retrospectively reviewed the effectiveness of a modified tumescent surgical technique in reducing blood transfusion requirements in burn patients. Their modified tumescent surgical technique included epinephrine tumescence at excision and donor sites, pneumatic tourniquets in the extremities, and epinephrine soaked pads for a dressing. The traditional surgical technique to which it was compared employed the use of epinephrine soaked pads only. The modified tumescent surgical technique decreased the total units of blood transfused from 15.7 to 7.9 units per patient and intraoperative units of blood transfused from 8.9 to 4.7 units per patient.101
These studies provide evidence that newer surgical techniques can decrease blood loss and transfusion rates. Specifically, these techniques have decreased acute blood loss anemia, because anemia of critical illness is not likely to be modified by surgical techniques.
Transfusion Restriction
Transfusion restriction to hemoglobin concentrations of 7 to 8 g/dl in patients without evidence of cardiac ischemia or active bleeding is now common practice. The Transfusion Requirements in Ctirical Care (TRICC) trial, in 1999, established the safety and benefit of transfusion restriction in a large prospective, randomized trial. By restricting transfusions to patients with hemoglobin concentrations of <7 g/dl, in comparison with the liberal strategy of maintaining hemoglobin levels >10 g/dl, the restrictive protocol was found to be safe and to decrease hospital mortality.102 However, challenging the anecdotal transfusion thresholds of hemoglobin of 10 g/dl and hematocrit of 30% occurred in burn care several years earlier. In 1994, Sittig and Deitch prospectively divided patients into a selective transfusion threshold group who were transfused at hemoglobin concentrations of 6 to 6.5 g/dl and a traditional group whose hemoglobin was maintained at 10 g/dl. Patients in the selective transfusion group received less blood (2.1 vs 7.4 units) and had no adverse hemodynamic outcomes as a result of the lower hemoglobin levels. Also, they found a significant decrease in the number of units transfused outside the OR in the selective group, indicating that many of these “maintenance transfusions” were excessive and not needed to maintain adequate hemodynamics or organ perfusion. Although this study was small (only 52 patients) and did not establish other outcome measures for the selective transfusion group, it was a daring study that safely allowed for additional work on lower transfusion thresholds in burn patients.103 Mann et al challenged the hematocrit of 30% rule. They noted that hematocrits of 15 to 20% in healthy patients with small burns, hematocrits as low as 25% in patients with more extensive burns, and hematocrits of 30% in patients who were critically ill or had preexisting cardiovascular disease were well tolerated. These guidelines decreased overall blood transfusion from 133 to 20 ml/%burn. Although the patients tolerated lower hematocrits and decreased transfusion rates, the authors did not address surgical techniques that may have developed during the 10-year time span that may have influenced transfusion rates. However, they demonstrated the efficacy of lower transfusion thresholds and stratifying patients based on oxygen carrying capacity needs.104
Prompted by the results of the TRICC trial, Kwan et al implemented a restrictive transfusion protocol of maintaining a hemoglobin concentration of 7 g/dl. They retrospectively compared this restrictive transfusion protocol with their earlier, liberal transfusion protocol. In the liberal group, the average hemoglobin at transfusion was 9.2 g/dl, whereas in the restrictive group, the average hemoglobin at transfusion was 7.1 g/dl. This lower transfusion threshold was felt to be physiologically safe as there were no differences in acute myocardial infarction rates between the two groups. There was a significant decrease in both 30-day mortality (38 for liberal vs 19% for restrictive) and overall hospital mortality (46 liberal vs 22% restrictive) with the restrictive transfusion strategy.105 Even though the TBICC trial and Kwan et al demonstrate the hemodynamic safety of a lower transfusion threshold, burn patients are transfused at a mean hemoglobin of 8.12 g/dl and hematocrit of 26%. This survey study by Palmieri and Greenhalgh found that the hemoglobin threshold was increased in patients with increased TBSA burn, cardiac disease, ARDS, and age.50 Testing a restrictive transfusion practice prospectively in burn patients, in a similar fashion to the TRICC trial, would help solidify the most appropriate and safest transfusion trigger for burn patients and may convince clinicians of the safety and benefit of transfusion restriction.
Similar benefits of transfusion restriction are seen in the pediatric population. In 2007, Palmieri et al retrospectively reviewed pediatric burn patients subjected to a traditional transfusion policy in which patients were transfused to maintain hemoglobin concentrations ≥10 g/dl and a restrictive policy to maintain hemoglobin concentrations ≥7 g/dl. In the traditional group, patients received an average of 12.3 ±1.8 units of blood, whereas in the restrictive group, patients received only 7.2 ± 1.2 units. There were fewer overall complications in the restrictive group and twice as many pulmonary complications in the traditional group. There were no differences in length of stay, ventilator days, number of operations, or mortality between groups.106
These studies clearly demonstrate both the safety and benefit of transfusion restriction with a goal of keeping the hemoglobin concentration greater than 7 g/dl in burn patients. Looking at transfusion from an opposing angle, transfusing above this threshold may actually be harmful to patient care. In the case of transfusion, “more may be less.”107 A large, multicentered, randomized control trial comparing a restrictive and liberal transfusion policy on outcome parameters is currently taking place. We expect their results will confirm both the safety and clinical benefit of a restrictive transfusion policy.
CONCLUSION
Great strides have been made to decrease transfusion in burn patients. Still, severely burned patients require massive transfusions to combat the acute blood loss from surgery and the blunted erythropoiesis of the anemia of critical illness. To make further headway into decreasing transfusion rates, viewing anemia of thermal injury not just as one entity but the combination of acute blood loss anemia and anemia of critical illness is key. This review has structured the mechanisms, trends, and treatments of anemia of thermal injury so that further research efforts can incorporate this thought process and terminology into practice.
References
- 1.Deitch EA, Sittig KM. A serial study of the erythropoietic response to thermal injury. Ann Surg. 1993;217:293–9. doi: 10.1097/00000658-199303000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes WA, Rogers PW, Beason JW, Pruitt BA., Jr The erythropoietin response to the anemia of thermal injury. J Lab Clin Med. 1976;88:584–92. [PubMed] [Google Scholar]
- 3.Harris RL, Cottam GL, Johnston JM, Baxter CR. The pathogenesis of abnormal erythrocyte morphology in burns. J Trauma. 1981;21:13–21. doi: 10.1097/00005373-198101000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Robinson H, Monafo WW, Saver SM, Gallagher NI. The role of erythropoietin in the anemia of thermal injury. Ann Surg. 1973;178:565–72. doi: 10.1097/00000658-197311000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders R, Garcia J, Sheldon GF, Schooley J, Fuchs R, Carpenter G. Erythropoietin elevation in anemia of thermal injury. Surg Forum. 1976;27:71–2. [PubMed] [Google Scholar]
- 6.Sheldon GF, Sanders R, Fuchs R, Garcia J, Schooley J. Metabolism, oxygen transport, and erythropoietin synthesis in the anemia of thermal injury. Am J Surg. 1978;135:406–11. doi: 10.1016/0002-9610(78)90075-2. [DOI] [PubMed] [Google Scholar]
- 7.Vasko SD, Burdge JJ, Ruberg RL, Verghese AS. Evaluation of erythropoietin levels in the anemia of thermal injury. J Burn Care Rehabil. 1991;12:437–41. doi: 10.1097/00004630-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Wallner S, Vautrin R, Katz J, Murphy J. The anemia of thermal injury: partial characterization of an erythroid inhibitory substance. J Trauma. 1987;27:639–45. [PubMed] [Google Scholar]
- 9.Wallner SF, Vautrin R. The anemia of thermal injury: mechanism of inhibition of erythropoiesis. Proc Soc Exp Biol Med. 1986;181:144–50. doi: 10.3181/00379727-181-42236. [DOI] [PubMed] [Google Scholar]
- 10.Moore FD, Peacock WC, Blakely E, Cope O. The anemia of thermal burns. Ann Surg. 1946;124:811–39. [PMC free article] [PubMed] [Google Scholar]
- 11.Birdsell DC, Birch JR. Anemia following thermal burns: a survey of 109 children. Can J Surg. 1971;14:345–50. [PubMed] [Google Scholar]
- 12.Loebl EC, Marvin JA, Curreri W, Baxter CR. Erythrocyte survival following thermal injury. J Surg Res. 1974;16:96–101. doi: 10.1016/0022-4804(74)90016-x. [DOI] [PubMed] [Google Scholar]
- 13.Kimber RJ, Lander H. The effect of heat on human red cell morphology, fragility, and subsequent survival in vivo. J Lab Clin Med. 1964;64:922–33. [PubMed] [Google Scholar]
- 14.Walsh TS, Saleh EE, Lee RJ, McClelland DB. The prevalence and characteristics of anaemia at discharge home after intensive care. Intensive Care Med. 2006;32:1206–13. doi: 10.1007/s00134-006-0213-7. [DOI] [PubMed] [Google Scholar]
- 15.Bateman AP, McArdle F, Walsh TS. Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med. 2009;37:1906–12. doi: 10.1097/CCM.0b013e3181a000cf. [DOI] [PubMed] [Google Scholar]
- 16.Moran KT, O’Reilly TJ, Furman W, Munster AM. A new algorithm for calculation of blood loss in excisional burn surgery. Am Surg. 1988;54:207–8. [PubMed] [Google Scholar]
- 17.Cartotto R, Musgrave MA, Beveridge M, Fish J, Gomez M. Minimizing blood loss in burn surgery. J Trauma. 2000;49:1034–9. doi: 10.1097/00005373-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Budny PG, Regan PJ, Roberts AH. The estimation of blood loss during burns surgery. Burns. 1993;19:134–7. doi: 10.1016/0305-4179(93)90036-8. [DOI] [PubMed] [Google Scholar]
- 19.Brown RA, Grobbelaar AO, Barker S, Rode H. A formula to calculate blood cross-match requirements for early burn surgery in children. Burns. 1995;21:371–3. doi: 10.1016/0305-4179(94)00012-3. [DOI] [PubMed] [Google Scholar]
- 20.Drew PJ, Ciampolini J, Dickson WA. Blood crossmatching for burn surgery: potential for reduced wastage using a modified dye formula. Burns. 1999;25:651–4. doi: 10.1016/s0305-4179(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 21.Dye DJ. Requirements for cross-matched blood in burns surgery. Burns. 1993;19:524–8. doi: 10.1016/0305-4179(93)90014-y. [DOI] [PubMed] [Google Scholar]
- 22.Janezic T, Prezelj B, Brcic A, Arnez Z, Zaletelj-Kragelj L. Intraoperative blood loss after tangential excision of burn wounds treated by subeschar infiltration of epinephrine. Scand J Plast Reconstr Surg Hand Surg. 1997;31:245–50. doi: 10.3109/02844319709051538. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg JL, Zawacki BE. Reduction of blood loss using tourniquets and ‘compression’ dressings in excising limb burns. J Trauma. 1986;26:47–50. doi: 10.1097/00005373-198601000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Hart DW, Wolf SE, Beauford RB, Lai SO, Chinkes DL, Herndon DN. Determinants of blood loss during primary burn excision. Surgery. 2001;130:396–402. doi: 10.1067/msy.2001.116916. [DOI] [PubMed] [Google Scholar]
- 25.Yogore MG, III, Boral L, Kowal-Vern A, Patel H, Brown S, Latenser BA. Use of blood bank services in a burn unit. J Burn Care Res. 2006;27:835–41. doi: 10.1097/01.BCR.0000245418.73538.25. [DOI] [PubMed] [Google Scholar]
- 26.Criswell KK, Gamelli RL. Establishing transfusion needs in burn patients. Am J Surg. 2005;189:324–6. doi: 10.1016/j.amjsurg.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Palmieri TL, Caruso DM, Foster KN, et al. Effect of blood transfusion on outcome after major burn injury: a multicenter study. Crit Care Med. 2006;34:1602–7. doi: 10.1097/01.CCM.0000217472.97524.0E. [DOI] [PubMed] [Google Scholar]
- 28.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT study: Anemia and blood transfusion in the critically ill-current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, Baron JE, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 30.von Ahsen N, Muller C, Serke S, Frei U, Eckardt KU. Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients. Crit Care Med. 1999;27:2630–9. doi: 10.1097/00003246-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Corwin HL, Krantz SB. Anemia of the critically ill: “acute” anemia of chronic disease. Crit Care Med. 2000;28:3098–9. doi: 10.1097/00003246-200008000-00079. [DOI] [PubMed] [Google Scholar]
- 32.Walsh TS, Saleh EE. Anaemia during critical illness. Br J Anaesth. 2006;97:278–91. doi: 10.1093/bja/ael189. [DOI] [PubMed] [Google Scholar]
- 33.Dale JC, Pruett SK. Phlebotomy-a minimalist approach. Mayo Clin Proc. 1993;68:249–55. doi: 10.1016/s0025-6196(12)60044-5. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Giron F, Alvarez-Mora F. Reduction of blood loss from laboratory testing in hospitalized adult patients using small-volume (pediatric) tubes. Arch Pathol Lab Med. 2008;132:1916–9. doi: 10.5858/132.12.1916. [DOI] [PubMed] [Google Scholar]
- 35.Smoller BR, Kruskall MS, Horowitz GL. Reducing adult phlebotomy blood loss with the use of pediatric-sized blood collection tubes. Am J Clin Pathol. 1989;91:701–3. doi: 10.1093/ajcp/91.6.701. [DOI] [PubMed] [Google Scholar]
- 36.Fowler RA, Berenson M. Blood conservation in the intensive care unit. Crit Care Med. 2003;31:S715–20. doi: 10.1097/01.CCM.0000099350.50651.46. [DOI] [PubMed] [Google Scholar]
- 37.Silver MJ, Li YH, Gragg LA, Jubran F, Stoller JK. Reduction of blood loss from diagnostic sampling in critically ill patients using a blood-conserving arterial line system. Chest. 1993;104:1711–5. doi: 10.1378/chest.104.6.1711. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care. 2001;16:36–41. doi: 10.1053/jcrc.2001.21795. [DOI] [PubMed] [Google Scholar]
- 39.Wallner SF, Warren GH. The haematopoietic response to burning: an autopsy study. Burns Incl Therm Inj. 1985;12:22–7. doi: 10.1016/0305-4179(85)90179-2. [DOI] [PubMed] [Google Scholar]
- 40.Wallner S, Vautrin R, Murphy J, Anderson S, Peterson V. The haematopoietic response to burning: studies in an animal model. Burns Incl Therm Inj. 1984;10:236–51. doi: 10.1016/0305-4179(84)90002-0. [DOI] [PubMed] [Google Scholar]
- 41.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 42.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–9. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 43.Finnerty CC, Jeschke MG, Herndon DN, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–60. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Means RT, Jr, Dessypris EN, Krantz SB. Inhibition of human colony-forming-unit erythroid by tumor necrosis factor requires accessory cells. J Clin Invest. 1990;86:538–41. doi: 10.1172/JCI114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Means RT, Jr, Krantz SB. Inhibition of human erythroid colony-forming units by tumor necrosis factor requires beta interferon. J Clin Invest. 1993;91:416–9. doi: 10.1172/JCI116216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi S, Dai CH, Price JO, Krantz SB. Interferon gamma downregulates stem cell factor and erythropoietin receptors but not insulin-like growth factor-I receptors in human erythroid colony-forming cells. Blood. 1997;90:2244–52. [PubMed] [Google Scholar]
- 47.Wang CQ, Udupa KB, Lipschitz DA. Interferon-gamma exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor cell development. J Cell Physiol. 1995;162:134–8. doi: 10.1002/jcp.1041620116. [DOI] [PubMed] [Google Scholar]
- 48.Jongen-Lavrencic M, Peeters HR, Rozemuller H, et al. IL-6-induced anaemia in rats: possible pathogenetic implications for anemia observed in chronic inflammations. Clin Exp Immunol. 1996;103:328–34. doi: 10.1046/j.1365-2249.1996.d01-622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graves TA, Cioffi WG, Mason AD, Jr, McManus WF, Pruitt BA., Jr Relationship of transfusion and infection in a burn population. J Trauma. 1989;29:948–52. doi: 10.1097/00005373-198907000-00007. discussion 952–4. [DOI] [PubMed] [Google Scholar]
- 50.Palmieri TL, Greenhalgh DG. Blood transfusion in burns: what do we do? J Burn Care Rehabil. 2004;25:71–5. doi: 10.1097/01.BCR.0000105094.25999.0D. [DOI] [PubMed] [Google Scholar]
- 51.Goodnough LT. Risks of blood transfusion. Crit Care Med. 2003;31:S678–86. doi: 10.1097/01.CCM.0000100124.50579.D9. [DOI] [PubMed] [Google Scholar]
- 52.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood. 2008;112:2617–26. doi: 10.1182/blood-2008-07-077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gianotti L, Pyles T, Alexander JW, Babcock GF, Carey MA. Impact of blood transfusion and burn injury on microbial translocation and bacterial survival. Transfusion. 1992;32:312–7. doi: 10.1046/j.1537-2995.1992.32492263443.x. [DOI] [PubMed] [Google Scholar]
- 54.Leal-Noval SR, Rincon-Ferrari MD, Garcia-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461–8. doi: 10.1378/chest.119.5.1461. [DOI] [PubMed] [Google Scholar]
- 55.Torchia MG, Danzinger RG. Perioperative blood transfusion and albumin administration are independent risk factors for the development of postoperative infections after colorectal surgery. Can J Surg. 2000;43:212–6. [PMC free article] [PubMed] [Google Scholar]
- 56.Bochicchio GV, Napolitano L, Joshi M, et al. Blood product transfusion and ventilator-associated pneumonia in trauma patients. Surg Infect (Larchmt) 2008;9:415–22. doi: 10.1089/sur.2006.069. [DOI] [PubMed] [Google Scholar]
- 57.Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68:566–72. [PubMed] [Google Scholar]
- 58.Croce MA, Tolley EA, Claridge JA, Fabian TC. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma. 2005;59:19–23. doi: 10.1097/01.ta.0000171459.21450.dc. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 59.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–74. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 60.Taylor RW, Manganaro L, O’Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30:2249–54. doi: 10.1097/00003246-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Taylor RW, O’Brien J, Trottier SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34:2302–8. doi: 10.1097/01.CCM.0000234034.51040.7F. quiz 2309. [DOI] [PubMed] [Google Scholar]
- 62.FDA. Fatalities reported to FDA following blood collection and transfusion: annual summary of fiscal year 2008. Rockville: Center for Biologics Evaluation and Research; 2008. [Google Scholar]
- 63.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–89. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 64.Webert KE, Blajchman MA. Transfusion-related acute lung injury. Curr Opin Hematol. 2005;12:480–7. doi: 10.1097/01.moh.0000177829.85904.39. [DOI] [PubMed] [Google Scholar]
- 65.Silliman CC, Fung YL, Bradley Ball J, Khan SY. Transfusion-related acute lung injury (TRALI): current concepts and misconceptions. Blood Rev. 2009;23:245–55. doi: 10.1016/j.blre.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Higgins S, Fowler R, Callum J, Cartotto R. Transfusion-related acute lung injury in patients with burns. J Burn Care Res. 2007;28:56–64. doi: 10.1097/BCR.0b013E31802C88EC. [DOI] [PubMed] [Google Scholar]
- 67.Silver GM, Klein MB, Herndon DN, et al. Standard operating procedures for the clinical management of patients enrolled in a prospective study of inflammation and the host response to thermal injury. J Burn Care Res. 2007;28:222–30. doi: 10.1097/BCR.0B013E318031AA44. [DOI] [PubMed] [Google Scholar]
- 68.Linden JV, Wagner K, Voytovich AE, Sheehan J. Transfusion errors in New York State: an analysis of 10 years’ experience. Transfusion. 2000;40:1207–13. doi: 10.1046/j.1537-2995.2000.40101207.x. [DOI] [PubMed] [Google Scholar]
- 69.Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin. 2004;20:187–212. doi: 10.1016/j.ccc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Fisher JW, Koury S, Ducey T, Mendel S. Erythropoietin production by interstitial cells of hypoxic monkey kidneys. Br J Haematol. 1996;95:27–32. doi: 10.1046/j.1365-2141.1996.d01-1864.x. [DOI] [PubMed] [Google Scholar]
- 71.Fried W. Erythropoietin and erythropoiesis. Exp Hematol. 2009;37:1007–15. doi: 10.1016/j.exphem.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–8. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 73.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–98. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 74.Erslev AJ, Wilson J, Caro J. Erythropoietin titers in anemic, nonuremic patients. J Lab Clin Med. 1987;109:429–33. [PubMed] [Google Scholar]
- 75.Rogiers P, Zhang H, Leeman M, et al. Erythropoietin response is blunted in critically ill patients. Intensive Care Med. 1997;23:159–62. doi: 10.1007/s001340050310. [DOI] [PubMed] [Google Scholar]
- 76.Krafte-Jacobs B, Levetown ML, Bray GL, Ruttimann UE, Pollack MM. Erythropoietin response to critical illness. Crit Care Med. 1994;22:821–6. doi: 10.1097/00003246-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 77.Hobisch-Hagen P, Wiedermann F, Mayr A, et al. Blunted erythropoietic response to anemia in multiply traumatized patients. Crit Care Med. 2001;29:743–7. doi: 10.1097/00003246-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Goodnough LT, Monk TG, Andriole GL. Erythropoietin therapy. N Engl J Med. 1997;336:933–8. doi: 10.1056/NEJM199703273361307. [DOI] [PubMed] [Google Scholar]
- 79.Rizzo JD, Seidenfeld J, Piper M, Aronson N, Lichtin A, Littlewood TJ. Erythropoietin: a paradigm for the development of practice guidelines. Hematology Am Soc Hematol Educ Program. 2001:10–30. doi: 10.1182/asheducation-2001.1.10. [DOI] [PubMed] [Google Scholar]
- 80.Fleming RY, Herndon DN, Vaidya S, et al. The effect of erythropoietin in normal healthy volunteers and pediatric patients with burn injuries. Surgery. 1992;112:424–31. discussion 431–2. [PubMed] [Google Scholar]
- 81.Still JM, Jr, Belcher K, Law EJ, et al. A double-blinded prospective evaluation of recombinant human erythropoietin in acutely burned patients. J Trauma. 1995;38:233–6. doi: 10.1097/00005373-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 82.Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357:965–76. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 83.Zarychanski R, Turgeon AF, McIntyre L, Fergusson DA. Erythropoietin-receptor agonists in critically ill patients: a meta-analysis of randomized controlled trials. CMAJ. 2007;177:725–34. doi: 10.1503/cmaj.071055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arcasoy MO. Erythropoiesis-stimulating agents in cancer. J Clin Oncol. 2008;26:3097–8. doi: 10.1200/JCO.2008.16.0531. author reply 3098–100. [DOI] [PubMed] [Google Scholar]
- 85.Burger D, Lei M, Geoghegan-Morphet N, Lu X, Xenocostas A, Feng Q. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res. 2006;72:51–9. doi: 10.1016/j.cardiores.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 86.Fantacci M, Bianciardi P, Caretti A, et al. Carbamylated erythropoietin ameliorates the metabolic stress induced in vivo by severe chronic hypoxia. Proc Natl Acad Sci U S A. 2006;103:17531–6. doi: 10.1073/pnas.0608814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu X, Xie W, Liu P, et al. Mechanism of the cardioprotection of rhEPO pretreatment on suppressing the inflammatory response in ischemia-reperfusion. Life Sci. 2006;78:2255–64. doi: 10.1016/j.lfs.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 88.Mori S, Sawada T, Okada T, Kubota K. Erythropoietin and its derivative protect the intestine from severe ischemia/reperfusion injury in the rat. Surgery. 2008;143:556–65. doi: 10.1016/j.surg.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 89.Warden GD, Saffle JR, Kravitz M. A two-stage technique for excision and grafting of burn wounds. J Trauma. 1982;22:98–103. doi: 10.1097/00005373-198202000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Marano MA, O’Sullivan G, Madden M, Finkelstein J, Goodwin CW. Tourniquet technique for reduced blood loss and wound assessment during excisions of burn wounds of the extremity. Surg Gynecol Obstet. 1990;171:249–50. [PubMed] [Google Scholar]
- 91.Smoot EC., III Modified use of extremity tourniquets for burn wound debridement. J Burn Care Rehabil. 1996;17:334–7. doi: 10.1097/00004630-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 92.O’Mara MS, Goel A, Redo P, et al. The use of tourniquets in the excision of unexsanguinated extremity burn wounds. Burns. 2002;28:684–7. doi: 10.1016/s0305-4179(02)00186-9. [DOI] [PubMed] [Google Scholar]
- 93.Robertson RD, Bond P, Wallace B, Shewmake K, Cone J. The tumescent technique to significantly reduce blood loss during burn surgery. Burns. 2001;27:835–8. doi: 10.1016/s0305-4179(01)00057-2. [DOI] [PubMed] [Google Scholar]
- 94.Kahalley L, Dimick AR, Gillespie RW. Methods to diminish intraoperative blood loss. J Burn Care Rehabil. 1991;12:160–1. doi: 10.1097/00004630-199103000-00015. [DOI] [PubMed] [Google Scholar]
- 95.Sheridan RL, Szyfelbein SK. Staged high-dose epinephrine dysis is safe and effective in extensive tangential burn excisions in children. Burns. 1999;25:745–8. doi: 10.1016/s0305-4179(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 96.Cartotto R, Kadikar N, Musgrave MA, Gomez M, Cooper AB. What are the acute cardiovascular effects of subcutaneous and topical epinephrine for hemostasis during burn surgery? J Burn Care Rehabil. 2003;24:297–305. doi: 10.1097/01.BCR.0000085847.47967.75. [DOI] [PubMed] [Google Scholar]
- 97.Missavage AE, Bush RL, Ken ND, Reilly DA. The effect of clysed and topical epinephrine on intraoperative catecholamine levels. J Trauma. 1998;45:1074–8. doi: 10.1097/00005373-199812000-00018. [DOI] [PubMed] [Google Scholar]
- 98.Papp AA, Uusaro AV, Ruokonen ET. The effects of topical epinephrine on haemodynamics and markers of tissue perfusion in burned and non-burned patients requiring skin grafting. Burns. 2009;35:832–9. doi: 10.1016/j.burns.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Barret JP, Dziewulski P, Wolf SE, Desai MH, Nichols RJ, II, Herndon DN. Effect of topical and subcutaneous epinephrine in combination with topical thrombin in blood loss during immediate near-total burn wound excision in pediatric burned patients. Burns. 1999;25:509–13. doi: 10.1016/s0305-4179(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 100.Sheridan RL, Szyfelbein SK. Trends in blood conservation in burn care. Burns. 2001;27:272–6. doi: 10.1016/s0305-4179(00)00110-8. [DOI] [PubMed] [Google Scholar]
- 101.Gomez M, Logsetty S, Fish JS. Reduced blood loss during burn surgery. J Burn Care Rehabil. 2001;22:111–7. doi: 10.1097/00004630-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 102.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 103.Sittig KM, Deitch EA. Blood transfusions: for the thermally injured or for the doctor? J Trauma. 1994;36:369–72. [PubMed] [Google Scholar]
- 104.Mann R, Heimbach DM, Engrav LH, Foy H. Changes in transfusion practices in burn patients. J Trauma. 1994;37:220–2. doi: 10.1097/00005373-199408000-00012. [DOI] [PubMed] [Google Scholar]
- 105.Kwan P, Gomez M, Cartotto R. Safe and successful restriction of transfusion in burn patients. J Burn Care Res. 2006;27:826–34. doi: 10.1097/01.BCR.0000245494.45125.3E. [DOI] [PubMed] [Google Scholar]
- 106.Palmieri TL, Lee T, O’Mara MS, Greenhalgh DG. Effects of a restrictive blood transfusion policy on outcomes in children with burn injury. J Burn Care Res. 2007;28:65–70. doi: 10.1097/BCR.0B013E31802C895E. [DOI] [PubMed] [Google Scholar]
- 107.Corwin HL, Carson JL. Blood transfusion-when is more really less? N Engl J Med. 2007;356:1667–9. doi: 10.1056/NEJMe078019. [DOI] [PubMed] [Google Scholar]
- 108.Djurickovic S, Snelling CF, Boyle JC. Tourniquet and subcutaneous epinephrine reduce blood loss during burn excision and immediate autografting. J Burn Care Rehabil. 2001;22:1–5. doi: 10.1097/00004630-200101000-00002. [DOI] [PubMed] [Google Scholar]


