Abstract
Background
Cocaine dependence involves problematic neuroadaptations that may be responsive to modulation of glutamatergic circuits. This investigation examined the effects of sub-anesthetic ketamine infusions on motivation for quitting cocaine and on cue-induced craving in cocaine dependent participants, 24 hours post-infusion.
Methods
Eight volunteers with active DSM-IV cocaine dependence not seeking treatment or abstinence were entered into this crossover, double-blind trial. Three 52 minute intravenous infusions were administered: ketamine (0.41 mg/kg or 0.71 mg/kg) or lorazepam 2 mg, counterbalanced into three orderings in which ketamine 0.41 mg/kg always preceded the 0.71 mg/kg dose. Infusions were separated by 48 hours, and assessments occurred at baseline and at 24 hours post-infusion. Outcomes were change between post-infusion and pre-infusion values for 1) motivation to quit cocaine scores using the University of Rhode Island Change Assessment (URICA), and 2) sums of visual analogue scale (VAS) craving ratings administered during cue exposure.
Results
Compared to the active control lorazepam, a single ketamine infusion (0.41 mg/kg) led to a mean 3.9 points gain in URICA (p=0.012), which corresponds to an approximately 60% increase over preceding values. There was a reduction of comparable magnitude in cue-induced craving (p=0.012). A subsequent ketamine infusion (0.71 mg/kg) led to further reductions in cue-induced craving compared to the control. Infusions were well tolerated.
Conclusions
Sub-anesthetic ketamine demonstrated promising effects on motivation to quit cocaine and on cue-induced craving, 24 hours post-infusion. Research is needed to expand on these preliminary results, and to evaluate the efficacy of this intervention in clinical settings.
Keywords: addiction, cocaine, craving, glutamate, ketamine, motivation
Cocaine dependence is a substantial global health problem (1) for which effective medications remain elusive, despite decades of research.(2) One disappointment has been the failure of strategies targeting glutamate to produce in humans the benefits predicted by preclinical studies.(3) Cocaine alters glutamate-mediated synaptic communication between prefrontal and limbic regions in animals; these changes in long-term depression (LTD) and long-term potentiation (LTP) are thought to attenuate the salience of natural reinforcers (e.g., food) while heightening drug-seeking and reactivity to drug cues.(3-7) Though modulation of the N-methyl-D-aspartate receptor (NMDAr), the predominant glutamate receptor involved in neural plasticity,(3) consistently disrupts the reinforcing effects of cocaine in rodents,(8-10) comparable modulators, such as memantine and D-cycloserine (DCS), have proven ineffective in humans in both laboratory and clinical settings.(11-13)
A glutamatergic adaptation that is also relevant to drug dependence is learned helplessness, a deficit typically characterized by depressive phenomena and prefrontal synaptic loss due to persistent, uncontrollable stress.(14-18,19-21) An important feature of drug dependence is compulsive drug use despite adverse consequences. This apparent lack of control in the midst of mounting stress may lead to demoralization or impaired motivation for change,(22) much as in depression-related learned helplessness.(14,15) Impaired motivation for changing drug use, as well as heightened sensitivity to stress or drug cues, may thus emerge from a cascade of adaptations, with the putative early disruptions in LTP and LTD evolving into prefrontal abnormalities similar in pathophysiology to those associated with stress-related or affective conditions.(4,19-21)
Recent investigations suggest that the NMDAr antagonist ketamine effectively addresses depression-related prefrontal alterations. A single sub-anesthetic dose (0.5 mg/kg over 40 minutes) has been shown to produce immediate and enduring improvements in depressive symptoms in treatment-resistant individuals, with benefits peaking 24 hours post-infusion.(16) This unique anti-depressant effect, unprecedented among glutamatergic modulators, has been attributed to improved prefrontal glutamate homeostasis, specifically of the anterior cingulate cortex (ACC), and to the promotion of prefrontal synaptic plasticity through various downstream effects.(16-18,23) These prefrontal effects may also serve to target complex neocortical functions(24) that might be unique to humans and difficult to approximate in animal models, as in a recent investigation suggesting that ketamine improves suicidality.(25) Ketamine may benefit cocaine-related cue reactivity and impaired motivation for changing drug use through comparable mechanisms, including improved glutamate homeostasis, ACC modulation, and prefrontal synaptic remodeling.(4,20,21) Though enhancement of motivation for change is recognized as an important goal in addiction-oriented psychotherapy,(22) it has never been studied as a focus of pharmacotherapy.
We therefore investigated the effects of sub-anesthetic ketamine infusions on cocaine dependence in this randomized, lorazepam-controlled, crossover trial. There were four aims. First, because this high-risk intervention has not been previously investigated in cocaine dependent subjects, we carefully assessed the tolerability of two doses of ketamine (0.41 mg/kg, 0.71 mg/kg), using a conservatively sized sample, close monitoring, and measures of abuse liability and behavioral toxicity. Second, we tested the hypothesis that a single ketamine infusion (0.41 mg/kg) leads to rapid effects on motivation to quit cocaine and on cue-induced craving, as measured 24 hours post-infusion. Third, we tested the hypothesis that an additional ketamine dose (0.71 mg/kg) has further effects on these outcomes. Fourth, we explored the relationship between ketamine-induced changes and reductions in cocaine use.
Methods
Overview of Study Procedures
Consenting participants were hospitalized at the New York State Psychiatric Institute (NYSPI) for 9 days. On Days 1 through 3, participants attained abstinence from cocaine and other intoxicants, and baseline measures of motivation to quit cocaine and cue-induced craving were collected on Day 1. On Day 4, participants were randomized to one of 3 infusion orders, and received the first infusion. Infusions were separated by 48 hours and were each followed by assessments that occurred the following afternoon, 24 hours post-infusion. Only a single participant was inpatient at a time, and efforts were taken to ensure that participants had no contact with one another to protect the various blinding procedures in the protocol. Following the last set of assessments on Day 9, participants were discharged from the research unit. They were then assessed weekly for 4 weeks. The Investigational New Drug application for ketamine and lorazepam was approved by the Food and Drug Administration in 2010. All procedures received approval from the Institutional Review Board of the NYSPI, and data were collected between February 2011 and March 2012.
Participants
Eight participants completed this trial. Participants consisted of adults recruited through advertisements for cocaine-administration studies at the NYSPI, and who underwent a Structured Clinical Interview for the Diagnostic Statistical Manual, 4th edition (DSM-IV) (SCID), as well as a thorough psychiatric examination. In addition to meeting DSM-IV criteria for current dependence on free-base (“crack”) cocaine, participants were between 21 and 52; were non-treatment and non-abstinence seeking; described a history of arousal and craving elicited by cocaine cues; and were not diagnosed with other past or current psychiatric disorders. All participants were in good health as assessed by medical history, physical examination, urinalysis, toxicology, serologic tests, vital sign assessment, and electrocardiogram. Participants were thus selected to be uninterested in abstinence or treatment, to report cue-induced craving, and to be sufficiently healthy as to safely participate in study procedures.
24 individuals were screened for the trial, 11 met eligibility criteria and were enrolled, 1 discontinued after undergoing baseline measures of cue-induced craving, 1 discontinued because he disliked the first infusion procedure (in which he received ketamine, without adverse events), and 1 was removed from the trial due to concerns about his veracity in regards to route of cocaine use and psychiatric co-morbidity, resulting in 8 individuals completing the trial.
Infusion Procedures
Participants were informed that they might receive any of 6 infused medications (amphetamine, buspirone, d-cycloserine, ketamine, lorazepam, and memantine) during study participation, and received full information about the expected effects and risks of each. Participants therefore anticipated receiving any of several psychoactive compounds with overlapping and/or distinct effects. This procedure served to blind participants to specific medication effects and addressed the possibility of participants expecting or identifying medications
Three 52-minute infusions separated by 48 hours were administered in a randomized, double blind manner to each participant (see Supplement). In the first infusion, ketamine 0.41 mg/kg (K1) or lorazepam 2 mg (LZP) was administered. Participants who received K1 in the 1st infusion received counter-balanced infusions of LZP or ketamine 0.71 mg/kg (K2) in the 2nd and 3rd infusions; those who received LZP first received K1 and K2 in the 2nd and 3rd respectively. These three orderings ensured that K2 was received after the tolerability of K1 was established. Ketamine dosages were based on previous reports of sub-anesthetic psychoactive potency and tolerability,(26) and were comparable to the anti-depressant dose (0.5 mg/kg over 40 minutes).(16) LZP was designated as the active control because it generates mild psychoactive effects, thereby helping to maintain the blind, without any known effects on cocaine dependence.(2) Accordingly, it was administered as a slow-drip infusion without an initial bolus dose (see Supplement) in order to generate psychoactive effects without putting participants immediately to sleep.
Prior to infusions, participants underwent a 10-minute mindfulness-based exercise that involved gently attending to their breathing, sensations, and thoughts while comfortably supine. Psychological preparation and pre-infusion relaxation exercises have been previously shown to reduce the anxiety and distress that may emerge with ketamine administration.(27)
Following infusions, a Clinician Administered Dissociative Symptoms Scale (CADSS) and other measures pertaining to acute drug effects were administered. The brief psychiatric rating scale (BPRS) for positive symptoms (suspiciousness, unusual ideas, hallucinations, or bizarre behavior) assessed psychotic phenomena. Participants were monitored for 2 hours after the infusion ended, and were interviewed 20 minutes and 1 hour post-infusion, with special attention given to persistent symptomatology that might merit psychiatric attention such as sedation, psychosis or dissociation.
Assessments
Motivation to stop cocaine use and cue-induced craving were assessed at baseline and at 24 hours after each infusion. The timing of measures reflects the anti-depressant effects of ketamine, which peak at 24 hours post-infusion and generally subside by 72 hours (though it may persist for weeks).(16)
Motivation to quit cocaine
Motivation scores were calculated at baseline and 24 hours post-infusion using the University of Rhode Island Change Assessment (URICA), a 32-item questionnaire validated to ascertain readiness for change in cocaine users.(28,29)
Cue-Induced Craving
Cue-induced craving was assessed at baseline and 24 hours post-infusion using the visual analogue scale (VAS) for cocaine craving. The VAS for cocaine craving is a 100 mm scale on which participants make a mark corresponding to the intensity of their wanting cocaine, from “none at all” on the left to “extremely” on the right. The VAS was administered at the start and every 3 minutes thereafter of the 15-minute exposure to cocaine cues (leading to 6 ratings for each session); sum VAS scores were calculated for each of the exposure sessions. Given the frequency of cue exposure (4 sessions over the course of 9 days), cue salience attenuation was addressed by employing a novel exposure paradigm aimed to maintain expectancy and to heighten cue intensity. In brief, participants were presented with cocaine cues – free-base cocaine, glass tube with filter (a “stem”), and lighter – at each session, and given the impression that they might be provided a choice to smoke the cocaine (see Supplement).
4-week Follow-Up
A Timeline Follow Back for the use of various drugs of abuse, including cocaine, ketamine, and benzodiazepines, was administered weekly, as was weekly urine toxicology. At each visit, participants were assessed by a psychiatrist for persistent adverse effects. No addiction treatment or psychotherapy was provided. If participants requested treatment, they were appropriately referred.
Statistical Methods
Efficacy
Efficacy was analyzed using within-subject Friedman's 2-way analyses of variance (ANOVA) [condition (LZP, K1, K2) by order (3 possible orders)] and Wilcoxon signed-rank tests in which the difference between the post-infusion score and the preceding score was the dependent variable. URICA and sum VAS scores were analyzed separately, and each analysis was Bonferroni-corrected. Difference from preceding score (change score), rather than difference from baseline, was used as the dependent variable to account for the possible persistence of ketamine effects up to 72 hours, as observed in others settings (16). Between-subjects analyses of first infusion effects (where carryover is not an issue) were also conducted. Change from baseline for LZP (n=3) and K1 (n=5) following the first infusion were compared with Wilcoxon tests for independent samples, with URICA and sum VAS scores analyzed separately.
Persistent effects; K2 efficacy
To fully assess order effects, change from baseline, as opposed to change from preceding score, was calculated for URICA and VAS and compared by order and condition using Friedman's 2-way ANOVA and Wilcoxon tests. To better evaluate K2 efficacy relative to LZP given apparent post-K1 carry-over effects, Friedman's and Wilcoxon tests compared change from preceding assessment for LZP and K2 in individuals who received K1 in the first infusion (n=5). URICA and sum VAS scores were analyzed separately in all tests.
Tolerability and follow-up
Friedman's and Wilcoxon tests compared CADSS and drug-liking scores by order and condition. Paired Wilcoxon tests compared drug use (amount in dollars for each use day and number of use days over 4 weeks) at baseline and during the 4-week follow-up period. All the above analyses were conducted using SPSS with 2-tailed α=0.05.(30)
Results
Participants were predominantly African American and male. Baseline cocaine use, VAS sum scores, and URICA scores were comparable (Table 1).
Table 1. Sociodemographic Characteristics and Baseline Morbidity (n=8).
| Age, years (SD) | 47.5 (5.5) |
| African American | 87.5% |
| Hispanic | 12.5% |
| Male | 87.5% |
| Unemployed | 75% |
| Education (>12 years, or HS equivalency) | 62.5% |
| Cocaine Amount, per occasion, $ (SD) | 157.83 (243.22) |
| Cocaine Frequency, occasions per 28 days, days (SD) | 22 (5.6) |
| Hamilton Depression Rating Scale (HAMD), score (SD) | 2.4 (1.7) |
| Alcohol, drinks/week (SD) | 2.9 (2.4) |
| Baseline University of Rhode Island Change Assessment (URICA), motivation score (SD) | 6.79 (1.49) |
| Baseline Cue-Induced Craving by Visual Analogue Scale (VAS), sum score (SD) | 282.13 (119.70) |
K1 Efficacy
Within-subject analyses of change from the preceding score found that K1 significantly increased motivation for changing cocaine use relative to LZP by URICA (median 0.15 vs. 3.6), p=0.012, and significantly decreased cue-induced cocaine craving by sum VAS change scores (median 65 vs. -126), p=0.012 (see Figures 1a and 1b for mean values and SEMs). This corresponded to a mean 3.9 gain in URICA scores after K1, and a mean 168 mm decrease in sum VAS scores, which represent a 60% change from baseline values.
Figure 1. K1 Effects, 24 hours Post-Infusion.
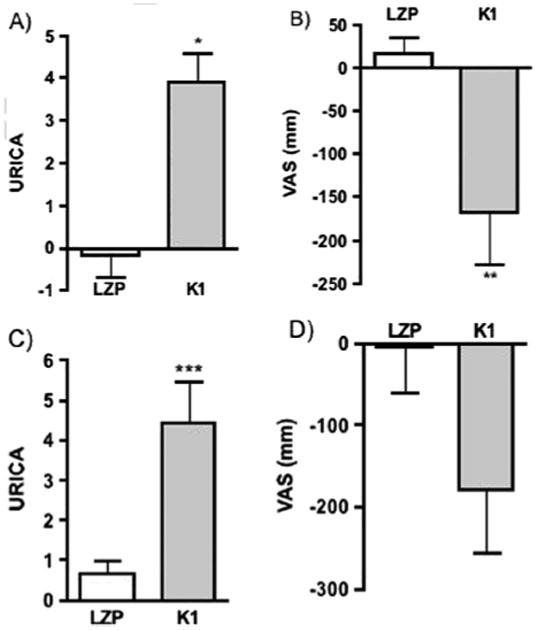
The difference across a condition between the post-infusion score and the immediately preceding score for LZP vs. K1 (change score). Bars represent mean difference; errors bars represent standard errors of the mean (SEMs). Median values are available in Findings. (a) paired comparison of URICA change scores, K1 vs. LZP, n=8, * p=0.012. (b) paired comparison of cue-induced craving (sum VAS score) change scores, K1 vs. LZP, n=8, **p=0.012. (c) independent comparison of URICA change scores following the first infusion, K1 (n=5) vs. LZP (n=3),*** p=0.051. (d) independent comparison of change scores for sum VAS scores following first infusion, p=0.1.
Difference from baseline for LZP (n=3) and K1 (n=5) were compared when both conditions were received in the first infusion. The effect of K1 on URICA scores was consistent with the within-subject analysis and nearly significant relative to LZP, despite small sample sizes (median 0.71 vs. 4.87), p=0.051. Similarly, a trend in craving reduction following K1 was observed when VAS scores were compared for the 1st infusion (median 7 vs. -169), p=0.10 (Figure 1c, 1d).
No order or interaction effects were found, suggesting that change scores were not significantly affected by the order (1st, 2nd, or 3rd) in which conditions were administered. However, because the effect of K1 may have persisted beyond 24 hours (see below) and because K2 was always received after K1 for safety reasons, order effects cannot be ruled out between K1 and K2. The change scores of K2 for both outcomes were not significantly different from those of K1 or LZP.
Persistent (∼72 hour) K1 effects
Change from baseline, as opposed to change from preceding assessment, was evaluated to further elucidate order effects. For LZP received in the 2nd and 3rd assessments, URICA scores were comparable, but URICA scores when LZP was 1st (before K1 was received) were significantly different from values when LZP was 2nd (post-K1) (median absolute change from baseline: 1.1 vs. 3.28, p=0.047), despite small samples. This comparison (see Figure 2a) suggests that the effect of K1 on URICA scores persisted beyond 72 hours. An evaluation of VAS scores suggests comparable post-K1 carryover effects (Figure 2b), but a comparison between the 1st and 2nd LZP assessments did not reach statistical significance (8 vs. -106, p=0.11).
Figure 2.
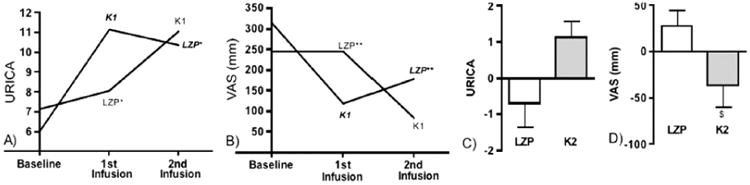
Persistent (>72 hr) K1 effects; K2 Effects, 24 hours Post-infusion: Figures (a) and (b) show baseline and post-infusion URICA and VAS scores by infusion order (1st or 2nd) for LZP (n=3) and K1 (n=3); Figures (c) and (d) show difference from preceding assessment, LZP vs K2, in subjects who received K1 in the 1st infusion (n=5) (mean values and SEMs shown; median values are provided in Findings).(a) URICA assessments for LZP were significantly different when LZP was administered 1st or 2nd, * p = 0.047, suggesting a post-K1 carry-over effect.(b) LZP order effects with sum VAS scores, ** p = 0.1.(c) paired within-subject comparison of URICA by condition, K2 vs. LZP, in those who received K1 in the first condition (n=5); non-significant, p=0.11. (d) paired within-subject comparison of sum VAS scores by condition in those who received K1 in the first infusion (n=5), K2 vs. LZP, $ p=0.046.
K2 efficacy
To better evaluate K2 efficacy in the setting of persistent K1 effects, differences between post- and pre-infusion scores for LZP and K2 were compared in individuals who received K1 first (n=5). K2 led to significant reductions in cue-induced craving relative to LZP (median 53 vs. -18), p=0.046, but not a significant change in URICA, p=0.11 (Figures 2c, 2d). Failure to detect K2 efficacy with URICA might be due to a post-K1 ceiling effect, as the mean URICA score following K1 was more than 90% of the highest possible score.(28,29)
Tolerability
Inspection of Figure 3a suggests an orderly increase in dissociation between LZP, K1, and K2, butonly K2 led to significantly higher CADSS scores relative to LZP (p=0.047). This suggests that LZP was mildly dissociative and served to maintain the blind in comparison with K1.
Figure 3. Acute Medication Effects.
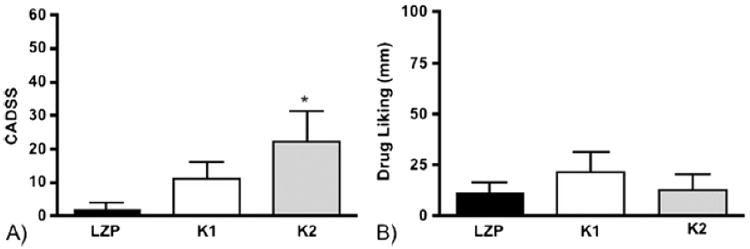
Acute medication effects for each condition, with bars demonstrating mean values and error bars SEMs. (a) level of dissociation assessed by the CADSS, with LZP (2±1.8) significantly different from K2 (22±7.2), *p=0.047. (b) drug liking scores, assessed by VAS, were comparable and low for all conditions.
Infusions were generally well tolerated. All participants reported mild to moderate sedation, which typically resolved by 2 hours post-infusion. There were no incidents of psychiatric disturbances, including positive symptoms of psychosis, during or after infusions. K1 and K2 led to modest and clinically insignificant systolic blood pressure elevation, but this did not merit medical attention and resolved within a ½ hour post-infusion. All infusions were associated with low and comparable drug-liking scores (Figure 3b), and there were no reports of initiation of ketamine or benzodiazepine use in the 4-week follow-up period.
Follow-up Drug use
There was a significant reduction in median amount and frequency of cocaine use in the 4-week follow-up period as compared to baseline (Baseline: $149.30/use day vs. Follow-up: $10.50/use day, p<0.001; Baseline: 22 days of use out of 28 days vs. Follow-up: 5/28 days, p=0.012). No participants entered into formal treatment in the follow-up period.
Correlations were explored between K1-associated benefits and changes in drug use. Participants who demonstrated 2 or greater weeks of abstinence (n=4), confirmed by urine toxicology, were also those with post-K1 URICA scores exceeding 11.5. This motivation score corresponds to maintenance, in which behavioral changes are actively maintained.(28,29) While the statistical significance of this correlation cannot be determined due to small samples, it suggests that the effects of K1 on motivation to stop cocaine may have influenced post-study abstinence.
Discussion
This study represents the first investigation of sub-anesthetic ketamine infusions in cocaine dependent individuals. We found that a single ketamine infusion (0.41 mg/kg) enhanced motivation to quit and dampened cue-induced craving. A subsequent dose (0.71 mg/kg) further reduced cue-induced craving. We also found that both ketamine doses could be safely given under controlled conditions to individuals with active cocaine dependence, without the emergence of ketamine misuse or unexpected psychiatric disturbances. In addition, though the effect of ketamine on cocaine use could not be clearly determined during the month after infusions because there was no control group that did not receive ketamine, self-reports of cocaine use decreased and some participants achieved urine-confirmed abstinence in follow-up, even though no outpatient treatment was provided.
This investigation had several limitations consistent with its preliminary nature. A major limitation is that the sample was small, psychiatrically uncomplicated and homogenous. Though these characteristics are typical of studies with non-treatment seeking cocaine dependent research volunteers,(31) they may limit the generalizability of this investigation. Second, ketamine doses were selected on the basis of safety and psychoactive potency,(26) as the effective dose for cocaine dependence is not known. Investigating a higher ketamine dose in the first infusion may have more greatly impacted primary outcomes. Third, K1 may have led to persistent effects lasting more than 72 hours, especially in relation to URICA scores. These order effects were not significant when differences between post- and pre-infusion scores were analyzed. However, because K2 was always received after K1 for safety reasons, this study cannot appropriately evaluate K2 efficacy nor ascertain a dose response. Future studies should consider spacing out infusions by a greater length of time, or using a between-subjects design to compare dose effects. Finally, it is not possible from this investigation to determine the effects of ketamine infusions on cocaine use. Further research is needed to elucidate the optimal ketamine dose and schedule, the temporal course of its apparent effects, and its effectiveness and safety in clinical settings.
Despite these limitations, our findings have novel and important therapeutic ramifications. Perhaps most remarkable is the rapid and apparently persistent effect of ketamine on motivation scores. The substantial improvement in URICA scores, which corresponds to a 60% increase from baseline values, represents the first demonstration to our knowledge that motivation for changing problematic behaviors may be enhanced pharmacologically. This robust finding was significant in the within-subjects analysis, and approached significance (p=0.051), despite small sample sizes, when a between-subjects analysis was conducted on assessments following the 1st infusion.
The low baseline levels of URICA scores in our research volunteers raise important questions about the relevance of the observed motivation enhancement to clinical settings, where motivation at the outset might be greater.(28) Impaired motivation for change, however, is not a problem unique to non-treatment seeking populations. It also presents a critical hurdle in the recovery of individuals already engaged in treatment, where it can undermine services utilization, treatment retention, self-efficacy and the maintenance of sobriety.(22) Further, motivation to quit in early recovery may be ephemeral, tenuous or highly sensitive to contextual factors. A pharmacological approach to motivation enhancement therefore represents a significant step forward in addiction treatment, and may be most helpful for individuals not responding adequately to conventional psychotherapy strategies or for whom motivation to quit might be particularly labile. Research is needed to clarify the temporal course of this effect, and how it might be utilized and sustained in clinical settings.
Our findings also suggest that cue-induced craving might be dampened by ketamine, as predicted by animal studies targeting glutamate.(3-6,8-10) Heightened reactivity to drug cues has been associated with a greater risk of relapse in cocaine dependent individuals,(20) and has long been recognized as an important target for pharmacotherapy.(2) The apparent effects of sub-anesthetic ketamine on this vulnerability have promising clinical applications for relapse prevention.
These improvements in cue reactivity may be secondary to a variety of effects, including ketamine-related enhancement of motivation to quit, with cocaine no longer having the value it previously enjoyed,(33) or modulation of prefrontal regions implicated in cue and stress sensitivity.(19-21) Also, given that cue and drug effect were uncoupled in the frequently administered exposure sessions, it is possible that ketamine served to dampen cue-induced craving by facilitating cue extinction learning, as has been suggested pre-clinically with other glutamatergic modulators, such as DCS, that promote neural plasticity.(34) Future studies can elucidate the mechanisms and clinical applicability of this promising effect.
Though their time-course cannot be clarified from our data, these improvements in cue-induced craving and motivation following K1 had a relatively rapid onset and were apparently persistent, consistent with the observed anti-depressant effects of ketamine.(16) Further, these effects were observed, at 24 hours post-infusion, well after ketamine and its active metabolites (e.g., norketamine and dyhydronorketamine) are expected to have been eliminated or at negligible serum levels.(32) Yet, these effects were not an anti-depressant response, as participants were neither depressed nor endorsing threshold symptoms (see Table 1). These findings therefore suggest that the therapeutic effects of sub-anesthetic ketamine extend to improving cocaine-related prefrontal adaptations. They also support the hypothesis that depressive and dependence disorders may have certain disruptions in common.(35)
The reported experiences of participants are interesting to consider, as they suggest a possible psychological component to the therapeutic mechanisms of ketamine. Participants found the cue-exposure sessions uncomfortable: they described frustration and disappointment when cocaine was denied them, particularly after the first cue exposure, and expressed feeling disconcerted before each “choice session” by the uncertainty of whether or not they would be provided a choice. These aversive experiences may have motivated individuals to alter their interest in and reactivity to cocaine so as to render the cue-exposure sessions less stressful. As such, it is possible that the discomfort elicited by the cue-exposure session served to make participants more amenable to finding benefit from the infusions, particularly in the domains of cue reactivity and readiness to stop using cocaine. This hypothesis is consistent with other research focused on “hallucinogenic” or dissociative agents, where the attitude and expectations of the participant concerning the psychoactive substance(s), as well as the context in which administration occurs, may impact on therapeutic efficacy and tolerability.(36,37)
Another finding of interest is the shared narrative articulated by participants regarding study involvement. A commonly expressed sentiment at the exit interview was the impression of having undergone a beneficial shift in outlook and values; participants described feeling redeemed, liberated, or provided an opportunity to start over. These commonalities were surprising given the absence of psychotherapy. Transformative experiences have received considerable attention in the literature on drug recovery due to the dramatic shift they signal in the lives of addicted individuals,(38) and the capacity for certain agents to precipitate them has been a focus of recent research.(36,39) Similarly, it may be that the benefits observed here involved a ketamine-facilitated “transformative” experience, even if this was not immediately apparent to study staff. Whether such experiences represent epiphenomena of neurobiological improvements, or whether they independently contribute to therapeutic efficacy in some crucial way, are relevant questions that future studies can address. In either case, the clinical framework by which the therapeutic potential of ketamine can be best harnessed and sustained is important to investigate,(36,39) as with early studies assessing Ketamine Psychedelic Therapy (KPT) for alcohol and opioid dependence.(40)
These findings have promising clinical implications. Both doses of ketamine were both well tolerated, suggesting that ketamine infusions may be given safely under controlled conditions to this population. The substantial reduction in cocaine use during follow-up, with abstinence rates greater than what is typically observed in these settings,(32) suggests that ketamine infusions may clinically improve cocaine dependence by producing sustained benefits in important deficits. Future trials can investigate this hypothesis by assessing the direct effects of ketamine infusions on clinical outcomes or use behavior. Investigators can also consider assessing the effectiveness of comparable compounds.(41,42)
In summary, these findings suggest that the glutamatergic actions of sub-anesthetic ketamine infusions extend beyond anti-depressant efficacy and may also address dependence-related adaptations. The novel effects on motivation to quit drug use are particularly noteworthy, as they suggest that this important deficit, typically addressed with psychotherapy alone, may be responsive to pharmacotherapy. Further research is needed to expand on these preliminary findings, and to clarify the effectiveness and safety of this intervention in clinical settings.
Acknowledgments
The authors thank the National Institute on Drug Abuse for supporting this project through the following grants: DA03476-23, DA007294-19, DA031749-01, DA031771-01, DA009236-18, DA029647-02, DA031749-02. The authors also acknowledge Kirsten Frazer, MS for her assistance in executing the experiments of this study; Sanjay Mathew, MD for his assistance with related experiments; Adam Bisaga, MD, PhD for his feedback on the methodology of this study; and the reviewers for improving this manuscript.
Footnotes
“Glutamatergic Modulation of Cocaine-related Deficits.” ClinicalTrials.gov Identifier: NCT01790490
Author Contributions: E.D. conceived, designed, and obtained funding for the study; E.D., C.H., and F.L. conducted the experiments; E.D., C.H., F.L., and E.N. provided supervision; E.D., C.H., and E.N. conducted statistical analyses; E.D. wrote the manuscript; and all authors edited the manuscript. All authors assume responsibility for the research, and all have approved the final manuscript.
Disclosures: F.L. is a consultant to GW Pharmaceuticals, and has received medication for a NIH funded study from US World Meds. The other authors have nothing to disclose. The authors have no competing financial interests to report.
References
- 1.UNODC. World Drug Report 2012. United Nations, New York: 2012. United Nations Office on Drugs and Crime. [Google Scholar]
- 2.Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, et al. New Treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacology. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature Neuroscience. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 5.Ulys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS Neurol Disord Drug Targets. 2008;7:428–91. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- 6.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addict Biol. 2012 Mar 28; doi: 10.1111/j.1369-1600.2012.00446.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popik P, Wrobel M, Rygla R, Bisaga A, Bespakov AY. Effect of memantine, an NMDA receptor antagonist, on place preference conditioned with drug and nondrug reinforces in mice. Behav Pharmacol. 2003;14:237–244. doi: 10.1097/00008877-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Myers KM, Carlezon WA., Jr Extinction of drug- and withdrawal paired cues in animal models: Relevance to the treatment of addiction. Neurosci Biobehav Rev. 2010;35:118–126. doi: 10.1016/j.neubiorev.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bespalov AY, Zvartau EE, Balster RL, Beardsely PM. Effects of N-methyl-D-aspartate receptor antagonists on reinstatement of cocaine-seeking behavior by priming injections of cocaine or exposures to cocaine-associated cues in rats. Behav Pharmacol. 2000 Feb;11(1):37–44. doi: 10.1097/00008877-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Collins ED, Ward AS, McDowell DM, Foltin RW, Fischman MW. The effects of memantine on the subjective, reinforcing, and cardiovascular effects of cocaine in human. Behav Pharmacol. 1998 Nov;9(7):587–598. doi: 10.1097/00008877-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, Nunes EV. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend. 2010;111(1-2):97–104. doi: 10.1016/j.drugalcdep.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price KL, McRae-Clark AL, Saladin ME. D-cycloserine and cocaine cue reactivity: Preliminary findings. A J Drug Alcohol Abuse. 2009;35:434–438. doi: 10.3109/00952990903384332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skolnick P, Popik P, Trullas P. Glutamate-based antidepressants: 20 years on. Trends in Pharmacological Sciences. 2009;30(11):563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Almeida RF, Thomazi AP, Godinho GF, Saute JA, Wofchuk ST, Souza DO, et al. Effects of depressive-like behavior of rats on brain glutamate uptake. Neurochem Res. 2010 Aug;35(8):1164–71. doi: 10.1007/s11064-010-0169-4. [DOI] [PubMed] [Google Scholar]
- 16.Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, et al. Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs. 2012 Mar 1;26(3):189–204. doi: 10.2165/11599770-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010 Aug 20;:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosten TA, SCanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153–9. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller WR, Rollnick S, editors. Motivational Interviewing, Third Edition: Preparing People for Change. New York: Guilford Publications, Inc; 2012. [Google Scholar]
- 23.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009 Feb 15;65(4):289–95. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009 Sep 1;66(5):522–6. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry EB, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology. 2007;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 27.Sklar GS, Zukin SR, Reilly TA. Adverse reactions to ketamine anesthesia: abolition by psychological technique. Anesthesia. 1981;36:183–187. doi: 10.1111/j.1365-2044.1981.tb08721.x. [DOI] [PubMed] [Google Scholar]
- 28.DiClemente CC, Hughes SO. Stages of change profiles in alcoholism treatment. Journal of Substance Abuse. 1990;2:217–235. doi: 10.1016/s0899-3289(05)80057-4. [DOI] [PubMed] [Google Scholar]
- 29.Siegal HA, Li L, Rapp RC, Saha P. Measuring readiness for change among crack cocaine users: a descriptive analysis. Subst Use Misuse. 2001 May-Jun;36(6-7):687–700. doi: 10.1081/ja-100104085. [DOI] [PubMed] [Google Scholar]
- 30.SPSS Inc. (2011). SPSS. Cary, NC.
- 31.Kalapatapu RK, Bedi G, Haney M, Evans SM, Rubin E, Foltin RW. Substance use after participation in laboratory studies involving smoked cocaine self-administration. Drug Alcohol Depend. 2012 Jan 1;120(1-3):162–7. doi: 10.1016/j.drugalcdep.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hijazi Y, Bodonian C, Bolon M, Salord F, Boulieu R. Pharmacokinetics and haemodynamics of ketamine in intensive care patients with brain or spinal cord injury. Br J Anaesth. 2003;90:155–60. doi: 10.1093/bja/aeg028. [DOI] [PubMed] [Google Scholar]
- 33.Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers KM, Carlezon WA., Jr D-cycloserine effects on extinction of conditioned responses to drug-related cues. Biol Psychiatry. 2012 Jun 1;71(11):947. doi: 10.1016/j.biopsych.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology Mar. 1998;18(3):135–74. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 36.Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci Sep. 2010;11(9):642–51. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- 37.Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008 Aug;22(6):603–20. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller WR, C'de Baca J. Quantum change: when epiphanies and sudden insights transform ordinary lives. New York: Guilford; 2001. [Google Scholar]
- 39.Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006 Aug;187(3):268–83. doi: 10.1007/s00213-006-0457-5. discussion 284-92. [DOI] [PubMed] [Google Scholar]
- 40.Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoactive Drugs. 1997 Apr-Jun;29(2):165–83. doi: 10.1080/02791072.1997.10400185. [DOI] [PubMed] [Google Scholar]
- 41.Machado-Vieira R, Manji HK, Zarate CA. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. 2009;15(5):525–39. doi: 10.1177/1073858409336093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preskhorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101.606, in patients with treatment refractory major depressive disorder. J Clin Psychopharmacology. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]


