Abstract
Adult stem cells are crucial for physiological tissue renewal and regeneration after injury. Prevailing models assume the existence of a single quiescent population of stem cells residing in a specialized niche of a given tissue. Emerging evidence indicates that both quiescent (out of cell cycle and in a lower metabolic state) and active (in cell cycle and not able to retain DNA labels) stem cell subpopulations may coexist in several tissues, in separate yet adjoining locations. Here, we summarize these findings and propose that quiescent and active stem cell populations have separate but cooperative functional roles.
Stem cells constitute a long-lived population of cells that possess the ability to self-renew (a process of duplication without losing developmental potential) and give rise to multiple cell types (differentiation) (1). In Drosophila and Caenorhabditis elegans, a single population of germ stem cells (GSCs) resides in a single niche (2, 3). The asymmetric architecture of the stem cell niche dictates that stem cells normally divide asymmetrically into a new stem cell (self-renewal) and a committed progenitor (differentiation). GSCs only divide symmetrically (self-renewal only) when expansion of the stem cell pool is required (3). The invertebrate GSCs normally undergo constant cycling (either asymmetric or symmetric division) (4, 5) and become quiescent when animals are challenged nutritionally (6). In contrast, mammalian adult stem cells are generally detected as in a predominantly quiescent state (7–9). How can long-term quiescent stem cells support rapidly regenerating tissues (e.g., producing billions of blood and intestinal cells daily) during normal physiology? The mechanism whereby quiescent stem cells give rise to transit amplifying (TA) cells, which in turn differentiate into mature cells, may not provide a satisfactory answer because TA cells are short-lived and cannot self-renew. Recently, populations of stem cells that are long-lived yet constantly cycling have been identified (10). Here, we integrate insights from bone marrow, intestinal epithelium, and hair follicle to formulate an alternative and complementary model in which subpopulations of quiescent and active adult stem cells coexist in the same tissue.
Hair Follicle
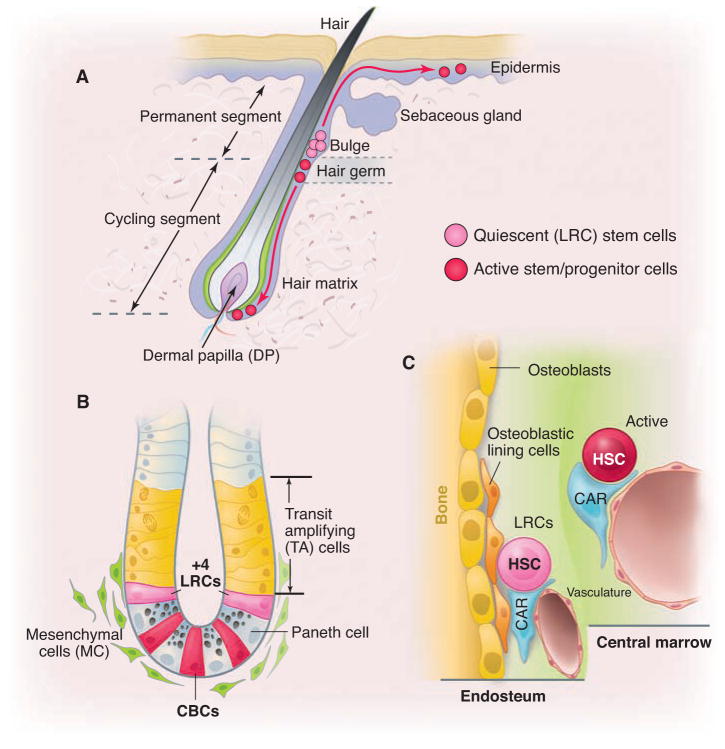
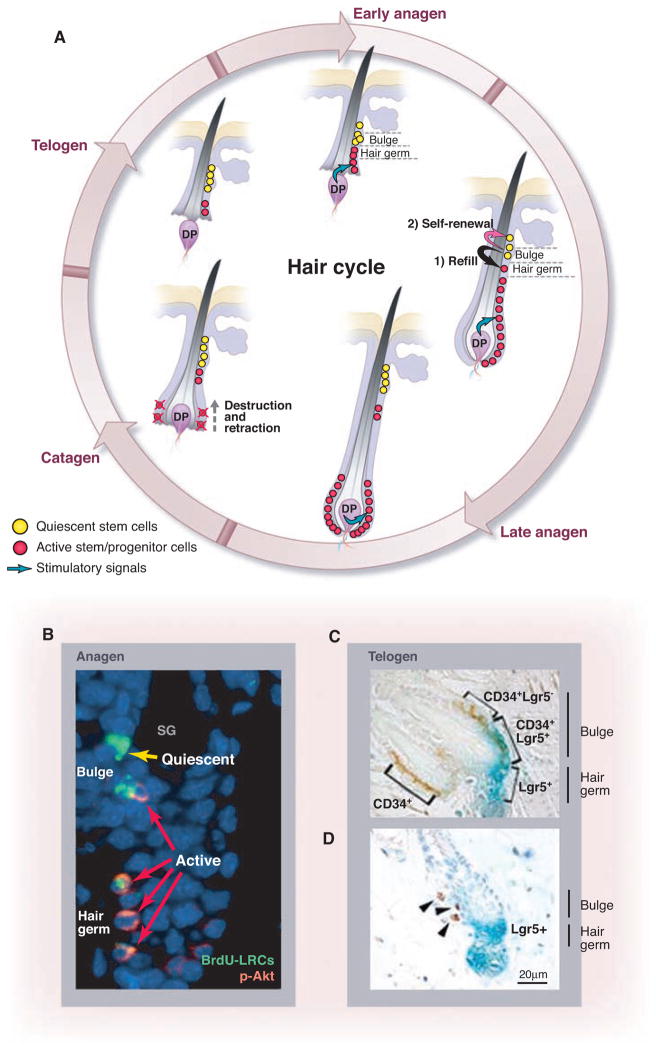
The hair follicle provides an excellent system for studying stem cell biology. Each hair follicle is composed of a permanent portion, which includes the sebaceous gland and the underlying bulge region, and a temporary portion (cycling segment) that cycles through anagen (active growth), catagen (apoptosis-driven retraction), and telogen (a resting period) (Figs. 1A and 2A) (11, 12). Signals emanating from the underlying dermal papilla (DP) induce activation of the stem cells in the hair germ (a cluster of cells located at the bottom of bulge) and the bulge (13). During anagen, the distance between the bulge and DP increases, which modifies the activation state of the stem cells (Fig. 2A).
Fig. 1.
Stem cell locations in hair follicle, gut, and bone marrow. (A) Hair follicle structure with quiescent (bulge) and active (hair germ) stem/progenitor cells. Bulge area typically maintains quiescent stem cells, whereas DP provides stimulatory signals. Only during development and under injury condition, bulge stem cells give rise to stem cells in epidermis. (B) Intestinal crypt structure with quiescent (+4) and active CBC (Lgr5+) stem cells, as well as TA and mesenchymal cells. (C) Quiescent HSCs located in the endosteal region where osteoblastic lining, endothelial, CAR, and other cells form the endosteal region and active HSCs located in the central marrow region, which lacks osteoblastic cells.
Fig. 2.
DP position determines state of stem/progenitor cells in hair follicle. (A) Hair cycle. At the start of anagen, DP is proximal to hair germ and bulge. Stem/progenitor cells in hair germ (a structure below the bulge) are activated by DP first, whereas stem cells remain quiescent in bulge. When hair germ cells enter the hair matrix, then stem cells in bulge are activated to replenish lost hair germ cells. During anagen, active hair germ stem cells give rise to TA cells, which in turn support hair growth. DP is pushed down from bulge because of fast expansion of progenitor cells. In catagen, DP retracts toward bulge. (B) Co-staining of BrdU with phosphorylated-Akt shows relationship between quiescent stem cells (yellow arrow) and active stem cells (red arrows) in bulge and hair germ. [Reprinted by permission from John Wiley and Sons, Incorporated, AlphaMed Press, p. 2834 of (20).] (C and D ) CD34+Lgr5− and CD34+Lgr5+ stem cells, respectively, located inside and next to bulge area (C), which is consistent with Lgr5+ stem cells located adjacent to LRCs (D). [Reprinted by permission from Macmillan Publishers, Limited, pp. 1292 and 1293 of (19).]
Cotsarelis and colleagues were the first to identify label-retaining cells (LRCs) in the bulge region (7). However, because of the inability to isolate live 5-bromo-2′-deoxyuridine–positive (BrdU+) LRCs, they could not determine whether bulge LRCs were stem cells. Fuchs and colleagues developed an elegant method to isolate live LRCs by generating a doxycycline-controlled histone2B–green fluorescent protein (H2B-GFP) mouse line (14). Expression of H2B-GFP was seen in almost all of the epidermal (including stem) cells without adding doxycycline. When doxycycline was administered to young mice for 1 month, most cells diluted out the H2B-GFP label by division during the chasing process. Some cells in the bulge, however, retained H2B-GFP and were thus identified as GFP-expressing LRCs. The majority of these H2B-GFP LRCs expressed the stem cell marker CD34 (14). Sorted and cultured CD34+ bulge cells from a keratin14-GFP transgenic mouse were transplanted and shown to generate the entire hair follicle (15). Taken together, these studies demonstrate that LRCs (here identified as CD34+ and K14+) in the hair follicle represent functional stem cells (15).
Although many bulge cells can be activated from the quiescent state to enter the cell cycle during anagen (15), some LRCs retain labels over many months, suggesting that they exist in a quiescent state and are not actively involved in hair regeneration (16) (Fig. 2B). These observations suggest that bulge cells are heterogeneous in terms of cycling state (17).
Bulge stem cells were recently found not to directly generate TA cells but rather give rise to an intermediate stem cell population located at the hair germ. These hair germ cells in turn produced the TA cells, which further differentiated into the hair shaft in the hair matrix (13, 18). Leucine-rich repeat–containing hetero-trimeric guanine nucleotide–binding protein (G protein)–coupled receptor (Lgr) 5 is expressed in the bulge region and the hair germ during late telogen/early anagen as well as in the hair matrix during late anagen (Fig. 2C) (19). In the bulge, the CD34+ compartment consists of both label-retaining Lgr5− cells and active Lgr5+ cells located side by side (Fig. 2C). This is consistent with the detection of Akt-phosphorylated (activated) LRCs in the lower bulge region and hair germ during anagen (Fig. 2B) (20). Indeed, the Lgr-5+ cells seem to be the first to proliferate upon induction of anagen, and some Lgr5+ cells persist through several hair cycles (19) as revealed by in vivo lineage tracing. Thus, the location of Lgr5+ cells in the lower bulge and extending into the hair germ is consistent with the recent finding that activation of bulge and hair germ stem cells occurs in two steps (13). Activation of bulge cells follows activation of hair germ cells, indicating that the bulge cells are replenishing hair germ cells that have entered the hair matrix to support hair growth (Fig. 2A) (18). On the basis of these observations, the hair follicle appears to contain both quiescent and active stem cell populations in separate yet adjacent locations.
Gut
In the intestinal epithelium, stem cells and their short-lived TA cells reside in crypts (Fig. 1B). Cells exiting the crypts and entering the villi terminally differentiate into enterocytes, goblet cells, or enteroendocrine cells. Paneth cells escape this course by migrating to crypt bottoms. The intestinal epithelium is renewed about every 5 days (21). Potten and others have proposed that LRCs (on average 2 to 4 out of 16 cells in a ring) at the +4 position located above the Paneth cells (Fig. 1B) represent stem cells (8). A recent study using in vivo lineage tracing has shown that cells expressing Bmi1 predominantly mark +4 position and are able to give rise to all four epithelial lineages (22). Although Bmi1+ cells were suggested to be slowly cycling, it is currently unknown whether they are LRCs (i.e., quiescent). Some cells at the +4 cell position that express the potential stem cells marker Musashi-1 are not sensitive to inactivation of the cell cycle protein CDC25, suggesting they have quiescent features (23).
In an alternative model, crypt-based columnar cells (CBCs) specifically expressing Lgr5 located at crypt bottoms among Paneth cells represent intestinal stem cells (ISCs) (Fig. 1B) (24). Lineage tracing has shown that Lgr5+ cells represent a long-lived and cycling multipotent stem cell population (25). A single Lgr5+ stem cell can form a long-lived, self-renewing “mini-gut” in culture (26). Lgr5+ stem cells are distinct from +4 LRCs in that Lgr5 stem cells do not retain DNA labels and are sensitive to CDC25 inactivation, supporting their proliferating feature (23). Thus, the intestine contains a quiescent stem cell population at the +4 position and a cycling Lgr5 stem cell population among the Paneth cells. Future studies are needed to determine the relationship between these cell populations.
Bone Marrow
Studies of hematopoietic stem cells (HSCs) have shaped our thinking on most basic features of mammalian stem cells (1). HSCs give rise to a hierarchically organized set of progenitors for erythroid, myeloid, lymphoid, and megakaryocyte lineages (1). The mainstay of HSC analysis has been marker-based cell sorting followed by transplantation in lethally irradiated recipients. This assay tests self-renewal as well as multi-lineage potential.
Most primitive HSCs are quiescent (27). Using DNA label BrdU and H2B-GFP incorporation respectively (14), LRCs were found to be predominantly located in the endosteum (Fig. 1C) (28) and subsequently confirmed to be a primitive HSC population (29, 30). The quiescent HSCs have superior long-term reconstitution potential; however, HSCs in this population cycle only once every 145 days on average and thus may not provide ongoing support for production of billions of blood cells, although they can be activated for this function under injury conditions (29). Other studies based on the DNA label BrdU and H2B-GFP incorporation have suggested that the majority of murine HSCs undergo more frequent cycling (29, 31–33). These seemingly disparate findings may be reconciled by postulating the existence of two subpopulations of HSCs, one being long-term quiescent (“reserved”) and the other being more actively cycling (“primed”) (Fig. 1C) (29, 33, 34). Primed HSCs may be the workhorse that supports the daily production of billions of blood cells, whereas reserved HSCs function as a “backup” (to replenish lost active stem cells under homeostasis and particularly in response to injury or pathological challenge) (29, 33).
Stem Cell Zones and Associated Microenvironmental Signals
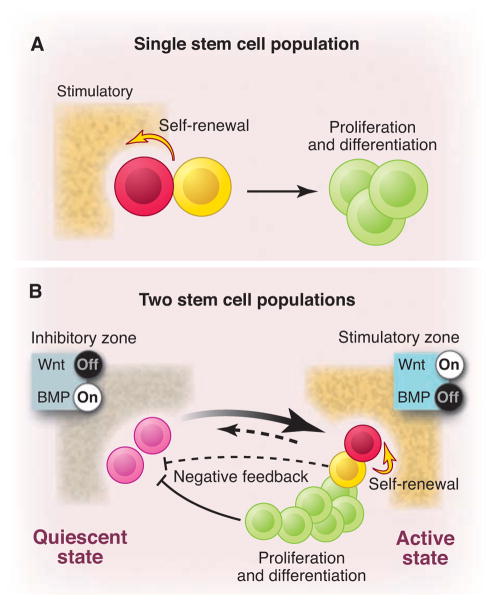
The observations described above do not easily conform to a model in which a single stem cell population maintains tissue self-renewal (Fig. 3A). An alternative “zoned” model, in which quiescent and active stem cells coexist within the same tissue, may better explain these observations (35). In the zoned stem cell model, active stem cells are the primed subpopulation that account for most of the replenishment of corresponding tissues, whereas quiescent stem cells function as a backup or reserved sub-population. This reserved population can be activated either by a stochastic mechanism (36) or by feedback upon loss of active stem cells or extensive tissue damage (Fig. 3B). For example, the cycling CBC stem cells and/or their progeny migrating upward from the crypt bottom and passing +4 may potentially provide a negative feedback to the quiescent stem cells. Thus, loss of CBCs or their progeny may trigger the activation of quiescent stem cells. Different assays may be biased in revealing one or the other population of stem cells: transplantation assays document “stem cell potential” and may favorably detect the reserved population, particularly in the serial transplantation assays, whereas in vivo lineage tracing assays visualize the primed population.
Fig. 3.
Two models for stem cell-based tissue self-renewal and regeneration. (A) Prevailing model of a single stem cell population located in the niche. Asymmetric division is the key mechanism to maintain balance between self-renewal and differentiation. (B) Proposed alternative and complementary model: co-existing quiescent and active stem cell populations located in adjacent zones with corresponding inhibitory and stimulatory signals. Quiescent stem cells replace damaged active stem cells (heavy arrow). Conversely, active stem cells may replace lost quiescent stem cells (light arrow). A negative feedback from either active stem cells (dashed line) or their progeny (solid line) may contribute to prevention of quiescent stem cells from activation.
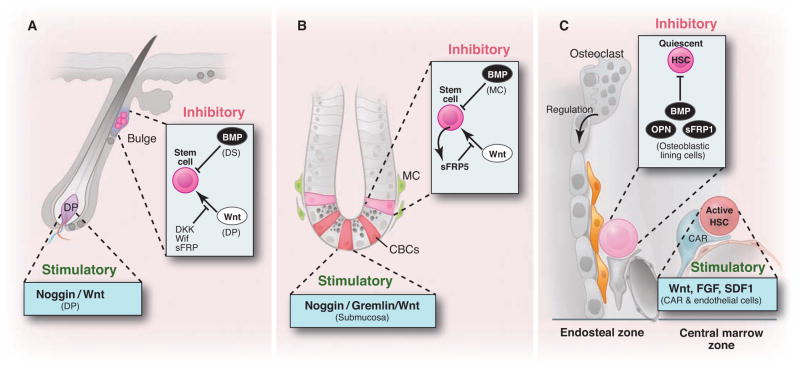
How can these two very different states of stem cell sub-populations be maintained at separate but adjoining locations? The nature of the locations of stem cell subpopulations in hair follicle and intestine suggests that there are segregated zones that determine the active or quiescent state of the stem cells. Individual stem cell niches are maintained by the secretion of specific proteins. For example, the hair follicle bulge constitutes a canonical Wnt-off/bone morphogenetic protein (BMP)–on microenvironment through the expression of secreted Dkk-1, sFRP, Wif, and BMP (14). In contrast, the DP secretes Wnt signals and noggin, an antagonist of BMP signaling (11) (Fig. 4A). Similarly, cycling Lgr5+ stem cells at the crypt bottom in intestine are in a microenvironment with high Wnt activity (10), whereas BMP signaling is inhibited by noggin and gremlin produced by sub-mucosal tissue below the crypts (37, 38). Stem cells at +4 positions encounter BMP4 and the Wnt inhibitor sFRP5 (37, 39) (Fig. 4B). In bone marrow, endosteal region [composed of osteoblastic, vascular (40), and CXCL12 abundant reticular (CAR) cells (41), as well as osteoclasts (42)] and central marrow region (lacking osteoblastic signals) have been shown to form inhibitory and stimulatory zones respectively (43, 44) (Fig. 4C). Intriguingly, HSCs in aged mice localized more distantly to the endosteum than in young mice, supporting the existence of two zones in bone marrow, with the central marrow zone favoring proliferation of HSCs (45). This correlates with the increasing number but decreasing function of aged HSCs (46). Under homeostatic conditions, BMPs (28), Osteopontin (47), Wnt signaling inhibitors sFRP1 (48), as well as potentially noncanonical Wnts are expressed in the endosteal zone, providing an inhibitory microenvironment. Fibroblast growth factors (FGFs) and canonical Wnts expressed from endothelial, megakaryocytes, and CAR cells may have a dominant and stimulatory influence on the central marrow zone under homeostatic conditions (33, 49, 50) (Fig. 4C). The locations of these cells and the associated signals might change, however, under stressed conditions (43).
Fig. 4.
Zoned stem cell population and the associated microenvironmental signals. (A) Bulge area typically provides Wnt-off and BMP-on signals thus maintaining quiescent stem cells, whereas DP provides stimulatory (Wnt-on and BMP-off) signals. (B) Similarly, in intestinal crypt (+4) ISCs are maintained by Wnt-off and BMP-on signals. In contrast, CBC (Lgr5+) stem cells are exposed to Wnt-on but BMP-off signals. (C) Quiescent HSCs located in the endosteal zone where osteoblastic lining cells provide dominant inhibitory signals including BMP, OPN, and sFRP1. In contrast, HSCs located in the central marrow zone are stimulated by endothelial, megakaryocyte, and CAR cells secreting Wnt, FGF, and SDF1.
Functional Significance of Zoned Stem Cell Subpopulations
What could be the advantage of maintaining zoned subpopulations of stem cells? We speculate that increasing life span and body size during evolution exerted selective pressure to increase the longevity and output of adult stem cell pools, particularly in the rapidly regenerating tissues, without increasing the risk for (tumorigenic) mutations. The adaptation of reserved quiescent stem cell pools may provide a mechanism to increase the longevity of adult stem cells through population replacement (Fig. 3B). Here, population replacement describes a mechanism by which one population of cells is replaced by another and should be distinguished from the classic mechanism of self-renewal at the individual cell level. This is exemplified by lost stem cells in hair germ being replenished by stem cells located in the bulge during each hair cycle (13). Normally, quiescent stem cells would be expected to replace damaged active stem cells (Fig. 3B). This would prevent the active stem cell pool from becoming exhausted and protect against accumulating potentially tumorigenic mutations during DNA replication. Conversely, active stem cells would possibly have the capacity to replace lost or damaged quiescent stem cells under very special circumstances (Fig. 3B). The combination of these reciprocal backup systems would provide a robust mechanism to ensure a high rate of physiological self-renewal as well as flexible damage repair, after which the original hierarchy could be reestablished.
Acknowledgments
We thank J. Kimble and E. Fuchs for their critical reading and very valuable comments; colleagues, including J. Grindley, D. Scoville, X. He, J. Haug, V. Greco, etc., for suggestions and discussions; and J. Perry and K. Tannen for proofreading. We regret that we are unable to cite other relevant studies because of space constraints. L.L. was supported by SIMR and by grant U01DK085507 from National Institute of Diabetes and Digestive and Kidney Diseases.
Contributor Information
Linheng Li, Email: lil@stowers.org.
Hans Clevers, Email: h.clevers@hubrecht.eu.
References and Notes
- 1.Weissman IL. Cell. 2000;100:157. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Kimble JE, White JG. Dev Biol. 1981;81:208. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- 3.Spradling A, Drummond-Barbosa D, Kai T. Nature. 2001;414:98. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 4.Crittenden SL, Leonhard KA, Byrd DT, Kimble J. Mol Biol Cell. 2006;17:3051. doi: 10.1091/mbc.E06-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song X, Call GB, Kirilly D, Xie T. Development. 2007;134:1071. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- 6.Narbonne P, Roy R. Development. 2006;133:611. doi: 10.1242/dev.02232. [DOI] [PubMed] [Google Scholar]
- 7.Cotsarelis G, Sun TT, Lavker RM. Cell. 1990;61:1329. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 8.Potten CS, Booth C, Pritchard DM. Int J Exp Pathol. 1997;78:219. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai F, et al. Cell. 2004;118:149. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Barker N, et al. Nature. 2007;449:1003. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs E, Segre JA. Cell. 2000;100:143. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- 12.Niemann C, Watt FM. Trends Cell Biol. 2002;12:185. doi: 10.1016/s0962-8924(02)02263-8. [DOI] [PubMed] [Google Scholar]
- 13.Greco V, et al. Cell Stem Cell. 2009;4:155. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tumbar T, et al. Science. 2004;303:359. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Cell. 2004;118:635. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Waghmare SK, et al. EMBO J. 2008;27:1309. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs E. Cell. 2009;137:811. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Cell Stem Cell. 2009;5:267. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaks V, et al. Nat Genet. 2008;40:1291. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, et al. Stem Cells. 2006;24:2826. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
- 21.Barker N, van de Wetering M, Clevers H. Genes Dev. 2008;22:1856. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangiorgi E, Capecchi MR. Nat Genet. 2008;40:915. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee G, White LS, Hurov KE, Stappenbeck TS, Piwnica-Worms H. Proc Natl Acad Sci USA. 2009;106:4701. doi: 10.1073/pnas.0900751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng H, Leblond CP. Am J Anat. 1974;141:537. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 25.Barker N, Clevers H. Gastroenterology. 2007;133:1755. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, et al. Nature. 2009;459:262. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 27.Arai F, et al. Cell. 2004;118:149. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, et al. Nature. 2003;425:836. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 29.Wilson A, et al. Cell. 2008;135:1118. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 30.Foudi A, et al. Nat Biotechnol. 2009;27:84. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheshier SH, Morrison SJ, Liao X, Weissman IL. Proc Natl Acad Sci USA. 1999;96:3120. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiel MJ, et al. Nature. 2007;449:238. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haug JS, et al. Cell Stem Cell. 2008;2:367. doi: 10.1016/j.stem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Abkowitz JL, et al. Proc Natl Acad Sci USA. 1990;87:9062. doi: 10.1073/pnas.87.22.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scoville DH, Sato T, He XC, Li L. Gastroenterology. 2008;134:849. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie JL, Gan OI, Doedens M, Wang JC, Dick JE. Nat Immunol. 2006;7:1225. doi: 10.1038/ni1393. [DOI] [PubMed] [Google Scholar]
- 37.He XC, et al. Nat Genet. 2004;36:1117. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 38.Kosinski C, et al. Proc Natl Acad Sci USA. 2007;104:15418. [Google Scholar]
- 39.Gregorieff A, Clevers H. Genes Dev. 2005;19:877. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 40.Kiel MJ, et al. Cell. 2005;121:1109. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Sugiyama T, Kohara H, Noda M, Nagasawa T. Immunity. 2006;25:977. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Kollet O, et al. Nat Med. 2006;12:657. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 43.Xie Y, et al. Nature. 2009;457:97. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 44.Lo Celso C, et al. Nature. 2009;457:92. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Köhler A, et al. Blood. 2009;114:290. doi: 10.1182/blood-2008-12-195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chambers SM, et al. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nilsson SK, et al. Blood. 2005;106:1232. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 48.Yokota T, et al. J Immunol. 2008;181:6061. doi: 10.4049/jimmunol.181.9.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antoine M, et al. Growth Factors. 2005;23:87. doi: 10.1080/08977190500096004. [DOI] [PubMed] [Google Scholar]
- 50.Wechezak AR, Coan DE. Exp Cell Res. 2003;288:335. doi: 10.1016/s0014-4827(03)00232-5. [DOI] [PubMed] [Google Scholar]