Abstract
Sulfamethoxazole (SMX) and trimethoprim (TMP) individually and a combination known as cotrimoxazole (SMX-TMP) are widely used for the treatment of protozoan and bacterial infections. SMX-TMP is also one of the widely used antibiotics administered orally in neonates, along with gentamicin injection, for treating pneumonia and sepsis by home-based healthcare providers in Asian countries. Although the use of this drug has successfully reduced neonate mortality, there is a concern for it causing neurotoxicity. Previous clinical studies with sulfisoxazole have demonstrated occurrence of kernicterus in neonates. This sulfonamide is thought to displace bilirubin from its albumin-binding sites in plasma leading to an elevation of plasma bilirubin, which crosses the blood-brain barrier, reaches central neurons to cause kernicterus. We performed an extensive review of clinical and animal studies with cotrimoxazole, which showed no reported incidences of kernicterus with SMX-TMP use in neonates. EndNote, BasicBiosis, Embase, PubMed and Toxline database searches were conducted using specific keywords yielding 74 full-length articles relevant to the review. This review has taken into account various factors, including the disease itself, direct effects of the drug and its metabolism through conjugation and acetylation through a thorough review of the literature to examine the potentials of SMX-TMP to cause kernicterus in neonates. SMX-TMP in oral doses administered to neonates for 7–10 days is unlikely to cause kernicterus. Also, this review recommends warranting the need of future studies using animal models and clinical studies in humans to address SMX-TMP toxicity.
Keywords: Hyperbilirubinemia, neonatal kernicterus, sulfamethoxazole, sepsis, trimethoprim
Introduction
The global mortality rate among infants and children of less than 5 years of age has declined significantly in recent years. However, the neonatal mortality rate still remains high as a result of inadequate healthcare facilities in developing countries. Recent advancement in home-based neonatal healthcare delivered by trained community health workers in villages and neonates receiving home-based antibiotic therapy has significantly reduced neonatal mortality (Bang et al., 1990, 1993; Bhutta et al., 2009; Khanal et al., 2011).
Currently, cotrimoxazole (SMX-TMP) is used, in developing countries, to treat pneumonia and sepsis in neonates by home-based healthcare providers (Bang et al., 1993, 1999). SMX-TMP is a combination of sulfamethoxazole (SMX) and trimethoprim (TMP) that is used for the treatment of protozoan and bacterial infections, particularly for urinary and respiratory tract infections (Darmstadt et al., 2009; Ladhani & Garbash, 2005; Ogra & Faden, 1985; Paap & Nahata, 1990; Salter, 1982; Thaver et al., 2009; Velvis et al., 1986). This combination is extensively used in the home--based neonate care setting in developing countries for treating pneumonia and sepsis in neonates (Bang et al., 1993, 1999; Bhutta et al., 2009). Between 1988 and 1990, pneumonia-specific mortality rate in the control area was 29.1 in 1000 in the postnatal period (0–29 days), whereas intervention managed by community health workers (CHWs) using the combination of gentamicin and SMX-TMP resulted in a reduction in mortality to 17.4 in 1000 (40% decline) (Bang et al., 1993). Similar intervention by trained CHWs reduced neonate sepsis-related mortality from 16.6% in the control area to 2.8% in areas where home-based neonate care was introduced (Bang et al., 1999). The use of SMX-TMP in the community-based setting has been recommended by an international group of experts met under the auspices of a World Health Organization (WHO) meeting in 2002 (WHO, 2002). Despite the safety label warning for the use of SMX-TMP in neonates less than 2 months of age, the combination is used in home-based neonatal care in developing countries with significant success (Bang et al., 1993, 1999). However, their use is not popular in western countries. This may be the result of several other side effects of SMX-TMP, such as blood dyscrasias, glossitis, renal or hepatic injury, rash, Steven Johnson syndrome and hemolysis in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency as well as the availability of better, cost-effective treatment options in western countries.
SMX-TMP is available as a pediatric suspension for oral use under various trade names, such as Bactrim, Septra and Sulfatrim. The SMX component belongs to the class of sulfonamides and is an antibacterial agent that exerts its antimicrobial activity by inhibiting the synthesis of folic acid in bacterium (O’Grady, 1975; Rollo, 1970). The use of TMP synergizes the pharmacological effects of SMX (Bushby, 1975; Gever, 1980; Harvey, 1982; Hitchings, 1973; Smilack, 1999). Published research work suggests that the potential for toxicity of SMX-TMP resides in its ability to displace bilirubin from its binding sites on serum albumin, resulting in hyperbilirubinemia in neonates and ultimately leading to kernicterus and associated brain damage (Wadsworth & Suh, 1988). However, literature evidence to correlate the incidences of kernicterus in neonates specifically with SMX-TMP treatment is scarce.
Despite the severe adverse reactions of SMX-TMP and hypersensitivity to SMX (Block et al., 1987; Gutman, 1984; Gimnig et al., 2006; Ho & Juurlink, 2011; Karpman & Kurzrock, 2004; Siegel et al., 1984), SMX-TMP is popularly used against infections in neonates and infants in home-based neonatal care. The low cost, effectiveness and broad spectrum of activity account for the reemergence of SMX-TMP’s use to treat neonatal infections in a home-based healthcare system (Crabbe et al., 2000; Mathew et al., 2011; Ryan et al., 2008). Further, the current knowledge of the safety of SMX-TMP in neonates is very limited. This review is focused to analyze the potential for the toxicity of this drug in neonates and the possibility of incidences of kernicterus in neonates. A thorough review of the published experimental and clinical evidence for toxicity was undertaken to determine whether the potential for toxicity was real or purely theoretical. The outcome of this review will fill in the knowledge gap that exists in the safety of SMX-TMP to treat infections among neonates.
Methods
Scope of literature search
Oral suspension of SMX-TMP has been available for therapeutic use since 1968. It is currently used in neonates and infants in some community-based settings to treat pneumonia and sepsis. Typically, the treatment lasts for a 5- to 7-day duration. The literature search conducted for this review specifically dealt with the potential for the toxicity of this drug in neonates and infants. This review sought to reveal published evidence related to the following issues:
Possibility that administration of SMX-TMP to neonates might aggravate jaundice and the associated toxicities in neonates.
If it does, perform database search for such case studies, case series or historic data for demonstrating evidence showing quantitative possibility.
Search for the evidence in animals and human clinical studies showing development of kernicterus after oral use of SMX-TMP syrup in neonates.
Search the literature for evidence of any long-lasting toxic effects that would warrant discontinuation of SMX-TMP usage in home-based neonatal care setting.
Because cotrimoxazole contains SMX as one of the components, the literature search was widened to include certain members of the sulfonamide family to see whether those caused any toxicity in neonates. Both experimental animal and human clinical studies were included. The literature search covered the period from the early 1940s to July 2012.
Search strategy
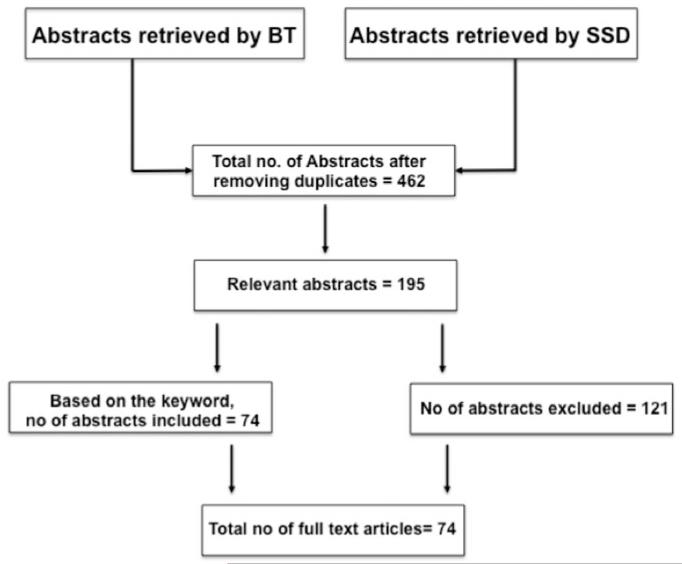
This review was performed according to the guidelines described by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Moher et al., 2009). Two authors (B.T. and S.S.D.) searched the databases independently. The individual keywords and their combination used for literature search, databases and the total number of records identified are shown in Figure 1, and the details of search terms, combination of keywords and limits applied in the advanced search are shown in Appendix 1.
Figure 1.
Total number of abstracts that are used as per the criteria defined for the review article to indicate the total number of full-length articles that were used for writing this review.
Databases that were searched included EndNote, BasicBiosis, EMBASE, PubMed and Toxline. B.T. used the following keywords: SMX-TMP; SMX and TMP; sulfonamides and neurotoxicity; sulfisoxazole; SMX and kernicterus; SMX and neonatal toxicity; SMX and neonatal jaundice; SMX-TMP and hyperbiliruinemia; sulfisoxazole and kernicterus; sulfisoxazole and neonatal jaundice; sulfonamides and kernicterus; SMX and sepsis and pneumonia; sepsis and kernicterus; pneumonia and kernicterus; hyperbilirubinemia and kenicterus; sulfonamide pharmacokinetics; SMX metabolism; pharmacokinetics of sulfisoxazole and SMX for the period from 1940 to July 2012. S.D. used the following keywords: SMX-TMP; TMP; SMX; sulfisoxazole and neonates and jaundice; TMP and neonates and jaundice; TMP and SMX and newborn babies; TMP, SMX and sulfisoxazole and neonate kernicterus for the period from 1950 to July 2012.
The criteria for the inclusion of articles that are referred in this review and the reasons for the exclusion of abstracts or full-text articles are given below.
Inclusion criteria
Human or animal studies, that are published in English, that showed SMX-TMP or SMX and sulfisoxazole toxicity in neonates, infants and children.
Human and animal studies (in vitro and in situ) demonstrating ability of SMX-TMP, SMX, sulfisoxazole and sulfonamides in general as a class of compounds to displace bilirubin from its binding sites on albumin and causing hyperbilirubinemia.
Animal or human studies specifically conducted in neonates, infants and children where a direct link could be established between SMX-TMP use (by any route of administration) and observed hepatotoxicity, development of kernicterus or long-term neurologic disorders.
Articles describing development of kernicterus in neonates caused by reasons other than SMX-TMP administration were considered.
Articles dealing with SMX-TMP absorption through blood, tissue distribution and elimination were included.
Field and clinical trials particularly under the home-based neonatal care setting where oral suspension of SMX-TMP was used to treat sepsis or pneumonia in neonates. Articles were examined particularly for documentation, if any, for adverse effects or toxicity.
Exclusion criteria
Articles showing efficacy and toxicity of SMX-TMP, SMX and sulfisoxazole in an adult male and female patient population.
Non-English articles were considered only if the abstracts were published in English and showed relevance to this review.
Articles where SMX-TMP, SMX or sulfisoxazole were used prophylactically or therapeutically in a human immunodeficiency virus (HIV)-infected adult male or female population.
Results
Sulfonamides discovered as early as 1908 are antibacterial agents used for the treatment of puerperal sepsis, septic sore throat and other bacterial infections. Sulfonamides are structural analogs and competitive antagonists of para-amino benzoic acid. This competitive antagonism results in the inhibition of dihydropteroate synthetase and therefore synthesis of folic acid, an enzyme that is crucial for DNA synthesis. Sulfonamides, because of their remarkable effectiveness against fatal pneumococcal and streptococcal infections coupled with lower toxicity, led to the development of other sulfonamides including SMX and sulfisoxazole (Shambaugh, 1966). Previous studies demonstrate that the toxicity of sulfonamides include skin rashes, Steven-Johnson syndrome, hemolytic anemia, serum sickness and kernicterus. However, the potential of sulfonamides as antibacterial drugs preserved their popularity to treat fatal infections as a result of sepsis (Andersen et al., 1956; Fox & Ottenberg, 1941; Howard & Howard, 1978; Schopf, 1987; Uhari et al., 1996; Wanat et al., 2009).
The birth of SMX-TMP in 1968 by combining SMX with TMP resulted in the synergism of antibacterial spectrum of SMX-TMP. TMP is an inhibitor of the enzyme, dihydrofolate reductase, which catalyzes the synthesis of tetrahydro folic acid. Thus, SMX-TMP provided a two-prong effect to inhibit two critical steps in the synthesis of folic acid and thereby exert their synergistic antibacterial activity.
Despite the popularity of SMX-TMP to successfully treat neonatal diseases, including sepsis, pneumonia, bacteremia and so on, in the home-based neonatal care setting by healthcare workers, the fear of toxic effects of SMX-TMP in developing neonates, especially kernicterus, has discouraged its use for treating neonate infections in western countries.
Sulfonamides directly linked to kernicterus development in neonates
Sulfisoxazole
The association between serum bilirubin levels and kernicterus in jaundiced newborn infants has been recognized for many years (Andersen et al., 1956; Silverman, 1959). The landmark study, however, was the one published in 1956 where a controlled clinical trial was conducted to test the relative effectiveness of two prophylactic regimens (penicillin/sulfisoxazole and oxytetracyclin) in premature infants (Andersen et al., 1956). Infants receiving sulfisoxazole treatment died at a significantly faster rate than those receiving oxytetracyclin. Kernicterus was confirmed by clinical diagnosis (opisthotonus, spasticity, bradypnea, oculogyric movements, convulsions and poor feeding) and postmortem as a yellow staining area of brain. Mortality rate (29% with sulfisoxazole vs. 9% with oxytetracyclin) and incidence of kernicterus were significantly higher (64% with sulfisoxazole vs. 0% with oxytetracyclin) in premature infants receiving sulfisoxazole and oxytetracyclin. In a follow-up study (Silverman, 1959) on 2-year-old children surviving premature birth and receiving the two treatment regimens, the neurologic deficit was not significantly different in the two groups, implying few, if any, infants survived the brain damage caused by penicillin/sulfisoxazole treatment. In 9 nonsurviving infants where kernicterus was confirmed at necropsy, the peak plasma bilirubin concentration was 15 mg/100 mL or less, except one with a concentration of 20 mg/100 mL (Silverman, 1959). According to the guidelines of the American Academy of Pediatrics, if the total serum bilirubin level is 25 mg/dL or higher, it should be considered as a medical emergency. Moreover, infants surviving penicillin/sulfisoxazole administration had peak plasma bilirubin levels significantly lower than those receiving oxytetracyclin (8 mg/100 mL on day 3 with the former vs. 16.4 mg/100 mL on day 4 with the latter), indicating these antibiotics having some effect on bilirubin metabolism (Silverman, 1959). These studies stimulated more research in plasma bilirubin levels and development of kernicterus in newborns.
In vitro studies
Displacement of bilirubin from albumin by sulfisoxazole
Plasma concentrations of bilirubin exceeding 20 mg/100 mL are considered levels at which kernicterus can occur (Crosse et al., 1955; Tuttle, 1955). In an in vitro study with sera from 20 newborn infants with high bilirubin content (18–28 mg%) using ultrafiltration and dialysis techniques, it was found that sulfisoxazole addition (clinically relevant blood levels) to these sera could uncouple protein-bound bilirubin and allow it to pass through the semipermeable membrane (Odell, 1959). Among the three sulfonamides that were examined, sulfisoxazole was the most potent, whereas sulfadiazine and sulfanilic acid required much higher molar concentrations. This article established the importance of binding of bilirubin to protein as well as the significance of diffusible, rather than total, bilirubin in the development of kernicterus.
Development of animal model for kernicterus
A report of sulfisoxazole-induced kernicterus in neonates prompted the researchers to look for an animal model for kernicterus, and that resulted in the work on Gunn rats, originally described by Gunn in 1938 (Gunn, 1938). These rats have an inherited defect in liver glucuronyl transferase (GT), which normally conjugates a glucuronoid group to bilirubin and renders it to be more water soluble, thereby easing excretion (Maisels, 2006). Lack of this enzyme conjugation results in hyperbilirubinemia. In an article published in 1959, based on the prevailing knowledge at that time, the pathogenesis of kernicterus and the various theories concerning its development are reviewed (Blanc & Johnson, 1959). The investigators propose that unconjugated bilirubin is itself the toxic agent producing nerve-cell necrosis. They envisaged kernicterus as being the result of a presence of a diffusible form of unconjugated bilirubin in plasma and a shift of this compound from the circulating blood to the brain and other tissues. The increase in the unconjugated bilirubin is, as stated earlier, a result of deficient activity of GT in the liver, and the formation of the diffusible compound is a result of inadequate protein binding of bilirubin. These rats develop kernicterus identical to that observed in neonates. Although lethality resulting from kernicterus was significantly higher in sulfisoxazole or sulfadiazine (Johnson et al., 1959) administration in these rats (97 vs. 29% in littermate controls receiving no drug), a puzzling finding was that indirect serum bilirubin level in rats receiving sulfa drugs was lower than the values noted in controls (Johnson et al., 1959). A similar finding in an earlier report (Odell, 1959) prompted the investigators to suggest competition between bilirubin and the sulfonamides for binding sites on the serum albumin. Although kernicterus observed in rats with congenital hypoglucuronyltransferasia is similar to the syndrome of human kernicterus, it is worth noting that administration of sulfonamides to normal newborn nits did not produce kernicterus nor did it alter the blood–brain barrier (BBB).
SMX-TMP and potential risk for kernicterus
SMX-TMP (combination of TMP and SMX) has been used in conjunction with gentamycin to combat sepsis and acute respiratory infections in newborns and infants very successfully in rural and tribal areas in India and elsewhere in developing countries under home-based neonatal care by CHWs. Typically, SMX-TMP is effective against both Gram-positive and -negative organisms (broad spectrum). It is used orally in conjunction with gentamycin (injectable), the latter being effective mostly against Gram-negative organisms and the Gram-positive Staphylococcus. In neonates (≥2500 g body weight) diagnosed with sepsis or pneumonia, the course of an oral dose of Cotrimoxazole syrup (200 mg of SMX plus 40 mg of TMP in 4 mL) was 1.25 mL twice a day for 7 days (Bang et al., 1999, 2005; Bhutta et al., 2009). Therefore, each neonate received 125 mg of SMX per day. In cases of pneumonia in older children (6–59 months), the dose was 5 mL/day for 5 days (250 mg of SMX/day). No untoward effects, including any signs of central nervous system (CNS) toxicity, were mentioned in these reports (Bang et al., 1999, 2005; Bhutta et al., 2009). However, these reports do not confirm the presence or existence of any toxicity.
In vivo toxicity studies
There are no reports of either blood levels of the drug or unconjugated serum bilirubin measurements in neonates receiving aforementioned therapeutic doses of SMX-TMP orally for the duration of 1 week. In a study conducted in neonates (Springer et al., 1982), the pharmacokinetic parameters of SMX-TMP were measured in 12 newborn infants of less than 3 days postnatal age following intravenous (i.v.) administration of a daily therapeutic dose of SMX-TMP (TMP, 5 mg/kg; SMX, 25 mg/kg) for 3 days to treat infection. Mean birth weight of neonates was 1800 g (range, 840–3100), and mean gestational age of 33 weeks (range, 28–40). Measurements included serum half-life of active TMP, metabolized SMX and creatinine clearance. Results showed that in serum, average SMX concentration measured at 24 hours was 20 μg/mL, whereas with repeated doses for 3 days, average drug level in serum measured 24 hours after the last dose was 42 μg/mL. No untoward side effects or kernicterus were noted in these neonates after repeated i.v. administration of the drug combination. Another study on 5 infants (7–17 months old) showed that daily administration of 185 mg of TMP and 925 mg of SMX in two divided doses produced peak blood level of SMX within 1–4 hours to approximately 15 μg/mL and was maintained for the next 12 hours. No untoward side effects were noted, except granulocytopenia in one child, a recipient of renal transplant receiving immunosuppressants (Lewin et al., 1973). The pharmacokinetics (PK) of SMX-TMP examined in a study in children from ages 3 months to 10 years found that after a single oral dose of SMX-TMP (20 mg of TMP/100 mg of SMX), mean serum concentration of SMX was 20 μg/mL within 4 hours with a half-life of 9–15 hours (Schwartz & Ziegler, 1969).
SMX-TMP is absorbed orally as efficiently as i.v. administration with volume of distribution of 0.21 L/kg and half-life of 8.6 hours (Gilman et al., 1980a, p. 1730). “When 800 mg of SMX is given with 160 mg of TMP (5:1) twice-daily, the peak conc. of the drugs in plasma are approx. 40 and 2 μg/ml, the optimal ratio that is sought.” “About 65% of SMX is bound to plasma protein” (Gilman et al., 1980b, p. 1117).
In vitro bilirubin binding
The investigators of in vivo article described above (Springer et al., 1982) also studied the effect of SMX on bilirubin-binding capacity (an assay done in vitro where various doses of SMX are added to the serum isolated from neonatal blood and the quantity of bilirubin displaced from its albumin binding site is measured) (Springer et al., 1982). It was observed that SMX concentration of 400 μg/mL (nearly 10–20 times the therapeutic concentration reached in neonates) was required to displace bilirubin and reduce serum bilirubin-binding capacity. It is extremely unlikely that dangerous concentrations of SMX in serum approaching 400 μg/mL would ever be reached in the clinical use of SMX-TMP orally for neonatal sepsis or pneumonia. This is supported by the theoretical calculations for the optimal plasma concentration for SMX after oral administration (given the volume of distribution [Vd] of SMX is 12 L and the dose 100 mg) would be 8.3 μg/mL. This is at least 50-fold lower than the concentration of SMX that would displace bilirubin.
Further, both TMP and SMX are moderately bound (approximately 40–60%) to plasma protein and that “SMX is particularly inefficient compared with other sulfonamides in displacing bilirubin from albumin” (Gutman, 1984, p. 353). In an in vitro study with pooled umbilical cord serum, bilirubin-displacing activity of various pharmacological agents was tested (Wadsworth & Suh, 1988). Using the ultracentrifugation method, the difference in total bilirubin concentration before and after centrifugation was used to determine the amount of bilirubin displaced by the drugs. Among sulfonamides tested, sulfisoxazole and SMX (10 and 50 μg/mL) (Wadsworth & Suh, 1988) were shown to displace bilirubin at a significant level when calculated as a ratio of total bilirubin concentration in the absence and in the presence of the drug. Sulfisoxazole was more potent than SMX.
Kernicterus caused by conditions other than drugs
Kernicterus, essentially a syndrome where unbound unconjugated bilirubin crosses the BBB and, because it is lipid soluble, penetrates neuronal and glial membranes. Excessive bilirubin deposition in the basal ganglia and brain-stem nuclei, are produced by a variety of conditions other than those described above. Among the risk factors, prematurity, low birth weight, hypothermia, hemolysis and sepsis are prominent (Korejo et al., 2010). In a recent report, 12 neonates (9 males) were identified with bilirubin levels at the time of diagnosis ranging from 405 to 825 μmol/L. Causes of these elevated levels included G6PD deficiency (7 patients), dehydration (3 patients), sepsis (1 patient) and was undetermined in 1 patient. Magnetic resonance imaging typically showed an increased signal in the posteromedial aspect of the globus pallidus and was therefore useful in the assessment of the structural changes of chronic bilirubin encephalopathy after kernicterus (Al Otaibi et al., 2005). It has been reported recently that major causes of hyperbilirubinemia and resultant kernicterus are hemolysis, G6PD deficiency, birth trauma, sepsis, excessive dehydration and excessive weight loss (Al Awad et al., 2012; Bhutani et al., 2004).
Evidence of toxicity of SMX-TMP in neonates and children as reported by case studies, case series or historic data
In a randomized, controlled, double-blind, multicenter study in children (2–59 months), both amoxicillin and SMX-TMP were equally effective in nonsevere pneumonia and no adverse effects with treatment were noted (Awasthi et al., 2008; Qazi, 2002). In a review article on severe and nonsevere pneumonia, amoxicillin demonstrated no superior efficacy over SMX-TMP for nonsevere pneumonia (Agweyu et al., 2012). SMX-TMP has been successfully used prophylactically in HIV-infected men and in pregnant mothers who pose a risk in transmitting infection to fetuses or newborns. Mortality has been significantly reduced with few, if any, side effects (Forna et al., 2006; Suthar et al., 2012). In another study, prophylactic SMX-TMP treatment was successfully used to reduce preterm delivery and neonatal mortality among HIV-infected women with low CD4 cell count (Walter et al., 2006). In a review article of newborn infections in a primary care setting, data from nine studies of community-based management of newborn sepsis or pneumonia were included. There was no mention about any toxicity attributable to SMX-TMP usage, although the effectiveness of the drug in reducing neonate mortality was quite significant (Bhutta et al., 2009). In a trial in Nepal by CHW-managed care of neonate infections where oral SMX-TMP was used, no adverse events were noted during the course of intervention (Khanal et al., 2011). Another study in Nepal showed that SMX-TMP administration in children less than 5 years of age produced a significant reduction in deaths resulting from pneumonia (no mention of adverse reactions) (Pandey et al., 1991). Any sign of kernicterus was not observed in the aforementioned studies in neonates. This supports the notion that SMX-TMP does not produce kernicterus either directly or through other yet-to-be-identified mechanisms in neonates. These data reiterate the WHO recommendation of SMX-TMP for the treatment of sepsis or pneumonia. In primary and many secondary healthcare facilities, it is sensible to consider neonatal pneumonia within the spectrum of neonatal sepsis. The WHO does not distinguish between these two forms as well as bacteremia or urinary tract infection because of overlap of clinical signs and organ involvement and that empirical treatment regimens are similar (Duke, 2005).
Discussion
Combination of the two antibiotics, TMP and SMX, happens to be an important advance in the antimicrobial therapy. This represented an application of two drugs acting on sequential steps in the pathway of an obligate enzymatic reaction in bacteria, resulting in a synergistic antibacterial effect (Gilman et al., 1980c, p. 1116; Hitchings, 1982).
The main reason for not using SMX-TMP in neonates is that the SMX part of the drug could exacerbate jaundice by increasing the risk of bilirubin displacement by the drug from albumin, resulting in neurotoxicity. The major causes of kernicterus include sepsis (Pearlman et al., 1980), liver malfunction, blood dyscrasias, malnutrition and so on. Although previous research studies have suggested kernicterus as one of the toxicities associated with sulfisoxazole in neonates, no studies have directly demonstrated the occurrence of kernicterus in neonates that are treated with SMX-TMP. Therefore, the idea that SMX-TMP treatment causes kernicterus in neonates still remains as a theory. On the other hand, SMX-TMP treatment has provided beneficial clinical outcomes in neonates suffering from sepsis and pneumonia. This review article aims to thoroughly analyze previously published literature to determine the potential of SMX-TMP to cause kernicterus in neonates. However, this review does not include details on other neurotoxic side effects, including ototoxicity caused by excessive serum bilirubin.
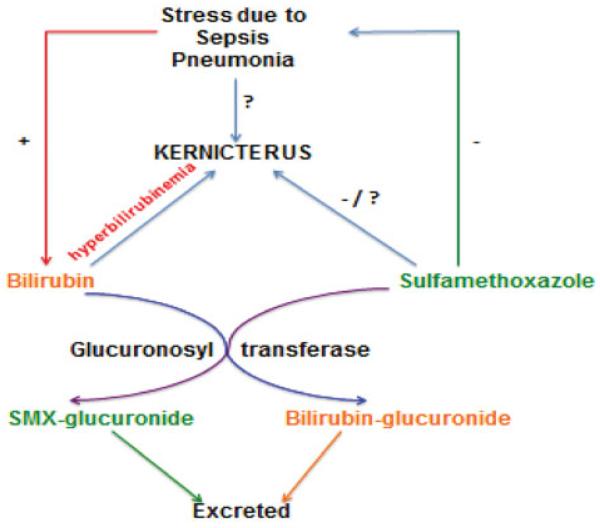
Interestingly, it was first demonstrated in 1959 that Gunn rat pups deficient in GT enzyme developed kernicterus when treated with sulfisoxazole. However, sulfisoxazole-untreated control pups also showed signs of kernicterus (Johnson et al., 1959). This suggests that hyperbilirubinemia may underlie the cause of kernicterus. GT is a liver enzyme that converts bilirubin into its glucuronide conjugate that is eliminated through bile. GT also converts several drugs, including sulfonamides, into harmless metabolites, which can be eliminated from the body as glucuronide conjugates (Figure 2).
Figure 2.
Hypothetical schema showing that stress resulting from sepsis/pneumonia leads to hyperbilirubinemia and causes kernicterus. The use of SMX for the treatment of sepsis/pneumonia inhibits stress, but may cause kernicterus by direct (yet-unknown) mechanisms. The enzyme, GT, metabolizes both bilirubin and SMX into their glucuronide conjugates for excretion.
Thus, sulfisoxazole treatment in Gunn’s rat pups that lack GT would result in the accumulation of the drug in the body and therefore result in kernicterus. Also, the possibility of direct effects of sulfisoxazole that are independent of hyperbilirubinemia to cause kernicterus cannot be ignored. Although neonates have been shown to have less-developed hepatic microsomal enzyme activity, thus far no studies have addressed the testing of the biotransformation of sulfonamides in neonates. Similarly, no published research work demonstrates that SMX treatment causes kernicterus in neonates.
The neonatal liver is capable of metabolizing many substrates by oxidation. Oxidative dealkylation is more efficient than hydroxylation. Reduction and hydrolysis are rudimentary in neonates and so is glucurodnation. Impaired conjugation of sulfonamides in the neonate can cause serious toxicity. Also, because of low renal plasma flow and glomerular filtration rate, the total body clearance rate of sulfonamides may be slow in the neonate. This may result in accumulation of unmetabolized drug in neonates, leading to toxicities. Yet another possibility is that repeated administration of sulfonamides to mothers at term can also cause neonatal toxicity. However, toxicity of SMX depends on the ability of the mother or neonates to metabolize the drug. Thus, the PK of sulfonamides in neonates, infants and children during development needs to be addressed to understand the potentials of SMX-TMP to cause kernicterus or any toxicity.
Ethnicity and race are tied to our genetics and environment. Acetylator phenotype is a useful predictor of fast and slow acetylators of drugs, and the population differences among fast and slow acetylators have been recognized. In a study of the Bangladesh population (Zaid et al., 2004), it was shown that approximately 67% of the patients (total, 517) fell in the category of fast acetylators of sulfadimidine.
This complexity presents a challenge for researchers to identify the specific connections between pharmacogenetics (PG) and race/ethnicity. PG refers to the genetic differences in drug disposition that can affect the individual responses to drugs as well as the therapeutic and adverse effects. Since the discovery of polymorphic N-acetylation of drugs, significant progress has been made to decipher the molecular genetics of acetylation. Acetylation is a process of phase II conjugation of sulfonamide biotransformation. The genetic variation affects the clinical consequences of being a rapid or slow acetylator (Zaid et al., 2004). The consequences of PG variation in these enzymes include (1) altered kinetics of specific drug substrates, (2) drug-drug interactions resulting from altered kinetics and (3) idiosyncratic adverse drug reactions. These can result clinically in an idiosyncratic hypersensitivity reaction, characterized by fever, skin rash, liver toxicity and CNS damage. Slow acetylation by NAT2 is a risk factor for such reactions to sulfonamides. Given the incidence of these severe adverse drug reactions (much less than 1 in 1000), slow acetylation cannot be the sole mechanism of predisposition in the population.
The reemerging use of SMX-TMP to treat neonatal sepsis and pneumonia is becoming popular in the home-based neonatal care setting in developing countries. This care, however, has received criticism because of the potential adverse reactions, and toxicities of SMX-TMP may be caused by the prediction of any toxicity resulting from SMX-TMP.
SMX-TMP is believed to cause kernicterus in neonates by displacing bilirubin from plasma albumin. The resultant hyperbilirubinemia serves as the cause for kernicterus. However, previous research studies demonstrate that neonatal sepsis and pneumonia serve as causes for hyperbilirubinemia and are often associated with jaundice. Further, SMX-TMP treatment has been shown to benefit the neonates from neonatal sepsis and pneumonia. If SMX-TMP causes hyperbilirubinemia by displacing bilirubin from plasma proteins, then this should increase bilirubin-mediated CNS toxicity leading to kernicterus. In the home-based neonatal care setting, total oral dose of SMX-TMP (200 mg of SMX plus 40 mg of TMP in 5 mL), 1.25 mL administered twice a day for 7 days was equivalent 100 mg per day. If SMX causes kernicterus, then the neonates that were treated with SMX-TMP syrup should have shown CNS toxicities leading to kernicterus. Conversely, SMX-TMP treatment has not been shown to increase the incidence of CNS toxicity and kernicterus in neonates, but, in fact, provided therapeutic benefits to treat neonatal sepsis and pneumonia. These data reinforce the observation that SMX-TMP treatment in neonates is beneficial and not detrimental.
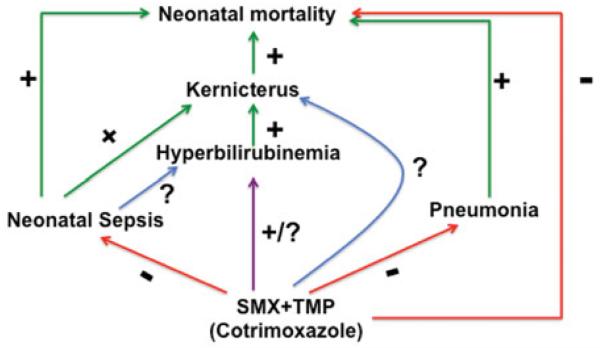
The model given below (Figure 3) describes the effects of SMX-TMP against neonatal mortality mediated by sepsis and pneumonia. Sepsis has been suggested as a leading cause of kernicterus in neonates because it increases the blood levels of bilirubin resulting from infection (Pearlman et al., 1980). This serves as a cause for kernicterus (Zuelzer & Mudgett, 1950). Under these conditions, SMX-TMP treatment should potentiate the toxicity of bilirubin, worsen the conditions of neonates and produce severe kernicterus. On the contrary, recent research demonstrates that SMX-TMP-treated neonates showed recovery from sepsis and pneumococcal infections (Bang et al., 1990, 1993; Mavalankar & Raman, 2006). Moreover, kernicterus was not observed in these neonates. However, this does not rule out any possibility for the presence of CNS toxicity in these neonates. Although the model below suggests a direct effect of SMX-TMP to cause kernicterus by hyperbilirubinemia, no evidences are currently available to support this. Therefore, further studies are needed to specifically address SMX-TMP toxicities.
Figure 3.
Hypothetical scheme illustrates the effects of neonatal sepsis and pneumonia on neonatal mortality. Hyperbilirubineria caused by sepsis results in kernicterus and increases neonatal mortality. Pneumococcal infections also facilitate this. SMX-TMP treatment significantly decreases neonatal mortality by controlling sepsis and pneumonia. Green arrows and plus (+) signs represent positive or facilitatory effects. Red arrows and minus (-) signs indicate negative or inhibitory effects. Question mark (?) represents undetermined effect(s). The purple arrow represents an uncovered direct effect of SMX-TMP to cause hyperbilirubinemia and kernicterus.
However, one cannot ignore the direct effects of SMX-TMP to produce CNS toxicities and kernicterus. This could be possible because SMX may cross the BBB and cause kernicterus by a direct, yet undetermined, mechanism. However, no published evidence is available to support this direct effect of the drug. One plausible reason for the lack of evidence for the direct effect of SMX to cause kernicterus could be that SMX does not accumulate in the body, but gets metabolized based on the PG.
Further studies are needed to critically address how the acetylator phenotypes affect SMX-TMP toxicity in neonates and adults. Recent clinical studies with sulfadoxin/pyrimethamine treatment in congenital taxoplasmosis and several large-scale clinical trials in Africa have not resulted in any cases of kernicterus. The report states, “although the pathogenesis of kernicterus remains unknown, it does not appear to be inducible by sulfonamides administered late in pregnancy or to term neonates” (Peters et al., 2007, p. 495). It is evident that when one considers risk versus benefits in the usage of SMX-TMP, the benefits of the drug far outweigh any theoretical risk of kernicterus and these concerns should not limit its use.
In general, many toxicology studies are conducted in adult and pregnant animals by pharmaceutical companies, chemical companies and U.S. Food and Drug Administration and other scientific investigators throughout the world, but it is striking that very few animal studies are performed utilizing newborn, infant and juvenile animals. “Also there are very few toxicology studies carried out on in children and that is why pregnant women and children are referred as therapeutic orphans” (Brent, 2004). Additionally, because of bacterial resistance to newer antibiotics (particularly MERSA strains), there is resurgence for the use of old-generation antibiotics, such as SMX-TMP (Falagas et al., 2008). Because no drug is completely safe, the toxicokinetics of drug molecules need to be studied to address long-term toxic profiles of the drug molecules. Because this takes a long time, one needs to consider cost-effectiveness and, more important, the risk-benefit factor in the usage of appropriate antibiotics in home-based neonatal care. Although SMX-TMP is used in home-based neonatal care to effectively treat sepsis and pneumonia, further in vivo animal studies and in vitro toxicity evaluation are needed to warrant the safe use of SMX-TMP to treat neonatal diseases. Even though this review demonstrates that no kernicterus was observed in neonates that are treated with SMX-TMP for up to 2 weeks, it does not exclude the possibility of the existence of any other CNS toxicities in neonates. This is because no clinical study has directly addressed the short- and long-term effects of oral SMX-TMP treatment in neonates. This is a limitation of this review.
Conclusion
In this report, we have attempted to analyze the neonatal toxicities of SMX-TMP in an unbiased manner. Our review of the literature indicates there is a clear lack of evidence showing the occurrence of kernicterus in neonates when SMX-TMP is used orally to treat pneumonia or sepsis. However, studies that demonstrate the occurrence of kernicterus resulting from sulfisoxazole warrants the necessity of follow-up studies to ensure the complete safety of SMX-TMP in neonatal population. To achieve this, there is a need for further focused clinical studies in humans as well as animal-based studies conducted in newborns to address the effect of SMX-TMP to test its ability to cause kernicterus and address the safety issues in neonates. Collectively, our review provides an analysis of the possibility of neonatal kernicterus after oral use of the SMX-TMP combination. Although it has been shown that SMX-TMP does not directly cause neonatal kernicterus, it does not rule out any other toxicity that could be caused by SMX-TMP. We therefore recommend that clinical studies that focus on evaluating the effects of short- and long-term use of SMX-TMP in home-based neonatal care need to be conducted. The review also warrants the need for future studies to analyze the safety profile of SMX-TMP.
Acknowledgments
Declaration of interest
The authors acknowledge the funding support provided by 1001070G NIH/DHHS COBRE, 1001481H NIH/DHHS INBRE and the American Association of Colleges of Pharmacy New Investigator Award (to B.T.).
Appendix 1
Summary of literature search by S.S.D.
Period of search: 1950-July 2012
From Embase: 3 articles of questionable relevance
From Chochrane database: 21 out of 7366
From CINAHL: 13 references
From PubMed search: 61 references
From Embase search: 29 references
Total number of references (abstracts) of possible use: 127
Summary of literature search by B.T.
Period of search: 1940-July 2012):
Total: 1088
Duplicate: 753
Total after subtracting duplicates: 335
Abstracts of possible use: 68
Total # abstracts from S.S.D. and B.T. search 127 + 68 = 195
Total # of relevant articles referred in the draft: 74
Details of search: includes search by B.T. and S.S.D.
Search included Medline, PubMed, Toxnet and information from WHO Bulletins. Also checked cross-references from published articles that gave even a slightest hint about CTX-induced toxicity in neonates.
Keywords and search terms: cotrimoxazole, trimethoprim, sulfamethoxazole, cotrimoxazole and neonates and jaundice, sulfamethoxazole and neonates and jaundice, trimethoprim and neonates and jaundice, cotrimoxazole-induced jaundice and neonates, trimethoprim and newborn babies, sulfamethoxazole and newborn babies, Trimethoprim and neonate kernicterus, sulphamethoxazole and neonate kernicterus
PubMed: cotrimoxazole[all fields] AND induced[all fields] AND (“infant, newborn”[MeSH terms] OR (“infant”[all fields] AND “newborn”[all fields]) OR “newborn infant”[all fields] OR “neonate”[all fields]) AND (“jaundice”[MeSH terms] OR “jaundice”[all fields])using newborn: cotrimoxazole[all fields] AND induced[all fields] AND (“jaundice, neonatal”[MeSH terms] OR (“jaundice”[all fields] AND “neonatal”[all fields]) OR “neonatal jaundice”[all fields] OR (“newborn”[all fields] AND “jaundice”[all fields]) OR “newborn jaundice”[all fields])
Embase search details
“Cotrimoxazole”/exp AND induced AND “jaundice”/exp AND ([newborn]/lim OR [infant]/lim) AND [humans]/lim
3 articles of questionable relevance
Cochrane database
There are 0 results out of 7366 records for: “cotrimoxazole AND induced AND jaundice AND neonates” in title, abstract or keywords in Cochrane Database of Systematic Reviews
There are 21 results out of 7366 records for: “cotrimoxazole AND induced AND jaundice AND infants” in title, abstract or keywords in Cochrane Database of Systematic Reviews
Using keywords: cotrimoxazole AND induced AND jaundice AND infants
Total results: 21
Database: CINAHL
Using keywords: cotrimoxazole AND induced AND jaundice AND neonates
Total results: 13
PubMed search:
Key words: cotrimaxazole AND cdverse effects AND neonates
Total results: 48
PubMed search:
Using keywords: cotrimaxazole AND hepatotaxicity AND infants Search details: “trimethoprim-sulfamethoxazole combination” [MeSH terms] OR (“trimethoprim-sulfamethoxazole”[all fields] AND “combination”[all fields]) OR “trimethoprim-sulfamethoxazole combination”[all fields] OR “cotrimoxazole”[all fields]) AND hepatotoxicity[all fields] AND (“infant”[MeSH terms] OR “infant”[all fields] OR “infants”[all fields])
Total results: 0 similar results for neonates
PubMed search:
Using keywords: cotrimaxazole AND hepatotoxicity.
Appendix 2
Some key references discussed in the review of the use and toxicity of SMX-TMP in neonates and animal studies.
| References | Subjects and type of study | Drug studied | Observations/conclusions |
|---|---|---|---|
| Khanal et al., 2011; Bhutta et al., 2009; Bang et al., 1990, 1993, 1999; Qazi, 2002 | Neonates, disease management | Oral SMX-TMP | Significant reduction in mor- tality from sepsis and pneu- monia; no untoward toxicities, including CNS toxicity, were mentioned |
| Wadsworth & Suh, 1988 | Pooled cord serum, in vitro study |
52 antimicrobial agents including SMX | Bilirubin displacement compared |
| Ho & Juurlink, 2011; Karpman & Kurzrock, 2004; Gutman, 1984; Gimnig et al., 2006; Block et al., 1987; Siegel et al., 1984 | Case reports, articles and reviews that include children ≤2 years of age |
Various drugs including SMX- TMP, oral |
Potentially adverse reactions and risk-benefit issues discussed |
| Silverman, 1959; Andersen et al., 1956 | Infants, clinical trial | Sulfisoxazole | Landmark study showing for the first time kernicterus associated with sulfisoxazole |
| Tuttle, 1955; Crosse et al., 1955; Odell, 1959 | Sera from infants, in vitro study | Sulfisoxazole | Used ultrafiltration and dialysis techniques to demonstrate uncoupling of protein-bound bilirubin by drug |
| Gunn, 1938; Blanc & Johnson, 1959; Maisels, 2006 | Rats, in vivo; also review | Sulfisoxazole, sulfadiazine | Development of rat model for kernicterus, unconjugated bilirubin causing neuronal toxicity |
| Springer et al., 1982 | Newborn infants; clinical and in vitro |
Intravenous SMX-TMP | Studied in vitro bilirubin bind- ing, serum drug levels and showed no side effects or kernicterus |
| Awasthi et al., 2008; Agweyu et al., 2012; Walter et al., 2006 | Clinical study | Amoxicillin and SMX-TMP comparison |
Lack of serious side effects or toxicity |
References
- Agweyu A, Opiyo N, English M. Experience developing national evidence-based clinical guidelines for childhood pneumonia in a low-income setting—making the GRADE? BMC Pediatr. 2012;12:1. doi: 10.1186/1471-2431-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Awad E, Obaid H, Mohammad K, et al. Staphylococcus aureus sepsis inducing severe hemolysis in glucose-6-phosphate dehydrogenase (G-6-PD)-deficient preterm infant causing severe neonatal jaundice and kernicterus. J Neonat Perinat Med. 2012;4:373–377. [Google Scholar]
- Al Otaibi SF, Blaser S, MacGregor DL. Neurological complications of kernicterus. Can J Neurol Sci. 2005;32:311–315. doi: 10.1017/s0317167100004182. [DOI] [PubMed] [Google Scholar]
- Andersen DH, Blanc WA, Crozier DN, Silverman WA. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Pediatrics. 1956;18:614–625. [PubMed] [Google Scholar]
- Awasthi S, Agarwal G, Singh JV, et al. Effectiveness of 3-day amoxycillin vs. 5-day co-trimoxazole in the treatment of non-severe pneumonia in children aged 2-59 months of age: a multi-centric open labeled trial. J Trop Pediatr. 2008;54:382–389. doi: 10.1093/tropej/fmn050. [DOI] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Baitule SB, et al. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999;354:1955–1961. doi: 10.1016/S0140-6736(99)03046-9. [DOI] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Morankar VP, et al. Pneumonia in neonates: can it be managed in the community? Arch Dis Child. 1993;68:550–506. doi: 10.1136/adc.68.5_spec_no.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang AT, Bang RA, Tale O, et al. Reduction in pneumonia mortality and total childhood mortality by means of community-based intervention trial in Gadchiroli, India. Lancet. 1990;336:201–206. doi: 10.1016/0140-6736(90)91733-q. [DOI] [PubMed] [Google Scholar]
- Bang AT, Reddy HM, Deshmukh MD, et al. Neonatal and infant mortality in the ten years (1993 to 2003) of the Gadchiroli field trial: effect of home-based neonatal care. J Perinatol. 2005;5(Suppl 1):S92–107. doi: 10.1038/sj.jp.7211277. [DOI] [PubMed] [Google Scholar]
- Bhutani VK, Johnson LH, Jeffrey Maisels M, et al. Kernicterus: epidemiological strategies for its prevention through systems-based approaches. J Perinatol. 2004;24:650–662. doi: 10.1038/sj.jp.7211152. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA, Zaidi AK, Thaver D, et al. Management of newborn infections in primary care settings: a review of the evidence and implications for policy? Pediatr Infect Dis J. 2009;28:S22–S30. doi: 10.1097/INF.0b013e31819588ac. [DOI] [PubMed] [Google Scholar]
- Blanc WA, Johnson L. Studies on kernicterus; relationship with sulfonamide intoxication, report on kernicterus in rats with glucuronyl transferase deficiency and review of pathogenesis. J Neuropathol Exp Neurol. 1959;18:165–187. doi: 10.1097/00005072-195901000-00011. discussion 187-9. [DOI] [PubMed] [Google Scholar]
- Block JM, Walstad RA, Bjertnaes A, et al. Ofloxacin versus trimethoprim-sulphamethoxazole in acute cystitis. Drugs. 1987;34:100–106. doi: 10.2165/00003495-198700341-00022. [DOI] [PubMed] [Google Scholar]
- Brent RL. Utilization of juvenile animal studies to determine the human effects and risks of environmental toxicants during postnatal developmental stages. Birth Defects Res B Dev Reprod Toxicol. 2004;71:303–320. doi: 10.1002/bdrb.20020. [DOI] [PubMed] [Google Scholar]
- Bushby SR. Synergy of trimethoprim-sulfamethoxazole. Can Med Assoc J. 1975;112:63–66. [PMC free article] [PubMed] [Google Scholar]
- Crabbe F, Vuylsteke B, De Clerck M, Laga M. Cost-effectiveness of management strategies for acute urethritis in the developing world. Trop Med Int Health. 2000;5:640–647. doi: 10.1046/j.1365-3156.2000.00616.x. [DOI] [PubMed] [Google Scholar]
- Crosse VM, Meyer TC, Gerrard JW. Kernicterus and prematurity. Arch Dis Child. 1955;30:501–508. doi: 10.1136/adc.30.154.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmstadt GL, Saha SK, Choi Y, et al. Population-based incidence and etiology of community-acquired neonatal bacteremia in Mirzapur, Bangladesh: an observational study. J Infect Dis. 2009;200:906–915. doi: 10.1086/605473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke T. Neonatal pneumonia in developing countries. Archives of disease in childhood. Arch Dis Child Fetal Neonatal Ed. 2005;90:F211–F219. doi: 10.1136/adc.2003.048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Grammatikos AP, Michalopoulos A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev Anti Infect Ther. 2008;6:593–600. doi: 10.1586/14787210.6.5.593. [DOI] [PubMed] [Google Scholar]
- Forna F, McConnell M, Kitabire FN, et al. Systematic review of the safety of trimethoprim-sulfamethoxazole for prophylaxis in HIV-infected pregnant women: implications for resource-limited settings. AIDS Rev. 2006;8:24–36. [PubMed] [Google Scholar]
- Fox CL, Ottenberg R. Acute hemolytic anemia from the sulfonamides. J Clin Invest. 1941;20:593–602. doi: 10.1172/JCI101252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gever LN. Two antibiotics in one. Nursing. 1980;10:49. doi: 10.1097/00152193-198003000-00012. [DOI] [PubMed] [Google Scholar]
- Gilman AG, Goodman LS, Gilman A, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 6th ed. Macmillan; New York: 1980a. p. 1730. [Google Scholar]
- Gilman AG, Goodman LS, Gilman A, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 6th ed. Macmillan; New York: 1980b. p. 1117. [Google Scholar]
- Gilman AG, Goodman LS, Gilman A, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 6th ed. Macmillan; New York: 1980c. p. 1116. [Google Scholar]
- Gimnig JE, MacArthur JR, M’bang’ombe M, et al. Severe cutaneous reactions to sulfadoxine-pyrimethamine and trimethoprim-sulfamethoxazole in Blantyre District, Malawi. Am J Trop Med Hyg. 2006;74:738–743. [PubMed] [Google Scholar]
- Gunn CK. Hereditary acholuric jaundice: in a new mutant strain of rats. J Hered. 1938;29:137. [Google Scholar]
- Gutman LT. The use of trimethoprim-sulfamethoxazole in children: a review of adverse reactions and indications. Pediatr Infect Dis. 1984;3:349–357. doi: 10.1097/00006454-198407000-00018. [DOI] [PubMed] [Google Scholar]
- Harvey RJ. Synergism in the folate pathway. Rev Infect Dis. 1982;4:255–260. doi: 10.1093/clinids/4.2.255. [DOI] [PubMed] [Google Scholar]
- Hitchings GH. Mechanism of action of trimethoprim-sulfamethoxazole. I. J Infect Dis. 1973;128:433–436. doi: 10.1093/infdis/128.supplement_3.s433. [DOI] [PubMed] [Google Scholar]
- Hitchings GH. A biochemical approach to chemotherapy. Drug Intell Clin Pharm. 1982;16:843–848. doi: 10.1177/106002808201601106. [DOI] [PubMed] [Google Scholar]
- Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183:1851–1858. doi: 10.1503/cmaj.111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JB, Howard JE., Sr Trimethoprim-sulfamethoxazole vs sulfamethoxazole for acute urinary tract infections in children. Am J Dis Child. 1978;132:1085–1087. doi: 10.1001/archpedi.1978.02120360041005. [DOI] [PubMed] [Google Scholar]
- Johnson L, Sarmiento F, Blanc WA, Day R. Kernicterus in rats with an inherited deficiency of glucuronyl transferase. AMA J Dis Child. 1959;97:591–608. doi: 10.1001/archpedi.1959.02070010593009. [DOI] [PubMed] [Google Scholar]
- Karpman E, Kurzrock EA. Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J Urol. 2004;172:448–453. doi: 10.1097/01.ju.0000130653.74548.d6. [DOI] [PubMed] [Google Scholar]
- Khanal S, Sharma J, Gc VS, et al. Community health workers can identify and manage possible infections in neonates and young infants: MINI—a model from Nepal. J Health Popul Nutr. 2011;29:255–264. doi: 10.3329/jhpn.v29i3.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korejo HB, Bhurgri GR, Bhand S, et al. Risk factors for kernicterus in neonatal jaundice. Gomal J Med Sci. 2010;8:12–15. [Google Scholar]
- Ladhani S, Garbash M. Staphylococcal skin infections in children: rational drug therapy recommendations. Paediatr Drugs. 2005;7:77–102. doi: 10.2165/00148581-200507020-00002. [DOI] [PubMed] [Google Scholar]
- Lewin EB, Klein JO, Finland M. Trimethoprim-sulfamethoxazole: absorption, excretion, and toxicity in six children. J Infect Dis. 1973;128:S618–S621. doi: 10.1093/infdis/128.supplement_3.s618. [DOI] [PubMed] [Google Scholar]
- Maisels JM. Neonatal jaundice. Pediatr Rev. 2006;27:443–454. doi: 10.1542/pir.27-12-443. [DOI] [PubMed] [Google Scholar]
- Mathew JL, Patwari AK, Gupta P, et al. Acute respiratory infection and pneumonia in India: a systematic review of literature for advocacy and action: UNICEF-PHFI series on newborn and child health, India. Indian Pediatr. 2011;48:191–218. doi: 10.1007/s13312-011-0051-8. [DOI] [PubMed] [Google Scholar]
- Mavalankar DV, Raman P. Case Study. Center for Management of Health Services (pre-publication results) Indian Institute of Management, Ahmedabad; Ahmedabad, India: 2006. ANKUR Project: a case study of replication of home based newborn care. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady F. Trimethoprim-sulfamethoxazole: a reappraisal. Can Med Assoc J. 1975;112:5–7. [PMC free article] [PubMed] [Google Scholar]
- Odell GB. Studies in kernicterus. I. The protein binding of bilirubin. J Clin Invest. 1959;38:823–833. doi: 10.1172/JCI103864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra PL, Faden HS. Urinary tract infections in childhood: an update. J Pediatr. 1985;106:1023–1029. doi: 10.1016/s0022-3476(85)80263-8. [DOI] [PubMed] [Google Scholar]
- Paap CM, Nahata MC. Clinical pharmacokinetics of antibacterial drugs in neonates. Clin Pharmacokinet. 1990;19:280–318. doi: 10.2165/00003088-199019040-00003. [DOI] [PubMed] [Google Scholar]
- Pandey MR, Daulaire NM, Starbuck ES, et al. Reduction in total under-five mortality in western Nepal through community-based antimicrobial treatment of pneumonia. Lancet. 1991;338:993–997. doi: 10.1016/0140-6736(91)91847-n. [DOI] [PubMed] [Google Scholar]
- Pearlman MA, Gartner LM, Lee K, et al. The association of kernicterus with bacterial infection in the newborn. Pediatrics. 1980;65:26–29. [PubMed] [Google Scholar]
- Peters PJ, Thigpen MC, Parise ME, Newman RD. Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 2007;30:481–501. doi: 10.2165/00002018-200730060-00003. [DOI] [PubMed] [Google Scholar]
- Qazi S. Clinical efficacy of co-trimoxazole versus amoxicillin twice daily for treatment of pneumonia: a randomised controlled clinical trial in Pakistan. Arch Dis Child. 2002;86:113–118. doi: 10.1136/adc.86.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollo IM. Dihydrofolate reductase inhibitors as antimicrobial agents and their potentiation by sulfonamides. CRC Crit Rev Clin Lab Sci. 1970;1:565–583. doi: 10.3109/10408367009043919. [DOI] [PubMed] [Google Scholar]
- Ryan M, Griffin S, Chitah B, et al. The cost-effectiveness of cotrimoxazole prophylaxis in HIV-infected children in Zambia. AIDS. 2008;22:749–757. doi: 10.1097/QAD.0b013e3282f43519. [DOI] [PubMed] [Google Scholar]
- Salter AJ. Trimethoprim-sulfamethoxazole in treatment of severe infections. Rev Infect Dis. 1982;4:338–350. doi: 10.1093/clinids/4.2.338. [DOI] [PubMed] [Google Scholar]
- Schopf E. Skin reactions to co-trimoxazole. Infection. 1987;15:S254–S258. doi: 10.1007/BF01643199. [DOI] [PubMed] [Google Scholar]
- Schwartz DE, Ziegler WH. Assay and pharmacokinetics of trimethoprim in man and animals. Postgrad Med J. 1969;45:32–37. [PubMed] [Google Scholar]
- Shambaugh GE. History of sulfonamides. Arch Otolaryn. 1966;83:27. [Google Scholar]
- Siegel SE, Wolff LJ, Baehner RL, Hammond D. Treatment of pneumocystis carinii pneumonitis. A comparative trial of sulfamethoxazole-trimethoprim v pentamidine in pediatric patients with cancer: report from the Children’s Cancer Study Group. Am J Dis Child. 1984;138:1051–1054. [PubMed] [Google Scholar]
- Silverman WA. The status of 2-year-old children who had received sulfisoxazole in the neonatal period after premature birth. J Pediatr. 1959;54:741–747. doi: 10.1016/s0022-3476(59)80140-2. [DOI] [PubMed] [Google Scholar]
- Smilack JD. Trimethoprim-sulfamethoxazole. Mayo Clinic Proc. 1999;74:730–734. doi: 10.4065/74.7.730. [DOI] [PubMed] [Google Scholar]
- Springer C, Eyal F, Michel J. Pharmacology of trimethoprim-sulfamethoxazole in newborn infants. J Pediatr. 1982;100:647–650. doi: 10.1016/s0022-3476(82)80778-6. [DOI] [PubMed] [Google Scholar]
- Suthar AB, Granich R, Mermin J, Van Rie A. Effect of cotrimoxazole on mortality in HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Bull World Health Org. 2012;90:128C–138C. doi: 10.2471/BLT.11.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaver D, Ali SA, Zaidi AK. Antimicrobial resistance among neonatal pathogens in developing countries. Pediatr Infect Dis J. 2009;28:S19–S21. doi: 10.1097/INF.0b013e3181958780. [DOI] [PubMed] [Google Scholar]
- Tuttle AH. Serum pigment studies in newborn infants. I. Erythroblastosis fetalis. AMA Am J Dis Child. 1955;89:544–552. [PubMed] [Google Scholar]
- Uhari M, Nuutinen M, Turtinen J. Adverse reactions in children during long-term antimicrobial therapy. Pediatr Infect Dis J. 1996;15:404–408. doi: 10.1097/00006454-199605000-00005. [DOI] [PubMed] [Google Scholar]
- Velvis H, Carrasco N, Hetherington S. Trimethoprim-sulfamethoxazole therapy of neonatal Proteus mirabilis meningitis unresponsive to cefotaxime. Pediatr Infect Dis. 1986;5:591–593. [PubMed] [Google Scholar]
- Wadsworth SJ, Suh B. In vitro displacement of bilirubin by antibiotics and 2-hydroxybenzoylglycine in newborns. Antimicrob Agents Chemother. 1988;32:1571–1575. doi: 10.1128/aac.32.10.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Mwiya M, Scott N, et al. Reduction in preterm delivery and neonatal mortality after the introduction of antenatal cotrimoxazole prophylaxis among HIV-infected women with low CD4 cell counts. J Infect Dis. 2006;194:1510–1518. doi: 10.1086/508996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat KA, Anadkat MJ, Klekotka PA. Seasonal variation of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with trimethoprim-sulfamethoxazole. J Am Acad Dermatol. 2009;60:589–594. doi: 10.1016/j.jaad.2008.11.884. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Meeting report of the Department of Child and Adolescent Health and Development, World Health Organization. World Health Organization; Geneva: 2002. Explore simplified antimicrobial regimens for the treatment of neonatal sepsis. [Google Scholar]
- Zaid RB, Nargis M, Neelotpol S, et al. Acetylation phenotype status in a Bangladeshi population and its comparison with that of other Asian population data. Biopharm Drug Dispos. 2004;25:237–241. doi: 10.1002/bdd.403. [DOI] [PubMed] [Google Scholar]
- Zuelzer WW, Mudgett RT. Kernicterus: etiologic study based on an analysis of 55 cases. Pediatrics. 1950;6:452–474. [PubMed] [Google Scholar]