Abstract
The rhesus macaque exhibits age-related brain changes similar to humans, making an excellent model of normal aging. Calorie restriction is a dietary intervention that reduces age-related comorbidities in short-lived animals, and its effects are still under study in rhesus macaques. Here, using deterministic fiber tracking method, we examined the effects of age and calorie restriction on a diffusion tensor imaging measure of white matter integrity, fractional anisotropy (FA), within white matter tracks traversing the anterior (genu) and posterior (splenium) corpus callosum in rhesus monkeys. Our results show: (1) a significant inverse relationship between age and mean FA of tracks traversing the genu and splenium; (2) higher mean FA of the splenium tracks as compared to that of genu tracks across groups; and (3) no significant diet effect on mean track FA through either location. These results are congruent with the age-related decline in white matter integrity reported in humans and monkeys, and the anterior-to-posterior gradient in white matter vulnerability to normal aging in humans. Further studies are warranted to critically evaluate the effect of calorie restriction on brain aging in this unique cohort of elderly primates.
Introduction
Calorie restriction (CR), in the absence of malnutrition, has been reliably demonstrated to protect against age-related diseases and to prolong healthy lifespan in several shorter-lived animal models [1,2]. Ongoing studies in rhesus macaques (Macaca mulatta) indicate that CR is effective in delaying the development of age-related diseases [2–5], although consensus has not been reached on its effect on lifespan. While the precise mechanisms underlying the effects of CR are not fully understood, it appears that CR, at least in part, may act through anti-oxidative and anti-inflammatory mechanisms [6]. Brain white matter (WM) is especially sensitive to inflammatory and oxidative damage due to its high metabolic activity [7,8]. Using diffusion tensor imaging (DTI), a type of magnetic resonance imaging (MRI) that is sensitive to WM fiber integrity by observing water diffusion [9], our group has previously demonstrated that there is an inverse relationship between age and DTI measures related to WM integrity in diverse association and projection fiber tracks [10]. This voxel-based morphometry (VBM) study was specifically suited for a whole brain approach, which compares DTI measures in each voxel between subjects. While this study was a first exploratory step in understanding the effects of CR on WM, additional work is clearly warranted.
In the present study, we examined the effect of age and CR on corpus callosum (CC) fiber integrity in rhesus monkeys using fiber tractography. Tractography is a post-processing method where tensors of cerebral WM water diffusion can be mathematically extrapolated from DTI. These tensors can track the three-dimensional fiber orientation in the brain macroscopically using various deterministic or probabilistic algorithms [11]. In tract-based analyses, an a priori region of interest (ROI) is used as seed region from which fibers are traced in the native space of each animal, thus precluding the need to register individual images to a common space and introducing error due to normalization, and fiber characteristics are compared between subjects. Unlike VBM, this method thus does not rely on effective between-subject registration and can be restricted to specific brain regions [12]. The rhesus monkey (Macaca mulatta) provides a valuable animal model for studying human aging because it exhibits several age-related brain and cognitive changes similar to humans [2,4,10,13,14]. In captivity, median life expectancy of macaques is approximately 26 years, 10% survive beyond 35 years, and maximum lifespan is approximately 40 years [15]; overall, macaques age at a rate of two and a half to three times that of humans [2]. In this study, we assessed age and diet effects on fractional anisotropy (FA), a measure of the proportion of diffusion within a voxel that is directional, which reflects tissue integrity and tends to decrease with aging [16,17]. We evaluated the average FA of fiber tracks traversing two distinct spherical ROIs: 1) the genu of the CC, and 2) the splenium of the CC. The CC is one of the largest and most widely studied compact WM regions using imaging methods in human aging and disease [16–20]. Choosing specific ROIs within the CC thus ensured consistent seed placement among subjects. Additionally, the extensive existing literature elucidating the effects of human aging and disease on CC provided a suitable comparison for our current findings in this nonhuman primate model of aging. The genu of the CC primarily contains interhemispheric prefrontal fibers, whereas the splenium contains fibers from the posterior parietal, occipital, and medial temporal cortices [21]. Our aim was to capture WM microstructural measures of fibers that project toward the cortex in native space at the single subject level. We evaluated these ROIs specifically because these regions have been previously reported to show age-related FA decreases in humans [17,22,23]. Additionally, this method also allowed us to assess any anterior to posterior differences in FA within the CC. For instance, FA is greater in posterior CC than anterior CC regions at all ages in humans [24,25].
We hypothesized that there would be a negative relationship between mean track FA and age, that posterior FA would be greater than anterior FA across all subjects, and that CR animals would exhibit higher track FA values than controls.
Materials and Methods
Subjects
Thirty-three rhesus macaques (controls = 15, CR = 18), aged 19 to 29 years, used in this study are part of the longitudinal "Dietary Restriction and Aging Study" at the Wisconsin National Primate Research Center. Four of these animals (controls = 2, CR = 2) were below the age of 20 (middle-aged), whereas the remaining animals were old (20 years or older) at the time of image acquisition. Complete details of the dietary manipulation and experimental setup have been described extensively elsewhere [26,27]; the reader is referred to these prior publications for further information. The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison. Animals were anesthetized using ketamine and xylazine prior to imaging, and all efforts were made to minimize suffering.
Image acquisition
Image acquisition parameters have been reported previously [10]; the reader is referred to this prior publication for a complete description of image acquisition methods. Briefly, MRI scans were acquired on a General Electric 3.0 T Signa MR Unit (GE Medical Systems, Milwaukee, WI, USA) with a quadrature transmit/receive volume coil (18 cm diameter). Animals were anesthetized and scanned in the morning. DTI was performed in the axial plane using a single-shot, spin-echo, diffusion-weighted echo-planar imaging sequence with diffusion gradients in 12 optimal directions. Imaging parameters include: b = 816 second/mm2, TR = 10000 ms, TE = 77.2 ms, NEX = 6, FOV = 160 mm, matrix = 120 × 120, section thickness = 2.5 mm, no gap. A higher-order shimming protocol was run before the DTI scan to minimize image distortion.
Image Processing and Tractography
DTI processing was done by personnel blind to the diet status of all the animals. Eddy current correction was completed using tools available in the FMRIB Software Library (FSL) Diffusion Toolbox [28]. We used the DTI software program Diffusion Toolkit (version 0.6.2.1) for calculating diffusion tensors and for mathematically reconstructing fiber tracks. Fiber tracking was then visualized using TrackVis (version 0.5.2.1, Ruopeng Wang, Van J. Wedeen, TrackVis.org, Martinos Center for Biomedical Imaging, Massachusetts General Hospital). Fiber tracks were generated from spherical seed regions of 5mm diameter placed in the genu and splenium of the CC. The genu was defined as the most anterior coronal slice containing crossing CC fibers, and the splenium as the most posterior coronal slice of the CC. Streamline tractography was performed by following the principal eigenvector (FACT method) [11], with the termination angle threshold set at 35° and FA threshold at 0.15. Average FA of all fibers traversing through these respective seed regions was then obtained. To evaluate the inter-rater reliability of the seed placement method, another experimenter (blind to the animals’ diet status) repeated seed region placement in 6 cases (n = 3 controls, 3 CR) using the same criteria.
Statistics
All statistical analyses were conducted using SPSS 21.0 software (IBM Software, Chicago, IL). Difference in age distribution between the two diet groups was determined using independent samples t-test and gender differences between the two diet groups were assessed using Chi-Square test. Paired sample t-test was used to examine differences in mean FA of tracks traversing the genu and splenium seeds for each s ubject. Linear regression models were used to study main effects of age and diet, controlling for sex as a covariate. Repeated measures analysis was used to evaluate interaction effect between location and diet, and location and age. Alpha was set at 0.05 (two-tailed) to be considered significant.
Results
Animal characteristics
Age and gender distributions did not differ significantly between the two diet groups (mean age ± SD, controls: 23.5 ± 3.0 years, CR: 23.7 ± 2.7 years, t = −0.23, p = 0.82; males/females, controls = 7/8, CR = 12/6, Pearson Chi-Square = 1.34, p = 0.25). The CR monkeys weighed significantly less than control animals (mean ± SD, controls: 11.7 ± 3.4 lbs, CR: 9.0 ± 1.7 lbs, t = 2.9, df = 31, p < 0.05) [10].
Fiber tracking inter-rater reliability
There was a high level of consistency in seed placement. The correlation coefficients of number of tracks and FA values for the 6 animals on which seed placement was completed at both locations by two experimenters was >0.98 (p < 0.001), demonstrating minimal discrepancies in seed placement between observers. A sample image of fiber tracking is shown in Figure 1.
Figure 1. Fiber tractography in rhesus macaque corpus callosum.
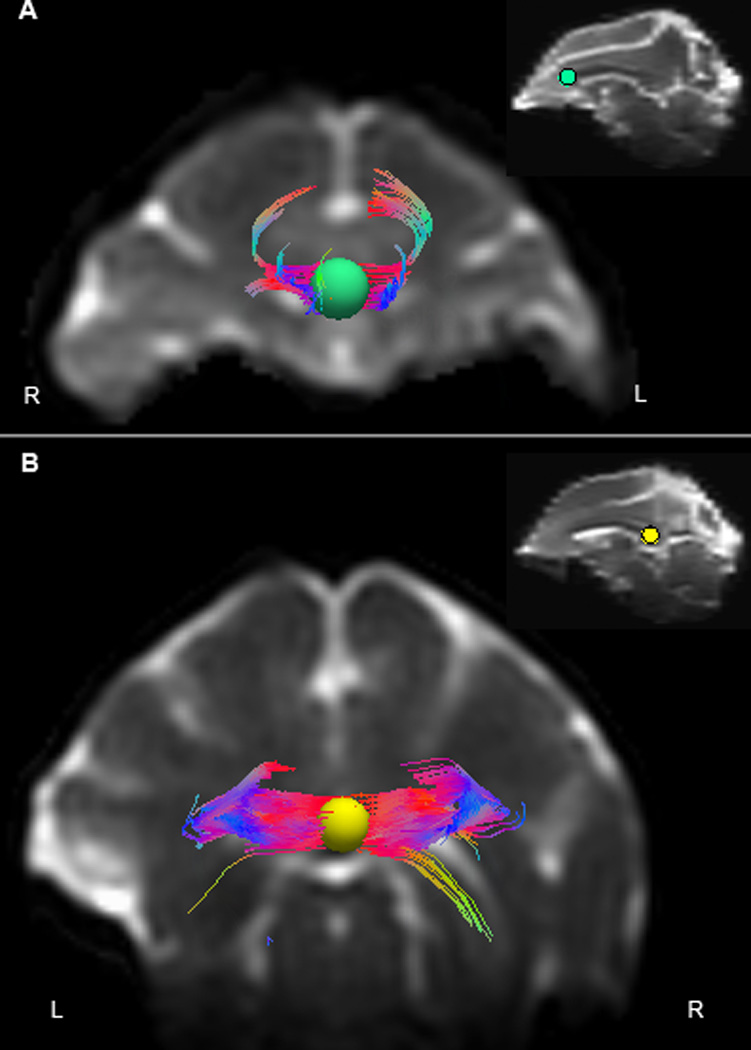
A representative image of deterministic fiber tractography from a 26-year-old calorie-restricted male rhesus monkey is shown in native space (T2-weighted image). Fibers traversing the 5mm spherical seed placed in the (A) genu (green) and (B) splenium (yellow) of corpus callosum in coronal view; the insets display the relative placement of the spheres in sagittal view. R = right, L = left. Fiber tracks are color coded based on directionality (anterior/posterior = blue, medial/lateral = red, superior/inferior = green).
Effect of location
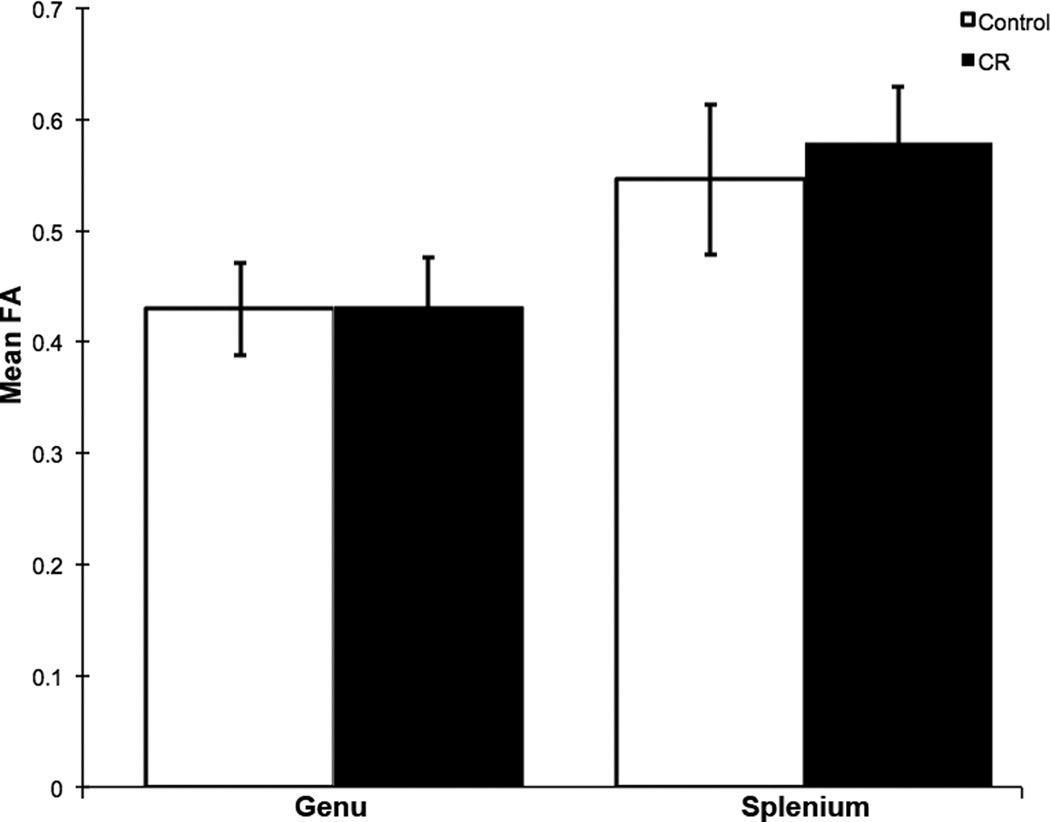
Mean FA of fiber tracks in the splenium was significantly greater than that of the genu of CC across all subjects (t = −15.4, p < 0.001; Figure 2).
Figure 2. Mean FA of genu and splenium fibers of corpus callosum in rhesus monkeys.
Mean FA within posterior corpus callosum (splenium) fiber tracks was significantly greater than that of anterior corpus callosum (genu) fiber tracks in a combined analysis of control and calorie-restricted animals (t = −15.4, p < 0.001). Error bars represent standard deviation.
Effect of age
Using linear regression analysis, we found a significant main effect of age on mean FA of fibers traversing the genu (t = −2.54, p = 0.017, Figure 3a) and splenium of the corpus callosum (t = −2.20, p = 0.036, Figure 3b), indicating loss of WM integrity with increasing age.
Figure 3. Correlation between age and mean FA of corpus callosal fibers.
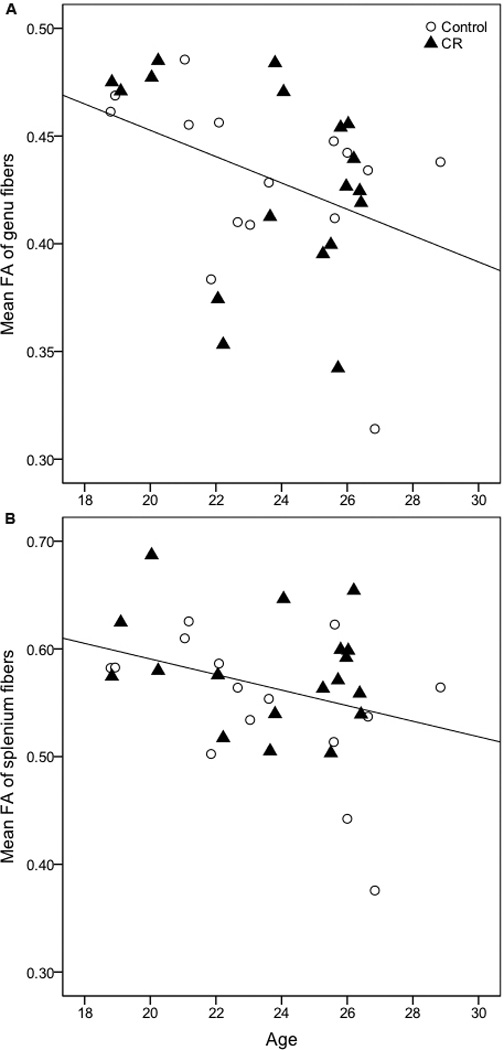
There is a significant inverse relationship between age and mean FA of fibers traversing the (A) genu (t = −2.54, p = 0.017) and (B) splenium of the corpus callosum (t = −2.20, p = 0.036) in a combined analysis of control and calorie-restricted animals.
Effect of CR
There was no significant main effect of diet on mean FA of fibers traversing the genu (t = −0.15, p = 0.88) or splenium (t = 1.48, p = 0.15) of the corpus callosum.
Interaction effect
There was no significant interaction effect between location and diet group while controlling for age (F = 2.54, p = 0.12), between location and age while controlling for diet (F = 0.07, p = 0.80), or between age and diet at either location independently (p > 0.05).
Discussion
In the present study, we show an inverse relationship between age and mean FA of tracks traversing the genu and splenium of CC, and a decline in mean track FA from posterior CC (splenium) to anterior CC (genu) across all animals. These findings are consistent with prior reports in humans and monkeys. For example, white matter FA declines significantly with increasing age in humans [17,23]. Brain imaging studies in macaques have also indicated a decline in forebrain WM with age, and microstructural alterations in several frontal WM bundles [29,30]. Parallel with our current finding, FA is greater in posterior CC regions than anterior CC regions across all ages in humans [24,25]. There is also an anterior-to-posterior gradient in WM aging in humans, with frontal regions showing greater age-related decline in anisotropy while posterior and inferior areas remain relatively unchanged [20,23,31,32]. We did not observe an interaction between age and location within the CC in the present study, possibly due to the cross-sectional nature of the current study and the relatively small sample size. Postmortem studies in macaques have demonstrated that age-related WM changes are a result of dense cytoplasmic inclusions, myelin sheath ballooning, redundant myelin formation, and circumferential splitting of thick sheaths in the anterior commissure, frontal lobe WM, and primary visual cortex [33,34].
We did not find a significant protective effect of CR on brain WM integrity in the present study, possibly due to the relatively small sample size. In the posterior CC, however, there was a nonsignificant trend (p = 0.15) such that CR animals had higher mean track FA compared to control subjects. While the mechanism underlying this finding is not known, one possibility is that CR benefits glucoregulation. Abnormal glucoregulation, including frank diabetes, is known to affect posterior brain regions, including the splenium [35,36]. We also previously found that increased levels of the vascular biomarker homocysteine predict lower FA in the splenium of CC, whereas CR monkeys did not show this relationship [37]. Thus vascular damage, which is reflective of and can exacerbate glucoregulatory dysfunction, is another potential mechanism for preserved FA in posterior transcallosal tracks of CR animals.
There are some limitations to the current study. This is a cross-sectional analysis of aging effects with a limited age range of the subjects included, thus only allowing interpretations of age effects over a short range. The limited resolution of the 12-directional diffusion gradient protocol, combined with the relatively small sample size, may have masked any significant CR effect on brain WM integrity. To further elucidate any underlying CR effects, a feasible alternative approach to the current method would be to perform probabilistic tractography along various tracts of interest. Finally, this study also does not provide any mechanistic insights into the aging associations observed in this non-human primate cohort. Nonetheless, these findings provide important parallels between human and non-human primate aging and pave the way for future studies at identifying causative events in brain aging and interventions for aging retardation.
Highlights.
Genu and splenium white matter integrity declines with aging in rhesus monkeys
There is an anterior-to-posterior gradient in white matter integrity in macaques
Calorie restriction has no effect on corpus callosal integrity in macaques
These age-related findings in monkeys are similar to those demonstrated in humans
Acknowledgements
The authors acknowledge the support of researchers and staff at the Waisman Center, where MR imaging took place. We also appreciate the assistance provided by the Animal Care, Veterinary and Pathology Staff of the Wisconsin National Primate Research Center. This study was supported by the National Institutes of Health grants RR000167, AG011915, and AG000213. The study was also supported with resources and facilities at the W.S. Middleton Memorial Veterans Hospital. AS researched the data, analyzed the data, and wrote the manuscript. CG, BBB, JMO, AAW, ALA, and JWK offered expertise and reviewed/edited the manuscript. RJC, RHW, and SCJ conceived the design of the project, contributed resources, offered expertise, and reviewed/edited the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors report no conflicts of interest or relevant financial interests related to this work.
References
- 1.Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colman RJ, Anderson RM. Nonhuman primate calorie restriction. Antioxid Redox Sign. 2011;14:229–239. doi: 10.1089/ars.2010.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A-Biol. 2003;58:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 4.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbet RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weindruch R. Caloric restriction, gene expression, and aging. Alzheimer Dis Assoc Disord. 2003;17(Suppl 2):S58–S59. doi: 10.1097/00002093-200304002-00008. [DOI] [PubMed] [Google Scholar]
- 7.Felts PA, Woolston AM, Fernando HB, Asquith S, Gregson NA, Mizzi OJ, Smith KJ. Inflammation and primary demyelination induced by the intraspinal injection of lipopolysaccharide. Brain. 2005;128:1649–1666. doi: 10.1093/brain/awh516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basser PJ, Pierpaoli C C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson Ser B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 10.Bendlin BB, Canu E, Willette A, Kastman EK, McLaren DG, Kosmatka KJ, Xu G, Field AS, Colman RJ, Coe CL, Weindruch RH, Alexander AL, Johnson SC. Effects of aging and calorie restriction on white matter in rhesus macaques. Neurobiol Aging. 2011;32:2319, e2311–e2311. doi: 10.1016/j.neurobiolaging.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T. MR tractography: a review of its clinical applications. Magnet Resonance Med Sci. 2009;8:165–174. doi: 10.2463/mrms.8.165. [DOI] [PubMed] [Google Scholar]
- 12.Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology. 2007;245:367–384. doi: 10.1148/radiol.2452060445. [DOI] [PubMed] [Google Scholar]
- 13.Sridharan A, Willette AA, Bendlin BB, Alexander AL, Coe CL, Voytko ML, Colman RJ, Kemnitz JW, Weindruch RH, Johnson SC. Brain volumetric and microstructural correlates of executive and motor performance in aged rhesus monkeys. Front Aging Neurosci. 2012;4:31. doi: 10.3389/fnagi.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sridharan A, Pehar M, Salamar MS, Pugh TD, Bendlin BB, Willette AA, Anderson RM, Kemnitz JW, Colman RJ, Weindruch RH, Puglielli L, Johnson SC. Calorie restriction attenuates astrogliosis but not amyloid plaque load in aged rhesus macaques: a preliminary quantitative imaging study. Brain Res. 2013;1508:1–8. doi: 10.1016/j.brainres.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemnitz JW. Calorie restriction and aging in nonhuman primates. ILAR J. 2011;52:66–77. doi: 10.1093/ilar.52.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang PJ, Saykin AJ, Flashman LA, Wishart HA, Rabin LA, Santulli RB, McHugh TL, McDonald JW, Mamourian AC. Regionally specific atrophy of the corpus callosum in AD, MCI and cognitive complaints. Neurobiol Aging. 2006;27:1613–1617. doi: 10.1016/j.neurobiolaging.2005.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Paola M, Di Iulio F, Cherubini A, Blundo C, Casini AR, Sancesario G, Passafiume D, Caltagirone C, Spalletta G. When, where, and how the corpus callosum changes in MCI and AD: a multimodoal MRI study. Neurology. 2010;74:1136–1142. doi: 10.1212/WNL.0b013e3181d7d8cb. [DOI] [PubMed] [Google Scholar]
- 20.Frederiksen KS, Garde E, Skimminge A, Ryberg C, Rostrup E, Baare WF, Siebner HR, Hejl AM, Leffers AM, Waldemar G. Corpus callosum atrophy in patients with mild Alzheimer's disease. Neurodegen Dis. 2011;8:476–482. doi: 10.1159/000327753. [DOI] [PubMed] [Google Scholar]
- 21.van der Knaap LJ, van der Ham IJ. How does the corpus callosum mediate interhemispheric transfer? A review. Behav Brain Res. 2011;223:211–221. doi: 10.1016/j.bbr.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- 24.Hasan KM, Kamali A, Iftikhar A, Kramer LA, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Res. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasan KM, Kamali A, Kramer LA, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain Res. 2008;1227:52–67. doi: 10.1016/j.brainres.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol. 1993;48:B17–B26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 28.Smith SM, Jenkinson M, Woolrich MW, Beckman CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 29.Makris N, Papadimitriou GM, van der Kouwe A, Kennedy DN, Hodge SM, Dale AM, Benner T, Wald LL, Wu O, Tuch DS, Caviness VS, Moore TL, Killiany RJ, Moss MB, Rosene DL. Frontal connections and cognitive changes in normal aging rhesus monkeys: a DTI study. Neurobiol Aging. 2007;28:1556–1567. doi: 10.1016/j.neurobiolaging.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Wisco JJ, Killiany RJ, Guttmann CR, Warfield SK, Moss MB, Rosene DL. An MRI study of age-related white and gray matter volume changes in the rhesus monkey. Neurobiol Aging. 2008;29:1563–1575. doi: 10.1016/j.neurobiolaging.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michielse S, Coupland N, Camicioli R, Carter R, Seres P, Sabino J, Malykhin N. Selective effects of aging on brain white matter microstructure: a diffusion tensor imaging tractography study. NeuroImage. 2010;52:1190–1201. doi: 10.1016/j.neuroimage.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 34.Sandell JH, Peters A. Disrupted myelin and axon loss in the anterior commissure of the aged rhesus monkey. J Comp Neurol. 2003;466:14–30. doi: 10.1002/cne.10859. [DOI] [PubMed] [Google Scholar]
- 35.Franc DT, Kodl CT, Mueller BA, Muetzel RL, Lim KO, Seaquist ER. High connectivity between reduced cortical thickness and disrupted white matter tracts in long13 standing type 1 diabetes. Diabetes. 2011;60:315–319. doi: 10.2337/db10-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–3089. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willette AA, Bendlin BB, Colman RJ, Kastman EK, Field AS, Alexander AL, Sridharan A, Allison DB, Anderson R, Voytko ML, Kemnitz JW, Weindruch RH, Johnson SC. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes. 2012;61:1036–1042. doi: 10.2337/db11-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]