Abstract
Due to their all-electrical nature, impedance biosensors have significant potential for use as simple and portable sensors for environmental studies and environmental monitoring. Detection of two endocrine-disrupting chemicals (EDC), norfluoxetine and BDE-47, is reported here by impedance biosensing, with a detection limit of 8.5 and 1.3 ng/mL for norfluoxetine and BDE-47, respectively. Although impedance biosensors have been widely studied in the academic literature, commercial applications have been hindered by several technical limitations, including possible limitations to small analytes, the complexity of impedance detection, susceptibility to nonspecific adsorption, and stability of biomolecule immobilization. Recent research into methods to overcome these obstacles is briefly reviewed. New results demonstrating antibody regeneration atop degenerate (highly doped) Si are also reported. Using 0.2 M KSCN and 10 mM HF for antibody regeneration, peanut protein Ara h 1 is detected daily during a 30 day trial.
Keywords: Electrochemical impedance spectroscopy, Biosensors, Sustainability, Endocrine-disrupting chemicals, Nanotechnology
Short abstract
This article describes the applicability of impedance biosensing to environmental contaminants, including new results for impedance detection of endocrine-disrupting chemicals.
Introduction
Biosensors that utilize electrochemical impedance spectroscopy for signal transduction have been widely studied in the academic literature, with several authoritative reviews.1−3 Impedance biosensing involves application of a small amplitude AC voltage to the sensor electrode and measurement of the in/out-of-phase current response as a function of frequency. Impedance biosensors are fabricated by immobilizing a biorecognition molecule onto a conductive and biocompatible electrode and then detecting the change in the interfacial impedance upon analyte binding.1−3 Biorecognition molecules may include antibodies, receptor proteins, single-stranded DNA, aptamer, or peptides. Although impedance biosensors have been well studied in the academic literature,1−3 commercial applications have not ensued, so technical challenges to usage of impedance biosensors are reviewed here.
For small molecule analysis, the actual structure targeted is approximately the size of the molecule that can be recognized by an antibody. Although small molecules (<1000 Da) alone cannot induce an immune response, they can be antigenic or recognized by an antibody.4 To render a small molecule immunogenic, it must be conjugated to a larger carrier molecule (i.e., a protein) via a functional group.5 The simplest and most convenient source of antibodies is the sera of an immunized animal (usually a mouse, rabbit, sheep, or goat). This sera contains a heterogeneous mixture of antibodies of varying affinity, termed polyclonal antibodies. Polyclonal antibodies can be employed as this mixture, or they can be further purified using standard techniques, such as immunoaffinity chromatography.6
Due to its all-electrical nature, impedance biosensors are simpler than other methods because they lack optical or acoustic components, offering significant advantages for portable applications.7 This makes impedance biosensors ideal for environmental monitoring of species such as endocrine-disrupting chemicals (EDCs),8 diarrhetic shellfish poisoning (DSP) toxins,9,10 polychlorinated biphenyls,11 and milk toxins such as veterinary drug residues and hormones.12,13 New results are presented here for impedance detection of norfluoxetine and BDE-47, demonstrating that even relatively small organic molecules can be detected by this method. Technical challenges that have hindered commercialization of impedance biosensors are briefly reviewed, including possible limitations to small analytes, the complexity of impedance detection, susceptibility to nonspecific adsorption, and stability of biomolecule immobilization. New results are also presented demonstrating antibody regeneration atop degenerate (highly doped) Si for a 30 day trial using 0.2 M KSCN and 10 mM HF in the denaturing solution.
Experimental Method
Electrode and Biosensor Preparation
Glass slides with a 100 nm Au film atop a 5 nm Ti adhesion layer were purchased from Evaporated Metal Films (Ithaca, NY). As-doped (n-type) degenerate silicon (111) wafers with a thickness of 500 μm and a diameter of 50 mm were purchased from University Wafers. 11-Mercaptoundecanoic acid (11-MUA) and 10 undecanoic acid were purchased from Aldrich. N-(3-(Dimethylamino)propyl)-N′-(ethylcarbodiimide hydrochloride) (EDC), potassium dihydrogen phosphate, and dipotassium dihydrogen phosphate were purchased from Sigma. N-hydroxysulfosuccinimide sodium salt (NHSS) was purchased from Pierce biotechnology, and potassium ferri/ferrocyanide was purchased from Acros Organics. Potassium thiocyante was purchased from Fisher Scientific. HF dip was purchased from J.T. Baker. Bovine serum albumin was purchased from Jackson Immnuo Research Laboratories. Peanut protein Arah 1 and its monoclonal antibody were purchased from Indoor Biotechnologies. Norfluoxetine was purchased from Santa Cruz Biotechnology, and its sheep polyclonal antibody was purchased from Abbiotec. BDE-47 was purchased from Chem Services, and rabbit polyclonal antibodies raised to BDE-47 were protein A purified and stored at 4 mg/mL in a phosphate-buffered saline.14
The n-type degenerate Si(111) electrode was embedded within a virgin Teflon mount with an electrode area of 0.19 cm2 and a cell volume of 1 mL, cleaned in ethanol and water, and etched in 10:1 HF dip to remove the native oxide. This was immediately immersed into 10% 10-undecanoic acid in deaerated toluene for 19 h, with exposure to 352 nm ultraviolet light for photoactivated alkene insertion into Si–H bonds, creating a carboxylate-terminated surface. The Au electrode was fixed by an O-ring onto an electrochemical cell constructed from virgin Teflon with an electrode area of 0.19 cm2 and a cell volume of 6 mL. The Au electrode was cleaned with ethanol, dried, and immersed for 17 h into 1 mM 11-MUA and 50 mM phosphate buffer solution (pH 10) to form a carboxylate-terminated self-assembled monolayer (SAM). The carboxylate groups on both Au and Si electrodes were then activated for 1 h in 75 mM EDC and 15 mM NHSS in 50 mM phosphate buffer solution (pH 7.3). The antibody-coated electrodes were created by immersion for 1 h into a solution containing 50 μg/mL antibody and 50 mM PBS at pH 7.3, forming amide bonds to amine groups on the protein surface, and then immersed into 0.1% BSA for 1 h to reduce the nonspecific adsorption.
Experimental Methods
All electrochemical measurements were performed with a three-electrode configuration using a Pt spiral counter electrode and a Ag/AgCl reference electrode. The background test solution contained 50 mM PBS and 5 mM K3Fe(CN)6/K4Fe(CN)6 at pH 7.4, with varying concentrations of the target analyte. Impedance measurements were performed using a Gamry instruments Reference 600 over the frequency range from 0.01 Hz to 15 kHz with an AC probe 120 amplitude of 5 mV. Each impedance spectrum takes about 2.8 min to acquire. The impedance results were obtained at a DC potential of +200 mV vs Ag/AgCl for the gold electrode and at a DC potential that is slightly cathodic to the open circuit potential (OCP) vs Ag/AgCl for Si electrode. In some systems, the impedance response may be unstable if the applied DC potential is too far from the open circuit potential.15
Results and Discussion
Technical Challenges for Impedance Biosensors
Impedance biosensors are widely considered to have some technical limitations that have hindered their commercial introduction, including (1) possible limitations for detection of small analytes, (2) difficulty of simplifying and miniaturizing AC impedance detection, (3) susceptibility to nonspecific adsorption in complex matrices, and (4) stability and reproducibility for biomolecule immobilization onto a conductive electrode material.
Many of these challenges are not specific to impedance biosensors. For example, nonspecific adsorption is a common problem for all biosensor transduction methods.
Impedance Detection of Small Analytes
Endocrine-disrupting compounds (EDCs) are an emerging class of contaminants that can disrupt the endocrine system in animals, resulting in a range of possible health problems, including birth defects and other developmental disorders.8 This has resulted in widespread concern about the presence of EDCs in the environment, particularly in water, wastewater, and agricultural irrigation. However, specific environmental regulations are still unclear, in part due to the large number of EDCs and the lack of detailed understanding of their source, distribution, and physiological effects.16−19 Simple portable biosensors that utilize impedance methods would be invaluable for understanding the source and distribution of EDCs, which are typically organic molecules of low molecular weight.
The detection limits reported for impedance biosensors in the academic literature generally range from 10–15 to 10–6 M.1−3 Impedance detection of small molecules is expected to be difficult due to the exponential increase of the charge transfer resistance (Rct) with tunneling distance (x) through the polymer–protein film to the underlying electrode20
| 1 |
where Rct0 and β are constants, with β typically in the range of 0.05–0.11/nm at Au–thiol interfaces.20 Thus, larger changes in x associated with binding of larger analytes are more easily detected. The fundamental underpinning for eq 1 is the exponential decline in the electron transfer rate (kET) with distance from the electrode surface at distances greater than atomic dimensions (∼0.3 nm)21
| 2 |
where α is a constant for a given redox couple, and λ is the Marcus reorganization energy. The charge transfer resistance (Rct) is inversely related to the rate of electron transfer (kET) through the polymer–protein film. Impedance detection of endocrine-disrupting chemicals has been previously reported by several groups,22−25 and two additional examples are given below for purposes of illustration.
Norfluoxetine is an active metabolite of fluoxetine and has been investigated by Eli Lilly as an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class, with a molecular weight of only 295.30. Figure 1 is a Nyquist plot illustrating impedance detection of norfluoxetine at an Au electrode onto which its sheep polyclonal antibody is immobilized.
Figure 1.
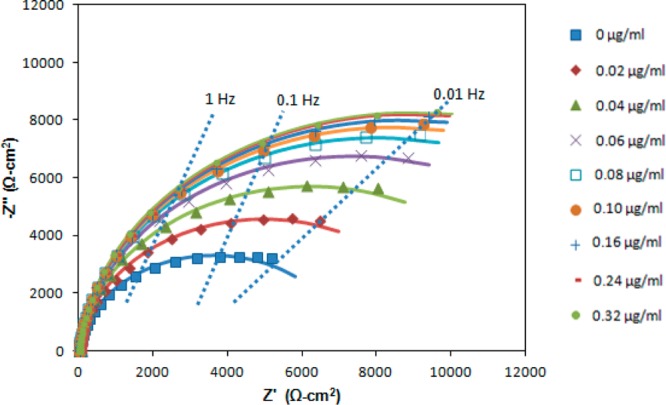
Nyquist plot of the interfacial impedance of the antibody-coated electrode after exposure to norfluoxetine.
The results from Figure 1 are fit to the Randles equivalent circuit shown in Figure 2 but with the differential capacitance (Cd) replaced by a constant phase element (CPE) whose impedance (Z) is26−28
| 3 |
The constant phase element can be viewed as a heuristic method to incorporate the effects of surface heterogeneity both along and through the electrochemical interface.26−28 The other circuit elements in Figure 2 are the charge transfer resistance (Rct) and solution resistance (RS). Although other applications of electrochemical impedance spectroscopy may involve complex equivalent circuits, the Randles equivalent circuit is almost always employed for impedance biosensors. The best-fit equivalent circuit parameters are given in Table 1 as a function of norfluoxetine concentration over the frequency range of 0.05 Hz to 15 kHz. Figure 3 illustrates the increase in the charge transfer resistance (Rct) with increasing concentration of norfluoxetine. Rct approaches a constant value at high norfluoxetine concentration as the antibody film gradually becomes saturated. Rct is typically the most sensitive of the equivalent circuit elements in Table 1 to analyte binding. Thus, many frequencies are insensitive to analyte binding, so impedance biosensors can be operated at only one or a few frequencies that are most sensitive to analyte binding.15 This also reduces the detection time, in some cases allowing for real time impedance biosensing.15
Figure 2.
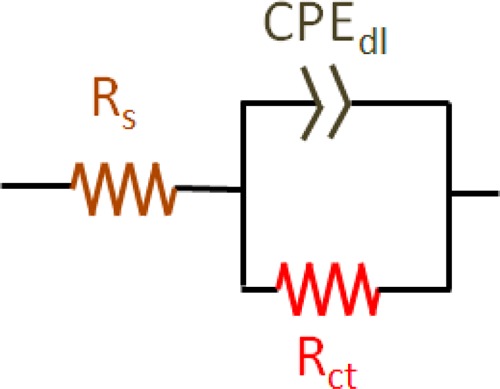
Randles equivalent circuit, with the differential capacitance (Cd) replaced with the constant phase element (CPE).
Table 1. Best-Fit Equivalent Circuit Parameters (Standard Errors) with Increasing Norfluoxetine Concentration.
| norfluoxetine concentration (μg/mL) | 0 | 0.02 | 0.04 | 0.06 | 0.08 | 0.1 | 0.16 | 0.24 | 0.32 |
|---|---|---|---|---|---|---|---|---|---|
| Rs (Ω cm2) | 19.4 (0.2) | 19.9 (0.2) | 21.6 (0.1) | 21.9 (0.1) | 21.8 (0.2) | 21.8 (0.1) | 21.9 (0.1) | 22.5 (0.1) | 23.3 (0.2) |
| CPE-T (μF cm–2 sn-1) | 2.27 (0.01) | 2.25 (0.01) | 2.23 (0.01) | 2.22 (0.01) | 2.21(0.01) | 2.20 (0.01) | 2.20 (0.01) | 2.20 (0.01) | 2.20 (0.01) |
| n | 0.95 (0.01) | 0.95 (0.01) | 0.95 (0.01) | 0.95 (0.01) | 0.95 (0.01) | 0.95 (0.01) | 0.95 (0.01) | 0.95 (0.01) | 0.95 (0.01) |
| Rct (kΩ cm2) | 7.21 (0.14) | 9.91 (0.15) | 12.4 (0.2) | 14.7 (0.2) | 16.1 (0.2) | 16.9 (0.2) | 17.4 (0.2) | 17.8 (0.2) | 18.0 (0.2) |
Figure 3.
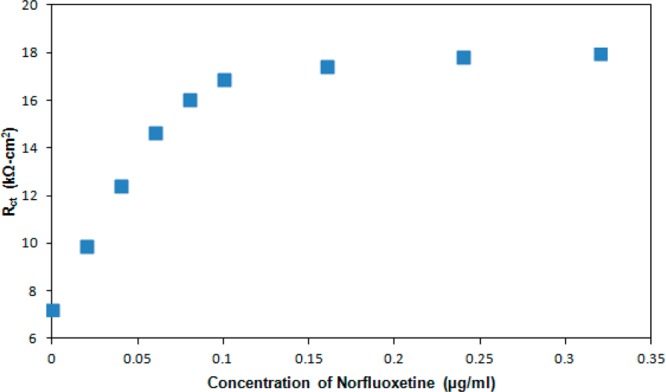
Variation of charge transfer resistance (Rct) with norfluoxetine concentration.
On the basis of the determination of Rct, the linear range extends approximately to 0.07 μg/mL, and the detection limit for norfluoxetine is approximately 8.5 ng/mL or 28 nM. This is comparable to the norfluoxetine detection limits reported from high pressure liquid chromatography (10 ng/mL) and gas chromatography (2 ng/mL) measurements.29,30 This is also well below physiological levels (72–258 ng/mL) reported by the U.S. Food and Drug Administration (FDA) following ingestion of fluoxetine hydrochloride.31
2,2′,4,4′-Tetrabromodiphenyl ether (BDE-47), with a molecular weight of 485.6, provides another demonstration of impedance detection of small organic analytes. Increasing concentrations of polybrominated diphenyl ethers (PBDEs) in the environment, human food chain, and human tissues raise concern about possible neurotoxic effects.32 PBDEs are used as flame retardants in a range of products, such as electronic equipment, furniture, construction materials, and textiles. In most cases, BDE-47 is the predominant congener. Accumulation of BDE-47 is more rapid in infants than adults due to their diet (breast feeding/relatively large intake) and contact with house dust.33 Behavioral studies have demonstrated adverse neurodevelopmental effects on learning and memory after BDE-47 exposure at the critical stage of neonatal brain development.34 As a demonstration, Figure 4 presents the Nyquist plot for the impedance detection of BDE-47 at a Au electrode on which its rabbit polyclonal antibody is immobilized. The best-fit equivalent circuit parameters are given in Table 2 as a function of BDE-47 concentration. Similar to norfluoxetine, the detection limit of BDE-47 was estimated as 1.3 ng/mL (2.7 nM) from the measurement of Rct and sensitivity.
Figure 4.
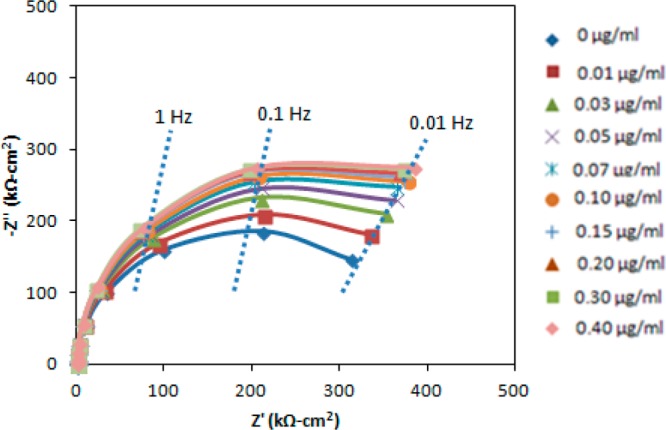
Nyquist plot of the interfacial impedance of the antibody-coated electrode after exposure to BDE-147.
Table 2. Best-Fit Equivalent Circuit Parameters (Standard Errors) Following Exposure to Different BDE-47 Concentrations.
| BDE-47 concentration (μg/mL) | 0 | 0.01 | 0.03 | 0.05 | 0.07 | 0.1 | 0.15 | 0.2 | 0.3 | 0.4 |
|---|---|---|---|---|---|---|---|---|---|---|
|
Rs (Ω cm2) |
57.07 (0.6) |
56.38 (0.6) |
56.32 (0.6) |
55.99 (0.6) |
55.59 (0.5) |
56.73 (0.6) |
56.67 (0.5) |
55.92 (0.6) |
56.67 (0.6) |
57.16 (0.6) |
| CPE-T (μF cm–2 sn-1) |
3.97 (0.04) |
3.97 (0.04) |
3.98 (0.04) |
3.97 (0.04) |
4.02 (0.04) |
3.97 (0.04) |
4.01 (0.04) |
4.05 (0.04) |
4.03 (0.04) |
3.96 (0.04) |
| n | 0.96 (0.01) |
0.96 (0.01) |
0.95 (0.01) |
0.96 (0.01) |
0.96 (0.01) |
0.96 (0.01) |
0.95 (0.01) |
0.96 (0.01) |
0.96 (0.01) |
0.96 (0.01) |
|
Rct (kΩ cm2) |
396 (6) |
449 (8) |
501 (6) |
537 (6) |
568 (7) |
586 (7) |
602 (7) |
613 (6) |
621 (7) |
625 (7) |
If impedance detection of low molecular weight EDCs proves challenging, then improved sensitivity can be attained in a direct impedance immunoassay format by analyte conjugation with electrochemically bright metal and semiconductor nanomaterials, particularly Au nanoparticles.35−37 In addition, enhanced sensitivity can be achieved with alternative formats such as an impedance displacement assay.38,39
The effect of BDE-47 binding at the biosensor interface can also be visualized by cyclic voltammetry of the antibody-coated electrode, as illustrated in Figure 5. As the BDE-47 concentration is increased, the increasing surface coverage of BDE-47 progressively blocks charge transfer to/from [Fe(CN)6]3–/4–, resulting in lower reduction/oxidation peak heights in Figure 5.
Figure 5.
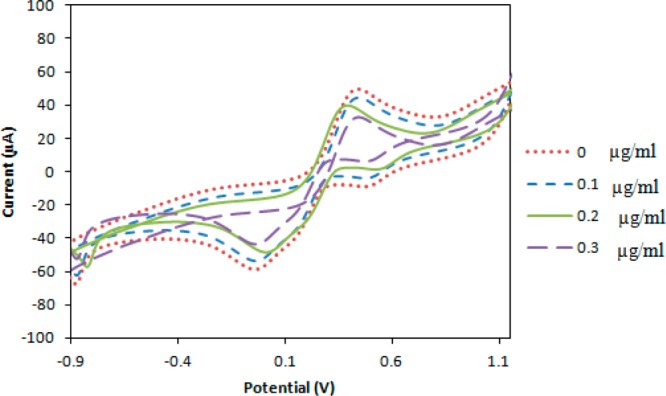
Cyclic voltammograms of antibody-coated electrode exposed to increasing concentrations of BDE-47.
Complexity of Impedance Detection Electronics
Another frequent concern with impedance biosensors is the more complex measurement circuitry relative to amperometry, which has already been miniaturized and commercialized for transdermal glucose biosensors.40,41 However, the research group of Dr. Suh-Moon Park has published extensively on Fourier transform electrochemical impedance spectroscopy (FTEIS) for a wide variety of electroanalytical applications, including electrocatalysis and corrosion.42,43 During FTEIS, impedance spectra are obtained by a relatively simple procedure that involves application of a potential step function, measurement of the current response at the electrochemical interface, differentiation, and fast Fourier transformation (FFT) back into frequency space.42,43 The first reports of FFT analyses of electrochemical systems by this type of probe–response approach actually date to Dr. Donald Smith in 1976.44,45 This approach has been criticized as being unable to obtain high frequency spectra due to limitations on sampling frequency and unable to obtain low frequency spectra due to limitations on experimental duration.46 However, inspection of Figures 1 and 4 reveals that information about analyte binding is contained mainly at intermediate frequencies, so these limitations do not significantly impact the utility of this method for impedance biosensing.
More recently, hardware simplifications for FTEIS have been reported to reduce the cost of biosensor applications.47 For situations where an electrochemical system is not at steady state, then dynamic FFTEIS may allow a more rapid snapshot of an electrochemical system.48,49 Impedance methods for biosensing can also be combined with other transduction methods, such as surface plasmon resonance biosensing.50,51
Susceptibility to Nonspecific Adsorption
The most frequently cited practical concern regarding impedance biosensors is the perception that this method is particularly susceptible to interference arising from nonspecific adsorption. Nonspecific adsorption is without question a common limitation for a wide variety of different biosensor methodologies.52−54 Nonspecific adsorption is typically ascribed to proteins contained in a complex test matrix binding to the sensor interface though an unwanted process not involving biomolecular recognition. Thus, nonspecific adsorption can be studied by control experiments using either complex test matrices or mixtures of different proteins or analytes.
While nonspecific adsorption may cause spurious signals during impedance biosensing, several methods have been employed to mitigate this, including sample dilution,55,56 adsorption of a blocking reagent such as bovine serum albumin (BSA),54 and use of a control electrode at which biomolecular recognition is unlikely.57,58 The utility of sample dilution depends on the particular application and the desired detection limit. When a monoclonal antibody is used for biomolecular recognition, a control electrode can be used with another antibody from the same animal and subtype whose antigen is unlikely to be found in the text matrix of interest. Recently, we reported impedance detection of Listeria monocytogenes in tomato pulp58 and demonstrated that nonspecific adsorption was unmeasurable. Nonspecific adsorption was quantified by comparing the impedance change at the measurement electrode (mouse monoclonal IgG1 antibody to L. monocytogenes) to that at a control electrode (mouse monoclonal IgG1 antibody to GAPDH). This approach depends on the availability of adequate control electrodes whose antigen is not present in the samples of interest and with no cross-reactivity to the analyte of interest. It should be noted that the use of multiple measurement antibodies with different binding epitopes and multiple control antibodies are both relatively straightforward.
Impedance detection of norfluoxetine and BDE-47, which is illustrated in Figures 1 and 4, respectively, has not yet been demonstrated in complex media. However, nonspecific adsorption is sometimes assessed by simply exposing the antibody-coated electrode to solutions of known proteins or to analyte mixtures. Electrodes at which the antibodies to norfluoxetine and BDE-47 have been immobilized were exposed to norfluoxetine, BDE-47, peanut protein Ara h 1, and Cyprinus carpio vitellogenin. In all cases, impedance changes were observed only for the analyte of interest, and none of the interfering species caused a change in the impedance spectrum. This is illustrated by the results of Figures 6A and B, where increasing concentrations of norfluoxetine (BDE-47) do not cause any interference at the BDE-47 (norfluoxetine) antibody-coated electrode, demonstrating both that nonspecific adsorption does not occur and that these antibodies are not cross-reactive. Similar results are obtained upon exposure of these electrodes to peanut protein Ara h 1 and Cyprinus carpio vitellogenin, demonstrating that nonspecific adsorption does not occur in this system, at least for these proteins. For low molecular weight analytes such as endocrine-disrupting chemicals (EDCs), antibody cross-reactivity may sometimes be observed.59,60
Figure 6.
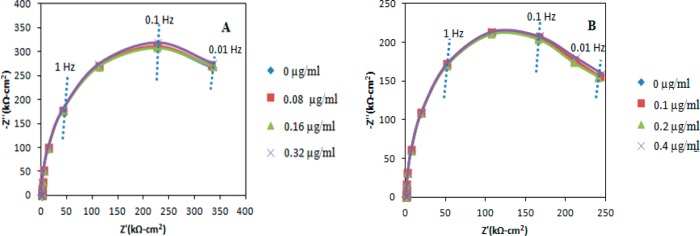
Nyquist plot of the impedance response of (A) the BDE-47 antibody-coated coated electrode after exposure to the Norfluoxetine, and (B) Norfluoxetine antibody-coated electrode after exposure to the BDE-47.
Stability of Biomolecule Immobilization onto a Conductive Electrode Material
For impedance biosensing, biomolecule immobilization onto a conductive and biocompatible electrode material is most commonly accomplished through Au–thiol self-assembly chemistry.61 However, the limited stability of Au–thiol self-assembly chemistry to date limits its application to impedance biosensors.62 Depending on storage conditions, the shelf life is limited to days to weeks. Durable chemistry for biomolecule immobilization is also needed for sensor calibration, which often involves the use of aggressive chemicals for antibody denaturation.
Other substrate materials that have been reported for impedance biosensors include carbon,63,64 Si,65,66 Pt,67,68 Ti,69,70 and ITO.71,72 Recently, degenerate (highly doped) Si was reported as an alternative electrode material for impedance biosensors.73 Degenerate Si behaves as an electrical conductor, albeit a poor one, rather than a semicondutor, preventing formation of a space charge layer during AC interrogation of the sensor interface. Figure 7 illustrates new results demonstrating the ability to regenerate a Si electrode during a 30 day trial period.74 During these experiments, the antibody-coated Si electrode was stored in 50 mM PBS buffer at pH 7.3. This was removed and tested every day for 30 days using the following procedure. The electrode was exposed to peanut protein Ara h 1 at a concentration of 0.04 μg/mL, exposed to 0.2 M KSCN and 10 mM HF to unfold the antibody film and release the analyte, and exposed to 0.1 M BSA and 50 mM PBS buffer to refold the antibody film. The inclusion of HF in the unfolding solution is necessary to dissolve Si oxide that forms during electrode storage. In order to avoid congestion, Figure 7 only illustrates the data taken every fifth day. While the response of the antibody film degrades, degraded, the response within any 1 day is consistent to within 2%. This illustrates the potential for this methodology to be used for storage of antibody-coated degenerate Si electrodes, with calibration on the day they are used. Although the results of Figure 7 have not been demonstrated for endocrine-disrupting chemicals (EDCs), the physical chemistry of different antibodies is quite similar, so this chaotropic agent (0.2 M KSCN and 10 mM HF) should work on other antibodies as well.
Figure 7.
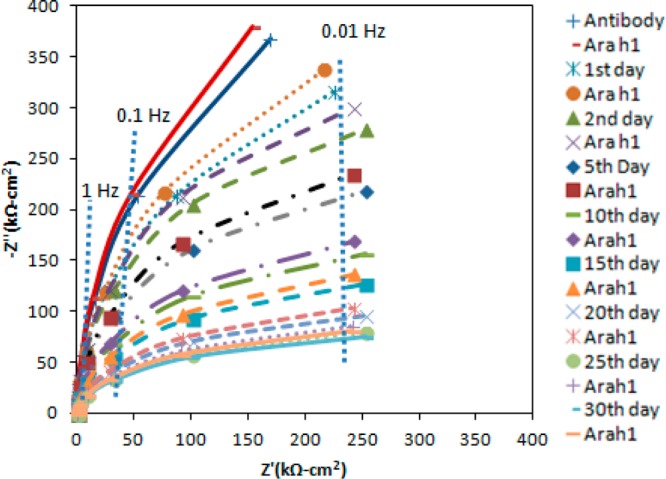
Nyquist plot of the interfacial impedance for the regeneration of Si electrode for 30 days. Test solution contains 50 mM PBS and 5 mM K3Fe(CN)6/K4Fe(CN)6 at pH 7.3.
Conclusions
Detection of two endocrine-disrupting chemicals (EDC), norfluoxetine and BDE-47, is reported here by impedance biosensing, with a detection limit of 8.5 and 1.3 ng/mL for norfluoxetine and BDE-47, respectively. Recent research into possible limitations of impedance biosensors are briefly reviewed, including possible limitations to small analytes, the complexity of impedance detection, susceptibility to nonspecific adsorption, and stability of biomolecule immobilization. New results demonstrating antibody regeneration atop degenerate (highly doped) Si are also reported. Using 0.2 M KSCN and 10 mM HF for antibody regeneration, peanut protein Ara h 1 is detected daily during a 30 day trial.
Acknowledgments
This research has been supported by National Science Foundation Grant #ECCS-1342618 and National Institute of Environmental Health Sciences Superfund Research Program, P42 ES04699.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Katz E.; Willner I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 2009, 1511913–947. [Google Scholar]
- Yang L.; Bashir R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 2008, 262135–150. [DOI] [PubMed] [Google Scholar]
- Prodromidis M. I. Impedimetric immunosensors: A review. Electrochim. Acta 2010, 55144227–4233. [Google Scholar]
- Erlanger B. F. The preparation of antigenic hapten-carrier conjucates: A survey. Methods Enzymol. 1980, 70A85–104. [DOI] [PubMed] [Google Scholar]
- Gooodrow M. H.; Sanborn D. W.; Stoutamire S. J.; Gee; Hammock B. D.. Strategies for Immunoassay Hapten Design. In Immnunoanalysis of Agrochemicals: Emerging Technologies; Nelson J. O., Karu A. E., Wong R. B., Eds.; ACS Symposium Series 586; American Chemical Society: Washington, DC, pp 119–139. [Google Scholar]
- Harlow E.; Lane D.. Antibodies: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, 1988. [Google Scholar]
- Laschi S.; Mascini M. Planar electrochemical sensors for biomedical applications. Med. Eng. Phys. 2006, 2810934–943. [DOI] [PubMed] [Google Scholar]
- Chang H. S.; Choo K. K.; Lee B. W.; Choi S. J. The methods of identification, analysis, and removal of endocrine-disrupting compounds (EDCs) in water. J. Hazard. Mater. 2009, 17211–12. [DOI] [PubMed] [Google Scholar]
- Stewart L. D.; Hess P.; Connolly L.; Elliott C. T. Development and single-laboratory validation of a pseudofunctional biosensor immunoassay for the detection of the okadaic acid group of toxins. Anal. Chem. 2009, 81, 10208–10214. [DOI] [PubMed] [Google Scholar]
- Aune T.; Larsen S.; Aasen J. A. B.; Rehmann N.; Satake M.; Hess P. Relative toxicity of dinophysistoxin-2 (DTX-2) compared with okadaic acid, based on acute intraperitoneal toxicity in mice. Toxicon 2007, 49, 1–7. [DOI] [PubMed] [Google Scholar]
- Tsutsumi T.; Miyoshi N.; Sasaki K.; Maitani T. Biosensor immunoassay for the screening of dioxin-like polychlorinated biphenyls in retail fish. Anal. Chim. Acta 2008, 61, 177–183. [DOI] [PubMed] [Google Scholar]
- Huet A. C.; Charlier C.; Singh G.; Godefroy S. B.; Leivo J.; Vehniainen M.; Nielen M. W. F.; Weigel S.; anDelahaut P. Development of an optical surface plasmon resonance biosensor assay for (fluoro)quinolines in egg, fish and poultry meat. Anal. Chim. Acta 2008, 623, 195–203. [DOI] [PubMed] [Google Scholar]
- Thompson C. S.; Traynor O. M.; Fodey T. L.; Crooks S. R. H. Improved screening method for the detection of a range of nitroimidazoles in various matrices by optical biosensor. Anal. Chim. Acta 2009, 637, 259–264. [DOI] [PubMed] [Google Scholar]
- Ahn K. C.; Gee S. J.; Tsai H. J.; Bennett D.; Nishioka M. G.; Blum A.; Fishman E.; Hammock B. D. Immunoassay for monitoring environmental and human exposure to the polybrominated diphenyl ether BDE 47. Environ. Sci. Technol. 2009, 43207784–7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Luck L. A.; Suni I. I. Immobilization of the glucose-galactose receptor protein onto an Au electrode through a genetically engineered cysteine residue. Electrochem. Solid-State Lett. 2007, 10, J33–J36. [Google Scholar]
- Bolong N.; Ismail A. F.; Salim M. R.; Matsuura T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 2391–3229–246. [Google Scholar]
- Basile T.; Petrella A.; Petrella M.; Boghetich G.; Petruzzelli V.; Colasuonno S.; Petruzzelli D. Review of endocrine-disrupting-chemical removal technologiesin water and wastewater treatment plans: An EU perspective. Ind. Eng. Chem. Res. 2011, 50148389–8401. [Google Scholar]
- Fatta-Kassinos D.; Kalavrouziotis I. K.; Koukoulakis P. H.; Vasquez M. I. The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci. Total Environ. 2011, 409193555–3563. [DOI] [PubMed] [Google Scholar]
- Jardim W. F.; Montagner C. C.; Pescara I. C.; Umbuzeiro G. A.; Di Dea Bergamasco A. M.; Eldridge M. L.; Sodré F. F. An integrated approach to evaluate emerging contaminants in drinking water. Separ. Purif. Technol. 2012, 84, 3–8. [Google Scholar]
- Bradbury C. R.; Zhao J. J.; Fermin D. J. Distance-independent charge-transfer resistance at gold electrodes modified by thiol monolayers and metal nanoparticles. J. Phys. Chem. C 2008, 1122710153–10160. [Google Scholar]
- Heller A. Electrical wiring of redox enzymes. Acc. Chem. Res. 1990, 235128–134. [Google Scholar]
- Yan F.; Sadik O. A. Enzyme-modulated cleavage of dsDNA for supramolecular design of biosensors. Anal. Chem. 2001, 73215272–5280. [DOI] [PubMed] [Google Scholar]
- Rahman M. A.; Shaddiky M. J. A.; Park J. S.; Shim Y. B. An impedimetric immunosensor for the label-free detection of bisphenol A. Biosens. Bioelectron. 2007, 22112464–2470. [DOI] [PubMed] [Google Scholar]
- Xia W.; Li Y. Y.; Chen T.; Wei J.; Lin Y.; Xu S. Q. Electrochemical biosensor for estrogenic substance using lipid bilayers modified by Au nanoparticles. Biosens. Bioelectron. 2010, 25102253–2258. [DOI] [PubMed] [Google Scholar]
- Gutes A.; Lee B. Y.; Carraro C.; Mickelson W.; Lee S. W.; Maboudian R. Impedimetric graphene-based biosensors for the detection of polybrominated diphenyl ethers. Nanoscale 2013, 5136048–6052. [DOI] [PubMed] [Google Scholar]
- Rammelt U.; Reinhard G. On the applicability of a constant phse element (CPE) to the estimation of roughness of solid metal electrodes. Electrochim. Acta 1990, 3561045–1049. [Google Scholar]
- Pajkossy T. Impedance of rough, capacitive electrodes. J. Electroanal. Chem. 1994, 3641–2111–125. [Google Scholar]
- Juttner K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35101501–1508. [Google Scholar]
- Nichols J. H.; Charlson J. R.; Lawson G. M. Automated HPLC assay of fluoxetine and norfluoxetine in serum. Clin. Chem. 1994, 4071312–1316. [PubMed] [Google Scholar]
- Fontanille P.; Jourdil N.; Villier C.; Bessard G. Direct analysis of fluoxetine and norfluoxetine in plasma by gas chtromatography with nitrogen-phosphorus detection. J. Chromatogr. B: Biomed. Sci. Appl. 1997, 6922337–343. [DOI] [PubMed] [Google Scholar]
- Prozac. U.S. Food and Drug Adminstration. http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-35-PROZAC-CLASS-LABELING.htm (accessed June 11, 2004). [Google Scholar]
- Hites R. A. Polybrominated diphenyl ethersin the environment and in people: A meta-analysis of concentrations. Environ. Sci. Technol. 2004, 38, 945–956. [DOI] [PubMed] [Google Scholar]
- Jones-Otazo H. A.; Clarke J. P.; Diamond M. L.; Archbold J. A.; Ferguson G.; Harner T. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate andhuman exposure to PBDEs. Environ. Sci. Technol. 2005, 39, 5121–5130. [DOI] [PubMed] [Google Scholar]
- Eriksson P.; Jackobsson E.; Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment?. Environ. Health Prospect. 2001, 109, 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suni I. I. Impedance methods for electrochemical sensors using nanomaterials. Trends Anal. Chem. 2008, 277604–611. [Google Scholar]
- Xu K.; Huang J. R.; Ye Z. H.; Ying Y. B.; Li Y. B. Recent developments of nanomaterials used in DNA biosensors. Sensors 2009, 9, 5534–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. D.; Ye Y. K.; Liu S. Q. Gold nanoparticle-based signal amplification for biosensing. Anal. Biochem. 2011, 41711–16. [DOI] [PubMed] [Google Scholar]
- Gao Q.; Zhang W. Y.; Guo Y. Y.; Qi H. L.; Zhang C. X. Highly sensitive impedimetric sensing of DNA hybridization based on the target DNA-induced displacement of gold nanoparticles attached to ssDNA probe. Electrochem. Commun. 2011, 134335–337. [Google Scholar]
- Ciani I.; Schulze H.; Corrigan D. K.; Henihan G.; Giraud G.; Terry J. G.; Walton A. J.; Pethig R.; Ghazal P.; Crain J.; Campbell J.; Bachmann T. T.; Mount A. R. Development of immunosensors for direct detection of three wound infection biomarkers at point of care using electrochemical impedance spectroscopy. Biosens. Bioelectron. 2012, 311413–418. [DOI] [PubMed] [Google Scholar]
- Heller A.; Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 10872482–2505. [DOI] [PubMed] [Google Scholar]
- Chen C.; Xie Q.; Yang D. W.; Xiao H. L.; Fu Y. C.; Tan Y. M.; Yao S. Z. Recent advanced in electrochemical glucose biosensors: A reivdew. RSC Adv. 2013, 3, 4473–4491. [Google Scholar]
- Yoo S. K.; Park S. M. An electrochemical impedance measurement technique employing Fourier transform. Anal. Chem. 2000, 7292035–2041. [DOI] [PubMed] [Google Scholar]
- Ko Y. H.; Singh I. B.; Park S. M. A novel method for corrosion reaction analysis by Fourier transform electrochemical impedance spectroscopy: Corrosion of 9Cr-1Mo ferritic steel in 0.0505 M H2SO4. Electroanalysis 2013, 2541035–1043. [Google Scholar]
- Smith D. E. The acquisition of electrochemical response spectra by online fast Fourier transform. Anal. Chem. 1976, 482221A–240A. [Google Scholar]
- Smith D. E. The acquisition of electrochemical response spectra by online fast Fourier transform. Anal. Chem. 1976, 486517A–526A. [Google Scholar]
- Jurczakowski R.; Lasia A. Limitations of the potential step technique to impedance measurements using discrete time Fourier transform. Anal. Chem. 2004, 76175033–5038. [DOI] [PubMed] [Google Scholar]
- Huang X. J.; Chen H. L.; Deng H. Q.; Wang L. S.; Liao S. J.; Tang A. M. A fast and simple electrochemical impedance spectroscopy technique and its application in portable, low-cost instrument for impedimetric biosensing. J. Electroanal. Chem. 2011, 1–2, 158–163. [Google Scholar]
- Slepski P.; Darowicki K.; Andrearczyzk K. Online measurement of cell impedance during charging and discharging process. J. Electroanal. Chem. 2009, 6331121–126. [Google Scholar]
- Krutunow A.; Darowicki K. DEIS evaluation of the relative effective surface area of AISI 304 stainless steel dissolution process in conditions of intergranular corrosion. Electrochim. Acta 2009, 5431034–1041. [Google Scholar]
- Lu J.; Wang W.; Wang S. P.; Shan X. N.; Li J. H.; Tao N. J. Plasmonic-based electrochemical impedance spectroscopy: Application to molecular binding. Anal. Chem. 2011, 841327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Foley K.; Shan X.; Wang S.; Eaton S.; Nagaraj V. J.; Wiktor P.; Patel U.; Tao N. Single cells and intracellular processes studied by a plasmonic-based electrochemical impedance microscopy. Nat. Chem. 2011, 33249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuni E.; Chapman R. G.; Holmlin R. E.; Takayama S.; Whitesides G. M. A survey of structure–property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [DOI] [PubMed] [Google Scholar]
- Bange A.; Halsall H. B.; Heineman W. R. Microfluidic immunosensor systems. Biosens. Bioelectron. 2005, 20, 2488–2503. [DOI] [PubMed] [Google Scholar]
- Shankaran D. R.; Gobi V. K.; Miura N. Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sens. Actuators, B 2007, 121, 158–177. [Google Scholar]
- Gaus K.; Hall E. A. Surface plasmon resonance sensor for heparin measurements in blood plasma. Biosens. Bioelectron. 1998, 13, 1307–1315. [DOI] [PubMed] [Google Scholar]
- Andersson L. I.; Hardenborg E.; Sandberg-Stall M.; Möller K.; Henriksson J.; Bramsby-Sjostrom I.; Olsson L.I.; Abdel-Rahim M. Development of a molecularly imprinted polymer based solid-phase extraction of local anaesthetics from human plasma. Anal. Chim. Acta 2004, 526, 147–154. [Google Scholar]
- Singh R.; Suni I. I. Minimizing non-specific adsorption in protein biosensors that utilize electrochemical impedance spectroscopy. J. Electrochem. Soc. 2010, 157, J334–J337. [Google Scholar]
- Radhakrishnan R.; Jahne M.; Rogers S. W.; Suni I. I. Detection of Listeria monocytogenes by electrochemical impedance spectroscopy. Electroanalysis 2013, 25, 2231–2237. [Google Scholar]
- Butler D.; Gilbault G. G. Disposable amperometric immunosensor for the detection of 17-estradiol using screen-printed electrodes. Sens. Actuat. B 2006, 1132692–699. [Google Scholar]
- Kim S. J.; Gobi K. V.; Harada R.; Shankaran D. R.; Miura N. Miniaturized portable surface plasmon resonance immunosensor applicable for on-site detection of low-molecular-weight analytes. Sens. Actuators, B 2006, 11351349–356. [Google Scholar]
- Love J. C.; Estroff L. A.; Kriebel J. K.; Nuzzo R. G.; Whitesides G. M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [DOI] [PubMed] [Google Scholar]
- Srimsobat L.; Jamison A. C.; Lee T. R. Stability: A key issue for self-assembled monolayers on gold as thin film coatings and nanoparticle protectants. Colloids Surf., B 2011, 390, 1–19. [Google Scholar]
- Brett C. M. A.; Oliveira-Brett A.M.; Serrano S. H. P. EIS study of DNA-modified electrodes. Electrochim. Acta 1999, 44, 4233–4239. [Google Scholar]
- Davis F.; Nabok A. V.; Seamus P. J. Species differentiation by DNA-modified carbon electrodes using AC impedimetric approach. Biosens. Bioelectron. 2005, 20, 1531–1538. [DOI] [PubMed] [Google Scholar]
- Cai W.; Peck J. R.; van der Weide D. W.; Hamers R. J. Direct electrical detection of hybridization at DNA-modified silicon surface. Biosens. Bioelectron. 2004, 19, 1013–1019. [DOI] [PubMed] [Google Scholar]
- Yang W. S.; Butler J. E.; Russell J. N.; Hamers R. J. Direct electrical detection of antibody-antigen binding on diamond and silicon substrates using electrical impedance spectroscopy. Analyst 2007, 132, 296–306. [DOI] [PubMed] [Google Scholar]
- DeSilva M. S.; Zhang Y.; Hesketh P. J.; Maclay G. J.; Gendel S. M.; Stetter J. R. Impedance based sensing of the of the specific binding reaction between Staphylococcus enterotoxin B and its antibody on an ultrathin Pt film. Biosens. Bioelectron. 1995, 10, 675–682. [DOI] [PubMed] [Google Scholar]
- Pak S. C.; Penrose W.; Hesketh P. J. An ultrathin platinum film sensor to measure biomolecular binding. Biosens. Bioelectron. 2001, 16, 371–379. [DOI] [PubMed] [Google Scholar]
- Mantzila A. G.; Prodromidis M. I. Performance of impedimetric biosensors based on anodically formed Ti/TiO2 electrodes. Electroanalysis 2005, 20, 1878–1885. [Google Scholar]
- Mantzila A. G.; Prodromidis M. I. Development and study of anodic Ti/TiO2 electrodes and their potential use as impedimetric immunosensors. Electrochim. Acta 2006, 51, 3537–3542. [Google Scholar]
- Ruan C. M.; Yang L.; Li Y. B. Immunobiosensor chips for detection of Escherichia coli O157:H57 using electrochemical impedance spectroscopy. Anal. Chem. 2002, 74, 4814–4820. [DOI] [PubMed] [Google Scholar]
- Corry B.; Janelle U.; Crawley C. Probing direct binding affinity in electrochemical antibody-based sensors. Anal. Chim. Acta 2003, 496, 103–116. [Google Scholar]
- Huang Y.; Suni I. I. Degenerate Si as an electrode material for electrochemical biosensors. J. Electrochem. Soc. 2008, 155, J350–J354. [Google Scholar]
- Radhakrishnan R.; Suni I. I.. Antibody regeneration during a thirty-day trial on a degenerate Si electrode, manuscript in preparation.


