Abstract
The study of perceptual decision-making offers insight into how the brain uses complex, sometimes ambiguous information to guide actions. Understanding the underlying processes and their neural bases requires that one pair recordings and manipulations of neural activity with rigorous psychophysics. Though this research has been traditionally performed in primates, it seems increasingly promising to pursue it at least partly in mice and rats. However, rigorous psychophysical methods are not yet as developed for these rodents as they are for primates. Here we give a brief overview of the sensory capabilities of rodents and of their cortical areas devoted to sensation and decision. We then review methods of psychophysics, focusing on the technical issues that arise in their implementation in rodents. These methods represent a rich set of challenges and opportunities.
Introduction
“Choices are the hinges of destiny.” – Pythagoras
The choices an organism makes define its existence, and many of those choices are based on sensory input. To understand the underlying circuits and computations, one must train animals to perform an appropriate sensory-guided behavior, measure neural responses during the behavior, and manipulate these responses to influence behavior. The gold standard for this approach is provided by studies performed in primates 1, 2. Increasingly, however, promising advances are obtained with experiments involving rats and mice. Rats and mice had long been considered ideal for probing spatial navigation, learning, memory, and the processing of rewards and punishments. More recently they have become popular for studies of perceptual function and of decision-making based on sensory input.
This new emphasis on rodents is fueled by large survey initiatives and by powerful techniques to identify and manipulate targeted groups of neurons. The Allen Brain Atlas, GENSAT, and the Mouse Brain Architecture Project provide surveys of gene expression in the mouse brain and maps of connections between brain regions. Thanks to a number of emerging technologies, a wide group of researchers -- not just expert molecular biologists -- can target gene expression to specific groups of neurons and monitor and manipulate their activity with high precision 3. These technologies include optogenetics 4–6, 2-photon microscopy 7, and transgenic mouse lines 8, such as those that allow targeting to specified neuronal cell types (e.g., Cre driver lines that target subclasses of inhibitory neurons 9).
Here we review some of the techniques for studying perceptual decisions in rodents. We highlight what are mainly open questions about rodent perceptual abilities and our ability to assess these experimentally. We focus mostly on mice and to some extent on rats, as these are the most common rodent species in neuroscience. Finally, due to our own backgrounds and interests, we perhaps unduly emphasize one brain region, the cerebral cortex, and one sensory modality, vision.
Sensory processing and the rodent cortex
Rodents are fairly close relatives to us, our common ancestors having lived not long before the last common ancestor of all primates 10, 10 million years after our common ancestors with “higher mammals” such as the cat (Fig 1a). Rodents and primates, as other mammals, share fundamental similarities in brain organization, including a basic common plan for the cortex 11 (Fig. 1a,b). This plan includes an assortment of multiple areas devoted to each of the senses (Fig. 1b). For instance, the mouse visual cortex contains at least 10 retinotopic areas 12–14 (Fig. 1c). These areas cannot correspond one-to-one to the tens of visual areas in the primate brain 15, but the principles that govern the processing of visual information along them might turn out to be similar.
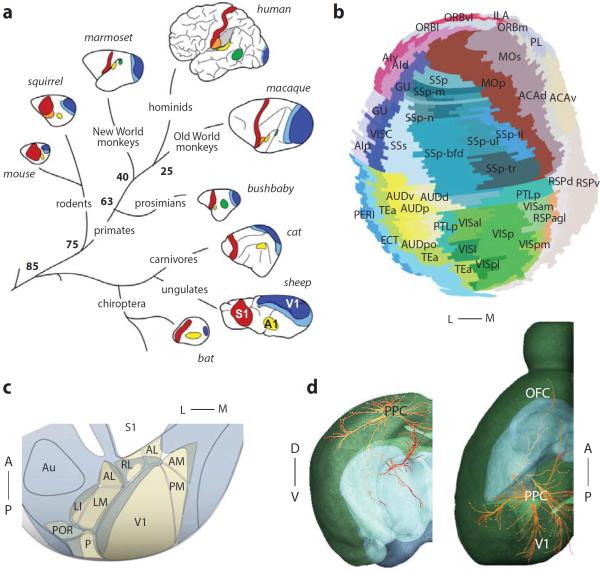
Fig. 1.
Areas and connections in the mouse cortex. a: A schematic of sensory cortical areas in eight mammalian species. Shown are the primary and secondary visual areas (V1 and V2), the primary auditory area (A1) and the primary somatosensory area (S1). Numbers at branch points indicate age of last common ancestors, in million of years 10. Adapted from Ref. 11. b: Flattened map of the mouse cortex. Adapted from Ref. 98. c: The mouse visual cortex contains at least 10 visual areas. Area LM is the region marked V2 in panel a. Modified from Ref. 12. d: The projections of mouse PPC. The brain is shown in transparency (green: cortex; blue: thalamus). Line color indicates projection intensity (red: strongest; yellow: weaker). Dots indicate termination sites. Only ipsilateral projections are shown; callosal fibers terminate in contralateral PPC (not shown). Images from the Allen Brain Atlas, visualized using Brain Explorer 2.
Despite the commonalities between rodent and primate brains, it is often thought that mice and rats rely on a different combination of senses from primates, giving particular weight to olfaction and somatosensation. Mice and rats have a large olfactory bulb and can use it to make delicate and reliable decisions 16, 17. Similarly, they make prodigious use of their whiskers (vibrissae) and devote to them relatively large regions of thalamus and cortex (barreloids and barrels). These regions represent rich opportunities for research into fundamental cortical circuitry 18 and the relationship between neural activity and behavior 19–21.
A notion that might be flawed, however, is that rodent behavior is only weakly influenced by vision. Spatial resolution in rats and mice is ~100 times lower than in primates 22, but vision is the primary sense rodents use to locate themselves in the environment during navigation 23. Indeed, the multiple cortical areas that rodents devote to vision (Fig. 1c) provide the opportunity for complex analyses of a visual scene. These analyses may include the recognition of 3D shape from 2D images 24, 25, which is the hallmark of spatial vision.
The view of mice as minimally visual might stem from the belief that they are nocturnal. Mice, however, are reliably nocturnal only if food is unlimited, as is typical in the laboratory; when food is scarcer, they sleep part of the night to lower body temperature and conserve energy 26. In nature, indeed, laboratory mice can become entirely diurnal 27. Accordingly, the eyes of rats and mice lack the reflective tapetum that would be expected in nocturnal animals. This evidence, contradicts the view that mice are nocturnal, a fact that has consequences for the design and interpretation of behavioral experiments.
Decision-making and the rodent cortex
Ultimately, when the goal is to understand perceptual decision-making, any differences between the details of sensory processing in rodents and primates might turn out to be unimportant. Indeed, many of the open questions in decision-making don't hinge on how the sensory information is initially processed in sensory areas. These questions include: how is the timescale for evidence integration determined and implemented 28? What neural circuits support optimal integration of multisensory information29? How do the salience and value of decisions interact 30? How are priors on sensory evidence incorporated into developing decisions31?
Multiple laboratories are finding it increasingly promising and advantageous to seek some of those answers in rodents. Indeed, in terms of perceptual decisions, the cognitive capabilities of rodents are far from trivial. For instance, rats combine multisensory information in a manner that approximates statistical optimality, just as humans do 29, 32. Moreover, they can accumulate information over time to make decisions based on an abstract quantity 33.
A cortical area that appears to play an intriguing role in decision-making is the Posterior Parietal Cortex (PPC, labeled PTLp in Fig. 1b). First, in rodents as in primates, PPC lies at the heart of a network of sensory areas, receiving inputs from auditory, visual and somatosensory areas 34, 35; it is therefore poised to integrate multisensory inputs. Second, PPC neurons carry signals related to navigation 36–38 and working memory 39. Third, the activity of PPC neurons gradually increases during accumulation of sensory information during decision-making tasks (Hanks, T.D., Duan, C.A., Erlich, J.C., Brunton, B.W. & Brody, C.D. Soc. Neurosci. Abstr. 699.17, 2012). Finally, PPC neurons are active while a mouse holds a decision in mind 40. This persistent activity is key to decision-making and higher brain function 2. An open and intriguing question is the degree to which cortical structures like PPC interact with subcortical pathways. Although the balance of cortical versus subcortical pathways in driving sensory-guided behavior may differ in rodents versus primates, growing evidence suggests a key role for subcortical structures in both species 17, 41, 42.
Among the regions that receive PPC input (Fig. 1d) is frontal cortex, a network of areas involved in cognitive processing. Rodent frontal cortex has been the subject of debate: some have argued, based on histology, that frontal regions are fundamentally different in primate and rodents 43. However, emerging evidence suggests that frontal cortex in rodents, as in primates, is essential for higher cognitive functions including working memory and categorization 44, 45.
Multiple areas in rodent frontal cortex appear to contribute to planning and decision-making. One such area is orbital frontal cortex (ORB, Fig. 1b), which plays a role in decisions made under uncertainty, both in primates 46, 47 and in rodents 16. ORB firing rates correlate with outcome prediction 48, confidence in perceptual decisions 16 and value 49. A second area is agranular cortex 50 (MOs in Fig. 1b), which may contribute to movement planning or preparation 51, 52. A final example is anterior cingulate cortex (ACC; ACAd and ACAv in Fig. 1b). Activity in ACC is maintained during a delayed motor response 53, and is implicated in foraging decisions in both rodents and primates 54, 55. These properties suggest a role in the temporal coordination of actions. Orbitofrontal cortex, in turn, has a multiple connections with the striatum, which are critical for habitual and action-based learning 56, 57. Work in rodents has identified a role for these connections in pathologies such as substance addiction 58.
Relating percepts to brain activity
To understand the neural circuits and computations giving rise to perceptual decisions, one must describe how these decisions depend on sensory input. Such a description should allow inferences about perception and decision based on observations of behavior. Fortunately, there is a mature field that was developed to do just that: psychophysics. Starting with Fechner's studies in 1860, psychophysics has developed standard experimental designs that are routinely used in humans, in primates, and, increasingly, in rodents.
The data resulting from these experiments is typically analyzed with a simple model of perceptual decisions called Signal Detection Theory 59 (SDT, Box 1). One plots the results in terms of “hit” rates and “false alarm” rates, or as full psychometric curves, which relate stimulus strength to the frequency of a specific response. One then interprets these plots to infer how stimulus presence or strength relates to internal “decision variables” and criteria.
Box: Signal Detection Theory.
Signal Detection Theory (SDT) allows one to interpret the results of psychophysical experiments to infer attributes of the subject's processes of perception and decision. Here we briefly describe its main concepts using two widespread psychophysical designs. More complete accounts can be found elsewhere 59, 62, 99.
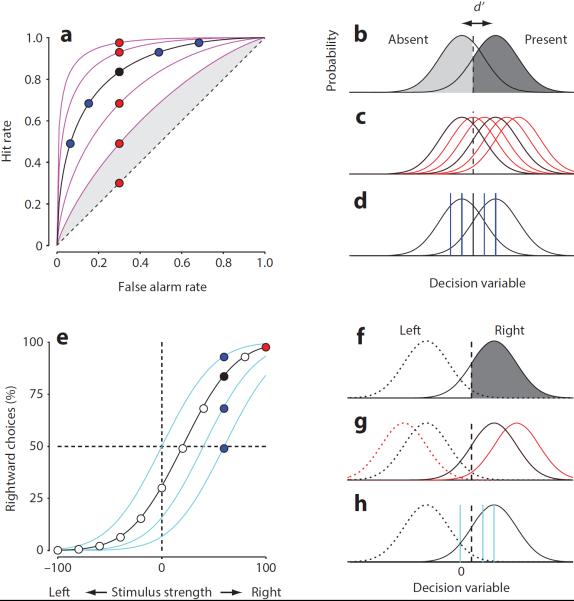
In a “go/no-go” design, the subject indicates whether a stimulus or a stimulus feature is present or absent (Box-Fig. a–d). The subject's performance is summarized by the fraction of “go” choices that are correct (“hits”) and incorrect (“false alarms”, Box-Fig. a). These two numbers, however, reflect not only the perceptual strength of the stimulus, but also the subject's bias to respond. SDT disambiguates these contributions. It postulates that the neural activity in each trial is drawn from one of two distributions depending on whether the stimulus is present or absent (Box-Fig. b). The subject places a threshold between the two, based on bias and other considerations, and decides “go” if the activity is larger than the threshold. The separation between the distributions, called d', measures the perceptual strength of the stimulus. For a given threshold, changing d' (Box-Fig. c) produces a family of points that differ only in hit rate (Box-Fig. a, red). At a given d', instead, changing threshold (Box-Fig. d) produces a family of points that vary along a curve, the “receiver operating characteristic” (ROC, Box-Fig. a, blue). Each pair of hit and false alarm rates constrains a unique ROC curve and yields a unique value of d': the perceptual strength of the stimulus.
In a more refined design, the stimulus is presented in one of two locations (say, left or right), and the subject indicates which one (Box-Fig. e–h). Varying stimulus strength gives rise to a “psychometric curve” relating the proportion of “rightward” choices to stimulus strength (Box-Fig. e). SDT interprets this curve by postulating that neural activity is drawn from one of two distributions depending on whether the stimulus is on the left or on the right (Box-Fig. f). The subject chooses rightward if activity is larger than a threshold. Increasing stimulus strength causes the distributions to become more dissimilar (Box-Fig. g) increasing the number of rightward choices (Box-Fig. e, red). Decreasing stimulus strength would have the opposite effect. Changing the threshold (Box-Fig. h) results in different psychometric curves, reflecting the subject's bias for making rightward decisions (Box-Fig. e, blue).
Box Figure. Analyzing psychophysical data with Signal Detection Theory.
a–d: Analysis of a “go/no-go” experiment. e–h: Analysis of a two-alternative forced choice experiment in which the subject chooses between two stimuli, one presented on the left and one presented on the right.
An increasingly popular alternative to SDT, the Drift Diffusion Model 2 postulates a decision variable that drifts in one direction or the other depending on the accumulating evidence. When the variable crosses a threshold, it triggers a decision. This model captures not only a subject's accuracy, but also the time required to respond.
These psychophysical methods provide a principled and quantitative link between perceptual decisions and brain activity, but only limited means are available to explore this link in humans. Fundamental insights have come from primates: macaques were trained to perform psychophysical tasks based on vision 60 or somatosensation 61, and their performance was related to neural activity measured during the task, typically from single neurons 1, 61, 62.
The success of studies of perceptual decisions in primates rests on a toolbox of standard techniques allowing precise control of stimulus delivery and precise measurement of motor output. For example, multiple researchers adopted the visual task based on random-dot motion 1, 2, 60. Those researchers have developed a shared and informal knowledge of this task's properties and the strategies it elicits. For instance, they know that one must present a balance of easy and difficult stimuli to discourage strategies where the animals selectively discard trials that are too hard.
As research into the neural basis of perceptual decisions progresses, new questions are arising that might be ideally answered in rodents. These questions concern the way neuronal populations work together, the degree to which their activity is causally related to perceptual decisions, and the structure of the underlying neural circuits. Therefore, there is great interest in establishing high-quality techniques to probe perceptual decisions in rodents, ideally to a level that is comparable to that available in primates. Below we review some of the techniques presently available, and some of the questions and opportunities that face researchers wishing to pursue this avenue.
Task design
A key question when designing a psychophysics experiment concerns the basic task structure: how many stimuli will the subjects be given in each trial, and how many kinds of response will they be allowed to give?
One stimulus, one response
The simplest kind of design to probe perceptual decisions involves a “go/no-go” task (sometimes called CS+/CS−, in the language of operant conditioning). In such a task, the subject reports the presence of a stimulus attribute (e.g. it is present or it is vertical) by performing or withholding a single action (e.g. release a bar or lick a spout). This design is often employed in rodent studies 20, 63–66, presumably because rodents learn it quickly.
A key issue with go/no-go tasks is that they are highly vulnerable to changes in the animal's motivation and criterion. For instance, over the course of a session the animal may respond progressively less often, because of decreased motivation to obtain a reward. This could lead to the false conclusion that their ability to detect the stimulus attribute has diminished.
Moreover, suppose one used a go/no-go design to compare a mouse's ability to detect a faint tone, with and without optogenetic stimulation. Suppose further that the stimulation increased the percentage of time the mouse indicated the presence of the tone. It would be premature to conclude that the optogenetic stimulation enhanced the neural representation of the tone: the increased response rate might simply reflect an increased willingness to perform the response action or to report the tone's presence, for instance because of a change in decision criterion.
Some of these difficulties can be overcome by applying SDT (Box 1). In SDT, one measures not only the rate of correct detections, but also the rate of false alarms: trials where the animal reported the stimulus or attribute was present when it wasn't. These rates yield a measure of detectability called d', which is independent of criterion, and thus immune to its changes. This measure is preferable to the difference between correct detections and false alarms (which has been used in some studies, e.g. 67), because the latter can change markedly following simple changes in criterion (Box 1).
However, applying SDT to data obtained in the go/no-go design is not trivial, especially when defining what constitutes a hit or a false alarm (e.g. Ref. 65). For instance, responses should not be counted as hits if they occurred too early to have been influenced by the stimulus. Moreover, some responses may have to be ignored even if they occurred later, as they may represent guesses 65.
One stimulus, two responses
A partial solution to many of these issues is given by tasks where the subject is given two choices of response, depending on whether or not a stimulus attribute is present. This design was originally called “yes/no” 59 but is increasingly called “two-alternative choice” (because of the choice between two responses). It is often applied to rodents, preferably with a symmetrical apparatus where the animal indicates the two choices in similar ways 19, 29, 68–71.
Data from this task design can be analyzed using SDT: if animals are choosing between left and right, for example, correct rightwards choices are categorized as hits and incorrect rightwards choices are categorized as false alarms. Unlike a go/no-go task, however, this design is immune to changes in a subject's willingness to respond, because the subject must report a decision on every trial. The requirement to respond on every trial, likewise, makes it clearer which responses constitute true decisions.. However, overall this task design is still liable to possible changes in decision criterion, especially if these changes happen within a session.
Two stimuli, two responses
A further refinement of the experimental design, used in a few rodent studies 31, 40, 72–74 involves presenting not one but two stimuli (simultaneously or in succession) and asking the subject which of the two has the attribute in question. This design is called “forced choice” or (since the choice is between two stimuli), “two-alternative forced choice”. Its key advantage is its immunity to the presence of an unknown (and potentially changing) decision criterion (Box 1). Indeed, in a task with two stimulus presentations subjects cannot have a bias for the presence or absence of a stimulus attribute: in each choice they must assign presence to a location and absence to the other. Again, animals can have a bias for one response or the other, but this is readily detectable in the data.
These issues underscore the importance of task design when interpreting the complex relationship between an animal's perception and its behavior. Although the experimenter might have the ultimate goal of understanding perceptual decisions, it is critical to control for any of a number of factors that might change an animal's behavior: not only a change in perception, but also changes in arousal or in motor bias or in perceptual criterion.
Choice of sensory environment
Another key question when designing a rodent psychophysics experiment concerns the choice of sensory environment and the apparatus for behavioral report. These choices depend intimately on how one plans to probe behavior and measure or influence neural activity.
In many cases, it may be preferable that the head be kept fixed: for instance, for two-photon imaging or intracellular recordings, or to monitor eye position. Multiple methods have been developed to obtain a behavioral report from head-fixed rodents 75. In a particularly simple one, a spout delivers a fluid reward if the animal licks it at appropriate times (Fig. 2a). This method is naturally suited for go/no-go tasks, but can be adapted to the other designs, e.g. by placing two spouts side by side (Gupta, P., Patel, H., Bhalla, U.S. & Albeanu, D.F. Soc Neurosci Abs 781.04, 2012). Other methods distinguish the actions that report a percept from those that obtain the rewards. For instance, mice can use their paws to operate one of two levers or a trackball 72 in one of two directions (Fig. 2b). The latter setups allow a continuous readout of decisions over time. For instance, they could reveal if an animal were to initially favor one decision but then change its mind (as humans commonly do 76).
Fig 2. Techniques for rodent psychophysics.
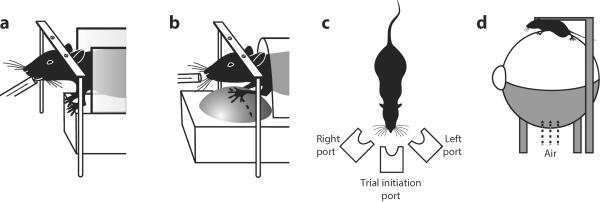
a. A custom apparatus keeps animals still during stimulus presentation. Animals lick a spout to report detection of a stimulus. b. Animals report decisions by moving a “track ball” to the left or right allowing a continuous monitoring of their developing decisions. c. A 3-port apparatus wherein animals freely move first to a center port, where stimuli are presented, and then to a left or right “reward port” where decisions about the stimuli are reported. d. A virtual reality set-up where movement of the animals' legs moves a floating Styrofoam ball that drives updating of a visual display.
In other cases it may be preferable to allow the animal to indicate its decision by walking in an environment. This approach is at the basis of the three-port setup developed at Cold Spring Harbor and adopted by a multitude of studies, both in rats 29, 69–71, 74, 77 and in mice 31 (Fig. 2c). In this setup, the subject initiates a trial by poking its nose into a central port, triggering the stimulus onset. The subject then reports a decision about the stimulus by going to one port or the other. This task can be paired with neural recordings, as long as these recordings don't require head fixation, and may even be feasible for two-photon imaging 78, especially if the head locks automatically every time the animal enters the central port (Kampff, A.R., Xie, K., Agrochao, M., Meister, M. & Ölveczky, B.P., Soc Neurosci Abs, 819.818, 2010; Scott, B.B., Brody, C.D. & Tank, D.W. Soc Neurosci Abs, 198.103, 2011). Allowing free movement can be an advantage when recording from regions involved in movement execution or planning: neurons there may signal a planned movement that cannot be executed if the head is fixed 79.
The advantages of these two approaches might be combined by using a hybrid solution, where a head-fixed rodent walks on a treadmill and the sensory environment is provided by virtual reality simulation (Fig 2d). If the treadmill is a ball floating on a jet of air, it will allow free virtual movement on a surface. The virtual reality simulation, in turn, can consist of a visual display or of tactile feedback. These methods are possible in rats 80, but are more readily usable with mice 23, 40, 81. These virtual reality approaches are proving invaluable to probe neural responses during locomotion and navigation 23, 40, 81, and may also be useful to probe perceptual decisions 40. However, mice require substantial training to learn to control the ball, especially in terms of rotation. Further, the visual feedback in the virtual environment may not entirely make up for the lack of vestibular feedback. This limitation might be ameliorated by providing not only visual feedback but also somatosensory feedback through the whiskers (Sofroniew, N.J., et al. Soc Neurosci Abs, 677.620, 2012).
Overall, the combination of head-fixing and virtual reality environments might strike an appealing balance between multiple demands. First, it facilitates the monitoring of neural activity. Second, it allows one to probe natural behaviors such as locomotion and navigation. Third, it affords fuller control of sensory stimulation, which is extremely desirable in experiments probing sensory decisions.
Training duration
Another key question when designing a rodent psychophysics experiment concerns the expected duration of training. Some tasks can be taught to mice in 2–10 days 82, 83 whereas others require 3–6 weeks 51, 63, 84. Rarely do rodent studies involve training longer than 2 months. These durations are substantially shorter than in typical primate studies. Most likely, these differences reflect the difficulty of the tasks: primates are routinely trained in eye fixation, dexterous manipulation, and context-dependent task switching. Tasks involving complex and non-stationary stimulus response contingencies take particularly long time for subjects to master 85, 86. It is common for a primate to be trained for 6–12 months before reaching proficiency in such tasks.
Intuitively, it seems advantageous to train rodents quickly not only because it saves time and effort but also because it prevents overtraining. The notion of “overtraining”, however, is poorly defined. Surely a brain that has learned even a simple task will have experienced some plasticity. Perhaps longer training schedules elicit more unwanted and uncontrolled plasticity than shorter ones, but it is not clear that this plasticity would complicate interpretation of behavioral and neural data more than the plasticity induced by shorter training schedules.
In fact, there are costs to a short training time. One of these costs is increased variability in behavioral parameters such as reaction times, movement planning times and movement directions. When these parameters fluctuate uncontrollably, the ability to understand variability in neural responses may be severely limited. Another cost of short training times involves error trials, which can be useful in distinguishing candidate explanations for neural activity 87. Errors are difficult to interpret during early stages of training: they may reflect confusion about the stimulus (“was that tone high or low?”) or confusion about the task (“what am I supposed to do for high tones?”). Well-trained animals make few errors of the latter sort (as can be confirmed by near-perfect performance on easy trials where stimuli have high intensity). Therefore, neural activity recorded during an error trial in a well-trained animal can be confidently interpreted as reflecting misperception of the stimulus. In summary, given a choice, it is arguably better to work with animals that are highly trained and give high quality psychophysical data.
These arguments point to another important design consideration: the number of stimulus intensities to be used. In the classic psychophysical approach, one presents stimuli of multiple intensities to obtain a full psychometric function (Fig. 3a). The steepness of this function measures perceptual sensitivity; its horizontal position measures sensory bias, and the levels of its tails is a measure of guessing (lapse rate), which reflects quality of training and degree of engagement. A well-performed psychophysical experiment yields a psychometric curve that spans most of the vertical axis (reflecting good training and high engagement) and is centered in the middle (reflecting minimal bias).
Fig 3. Interpreting psychometric curves.
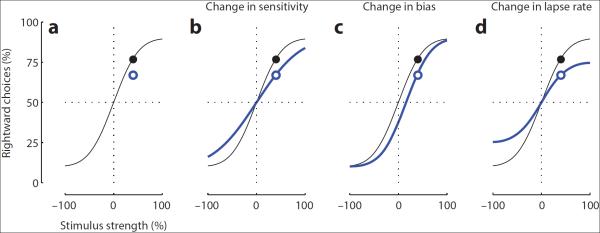
a. Psychometric functions for an experiment involving 2 stimuli and 2 responses, relating the rate of one of the responses (ordinate) to the relative strength of the corresponding stimulus (abscissa). Black dot is one of the underlying measurements. Blue dot is a measurement made in a different experimental condition, where performance is reduced. b–d: Three interpretations of the new measurement (blue dot): it may reflect a change in sensitivity (b), a change in bias (c) or a change in lapse rate (d). These possibilities would be disambiguated if one measured a full psychometric function (blue curves). In all plots, the black curve is the same and so are the two data points.
Obtaining behavioral data at only a single intensity (as is often done in rodent studies) would not allow one to distinguish the effects of sensitivity, bias, and lapse rate. Consider an experiment where one measures behavioral performance only at a single strength, for instance the hypothetical optogenetic experiment we described earlier. Imagine that the optogenetic manipulation hinders performance, and that this effect is evaluated only at a single stimulus intensity and location (Fig. 3a). A tempting interpretation is that the manipulation decreased the subject's sensitivity (Fig. 3b). However, two other changes would lead to identical changes at that stimulus strength: a change in bias (Fig. 3c) and a change in lapse rate (Fig. 3d). In other words, the manipulation may have simply affected the animal's willingness to give one of the responses, or to engage in the task at all (rather than simply guessing). These effects can be distinguished easily using a design with two possible stimuli and with multiple stimulus strengths, so that one can obtain a full psychometric curve.
Researchers, therefore, need to balance the wish for speedy training and testing with an appreciation of the advantages of an animal under strict behavioral control. A middle ground may be achievable: by using multiple stimulus intensities and allowing enough training time to reduce exploratory behavior, researchers have been successful in generating stable, reliable behavior that affords insight into sensory capabilities and decision-making strategies 16, 31, 65, 71.
Reward and punishment
A key factor that constrains the length of training and the duration of each test session is the form of reward. Many studies of rodent behavior use punishments rather than rewards, e.g. by creating negative associations with certain stimuli via electrical shocks 88, 89. Other studies, such as the water-maze 90 and its variations 91 use implied danger: the risk of drowning. These methods may be advantageous as they can lead to very fast learning of simple tasks, but they may create stress, which might prevent learning of more complex tasks. Therefore, following an established tradition with primates, an increasing number of studies of perceptual decisions in rodents use positive reward, and specifically fluid reward17, 21, 29, 31–33, 40, 55, 71.
Fluid reward is usually administered in a regime of water control, where the experimenters control the amount of water that the animals can obtain outside the task. The details of this regime may be very important to obtain reliable behavior and to maintain high levels of training. Up until recently, the regime of water control was typically implemented 5 days out of 7, with unlimited free water available during weekends (e.g. Ref. 31). However, this regime causes substantial variations in motivation across days of the week, with the animals being insufficiently motivated and proficient early in the week. The difference can be as large as a factor of 4, with mice performing ~100 trials/session on Mondays vs. ~400 trials/session on Fridays (M.C., unpublished observations). Typical contemporary training regimes obviate this problem by aiming for more constant water intake across days of the week (e.g. 25 ml/kg/day 65), and by training the animals 7 days a week.
These methods are continuously being refined. For instance, it may be possible to obtain faster learning by contrasting fluid rewards with mildly aversive stimuli such as air puffs 20, or by supplementing the fluid rewards with sugar or other appetitive tastes (as is sometimes done in primates with juice rewards). A final possibility would be to replace all physical rewards with rewards generated internally by the brain, e.g. by stimulating the ventral tegmental nucleus to elicit the release of dopamine 92.
Mice or rats?
Because mice are a more developed species for genetic manipulation than rats, they may become the species of choice for perceptual studies. However, the advantage in genetic tractability that mice currently enjoy may not be permanent. Transgenic rats are beginning to be available and gene expression can be driven by genetically restricted recombinase-drivers 93. In addition, multiple rat disease models suggest an opportunity for translational research: these include retinal degeneration 94, schizophrenia 95 and autism 96.
Further, probing perceptual decisions in rats may offer some advantages: a commonly accepted view is that rats might learn perceptual tasks more quickly, are better suited for complex task designs and can complete a larger trial load in a single session compared to mice. As far as we know, however, this view has not been directly tested. In fact, rats may only appear to be easier to train simply because mice require different training techniques. Once optimal training procedures are developed for mice, their cognitive abilities may prove to rival those of rats. This might be truer for some mouse strains than for others, and future research may seek to identify such strains.
In the extreme scenario, any advantage rats do enjoy might come down to body weight: even in mice, there is a strong correlation between body weight and number of trials performed per day 31. This difference can be very important: decision-making experiments, particularly those with multisensory stimuli 97, frequently require many stimulus conditions, and therefore many trials per session. Pooling data across sessions is often difficult, especially if one concurrently measures neural data with methods that sample different neurons in different sessions.
Conclusions
Mice and rats are becoming an important model system in the study of perceptual decision-making. Though their brains are smaller and less complex than primates, they offer key advantages, particularly in terms of available technologies and bodies of data provided by survey initiatives. As a result, they have the potential to afford major insights both into how decision-making circuits operate in healthy humans and also how they are disrupted in disease.
Research probing perceptual decisions in rodents is at an early stage, but it is already contributing methods that could be useful to understand perceptual decisions in primates and humans. These methods include mathematical descriptions of the factors contributing to decisions over time, including the accumulation of evidence 74, the near-optimal weighting of information from different senses 32, and the suboptimal use of previous decisions when evaluating current evidence 31. As with established models such as SDT or drift diffusion, these mathematical descriptions are essential because they provide variables that can be correlated with the activity of neurons.
However, some questions remain as to how this research will develop. First, will a few sensory environments be adopted as industry standards or will multiple environments be used in parallel? Second, will experimental designs in rodents be able to take advantage of rigorous psychophysical methods, as much as those that were developed for primates? Some aspects of experimental design, such as training time and number of stimulus strengths, affect both the feasibility of an experiment and its interpretability. This is a tradeoff that each experimenter must take into account. Finally, how will rodent studies complement ongoing work in primates? Although some kinds of decision-making task are ported easily from primate to rodent, others may not be transferable. For this and other reasons, including the closer similarity with the human brain, it would be a mistake to consider rodents as a complete replacement for primates in investigations of perceptual decision-making.
These questions notwithstanding, it is likely that rodents will play a major role in the coming years in our efforts to understand the neural circuits and computations underlying perception and decision-making. Much of what a brain does is defined by the decisions it makes about incoming information. Understanding how the rodent brain makes perceptual decisions will provide a window into these fundamental brain operations.
Acknowledgments
We thank Adam Kepecs, Anthony Zador, and Laura Busse for useful comments. MC's research is supported by the European Research Council, by the Wellcome Trust, and by the GlaxoSmithKline / Fight for Sight Chair in Visual Neuroscience. AKC's research is supported by the National Eye Institute (grants EY022979, and EY019072), the National Science Foundation, the McKnight Foundation, the John Merck Fund, the Chapman Foundation, and the Marie Robertson Memorial Fund of Cold Spring Harbor Laboratory.
References
- 1.Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci. 1998;21:227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- 2.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 3.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanziani M, Hausser M. Electrophysiology in the age of light. Nature. 2009;461:930–939. doi: 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- 5.Peron S, Svoboda K. From cudgel to scalpel: toward precise neural control with optogenetics. Nature methods. 2011;8:30–34. doi: 10.1038/nmeth.f.325. [DOI] [PubMed] [Google Scholar]
- 6.Prakash R, et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nature methods. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nature methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 8.Zeng H, Madisen L. Mouse transgenic approaches in optogenetics. Prog Brain Res. 2012;196:193–213. doi: 10.1016/B978-0-444-59426-6.00010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi H, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawkins R. The ancestor's tale : a pilgrimage to the dawn of evolution. Houghton Mifflin; Boston: 2004. [Google Scholar]
- 11.Krubitzer L. The magnificent compromise: cortical field evolution in mammals. Neuron. 2007;56:201–208. doi: 10.1016/j.neuron.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Burkhalter A. Area map of mouse visual cortex. J Comp Neurol. 2007;502:339–357. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- 13.Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RC. Functional specialization of mouse higher visual cortical areas. Neuron. 2011;72:1025–1039. doi: 10.1016/j.neuron.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshel JH, Garrett ME, Nauhaus I, Callaway EM. Functional specialization of seven mouse visual cortical areas. Neuron. 2011;72:1040–1054. doi: 10.1016/j.neuron.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 16.Kepecs A, Uchida N, Zariwala HA, Mainen ZF. Neural correlates, computation and behavioural impact of decision confidence. Nature. 2008;455:227–231. doi: 10.1038/nature07200. [DOI] [PubMed] [Google Scholar]
- 17.Felsen G, Mainen ZF. Midbrain contributions to sensorimotor decision making. J Neurophysiol. 2012;108:135–147. doi: 10.1152/jn.01181.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Huber D, et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor DH, et al. Vibrissa-based object localization in head-fixed mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:1947–1967. doi: 10.1523/JNEUROSCI.3762-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Huberman AD, Niell CM. What can mice tell us about how vision works? Trends Neurosci. 2011;34:464–473. doi: 10.1016/j.tins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G, King JA, Burgess N, O'Keefe J. How vision and movement combine in the hippocampal place code. P Natl Acad Sci USA. 2013;110:378–383. doi: 10.1073/pnas.1215834110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoccolan D, Oertelt N, DiCarlo JJ, Cox DD. A rodent model for the study of invariant visual object recognition. P Natl Acad Sci USA. 2009;106:8748–8753. doi: 10.1073/pnas.0811583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tafazoli S, Di Filippo A, Zoccolan D. Transformation-tolerant object recognition in rats revealed by visual priming. The Journal of Neuroscience. 2012;32:21–34. doi: 10.1523/JNEUROSCI.3932-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hut RA, Pilorz V, Boerema AS, Strijkstra AM, Daan S. Working for food shifts nocturnal mouse activity into the day. PLoS One. 2011;6:e17527. doi: 10.1371/journal.pone.0017527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daan S, et al. Lab mice in the field: unorthodox daily activity and effects of a dysfunctional circadian clock allele. J Biol Rhythms. 2011;26:118–129. doi: 10.1177/0748730410397645. [DOI] [PubMed] [Google Scholar]
- 28.Kiani R, Hanks TD, Shadlen MN. Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment. J Neurosci. 2008;28:3017–3029. doi: 10.1523/JNEUROSCI.4761-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raposo D, Sheppard JP, Schrater PR, Churchland AK. Multisensory decision-making in rats and humans. The Journal of neuroscience. 2012;32:3726–3735. doi: 10.1523/JNEUROSCI.4998-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navalpakkam V, Koch C, Rangel A, Perona P. Optimal reward harvesting in complex perceptual environments. P Natl Acad Sci USA. 2010;107:5232–5237. doi: 10.1073/pnas.0911972107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busse L, et al. The detection of visual contrast in the behaving mouse. The Journal of neuroscience. 2011;31:11351–11361. doi: 10.1523/JNEUROSCI.6689-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheppard JP, Raposo D, Churchland AK. Dynamic weighting of multisensory stimuli shapes decision-making in rodents and humans. Journal of Vision. doi: 10.1167/13.6.4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunton BW, Botvinick MM, Brody CD. Rats and humans can optimally accumulate evidence for decision-making. Science. 2013;340:95–98. doi: 10.1126/science.1233912. [DOI] [PubMed] [Google Scholar]
- 34.Kolb B, Walkey J. Behavioural and anatomical studies of the posterior parietal cortex in the rat. Behav Brain Res. 1987;23:127–145. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- 35.Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- 36.Save E, Poucet B. Role of the parietal cortex in long-term representation of spatial information in the rat. Neurobiol Learn Mem. 2009;91:172–178. doi: 10.1016/j.nlm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Nitz DA. Spaces within spaces: rat parietal cortex neurons register position across three reference frames. Nat Neurosci. 2012 doi: 10.1038/nn.3213. [DOI] [PubMed] [Google Scholar]
- 38.Whitlock JR, Pfuhl G, Dagslott N, Moser MB, Moser EI. Functional split between parietal and entorhinal cortices in the rat. Neuron. 2012;73:789–802. doi: 10.1016/j.neuron.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K. Auditory spatial discriminatory and mnemonic neurons in rat posterior parietal cortex. J Neurophysiol. 1999;82:2503–2517. doi: 10.1152/jn.1999.82.5.2503. [DOI] [PubMed] [Google Scholar]
- 40.Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horwitz GD, Batista AP, Newsome WT. Representation of an abstract perceptual decision in macaque superior colliculus. J Neurophysiol. 2004;91:2281–2296. doi: 10.1152/jn.00872.2003. [DOI] [PubMed] [Google Scholar]
- 42.Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2991–3007. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preuss TM. Do rats have a prefrontal cortex? The Rose-Woosley-Akert program reconsidered. Journal of Cognitive Neuroscience. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49:757–763. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 47.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 48.Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinnamon HM, Galer BS. Head movements elicited by electrical stimulation of the anteromedial cortex of the rat. Physiol Behav. 1984;33:185–190. doi: 10.1016/0031-9384(84)90098-2. [DOI] [PubMed] [Google Scholar]
- 51.Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron. 2011;72:330–343. doi: 10.1016/j.neuron.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sul JH, Jo S, Lee D, Jung MW. Role of rodent secondary motor cortex in value-based action selection. Nat Neurosci. 2011;14:1202–1208. doi: 10.1038/nn.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platt ML, Hayden B. Learning: not just the facts, ma'am, but the counterfactuals as well. PLoS biology. 2011;9:e1001092. doi: 10.1371/journal.pbio.1001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kvitsiani D, Kepecs A. Distinct behavioural correlates and network interactions of two interneuron classes in mouse prefrontal cortex. Nature. doi: 10.1038/nature12176. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 58.Everitt BJ, et al. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann N Y Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- 59.Green DM, Swets JA. Signal detection theory and psychophysics. Wiley; New York: 1966. [Google Scholar]
- 60.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romo R, Salinas E. Touch and go: decision-making mechanisms in somatosensation. Annu Rev Neurosci. 2001;24:107–137. doi: 10.1146/annurev.neuro.24.1.107. [DOI] [PubMed] [Google Scholar]
- 62.Stuttgen MC, Schwarz C, Jakel F. Mapping spikes to sensations. Front Neurosci. 2011;5:125. doi: 10.3389/fnins.2011.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andermann ML, Kerlin AM, Reid RC. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front Cell Neurosci. 2010;4:3. doi: 10.3389/fncel.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smear M, Shusterman R, O'Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature. 2011;479:397–400. doi: 10.1038/nature10521. [DOI] [PubMed] [Google Scholar]
- 65.Histed MH, Carvalho LA, Maunsell JH. Psychophysical measurement of contrast sensitivity in the behaving mouse. J Neurophysiol. 2012;107:758–765. doi: 10.1152/jn.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abraham NM, et al. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Houweling AR, Brecht M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature. 2008;451:65–68. doi: 10.1038/nature06447. [DOI] [PubMed] [Google Scholar]
- 68.Meier P, Reinagel P. Rat performance on visual detection task modeled with divisive normalization and adaptive decision thresholds. Journal of Vision. 2011;11 doi: 10.1167/11.9.1. [DOI] [PubMed] [Google Scholar]
- 69.Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- 70.Jaramillo S, Zador AM. The auditory cortex mediates the perceptual effects of acoustic temporal expectation. Nat Neurosci. 2011;14:246–251. doi: 10.1038/nn.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Znamenskiy P, Zador AM. Striatal projection neurones in auditory cortex drive decisions during auditory discrimination. Nature. doi: 10.1038/nature12077. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanders JI, Kepecs A. Choice ball: a response interface for two-choice psychometric discrimination in head-fixed mice. J Neurophysiol. 2012;108:3416–3423. doi: 10.1152/jn.00669.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lepora NF, et al. Optimal decision-making in mammals: insights from a robot study of rodent texture discrimination. J R Soc Interface. 2012;9:1517–1528. doi: 10.1098/rsif.2011.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunton BW, Botvinick MM, Brody CD. Rats and Humans can Optimally Accumulate Evidence for Decision-making. Science. doi: 10.1126/science.1233912. in press. [DOI] [PubMed] [Google Scholar]
- 75.Schwarz C, et al. The head-fixed behaving rat--procedures and pitfalls. Somatosens Mot Res. 2010;27:131–148. doi: 10.3109/08990220.2010.513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature. 2009;461:263–266. doi: 10.1038/nature08275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci. 2009;12:646–654. doi: 10.1038/nn.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerr JN, Nimmerjahn A. Functional imaging in freely moving animals. Current opinion in neurobiology. 2012;22:45–53. doi: 10.1016/j.conb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- 80.Holscher C, Schnee A, Dahmen H, Setia L, Mallot HA. Rats are able to navigate in virtual environments. The Journal of experimental biology. 2005;208:561–569. doi: 10.1242/jeb.01371. [DOI] [PubMed] [Google Scholar]
- 81.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komiyama T, et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- 83.Lee SH, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kato HK, Chu MW, Isaacson JS, Komiyama T. Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron. 2012;76:962–975. doi: 10.1016/j.neuron.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Merten K, Nieder A. Active encoding of decisions about stimulus absence in primate prefrontal cortex neurons. P Natl Acad Sci USA. 2012;109:6289–6294. doi: 10.1073/pnas.1121084109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bennur S, Gold JI. Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:913–921. doi: 10.1523/JNEUROSCI.4417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Newton JR, Ellsworth C, Miyakawa T, Tonegawa S, Sur M. Acceleration of visually cued conditioned fear through the auditory pathway. Nat Neurosci. 2004;7:968–973. doi: 10.1038/nn1306. [DOI] [PubMed] [Google Scholar]
- 89.Letzkus JJ, et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- 90.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 91.Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res. 2000;40:2201–2209. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- 92.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Witten IB, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bourne MC, Campbell DA, Tansley K. Hereditary Degeneration of the Rat Retina. Br J Ophthalmol. 1938;22:613–623. doi: 10.1136/bjo.22.10.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- 96.Umeda T, et al. Evaluation of Pax6 mutant rat as a model for autism. PLoS One. 2010;5:e15500. doi: 10.1371/journal.pone.0015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fetsch CR, Turner AH, DeAngelis GC, Angelaki DE. Dynamic reweighting of visual and vestibular cues during self-motion perception. J Neurosci. 2009;29:15601–15612. doi: 10.1523/JNEUROSCI.2574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ng L, et al. Methods. Vol. 50. San Diego; Calif: 2010. Surface-based mapping of gene expression and probabilistic expression maps in the mouse cortex; pp. 55–62. [DOI] [PubMed] [Google Scholar]
- 99.Macmillan NA, Creelman CD. Detection theory : a user's guide. Lawrence Erlbaum Associates; Mahwah, N.J.: 2005. [Google Scholar]