Abstract
Background
The PAM50, a gene expression assay to categorize breast tumors into intrinsic subtypes, has not been previously used to examine short- and long-term prognostication in a population-based cohort where treatment patterns and time of initial follow-up vary.
Methods
In a stratified case-cohort design of 1,691 women from the LACE and Pathways breast cancer survivor cohorts, we used PAM50 to categorize tumors into Luminal A, Luminal B, HER2-enriched (E), Basal-like and Normal-like, and to examine risk of early and late recurrence and mortality by Cox proportional hazards regression.
Results
Compared with Luminal A, cumulative risk of recurrence and breast cancer (BC) death was higher for Luminal B, Her2-E and Basal-like tumors at 2, 5 and 10 years. However, hazard ratios (HR) of BC death varied over time (<5 years (early) vs. >5 years (late)) for both Basal-like (HR 6.23 early vs.0.63 late) and HER2-E tumors (HR 2.97 early vs. HR 0.73 late) but not for Luminal B tumors where risk was elevated consistently (HR 2.67 early vs. HR 1.47 late). The contrast between Luminal B, HER2-E and Basal-like compared with Luminal A on early recurrence was stronger when subtype was defined by PAM50 than by immunohistochemistry markers (IHC).
Conclusions
The PAM50 categorized intrinsic subtypes in a manner that more accurately predicts recurrence and survival, especially for luminal tumors, compared with commonly used methods that rely on traditional IHC clinical markers.
Impact
The PAM50 is robust for use in epidemiological studies and should be considered when archived tumor tissues are available.
Keywords: breast cancer, prognosis, cancer survivors, intrinsic subtypes, PAM50
INTRODUCTION
Background
Breast cancer is a heterogeneous disease with respect to molecular alterations, cellular composition, and clinical outcomes(1-4), and this heterogeneity should be considered in the search for risk factors leading to initiation and progression. Gene expression profiling has given us insight into the molecular complexity of breast tumors(3) and improves prognostication. As a result, many different gene expression tests have been developed. For example, the 21-gene OncotypeDx assay (Genome Health Inc, Redwood City, CA) can be used to risk stratify early-stage estrogen receptor (ER) positive breast cancer(5;6), and the 70-gene MammaPrint (Agendia, Huntington Beach, CA) microarray assay has shown prognostic significance in ER-positive and ER-negative, early-stage, node-negative breast cancer(7;8). While these tests appear to accurately predict prognosis or response to chemotherapy, they are applicable only to clinically defined subgroups of breast cancers. There is still controversy over the value that such assays add to clinicopathologic characteristics and the practicing clinician to make informed treatment decisions(9).
To date, however, most research applying gene expression-based assays has been from clinical trial study populations and not breast cancers treated in the community or prospective epidemiological cohorts. Beyond their potential utility for individual care, molecular subtyping may be useful to incorporate in epidemiologic research. Subtyping may enable us to understand underlying factors specific to biological pathways and how behavioral and lifestyle risk factors differ by molecular subgroup.
One of the more recently developed genomic assays, the PAM50, is based on the intrinsic subtypes that have become commonly known as Luminal A (LumA), Luminal B (LumB), HER2-enriched (HER2-E), Basal-like, and Normal-like(3;10;11) and can be applied across all clinical subgroups of breast cancer. The test can be performed on formalin-fixed, paraffin-embedded (FFPE) tissues, and thus is particularly useful for epidemiological studies where fresh tissue is typically not available. Previous studies with the PAM50 have been done to assess “pure” prognosis (i.e., in patients who received no systemic therapy)(3), and in randomized clinical trials to assess subtype response to different chemotherapy regimens(12;13). Furthermore, follow-up time in these studies have been relatively short and did not examine prognostication in the latter years post-diagnosis.
Our goal was to evaluate the performance of the PAM50 in a population-based study in a combined group of breast cancer survivors from two cohort studies where treatment patterns and time of initial follow-up varied among patients. The parent cohorts were diverse in terms of race and ethnicity, and included a broad range of ages at diagnosis and disease severity. Additionally, we were interested in the performance of the PAM50 in predicting early versus late outcomes, and how the performance of the PAM50 in this population differed from subtype classifications utilizing immunohistochemical (IHC)and pathologic markers routinely collected in community settings.
MATERIALS AND METHODS
Study population
Women were breast cancer patients from two population-based cohorts, the LACE and Pathways cohorts. LACE participants were 18-79 years old at breast cancer diagnosis, diagnosed with early stage breast cancer from 1996-2000 (AJCC Stage I with tumor size ≥1 cm, Stage II or Stage IIIA) and at the time of entry into the cohort had completed chemotherapy or radiation therapy, and were within 39 months of diagnosis (median time from diagnosis to enrollment = 23 months, 61% between 12 and 24 months). At the time of enrollment into the LACE cohort, women were required to be free of recurrence. The majority of women were identified from the Kaiser Permanente Northern California (KPNC, 83%)and the University of Utah Cancer Registries (12%). LACE study methods and baseline characteristics of participants have been described(14). The Pathways Study enrolled women diagnosed with invasive breast cancer from 2005–2013 in KPNC with no previous diagnosis of other invasive cancer, and were at least 21 years of age at diagnosis; most women were enrolled within two months of diagnosis (mean time from diagnosis to enrollment = 1.8 months, maximum 7.2 months). Women were identified from daily review of pathology reports. Details of the study methods have been previously described(15). For this study, we included Pathways women diagnosed through 2008. Additional exclusions for this study were invasive tumor < 0.5 cm diameter, bilateral disease, or neoadjuvant therapy. Participants provided informed consent under protocols approved by institutional review boards at KPNC and the University of Utah.
Patient and disease characteristics at the time of diagnosis, including age, disease stage, tumor size, node status, and histologic grade, were abstracted from tumor registry data and medical records review. Ethnicity was based on self-report. Hormone receptor status (ER and PR) and Her2 expression were obtained from medical record review and either the KPNC Cancer Registry (KPNC cases) or Utah Cancer Registry (Utah cases). For all breast surgical specimens at KPNC, ER, PR, and Her2 status were determined by immunohistochemistry (IHC) at the KPNC regional IHC lab; at Utah, by hospital pathology departments or ARUP Laboratories, Inc. (Salt Lake City, UT).
A total of 1,691 women were selected for PAM50 molecular subtyping. We used a stratified case-cohort study design, with strata based on IHC results of ER, PR and Her2. The case-cohort design is an efficient alternative to the nested case-control study design in studies examining multiple outcomes (e.g., recurrence and survival)(16). This design consists of a random sample of a subcohort from the parent cohort with follow-up for all outcomes of interest. In addition, all non-subcohort members of the parent cohort with any outcome of interest during follow-up are selected into the study. Rather than simple random sampling of the subcohort, we selected a stratified random sample given the potential for increasing statistical efficiency in analyses. ER, PR and Her2 status based on IHC (and/or FISH for HER2) defined the strata used for sampling, with an 18% random sample selected among cases of the common breast cancer phenotype that is positive for ER or PR expression and negative for Her2 (and has low risk of recurrence) and a 100% sample of tumors that were ER- and PR- or Her2+. The table below describes details of the sample selection:
| Clinical Subtype | Population N | Sampled N |
Sample Fraction |
Extra Cases N |
Subcohort After Exclusions |
Extra Cases After Exclusions |
Analytic Sample |
| ER+ or PR+, Her2- | 3018 | 500 | 18% | 467 | 435 | 372 | |
| ER+ or PR+, Her2+ | 439 | 439 | 100% | 343 | |||
| ER-, PR-,Her2- | 505 | 505 | 100% | 405 | |||
| ER-, PR-, Her2+ | 177 | 177 | 100% | 136 | |||
| Totals | 4139 | 1621 | 467 | 1319 | 372 | 1691 |
After exclusions, there were 372 non-subcohort members with an event of interest (recurrence, second breast cancer, death) that were included in the study, all of whom were from the non-sampled remaining women with ER+ or PR+, Her2-tumors. The cohort was followed for recurrence and survival through August, 2012. Among all events,370 women had a recurrence and 510 died of any cause, with 274 (53.7%) from breast cancer.
Outcomes were ascertained by self-report and regular linkage to medical records and KPNC mortality files and verified by medical record review. Cause of death was determined from death certificates and supplemented with medical records if necessary. Primary analytic outcomes were: new breast cancer event, defined as a first recurrence/metastasis or new primary breast cancer (hereafter referred to as recurrence)and breast cancer-specific mortality.
Tissue samples
For cohort members who were selected for the analytic sample, we contacted the hospital where surgery for resection of the primary tumor was performed, or the institution’s pathology storage facility, to obtain FFPE tissue blocks from the procedure and corresponding slides. Slides were reviewed by one pathologist (R.E.F.). The pathologist marked an area of representative tumor tissue on a slide.
From the 2088 women selected for the case-cohort (1621 subcohort and 467 extra cases), 150(7.2%) had no suitable tumor block available. For an additional 67(3.2%), we were unable to obtain consent to retrieve the tumor block and in 24 women (1.2%), the Pam 50 assay failed. An additional 155 women were ineligible if the area of invasive tumor was observed to be smaller than 0.5 cm in diameter, if the appearance of the primary tumor tissue in the slides indicated to the pathologist that neoadjuvant therapy had been used before resection or if the PAM50 the case was classified as ineligible. For eligible cases, tissue punches 1 mm in diameter were obtained from the area of the FFPE tissue block corresponding to the marked slide. Two punches per case (or one punch if the primary tumor was less than 0.7 cm in diameter) were placed in plastic tubes labeled with a sample identifying number.
Gene expression assay and IHC categorization
Tissue punches were deparaffinized and digested for RNA extraction as described previously(17). Reverse-transcriptase polymerase chain reaction (RT-PCR) was conducted for the 50 target genes (i.e. PAM50) and 5 control genes(3). Details of RT-PCR methods have been provided elsewhere(17;18). Laboratory personnel were blinded to clinical information and received only a study identifying number to track the sample. Each batch of tissue samples sent to the laboratory for assay work included a mix of clinicopathologic types.
Surrogate subtyping was done using available clinical IHC results for ER and PR, and clinical IHC and/or fluorescence in-situ hybridization (FISH) for Her2. Scoring criteria for each clinical marker followed the standard at the time of diagnosis. Subtyping by IHC using the three standard markers was the foundation for the sampling and weighting in the cohort (Table 1). Recent recommendations for clinicopathologic categorization of breast tumor subtypes incorporate these three markers with the addition of Ki-67 proliferation score or histologic tumor grade(19) and PR status(20) to distinguish low and high risk endocrine positive tumors. Accordingly, for data analysis, we used two different methods of subtype classification, that were both modifications of more commonly used methods. The first classification was an adaptation of the Carey method(21), herein called the 3-marker IHC method adapted from Carey which categorized tumors that were ER+ or PR+ and Her2- as ‘luminal A’, ER+ or PR+ and Her2+ as ‘luminal B’, ER-, PR- and Her2 + as ‘Her2+/ER-’, and ER- and PR- and Her2- as Triple negative (TNBC). The original Carey method incorporated CK5/6 and HER1 which were not available to us. The second classification incorporated grade in place of Ki-67 and defined subtype according to categories adopted by the St. Gallen’s Consensus Conference(19) with a further adaptation by Prat(20). We herein referred to this as the 3-marker IHC plus grade adapted from St. Gallen’s and is defined as follows : low risk endocrine positive or surrogate ‘luminal A’ as ER+ and PR+ and Her2- (well- or moderately-differentiated); high risk endocrine positive or surrogate ‘luminal B’ as ER+ or PR+ and any of PR-, Her2+ or tumor grade of poorly or un-differentiated; ‘Her2-positive, endocrine negative’ as ER-, PR-, and Her2+; and TNBC as ER-, PR-, and Her2-.
Table 1.
Characteristics of the stratified case-cohort study population
| LACE (n=872) Weighted % | Pathways (n=766) Weighted % | Total (n=1638) Weighted % | p-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Age at Breast Cancer Diagnosis | ||||||
|
| ||||||
| < 50 | 22.2 | 22.9 | 22.5 | 0.82 | ||
| 50-64 | 45.2 | 46.5 | 45.9 | |||
| 65+ | 32.6 | 30.7 | 31.5 | |||
|
| ||||||
| Race/Ethnicity | ||||||
|
| ||||||
| White | 81.6 | 66.3 | 73.3 | <0.0001 | ||
| Black/AA | 5.5 | 6.7 | 6.2 | |||
| Hispanic | 6.2 | 10.8 | 8.7 | |||
| Asian/PI | 5.5 | 12.8 | 9.5 | |||
| Other | 1.2 | 3.4 | 2.4 | |||
|
| ||||||
| Education | ||||||
|
| ||||||
| HS or less | 26.1 | 16.4 | 20.8 | <0.0001 | ||
| Some college | 39.6 | 39.4 | 39.5 | |||
| College grad | 15.2 | 25.2 | 20.6 | |||
| Post-college | 19.0 | 19.0 | 19.0 | |||
|
| ||||||
| Family History | ||||||
|
| ||||||
| No | 75.7 | 80.3 | 78.2 | 0.12 | ||
| Yes | 24.3 | 19.7 | 21.8 | |||
|
| ||||||
| Smoking History at Breast Cancer Diagnosis | ||||||
|
| ||||||
| Never | 51.9 | 52.7 | 52.3 | 0.92 | ||
| Past | 41.7 | 41.5 | 41.6 | |||
| Current | 6.4 | 5.8 | 6.0 | |||
|
| ||||||
| Menopausal Status at Breast Cancer Diagnosis | ||||||
|
| ||||||
| Postmenopausal | 66.5 | 71.8 | 69.4 | <0.0001 | ||
| Premenopausal | 19.4 | 28.2 | 24.2 | |||
| Unknown | 14.0 | 0.0 | 6.4 | |||
|
| ||||||
| BMI | ||||||
|
| ||||||
| Under/Normal | 46.8 | 35.3 | 40.5 | 0.001 | ||
| Overweight | 28.8 | 30.7 | 29.8 | |||
| Obese | 24.5 | 34.1 | 29.7 | |||
|
| ||||||
| AJCC Stage | ||||||
|
| ||||||
| I | 45.7 | 51.6 | 48.9 | <0.0001 | ||
| II | 51.5 | 37.0 | 43.6 | |||
| III | 2.8 | 10.4 | 6.9 | |||
| IV | 0.0 | 1.0 | 0.5 | |||
|
| ||||||
| Tumor Size | ||||||
|
| ||||||
| <2cm | 62.3 | 65.2 | 63.9 | 0.001 | ||
| >= 2cm | 37.7 | 31.6 | 34.4 | |||
| Missing | 0.0 | 3.2 | 1.7 | |||
|
| ||||||
| Number of Positive Nodes | ||||||
|
| ||||||
| 0 | 65.2 | 64.1 | 64.6 | 0.001 | ||
| 1-4 | 27.0 | 24.6 | 25.7 | |||
| 5+ | 7.8 | 7.5 | 7.6 | |||
| Missing | 0.0 | 3.8 | 2.1 | |||
|
| ||||||
| Tumor Grade | ||||||
|
| ||||||
| I | 19.3 | 23.5 | 21.6 | 0.11 | ||
| II | 40.1 | 43.5 | 42.0 | |||
| III-IV | 31.5 | 26.8 | 28.9 | |||
| Missing | 9.1 | 6.1 | 7.5 | |||
|
| ||||||
| Breast Cancer Surgery | ||||||
|
| ||||||
| None | 0.0 | 1.3 | 0.7 | <0.0001 | ||
| Lumpectomy | 50.1 | 62.5 | 56.8 | |||
| Mastectomy | 49.9 | 36.3 | 42.5 | |||
|
| ||||||
| Chemotherapy | ||||||
|
| ||||||
| No | 46.5 | 52.6 | 49.8 | 0.08 | ||
| Yes | 53.5 | 47.4 | 50.2 | |||
|
| ||||||
| Radiation | ||||||
|
| ||||||
| No | 38.7 | 66.0 | 53.5 | <0.0001 | ||
| Yes | 61.3 | 34.0 | 46.5 | |||
|
| ||||||
| Hormonal Therapy | ||||||
|
| ||||||
| No | 22.6 | 27.3 | 25.2 | 0.06 | ||
| Yes | 77.4 | 72.7 | 74.8 | |||
|
| ||||||
| Any Comorbidity (Charlson comorbidity index) | ||||||
|
| ||||||
| No | 87.0 | 85.2 | 86.0 | 0.45 | ||
| Yes | 13.0 | 14.8 | 14.0 | |||
|
| ||||||
| ER Status | ||||||
|
| ||||||
| Negative | 18.4 | 16.8 | 17.5 | 0.36 | ||
| Positive | 81.6 | 83.2 | 82.5 | |||
|
| ||||||
| PR Status | ||||||
|
| ||||||
| Negative | 35.4 | 31.6 | 33.3 | 0.20 | ||
| Positive | 64.6 | 68.4 | 66.7 | |||
|
| ||||||
| Her2 Status | ||||||
|
| ||||||
| Negative | 83.4 | 87.0 | 85.3 | 0.02 | ||
| Positive | 16.6 | 13.0 | 14.7 | |||
To determine molecular subtypes from the PAM50 data, we used centroids from an independent RT-qPCR training set(17). For each sample, this algorithm generates a categorical subtype call, a Pearson correlation to each subtype in the training set, and a continuous quantitative score (between 1 and 10) for the expression of ESR1, PGR, ERRB2, and proliferation.
Data analysis
All analyses incorporated sampling weights and the stratified sampling design for unbiased estimation of population parameters and valid estimates of standard errors using the ‘svy’ commands in Stata software, StataCorp, College Station, TX. This method includes estimates of frequency distributions of baseline characteristics. The Cox proportional hazards regression model was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the associations of PAM50 subtype with recurrence and breast cancer-specific (BC) mortality, adjusted for age at diagnosis, race/ethnicity, tumor size, number of positive nodes and grade. Time since diagnosis was the time scale used in the regression models, allowing for delayed entry into the cohort (i.e., left truncation, with time of entry into the study ranging from 0 to 3.2 years post-diagnosis). Point and interval estimation of regression parameters accounted for the case-cohort study design with stratified sampling of the subcohort using the methods of Borgan and Langholz et al, as implemented in SAS subroutines developed by Langholz and Jia(22). We further conducted analyses stratified by PAM50 subtype and IHC categorization as defined by an adaptation of the Carey method(21) and an adaption by Prat(20) of the recommendation from the St. Gallen’s Consensus Conference(17). In addition, we examined heterogeneity in strength of association between PAM50 subtype and risk over time (<5years, 5-10years) via introduction of cross-product terms between time and subtype.
RESULTS
We obtained PAM50 assay results from the tumors of 1,691 women within a combined cohort of 4,139 breast cancer survivors participating in the LACE and Pathways studies. The 53 tumors classified as Normal-like by PAM50 were excluded from the analysis, for a final analytical sample size of 1,638. During a mean follow-up of 7.4 years, we identified 370 recurrences and 274 BC deaths. A total of 243 recurrences and 115 BC deaths occurred in the first 5 years after diagnosis.
LACE and Pathways women were similar in age, family history of breast cancer, smoking history and receipt of chemotherapy. Pathways enrolled a higher percentage of women who were minority (33.7%vs. 18.4%), college-educated (83.6% vs. 73%) and who were obese (BMI > 30; 34.1% vs. 24.5%). Table 1 provides distributions of selected characteristic for the LACE and Pathway cohorts, based on applying the sampling weights for the case-cohort patients included in this analysis. These distributions are very similar to what has been reported on the full cohort(23) (Table 1). With respect to tumor characteristics, Pathways had a higher percentage of women with Stage III tumors (10.4% vs. 2.8%), and had a lower percentage of women who were Her2+ (54% vs. 44.0%). Treatment also varied between Pathways and LACE women: LACE women, diagnosed in 1996–2000, more frequently had a mastectomy (49.9% vs. 36.3%) and more frequently had radiation therapy (61.3% vs. 34.0%) than the more recently diagnosed (2005–2008) Pathways women.
BC mortality in the cohort differed markedly by PAM50 subtype. Among women with Luminal A tumors, the cumulative probability of dying from breast cancer increased from <1% at 2 years to 7.1% at 10 years but remained considerably lower than all other subtypes at every time point examined. Risk of dying from breast cancer was highest for Basal-like tumors, most markedly at 2 and 5 years. Luminal B and HER2-enriched tumors were similar at 2 and 5 years, but Luminal B had a worse cumulative mortality after 10 years. At 10 years, cumulative risk of BC death was highest for those with Basal-like (17.0%) and Luminal B (16.2%) subtypes (Figure 1).
Figure 1.
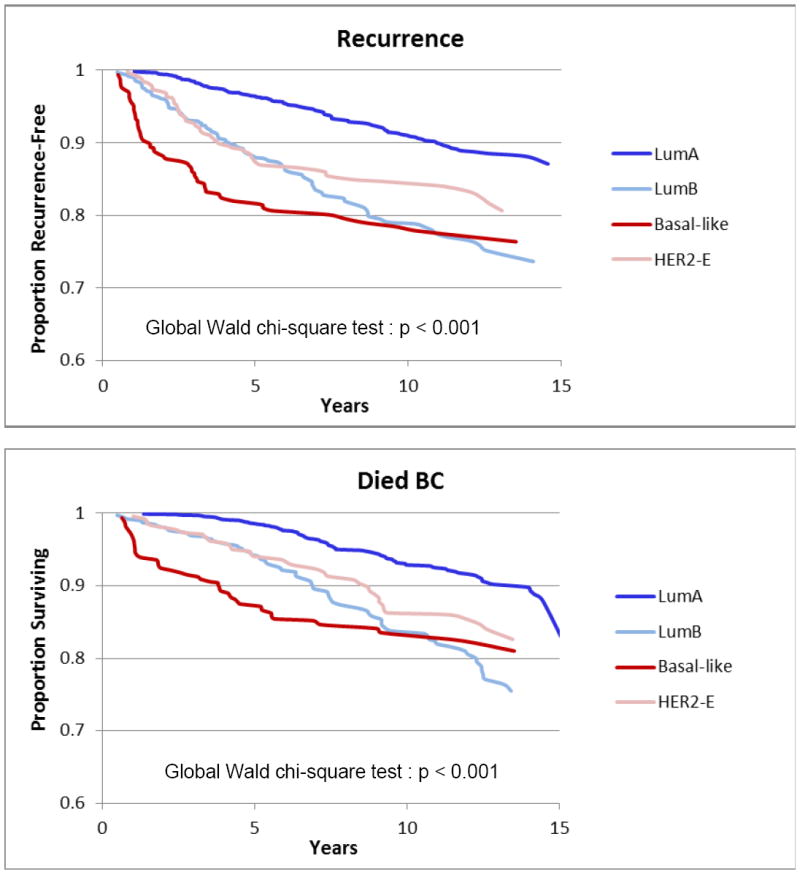
Kaplan-Meier Estimates for Breast Cancer Survival and Recurrence, by PAM50 Subtype
We also examined the difference in survival from Basal-like tumors by time of recruitment into the cohort (range: 0.3 to 3.1 years) to determine the impact of delayed follow-up commonly observed in epidemiological cohorts. Figure 2 demonstrates the difference in cumulative hazard for Basal-like and Luminal B tumors in Pathways women who entered the cohort close to diagnosis (average 2 months post-diagnosis) versus LACE participants who entered the cohort after completion of surgery and chemotherapy (average 22 months post-diagnosis). For the LACE women who needed to survive this 22 month period on average and be recurrence-free at the time of study follow-up, the subsequent risk of dying of breast cancer among women with Basal-like tumors appeared similar to risk of women with Luminal A tumors, whereas Pathways women who only needed to survive a couple of months and not be free of recurrence and had basal-like tumors had a significantly elevated mortality risk compared with those with Luminal A. For Luminal B tumors, risk was slightly higher in Pathways than in LACE compared to Luminal A but risk from the LACE and Pathways cohorts appeared to converge at approximately 5 years.
Figure 2.
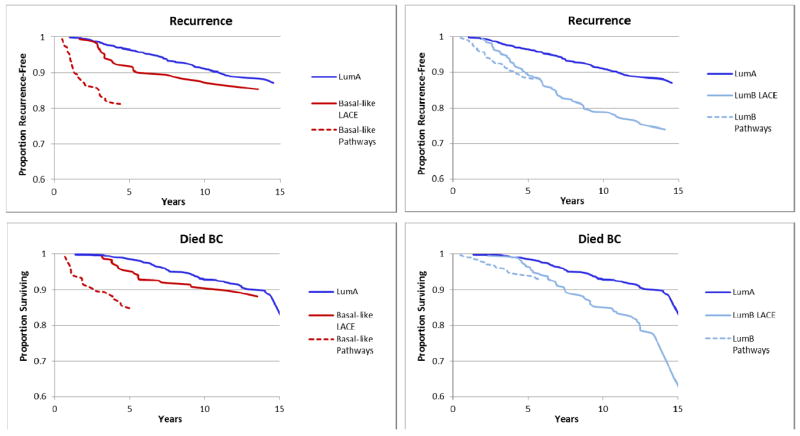
Kaplan-Meier Estimates for Breast Cancer Survival and Recurrence, by Early Entry (Pathways Study) vs. Late Entry (LACE Study) for Basal-like and Luminal B Tumors compared with Luminal A Tumors
Table 2 shows the examination of PAM50 subtypes on risk of recurrence adjusted for age, race/ethnicity, tumor size, number of positive nodes and grade. Women who were classified by PAM50 as having Luminal B (HR 1.94, 95% CI 1.36, 2.77), HER2-enriched (HR 1.63 95% CI 1.10, 2.41) or Basal-like (HR 2.10 95% CI 1.37, 3.22) subtypes all had a significantly greater risk of recurrence compared with those who were classified as Luminal A when both early and late recurrence events were considered together. Women who were HER2-enriched and treated with Herceptin had fewer recurrences and better survival than those not treated with Herceptin when both were compared to Luminal A (data not shown). When the risks for only early recurrences (relapse in the first 5 years after diagnosis) were considered, HRs for each subtype compared with Luminal A were of considerably higher magnitude than HRs for events occurring at all timepoints combined. The risk of early recurrence for Basal-like (HR 3.98, 95% CI 2.47, 6.42)was almost twice as high as for all timepoints combined, and for HER2-enriched (HR 2.83, 95% CI 1.83, 4.38) approximately 75% higher. The risk for Luminal B for early events (HR 2.55 95% CI 1.68, 3.88) was similar to risk for all timepoints, indicating that risk remained relatively constant over time.
Table 2.
Risk of Recurrence by PAM50 Intrinsic Subtype
| All Recurrences n=370 | Early Recurrences (<5 years) n=243 | Late Recurrences (5-10 years) n=127 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| PAM50 Subtype | N Events | HR | Lower 95 CI | Upper 95 CI | N Events | HR | Lower 95 CI | Upper 95 CI | N Events | HR | Lower 95 CI | Upper 95 CI |
| Luminal A | 127 | ref | 61 | ref | 66 | ref | ||||||
| Luminal B | 120 | 1.94 | 1.36 | 2.77 | 82 | 2.55 | 1.68 | 3.88 | 38 | 1.34 | 0.79 | 2.28 |
| Luminal B PATHWAYS | 53 | 1.54 | 0.99 | 2.41 | 52 | 2.39 | 1.48 | 3.86 | 1 | 0.14 | 0.02 | 1.08 |
| Luminal B LACE | 67 | 2.42 | 1.54 | 3.82 | 30 | 2.93 | 1.67 | 5.14 | 37 | 1.73 | 0.99 | 3.04 |
| HER2-E | 67 | 1.63 | 1.10 | 2.41 | 54 | 2.83 | 1.83 | 4.38 | 13 | 0.55 | 0.28 | 1.09 |
| Basal-like | 56 | 2.10 | 1.37 | 3.22 | 46 | 3.98 | 2.47 | 6.42 | 10 | 0.59 | 0.28 | 1.23 |
| Basal-like PATHWAYS | 33 | 3.83 | 2.33 | 6.28 | 33 | 5.44 | 3.23 | 9.15 | 0 | |||
| Basal-like LACE | 23 | 1.29 | 0.75 | 2.23 | 13 | 2.41 | 1.23 | 4.75 | 10 | 0.63 | 0.30 | 1.33 |
Adjusted for age, race/ethnicity, tumor size, number of positive nodes and grade
Table 3 shows the risk of BC death by PAM50 subtype adjusted for race/ethnicity, tumor size, number of positive nodes and grade. Risk of BC death for women with Basal-like tumors varied most by time. For events occurring in the first 5 years, the risk of BC death is more than 6 times higher than that of Luminal A (HR 6.23 95% CI 3.31, 11.73). However, once a woman survives to 5 years without an event, those with Basal-like tumors were no longer at increased risk for BC death (HR 0.61, 95% CI 0.30, 1.23) compared with Luminal A subtype. The risk for HER2-enriched subtype compared with Luminal A also varied by time of BC death, but differences in HRs for risk of BC death (HR 2.97 early vs. HR 0.73 late) were not as pronounced as for Basal-like tumors. Differences by time are least pronounced in Luminal B tumors (HR 2.67 early vs. HR 1.47 late); risk of BC death was only slightly lower in events occurring > 5 years compared to events <5 years.
Table 3.
Risk of Breast Cancer Death by PAM50 Intrinsic Subtype
| All BC Deaths n=274 | Early BC Deaths (<5 years) n=115 | Late BC Deaths (5-10 years) n=154 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| PAM50 Subtype | N Events | HR | Lower 95 CI | Upper 95 CI | N Events | HR | Lower 95 CI | Upper 95 CI | N Events | HR | Lower 95 CI | Upper 95 CI |
| Luminal A | 96 | ref | 23 | ref | 73 | Ref | ||||||
| Luminal B | 86 | 1.77 | 1.16 | 2.70 | 35 | 2.67 | 1.46 | 4.88 | 49 | 1.47 | 0.87 | 2.48 |
| Luminal B PATHWAYS | 29 | 1.42 | 0.81 | 2.47 | 26 | 3.01 | 1.55 | 5.85 | 3 | 0.43 | 0.12 | 1.49 |
| Luminal B LACE | 57 | 2.03 | 1.23 | 3.35 | 9 | 2.03 | 0.86 | 4.81 | 48 | 1.74 | 1.01 | 3.01 |
| HER2-E | 46 | 1.27 | 0.79 | 2.02 | 24 | 2.97 | 1.58 | 5.59 | 22 | 0.73 | 0.40 | 1.33 |
| Basal-like | 46 | 1.84 | 1.12 | 3.01 | 33 | 6.23 | 3.31 | 11.73 | 13 | 0.61 | 0.30 | 1.23 |
| Basal-like PATHWAYS | 25 | 4.74 | 2.64 | 8.51 | 24 | 8.60 | 4.38 | 16.88 | 1 | 0.86 | 0.11 | 6.61 |
| Basal-like LACE | 21 | 1.05 | 0.58 | 1.93 | 9 | 3.63 | 1.54 | 8.56 | 12 | 0.59 | 0.29 | 1.22 |
Adjusted for age, race/ethnicity, tumor size, number of positive nodes and grade
To exemplify how results may differ depending on methods used to classify tumors into subtypes, we used the risk of early recurrence as the outcome (see Table 4) to present differences in risk of early recurrence by two subtype classification methods defined by IHC and IHC and grade compared with PAM50. For each classification method the non-luminal subtype was compared to the luminal A subtype which served as the reference group.
Table 4.
Early Recurrences: Comparison of PAM50 and IHC Tumor Subtype Classifications
| Early Recurrences (n=243)
|
||||
|---|---|---|---|---|
| N Events | HR | Lower 95 CI | Upper 95 CI | |
|
PAM50 Subtype*
| ||||
| Luminal A | 61 | ref | ||
| Luminal B | 82 | 2.55 | 1.68 | 3.88 |
| HER2-E | 54 | 2.83 | 1.83 | 4.38 |
| Basal | 46 | 3.98 | 2.47 | 6.42 |
|
| ||||
|
Carey Subtype defined by IHC*
| ||||
| “luminal A” surrogate | 142 | ref | ||
| “luminal B” surrogate | 31 | 1.19 | 0.79 | 1.81 |
| Her2+/ER- | 16 | 1.37 | 0.78 | 2.38 |
| TNBC | 54 | 2.13 | 1.46 | 3.11 |
|
| ||||
|
Adapted by Prat from St. Gallens Consensus conference: Subtype defined by IHC**
| ||||
| “luminal A” surrogate | 69 | ref | ||
| “luminal B” surrogate | 104 | 1.77 | 1.22 | 2.56 |
| Her2+/ER- | 16 | 2.05 | 1.16 | 3.62 |
| TNBC | 54 | 3.25 | 2.22 | 4.77 |
Adjusted for age, race/ethnicity, tumor size, number of positive nodes and grade
Adjusted for age, race/ethnicity, tumor size, and number of positive nodes
Risk associated for Luminal B subtype (PAM50) or IHC proxy for luminal B varied by method used. When defined by PAM50 (HR 2.55, 95% CI 1.68, 3.88), risk of early recurrence was statistically significant compared with Luminal A and was approximately double the risk defined by the 3-marker IHC adaptation of the Carey method (HR 1.19 95% CI 0.79, 1.81)(21), but estimates were closer to, but still higher than, the 3-marker IHC plus grade method adapted from the St.Gallen’s Consensus Conference (HR 1.77, 95% CI 1.22, 2.56)(20). For both HER2-enriched and Basal-like tumor subtypes, risk of an early recurrence was again higher when defined by PAM50 than when defined by the 3-marker IHC adaptation of the Carey method and closer to, but still higher than, the 3-marker IHC plus grade adaptation of the St. Gallen’s method. Triple negatives defined by the 3-marker IHC adaptation of the Carey method had slightly more than half the risk of early recurrence (HR 2.13 95% CI 1.46, 3.11) than PAM50 Basal-like tumors (HR 4.07 95% CI 2.53, 6.56).
DISCUSSION
This study is innovative in using PAM50, a gene expression assay, rather than IHC, to investigate the prognostication of intrinsic subtypes in a population-based epidemiologic cohort of breast cancer survivors. The cohort represents a heterogeneous group in which cases vary by ER, PR, and Her2 status, pathological characteristics, adjuvant therapy, and initiation and length of follow-up. We demonstrated that when using PAM50 to characterize tumors, women with Luminal A tumors had significantly lower risk of recurrence and BC death than women with more aggressive tumor subtypes (Luminal B, HER2-enriched and Basal-like) and higher risks for poor outcomes. We also demonstrated that the PAM50 when compared to the routinely collected clinical IHC markers (ER, PR and Her2) and tumor grade to categorize women, appeared better able to distinguish risk groups within luminal subtypes, specifically those with the probability of the lower risk of recurrence and BC death (Luminal A) from those with higher risk (Luminal B). This is consistent with improved risk prediction observed for PAM50 in clinical study populations(12;24).
In a study of 786 women who were ER-positive by IHC, Nielsen et al. demonstrated that when subtyped subsequently by PAM50, 9% were reassigned to non-Luminal subtypes(24) and women reassigned to either Luminal B, or the non-Luminal subtypes (HER2-enriched and Basal-like), had significantly worse prognosis than women who remained in the Luminal A category. Similarly, in another study of 476 node positive premenopausal women diagnosed between 1989 and 1993 and randomized to receive either adjuvant CEF or CMF(12), patients with HER2-enriched, Basal-like and Luminal B subtypes by PAM50 all had significantly higher risk of a poor clinical outcome compared with women with Luminal A tumors, regardless of treatment arm.
Our study was able to demonstrate that PAM50 was most useful in risk prediction for the early period post-diagnosis (0-5 years) and less useful in the later period post-diagnosis (5-10 years). We demonstrated elevated risk of all subtypes compared with Luminal A in the first 5 years, with the Basal-like tumors conferring the highest magnitude of risk and higher than other subtypes. Additionally, after 5 years, risks for HER2-enriched and Basal-like subtypes compared with Luminal A were no longer increased, and only risk for Luminal B remained significantly increased.
Using estimates from Adjuvant! Online to determine disease-specific survival (similar to our risk of death from breast cancer), Nielson et al.(24) reported risks stratified by early risk (0-5 years post-diagnosis) and late risk (5-10 years post-diagnosis) by intrinsic subtype. They found increased risks of similar magnitude to our findings. Women with HER2-enriched (RR 3.65) and Luminal B (RR 1.99) had increased risks of disease-specific survival compared with Luminal A, and Basal-like tumors had the largest increased risk (RR 17.71), which was similar to our findings (RR 8.60 for those entering the cohort closer to diagnosis). They also reported that the risk of Luminal B compared with Luminal A remained significantly elevated in the 5-10 year period and only slightly decreased from risk estimates of 0-5 years, while the significantly increased risk observed for HER2-enriched in the early post-diagnosis period was no longer increased in the 5-10 year period post-diagnosis. This is also consistent with data from another study from Bianchini et al.(25) of over 1500 ER positive women which demonstrated that those tumors with high proliferation as well as high estrogen gene expression were those who were at highest risk for late relapses after 5 years [HR 3.86; p = 0.007] when compared to those with high proliferation and low estrogen expression
Other studies have also found that subtypes from PAM50 appear better able to predict those with poorer outcomes compared with using IHC markers (ER, PR and Her2) and tumor grade to categorize women(3;17). In a study by Parker et al(3) where they examined the risk of relapse models using PAM50 and compared them to models using pathologic stage, grade, and routine IHC biomarker status (ER and Her2), there was clear improvement in prediction with subtype relative to the model employing clinical variables only. Furthermore, a combination of clinical variables and subtype was also a significant improvement over either individual predictor variables or subtype alone. However, information on grade did not significantly improve risk of relapse in the combined model, indicating that the prognostic value of grade had been superseded by information provided by the intrinsic subtype.
Lastly of relevance in epidemiological studies where all follow-up may not start at diagnosis and the PAM50 is used, we found that the distribution of subtypes may be biased towards less aggressive tumor subtypes because women with tumors that recurred early or died from breast cancer are likely excluded from the study sample. This “survivor bias” impacts the prognostic value of the PAM50 since tumors that categorized into the more aggressive subtypes of HER2-enriched and Basal-like are likely to be comprised of the least aggressive tumors within that subtype.
In conclusion, the PAM50 had excellent prognostic value in a population-based sample where treatment, demographic, and clinicopathologic characteristics are not uniform, where a portion of the most aggressive tumors resulting in early recurrence/death were likely to be excluded because of delayed follow-up and where treatment with Herceptin varied because some women were diagnosed before it became available. Our results suggest the utility of this assay is robust for defining molecular tumor subtypes in this situation. While a recent task force(9) on use of molecular subtypes for clinical practice concluded that there is not adequate evidence to use PAM50 subtypes to make treatment decisions, the PAM50 appears well-suited for incorporation into population-based epidemiological studies of breast cancer survivorship. In particular, using the PAM50 should be considered in such studies when archived (FFPE)tumor samples are available to help examine if risk factors for breast cancer survival vary by tumor subtype. For epidemiological studies, the test appears to categorize intrinsic subtypes in a manner that more accurately predicts survival compared with commonly used methods that rely on traditional IHC clinical markers.
Acknowledgments
Financial Support: Supported by U.S. National Institutes of Health awards R01CA129059 (B.J. Caan, PI) and R01CA105274 (L.H. Kushi, PI). Additional support from Bioinformatics and Biostatistics core resources of the Huntsman Cancer Institute, P30CA042014. The Utah Cancer Registry is funded by Contract No. HHSN261201000026C from the National Cancer Institute’s SEER Program with additional support from the Utah State Department of Health and the University of Utah. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Potential conflict of interest: PSB has an interest in Bioclassifier LLC. The other authors declare that they have no competing interests.
References
- 1.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis P. WHO Classification of Tumours. Pathology and Genetics of Tumours of Breast and Female Genital Organs Tumours. 2003 [Google Scholar]
- 3.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6:718–30. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 6.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 7.van’t Veer LJ, Paik S, Hayes DF. Gene expression profiling of breast cancer: a new tumor marker. J Clin Oncol. 2005;23:1631–5. doi: 10.1200/JCO.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.van ’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 9.Azim HA, Jr, Michiels S, Zagouri F, Delaloge S, Filipits M, Namer M, et al. Utility of prognostic genomic tests in breast cancer practice: The IMPAKT 2012 Working Group Consensus Statement. Ann Oncol. 2013;24:647–54. doi: 10.1093/annonc/mds645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perou CM, Sorlie T, Eisen MB, van de RM, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 11.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheang MC, Voduc KD, Tu D, Jiang S, Leung S, Chia SK, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res. 2012;18:2402–12. doi: 10.1158/1078-0432.CCR-11-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin M, Prat A, Rodriguez-Lescure A, Caballero R, Ebbert MT, Munarriz B, et al. PAM50 proliferation score as a predictor of weekly paclitaxel benefit in breast cancer. Breast Cancer Res Treat. 2013;138:457–66. doi: 10.1007/s10549-013-2416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caan B, Sternfeld B, Gunderson E, Cates A, Quesenberry C, Slattery M. Life After Cancer Epidemiology (LACE) Study: A cohort of early stage breast cancer survivors. Cancer Causes and Control. 2005;16:545–56. doi: 10.1007/s10552-004-8340-3. [DOI] [PubMed] [Google Scholar]
- 15.Kwan ML, Ambrosone CB, Lee MM, Barlow J, Krathwohl SE, Ergas IJ, et al. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19:1065–76. doi: 10.1007/s10552-008-9170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacholder S. Practical considerations in choosing between the case-cohort and nested case-control designs. Epidemiology. 1991;2:155–8. doi: 10.1097/00001648-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Bastien RR, Rodriguez-Lescure A, Ebbert MT, Prat A, Munarriz B, Rowe L, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. 2012;5:44. doi: 10.1186/1755-8794-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebbert MT, Bastien RR, Boucher KM, Martin M, Carrasco E, Caballero R, et al. Characterization of uncertainty in the classification of multivariate assays: application to PAM50 centroid-based genomic predictors for breast cancer treatment plans. J Clin Bioinforma. 2011;1:37. doi: 10.1186/2043-9113-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, Caballero R, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31:203–9. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Langholz B, Jiao J. Computational methods for case-cohort studies. Computational Statistics and Data Analysis. 2007;51:3737–48. [Google Scholar]
- 23.Sweeney C, Bernard PS, Factor R, Shakespear K, Kwan ML, Habel LA. Molecular subtypes from PAM50 in a breast cancer cohort: differences by patient characteristics. American Association for Cancer Research Annual Meeting; Chicago, IL. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–32. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchini G, Pusztai L, Karn T, Iwamoto T, Rody A, Kelly CM, et al. Proliferation and estrogen signaling can distinguish patients at risk for early versus late relapse among estrogen receptor positive breast cancers. Breast Cancer Res. 2013;15:R86. doi: 10.1186/bcr3481. [DOI] [PMC free article] [PubMed] [Google Scholar]


