Abstract
Objective
The debilitating and persistent effects of intensive care unit (ICU)-acquired delirium and weakness warrant testing of prevention strategies. The purpose of this study was to evaluate the effectiveness and safety of implementing the Awakening and Breathing Coordination, Delirium monitoring/management, and Early exercise/mobility (ABCDE) bundle into everyday practice.
Design
Eighteen-month, prospective, cohort, before-after study conducted between November 2010 and May 2012.
Setting
Five adult ICUs, one step-down unit, and one oncology/hematology special care unit located in a 624-bed tertiary medical center.
Patients
Two hundred ninety-six patients (146 pre- and 150 post-bundle implementation), age ≥ 19 years, managed by the institutions’ medical or surgical critical care service.
Interventions
ABCDE bundle.
Measurements
For mechanically ventilated patients (n = 187), we examined the association between bundle implementation and ventilator-free days. For all patients, we used regression models to quantify the relationship between ABCDE bundle implementation and the prevalence/duration of delirium and coma, early mobilization, mortality, time to discharge, and change in residence. Safety outcomes and bundle adherence were monitored.
Main Results
Patients in the post-implementation period spent three more days breathing without mechanical assistance than did those in the pre-implementation period (median [IQR], 24 [7 to 26] vs. 21 [0 to 25]; p = 0.04). After adjusting for age, sex, severity of illness, comorbidity, and mechanical ventilation status, patients managed with the ABCDE bundle experienced a near halving of the odds of delirium (odds ratio [OR], 0.55; 95% confidence interval [CI], 0.33–0.93; p = 0.03) and increased odds of mobilizing out of bed at least once during an ICU stay (OR, 2.11; 95% CI, 1.29–3.45; p = 0.003). No significant differences were noted in self-extubation or reintubation rates.
Conclusions
Critically ill patients managed with the ABCDE bundle spent three more days breathing without assistance, experienced less delirium, and were more likely to be mobilized during their ICU stay than patients treated with usual care.
Keywords: ABCDE bundle, ventilator-free days, delirium, intensive care unit
INTRODUCTION
Growing evidence suggests that there is an iatrogenic component to intensive care unit practice (ICU) that influences critically ill patients’ likelihood of experiencing ICU-acquired delirium and weakness. These comorbidities are common in adult critically ill patients (1–7) and independently predict increased mortality (1, 8–11), mechanical ventilator days (5, 10–12), ICU length of stay (12–14), and use of continuous sedation and physical restraints (15–16). The effects of both conditions are often persistent and include functional decline (17) and long-term cognitive impairment (18). Strategies are needed to prevent and/or treat ICU-acquired delirium and weakness.
Mechanical ventilation, sedative medications, and immobilization are known risk factors for ICU-acquired delirium and weakness (6–7, 19). When these factors interact with other known predisposing factors, the likelihood of developing delirium and weakness rises (6–7, 20). Multicomponent approaches targeted to modifiable risk factors have effectively prevented delirium among older hospitalized medical patients (21). Such multifaceted interventions, however, are understudied in the ICU setting.
A multicomponent liberation and animation strategy aimed at reducing delirium and weakness has recently been proposed (22–25). Liberation refers to reducing exposure to mechanical ventilation and sedative medications through use of coordinated, target-based sedation protocols, spontaneous awakening trials (SATs) (26), and spontaneous breathing trials (SBTs) (27). Animation refers to early mobilization, which reduces delirium (28–30). This evidence-based strategy is referred to as the ABCDE bundle: Awakening and Breathing Coordination, Delirium monitoring/management, and Early exercise/mobility (22–25).
A bundle is a small set of evidence-based practices that, when performed collectively and reliably, have been proven to improve patient outcomes (31). Bundles are used in the ICU setting to address a number of serious iatrogenic conditions (e.g., ventilator-associated pneumonia, central line infections). The use of bundles, as suggested in the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the Intensive Care Unit (32), may be similarly beneficial for developing patient-centered protocols for preventing and treating PAD in critically ill patients.
While many ABCDE bundle components improved important clinical outcomes in rigorously-designed randomized controlled trials (RCTs), most of these RCTs evaluated the safety and efficacy of these interventions in isolation, excluded many important ICU populations, and generally relied on research staff to implement the intervention. Additionally, the evidence supporting both the ABCDE bundle and the new PAD guideline recommendations was based predominately on data derived from RCTs in mechanically ventilated patients. Given these circumstances, there is great interest on the part of ICU clinicians to know if the ABCDE approach will improve patient outcomes and which patients the bundle should be applied to (e.g., intubated vs. non-intubated patients). These are relevant questions considering that the vast majority of ICU patients are not mechanically ventilated (33).
This study was designed to better understand these important aspects of the ABCDE management strategy. Our goal was to determine if implementing the ABCDE components as a bundle would prove safe and effective if applied to every critically ill patient, every day, regardless of mechanical ventilation status, as well as to identify successes and pitfalls in bundle implementation. Some results of the current study have been previously reported in abstract form (34–36).
METHODS
Additional information about the methods is provided in the online supplement.
Overview of Study Development and Adoption of ABCDE Bundle Policy
We recently described in detail our experience implementing the ABCDE management strategy into everyday practice (37). In brief, over an 18-month period, members of the research team and study site collaborated on the development of an institutional ABCDE bundle policy and numerous ABCDE bundle-related educational opportunities (Table E1 online supplement). The ABCDE bundle was officially implemented on October 3, 2011.
Usual Care (Pre-ABCDE Bundle Implementation)
Prior to ABCDE bundle implementation, clinicians at the participating institution had some experience with SATs and SBTs. The performance of both procedures, however, was inconsistent and identified as a needed area of quality improvement. There were no official policies in place to guide the SAT or SBT process (e.g., no checks to see if it was safe to perform a SAT or SBT, no guidance as to what defined success or failure). Additionally, SATs and SBTs were rarely coordinated, and interprofessional rounding depended on the individual ICU physicians’ practice. No delirium monitoring or management policies were in place. One ICU was in the beginning phase of an early mobility program, but patients were not routinely assisted out of bed in the ICU setting.
ABCDE Bundle Intervention (Post-ABCDE Bundle Implementation)
In the post-implementation period, the stated institutional policy was that the ABCDE bundle was to be applied to every adult patient receiving ICU level of care. All patients were to receive the intervention on a daily basis unless a licensed prescriber wrote an order not to have the patient participate in certain components of the ABCDE bundle (opt-out method). The five distinct components of the ABCDE bundle, along with safety screen and success failure criteria used in this study, are provided in Figure 1 and Table 1.
Figure 1. ABCDE Bundle Policy.
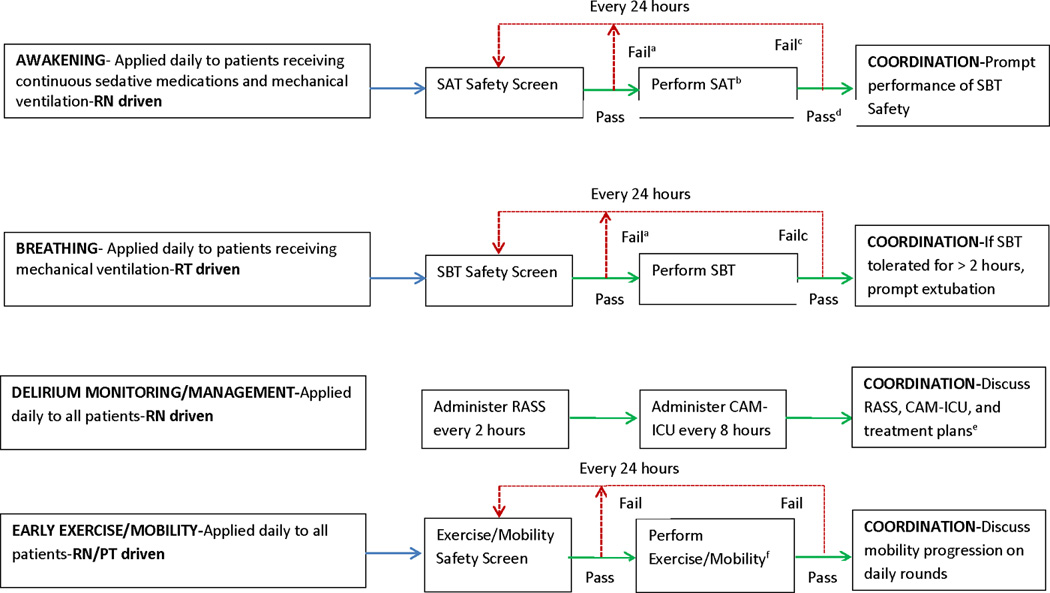
RN = Registered Nurse, RT = Respiratory Therapist, PT = Physical Therapist, SAT = Spontaneous Awakening Trial; SBT = Spontaneous Breathing Trial; RASS = Richmond Agitation-Sedation Scale; CAM-ICU = Confusion Assessment Method for the Intensive Care Unit.
aContinuous sedative medications maintained at previous rate if SAT safety screen failure. Mechanical ventilation continued, and continuous sedative medications restarted at half the previous dose only if needed due to SBT safety screen failure.
bContinuous sedative infusions stopped, and sedative boluses held. Bolus doses of opioid medications allowed for pain. Continuous opioid infusions maintained only if needed for active pain.
cContinuous sedative medications restarted at half the previous dose, and then titrated to sedation target if SAT failed. Interdisciplinary team determines possible causes of SAT/SBT failure during rounds. Mechanical ventilation restarted at previous settings, and continuous sedative medications restarted at half the previous dose only if needed if SBT failed.
dSAT pass if the patient is able to open his/her eyes to verbal stimulation without failure criteria (regardless of trial length) or does not display any of the failure criteria after four hours of shutting off sedation.
eEach day on interdisciplinary rounds, the RN will inform the team of the patient’s target RASS score, actual RASS score, CAM-ICU status, and sedative and analgesic medications the patients is receiving. If delirium is detected, team will discuss possible causes, eliminate risk factors, and employ non-pharmacologic management strategies.
fEach eligible patient is encouraged to be mobile at least once a day, with the specific level of activity geared to his or her readiness. Patients progress through a three-step process, embarking on the highest level of physical activity they can tolerate. Progress includes sitting on edge of bed, standing at bedside and sitting in chair, and walking a short distance. Use of the protocol ends when the patient is discharged from the ICU.
Table 1.
ABCDE Bundle Safety Screen Questions and Success/Fail Criteria
| ABCDE Bundle Component |
Safety Screen Criteria- Conditions for exclusion |
Pass/Fail Criteria- Conditions denoting failure |
|---|---|---|
| Spontaneous Awakening Trial |
|
|
| Spontaneous Breathing Trial |
|
|
| Early Exercise/Mobility |
|
|
ABCDE = Awakening and Breathing Coordination, Delirium Monitoring/Management, and Early Mobility Bundle; Richmond Agitation-Sedation Scale; ICP = Intracranial Pressure; ECMO = Extracorporeal Membrane Oxygenation; MI = myocardial ischemia; BPM = Beats per Minute; FiO2 = Fraction of inspired oxygen; PEEP = positive end expiratory pressure; Pulse-ox = Pulse oximetry reading.
Study Design, Setting, and Participants
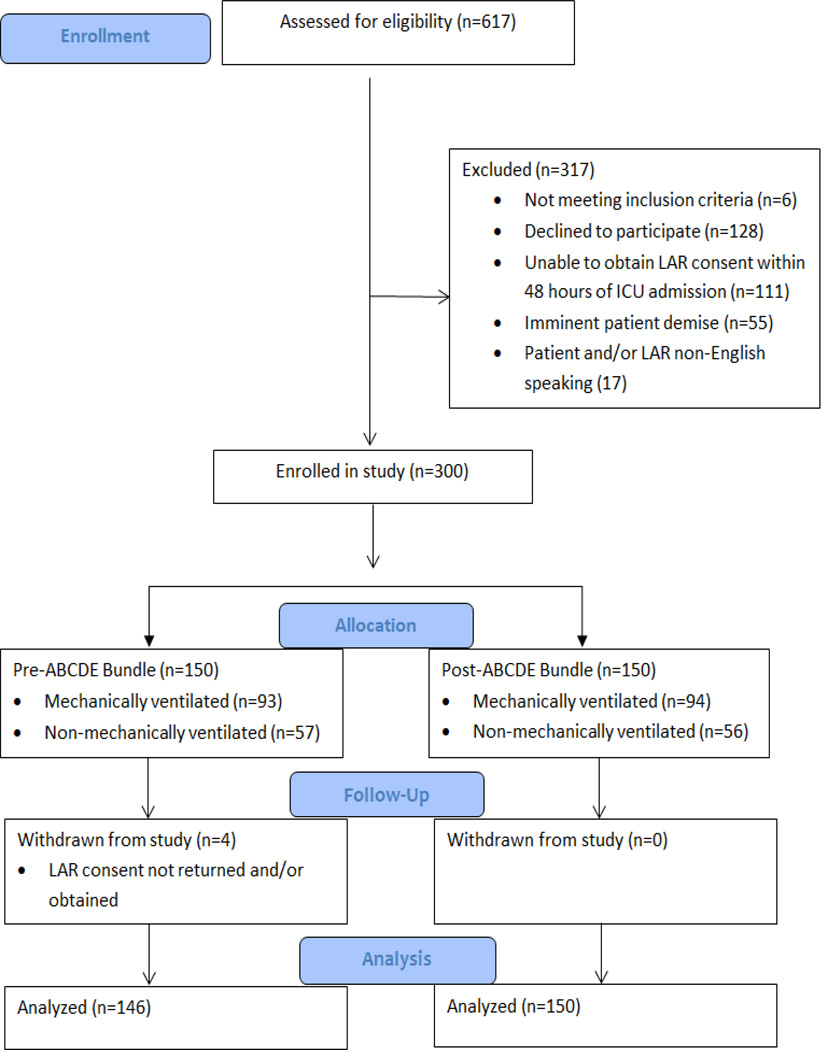
This prospective, before-after study was conducted at a 624-bed tertiary medical center. Eligible patients included adult patients (≥19 years old) admitted to the institution’s medical or surgical critical care service, regardless of mechanical ventilation status. Critically ill patients were recruited consecutively from five adult ICUs, one step down unit, and an oncology/hematology special care unit. Patients were excluded if they did not have a legally authorized representative (LAR) to provide consent within 48 hours of ICU admission. “Pre” patients were enrolled from February to October 2011 and received “usual care.” “Post” patients were enrolled from October 2011 to April 2012. The institutional review board approved the study protocol, and written informed consent was obtained from all patients’ LAR.
Study Procedures
To ensure reliability of outcomes assessment in the pre- and post- implementation period, trained research personnel (all RNs) were hired to enroll patients, perform daily sedation/agitation and delirium assessments, and conduct standardized medical record reviews. Inter-rater reliability checks for delirium and sedation/agitation screenings were 100% for all four research personnel. The research personnel had no role in administering any parts of the ABCDE bundle. The decision to perform daily SATs, SBTs, delirium monitoring/management, and early exercise/mobility was made solely by the ICU team.
At enrollment, we collected demographic data, admission source, and primary diagnosis. Severity of illness and comorbidity were measured by the Acute Physiology and Chronic Health Evaluation II (APACHE II) (38) score and Charlson Comorbidity Index (39), respectively. We recorded the cumulative amount of sedative medications administered from ICU admission until study enrollment, including operating room, post-anesthesia care unit, and procedural sedation.
Awakening and Breathing Coordination
We recorded daily the total 24-hour dose of sedative medications as we did at study enrollment. If a patient received a continuous infusion of sedative medications and/or mechanical ventilation within the preceding 24-hour period, we recorded whether that patient received a SAT/SBT and the documented reasons for safety screen or trial failure. We recorded the date and time of intubation and extubation, any unplanned extubations, reintubations, new tracheostomies, and any hospital discharges on mechanical ventilation.
Delirium Monitoring/Management
The patients’ level of arousal was assessed daily by research personnel with the Richmond Agitation-Sedation Scale (RASS) (40–41). Subjects with a RASS score of −3 or higher underwent delirium screening with the Confusion Assessment Method for the ICU (CAM-ICU) (2, 42). If the subject was unavailable, additional attempts were made to evaluate their neurologic status that day. We also recorded the results of the ICU clinicians’ every-8-hour CAM-ICU and RASS assessments.
Early Exercise/Mobility
We recorded daily whether patients received physical therapy consultation and if they were mobilized out of bed anytime in the previous 24 hours.
Outcome Definitions
Our primary outcome for mechanically ventilated patients was ventilator-free days (VFDs). We defined VFDs as the number of days patients were breathing without mechanical ventilator assistance during a 28-day period which began at the time of study enrollment. A period of unassisted breathing began with extubation (or removal of mechanical ventilation support for patients with tracheostomies) if the period of unassisted breathing lasted at least 48 consecutive hours. Patients who died during the study period were assigned 0 VFDs.
We secondarily examined outcomes across the entire ICU population (i.e., mechanically ventilated and non-mechanically ventilated patients), including the prevalence, duration, and percent of ICU days of delirium and coma. Duration of delirium was defined as the number of ICU days in which patients were CAM-ICU-positive and not comatose. Duration of coma was defined as the number of ICU days that patients had a RASS score of −4 or −5. We additionally explored the number of patients mobilized out of bed during their ICU stay. Finally, we examined 28-day ICU and total hospital mortality, time to discharge from the ICU and hospital, and the number of patients who experienced a change in residence. Change in residence was defined as discharge from the hospital to a place other than home in subjects residing at home prior to admission. Unplanned extubations, reintubations, tracheostomy placement, percent of ICU time in physical restraints, and the use of imaging for “changes in mental status” were tracked as safety endpoints. Reintubation was defined as a second intubation that occurred during the patient’s initial ICU stay.
Statistical Analysis
Demographic and clinical characteristics were compared between both sets of subjects and by mechanical ventilation status. Mean and standard deviation (median and interquartile range [IQR] for skewed distributions) or frequencies and percentages are presented for continuous or categorical variables, respectively. Initial comparisons between pre- and post-groups were made using t tests (or Wilcoxon test) for continuous variables, chi-square (or Fisher’s exact test) for categorical variables and log-rank tests for time-to-event variables. Differences in outcomes between pre and post groups were analyzed after adjusting for age, sex, mechanical ventilation status, APACHE II, and Charlson Comorbidity Index using logistic regression for binary outcomes and Cox regression for time-to-event outcomes. SAS version 9.2 was used for all summaries and analyses. The statistical level of significance was set at < 0.05 (2-sided alpha).
RESULTS
Patient Characteristics
A total of 146 patients were enrolled before ABCDE bundle implementation (“pre” group) and 150 after implementation (“post” group) (Figure 2). Patients in the pre-implementation phase were older (pre-age mean 59.2 +/−16.1 vs. post-age mean 55.6 +/−14.9; p = 0.05), but otherwise shared similar baseline characteristics to patients in the post-implementation period (Table 2). Patients in both groups were lightly sedated at the time of study enrollment (median RASS score of −1) and received similar doses and types of sedative medications prior to study enrollment. Patients were admitted to the ICUs with a variety of medical and surgical diagnoses, with more than 40% of the sample having surgery on or during their ICU admission. When we examined baseline characteristics by mechanical ventilation status, no significant differences pre- and post-implementation were noted (Table E2 online supplement).
Figure 2. PATIENT FLOW DIAGRAM.
LAR = Legally Authorized Representative.
Table 2.
Baseline Patient Characteristics
| Characteristic | Pre-ABCDE Bundle N = 146 |
Post-ABCDE Bundle N = 150 |
p-value |
|---|---|---|---|
| Agea, mean (SD) yr | 59.2 (± 16.1) | 55.6 (± 14.9) | 0.05 |
| Female, n (%) | 67 (45.9) | 64 (42.7) | 0.58 |
| Caucasian, n (%) | 134 (93.1) | 133 (89.3) | 0.25 |
| Residence preadmissionb, n (%) | 0.09 | ||
| Home | 118 (80.8) | 132 (88.0) | |
| Nursing home | 7 (4.8) | 7 (4.7) | |
| Skilled nursing facility | 4 (2.7) | 6 (4.0) | |
| Rehabilitation center | 5 (3.4) | 0 (0) | |
| Other hospital | 9 (6.2) | 1 (0.7) | |
| Other | 3 (2.1) | 4 (2.7) | |
| APACHE II score, median (IQR) | 23.5 (17 to 29) | 21 (16 to 28) | 0.08 |
| Charlson Comorbidity Index, median (IQR) | 2 (1 to 5) | 2 (1 to 4) | 0.48 |
| Admitting ICU diagnosis, n (%) | NT | ||
| Medicalc | |||
| Shock | 20 (13.7) | 20 (13.3) | |
| Respiratory | 37 (25.3) | 35 (23.3) | |
| Cardiac | 6 (4.1) | 5 (3.3) | |
| Neurologic/other | 25 (17.1) | 34 (22.7) | |
| Surgicald | |||
| Neurosurgical | 29 (19.9) | 24 (16.0) | |
| Cardiothoracic/vascular | 6 (4.1) | 20 (13.3) | |
| General surgery/trauma | 21 (14.4) | 11 (7.3) | |
| Other | 2 (1.4) | 1 (0.7) | |
| Admission type (elective), n (%) | 30 (20.6%) | 39 (26.0%) | 0.27 |
| Sedation before enrollment median (IQR) | |||
| Benzodiazepinese (mg) | 7.2 (2 to 24) | 8.8 (2 to 26.8) | 0.91 |
| Opiate (mg)f | 16.7 (6.7 to 42.7) | 26.7 (10 to 47) | 0.27 |
| Propofol (mg) | 230 (100 to 1260) | 200 (100 to 480) | 0.48 |
| Dexmedetomidine (ug) | 1034 (748 to 1320) | 78 (35 to 184) | 0.06 |
| Haloperidol (mg) | 5 (n = 1) | 1 (n = 1) | NT |
| Surgery on/during ICU admission | 63 (44.4%) | 70 (46.7%) | 0.69 |
| RASS on first study day | −1 (−3 to 0) N = 121 | −1 (−3 to 0) N = 131 | 0.99 |
APACHE = Acute Physiology and Chronic Health Evaluation; ICU = intensive care unit; IQR = interquartile range; N = number; NT = not tested (not enough subjects); mg = milligram; RASS = Richmond Agitation-Sedation Scale; SD = standard deviation; yr = year; ug = microgram
When age was examined by mechanical ventilation status, no significant differences were noted (Mechanically ventilated patients age pre 57.7 +/−16.2 vs. post 55.4 +/−14.5, p = 0.30; Non mechanically ventilated patients age pre 61.7 +/−15.8 vs. post 56 +/−15.7, p = 0.06).
Data were re-categorized as home/other for purposes of statistical analysis.
Medical category described fully in online supplement.
Surgical category described fully in online supplement.
Expressed in lorazepam equivalents. Includes the following medications: lorazepam, midazolam, clonazepam, diazepam, temazepam. The total dose includes continuous infusions and bolus doses given intravenously, intramuscularly, and orally.
Expressed in morphine equivalents. Includes the following medications: morphine, hydromorphone, and fentanyl. The total dose includes continuous infusions and bolus doses given intravenously, intramuscularly, and orally.
Outcomes-Effectiveness
Mechanically ventilated patients post-implementation spent more days breathing without mechanical ventilator assistance than those pre-implementation (pre-median 21 days [IQR 0 to 25] vs. post-median 24 days [IQR 7 to 26]; p = 0.04) (Table 3). Three patients in the post-implementation and five in the pre-implementation period were discharged from the hospital on mechanical ventilation (p = 0.50).
Table 3.
Main Study Outcomes
| ABCDE Bundle Component Outcome |
Pre ABCDE Bundle N = 146 |
Post ABCDE Bundle N = 150 |
Unadjusted p value |
Adjusted Odds Ratio |
Adjusted p- value |
|---|---|---|---|---|---|
| Awakening and Breathing Coordinationa | |||||
| Ventilator-free daysa | |||||
| Mean (SD) | 15 (11.4) | 18 (10.6) | |||
| Median (IQR) | 21 (0 to 25) | 24 (7 to 26) | 0.04 | ||
| Delirium Monitoring/Management | |||||
| Delirium anytime, n (%) | 91 (62.3%) | 73 (48.7%) | 0.02 | 0.55b (0.33–0.93) | 0.03 |
| Duration of delirium, days, median (IQR) | 3 (1 to 6) | 2 (1 to 4) | 0.52 | ||
| Percent ICU days spent delirious, median (IQR) | 50 (30 to 64.3) | 33.3 (18.8 to 50) | 0.003 | ||
| Coma anytime, n (%) | 41 (28.1%) | 43 (28.7%) | 0.91 | 1.00b | 0.99 |
| Coma days, median (IQR) | 2 (1 to 4) | 2 (1 to 5) | 0.35 | ||
| Percent ICU days spent in coma, median (IQR) | 25 (18.2 to 44.4) | 25 (12.5 to 42.9) | 0.89 | ||
| Richmond Agitation-Sedation Scale Score, mean (SD) | 0.02 (1.4) | −1.03 (1.2) | 0.38 | ||
| Early Exercise/Mobility | |||||
| Mobilized out of bed anytime in ICU, n (%) | 70 (48%) | 99 (66.0%) | 0.002 | 2.11b (1.30–3.45) | 0.003 |
| 28-day Mortalityc | |||||
| Hospital mortality (ICU and post-ICU), n (%) | 29 (19.9%) | 17 (11.3%) | 0.04 | 0.56b (0.28–1.10) | 0.09 |
| ICU mortality, n (%) | 24 (16.4%) | 14 (9.3%) | 0.07 | ||
| Time to discharged (days) | |||||
| From ICU, median (IQR) | 5 (3, 8) | 4 (3, 5) | 0.21 | 1.16e (0.89–1.50) | 0.27 |
| From hospital, median (IQR) | 13 (9, 15) | 11 (9, 13) | 0.99 | 1.01e (0.77–1.31) | 0.96 |
| Residence at hospital dischargef, n (%) | 0.86 | ||||
| Home | 51 (44%) | 60 (45.1%) | |||
| Nursing home | 9 (7.8%) | 8 (6%) | |||
| Skilled nursing facility | 13 (11.2%) | 16 (12%) | |||
| Rehabilitation center | 29 (25%) | 27 (20.3%) | |||
| Home with hospice | 1 (0.9%) | 2 (1.5%) | |||
| Hospice center | 2 (1.7%) | 4 (3%) | |||
| Swing bed/other hospital | 8 (6.9%) | 6 (4.5%) | |||
| Other | 3 (2.6%) | 10 (7.5%) | |||
| Change in residence for those who came from homeg, n (%) | 72 (61%) | 72 (54.6%) | 0.30 | 1.16b (0.66–2.03) | 0.60 |
ICU = intensive care unit; IQR = interquartile range; n = number; SD = standard deviation.
For those subjects that received mechanical ventilation only pre N = 93 post N = 94.
Odds ratio and 95% Wald confidence limits.
All subjects who died did so within 28 days post-enrollment.
Time to ICU discharge is calculated only for the 28-day interval post enrollment. If a subject was not discharged within the first 28 days or died within 28 days, then she/he was considered censored.
Hazard ratio and 95% Hazard ratio confidence limits.
Data were categorized as home/other for purposes of statistical analysis.
Change in residence was defined as discharge from the hospital to a place other than home in those subjects residing at home prior to hospital admission.
Fewer patients treated with the ABCDE bundle experienced delirium (pre 62.3% vs. post 48.7%; p = 0.02) (Table 3). Delirium duration was reduced by one day in the post-implementation period, and the percent of ICU days spent delirious decreased by 17% (pre 50% [IQR 30 to 64.3] vs. post 33.3% [IQR 18.8 to 50]; p = 0.003). After adjusting for age, APACHE II score, sex, Charlson Comorbidity Index, and mechanical ventilation, there continued to be a significant effect of the ABCDE bundle on prevalence of delirium (p = 0.03), and the odds of delirium were reduced by almost half (OR: 0.55; 95% CI, 0.33–0.93). No significant difference was noted in coma prevalence, coma duration, percentage of ICU days spent in coma, or mean RASS score between the pre- and post-implementation period in unadjusted or adjusted analyses.
Following multivariable adjustment, a significant effect of the ABCDE bundle was observed on the percentage of patients (pre 48% vs. post 66%; p = 0.002) who were mobilized during their ICU stay. Patients treated with the ABCDE bundle had twice the odds (95% CI, 1.30–3.45; p = 0.003) of mobilizing out of bed at least once during their ICU stay compared to patients in the ICU prior to bundle implementation (Table 3).
Unadjusted hospital mortality was significantly lower in the post-implementation group (p = 0.04), while ICU mortality showed a non-statistically significant reduction (p = 0.07). The hospital mortality rate was 19.9% in the pre-implementation period vs. 11.3% in the post-implementation period, yielding an odds ratio of 0.56 (95% CI 0.28–1.10; p = 0.09) after adjustment for age, APACHE II score, sex, and comorbidity (Table 3).
No significant difference was observed in the time to ICU or hospital discharge between the pre- and post-implementation periods (Table 3). Few ICU patients who were admitted from home returned to this setting at hospital discharge in either the pre- or post-implementation period. No significant differences, however, were noted in change in residence in either the unadjusted or adjusted analysis.
Outcomes-Safety
No significant differences were found in unplanned extubations, reintubation rates, tracheostomy placements, percent time spent in physical restraints, or the use of imaging for mental status changes pre versus post ABCDE bundle implementation (Table 4).
Table 4.
Safety Outcomes
| Safety Outcome | Pre ABCDE Bundle N = 93 |
Post ABCDE Bundle N = 94 |
p-Value |
|---|---|---|---|
| Any unplanned extubation | 7 (7.5%) | 7 (7.5%) | 0.98 |
| Any self-extubationa | 6 (6.5%) | 5 (5.3%) | 0.74 |
| Self-extubation requiring re-intubationa | 1 (1.1%) | 1 (1.1%) | 0.99 |
| Any re-intubation | 16 (17.2%) | 11 (11.7%) | 0.28 |
| Tracheostomy | 15 (16.1%) | 14 (14.9%) | |
| Underwent imaging related to change in mental statusb | 21 (14.4%) | 17 (11.3%) | 0.43 |
| Percent of ICU time in physical restraints (median, interquartile range)b | 12.7% (0–51.4%) | 6.9% (0–50%) | 0.29 |
Note:
Defined as an extubation documented to be done by patient.
For all patients included in study: pre n = 146 post n = 150.
ABCDE Bundle Compliance and Sedative Medication Use
Most patients requiring mechanical ventilation received a continuous infusion of either sedative or opioid medications sometime during their ICU stay (pre 77.4% vs. post 70.2%, p = 0.26) (Table 5). Post ABCDE bundle implementation, there was a significant increase in the number of patients who had their continuously infused sedative medication held at least once for a SAT (pre 53% vs. post 71%; p = 0.04). The percentage of ICU days on which patients received a SAT while on a continuously infused opioid medication also doubled in the post-implementation period (pre 25% vs. post 50%; p = 0.001). Patients in the post-implementation period were significantly more likely to undergo a SBT at least once during their ICU stay (pre 71% vs. post 84%; p = 0.03). In the post-implementation period, clinicians documented a variety of reasons for not performing both SATs and SBTs (Table E3 online supplement).
Table 5.
Spontaneous Awakening and Breathing Trial Implementation
| Mechanically Ventilated Patients |
|||
|---|---|---|---|
| Variable | Pre-ABCDE Bundle N = 93 |
Post-ABCDE Bundle N = 94 |
p-value |
| Received a continuously infused sedative medicationa anytime during ICU stay, n (%) | 67 (72%) | 59 (62.8%) | 0.18 |
| Had continuously infused sedative medicationa held at least once for a spontaneous awakening trial (SAT), n (%) | 35 (53%) | 42 (71.2%) | 0.04 |
| SATs performed on eligible days (sedative medicationa only), median % (IQR) | 42.9 (25, 66.7) | 50 (33.3, 55.6) | 0.38 |
| Received a continuously infused opioid medicationb anytime during ICU stay, n (%) | 34 (36.6%) | 30 (31.9%) | 0.50 |
| Had continuously infused opioid medicationb held at least once for a SAT, n (%) | 15 (45.5%) | 18 (60%) | 0.25 |
| SATs performed on eligible days (opioid medicationb only), median % (IQR) | 25 (14.3, 40) | 50 (45.2, 66.7) | 0.001 |
| Received a continuously infused sedativea or opioidb medication anytime during ICU stay, n (%) | 72 (77.4%) | 66 (70.2%) | 0.26 |
| Had continuously infused sedativea or opioidb medication held at least once for a SAT, n (%) | 36 (50.7%) | 42 (63.6%) | 0.13 |
| SATs performed on eligible days (sedativea or opioidb medication held), median % (IQR) | 33.3 (24.4, 52.8) | 50 (33.3, 50) | 0.18 |
| Underwent a spontaneous breathing trial (SBT) anytime during ICU stay, n (%) | 65 (70.7%) | 79 (84%) | 0.03 |
| SBT/mechanical ventilation days, median (IQR) | 50 (31.8, 66.7) | 50 (33.3, 66.7) | 0.94 |
ICU = intensive care unit; IQR = interquartile range; n = number; SAT = spontaneous awakening trial; SBT = spontaneous breathing trial.
Includes the following medications: lorazepam, midazolam, propofol, and dexmedetomidine.
Includes the following medications: morphine, hydromorphone, and fentanyl.
While there was a trend toward decreased benzodiazepine use and increased opiate use in the post-intervention period, the number of patients treated and total average daily doses of these medications did not differ significantly pre- and post-implementation (Table 6). CAM-ICU and RASS score were documented in the post-implementation period every 8 hours by bedside nurses 50% and 68% of the time, respectively (Table 6). Delirium was identified as present by bedside nurses in 51% of patients in the post-implementation period. While nearly two-thirds of the patients received a physical therapy consultation while they were in the ICU, approximately one-third of ICU days were spent out of bed.
Table 6.
Sedative Medication Utilization and Delirium Monitoring/Management and Early Exercise/Mobility Implementation
| Variable | Pre ABCDE Bundle N = 146 |
Post ABCDE Bundle N = 150 |
p-value |
|---|---|---|---|
| Sedation received post-enrollment | |||
| Benzodiazepinesa | |||
| Patients treated, n (%) | 91 (62.3%) | 77 (51.3%) | 0.06 |
| Total dose (mg), median (IQR) | 21.2 (3, 87.6) | 17.4 (3, 56.1) | 0.41 |
| Average daily doseb (mg), median (IQR) | 2.8 (1, 12.7) | 1.7 (0.4, 7.8) | 0.09 |
| Opiatesc | |||
| Patients treated, n (%) | 124 (84.9%) | 134 (89.3%) | 0.26 |
| Total dose (mg), median (IQR) | 26.3 (10, 147.2) | 35.8 (14, 126) | 0.70 |
| Average daily doseb (mg), median (IQR) | 5.8 (2, 16.7) | 5.5 (2.2, 14.3) | 0.97 |
| Propofol | |||
| Patients treated, n (%) | 25 (17.1%) | 31 (20.7%) | 0.44 |
| Total dose (mg), median (IQR) | 1003 (150, 5305) | 410 (140, 2310) | 0.50 |
| Average daily doseb (mg), median (IQR) | 83.3 (10, 499.8) | 66.7 (7.7, 419) | 0.64 |
| Dexmedetomidine (ug) | |||
| Patients treated, n (%) | 12 (8.2%) | 16 (10.7%) | 0.47 |
| Total dose (mg), median (IQR) | 1538 (566, 5820.3) | 2500 (332, 3726) | 0.69 |
| Average daily doseb (mg), median (IQR) | 140.3 (88.4, 269.3) | 185.7 (28.4, 294.9) | 0.87 |
| Haloperidol | |||
| Patients treated, n (%) | 11 (7.5%) | 12 (8.0%) | 0.88 |
| Total dose (mg), median (IQR) | 6 (2.5, 19.5) | 17.5 (3.8, 39.3) | 0.24 |
| Average daily doseb (mg), median (IQR) | 0.5 (0.3, 1.3) | 1.3 (0.4, 4.1) | 0.20 |
| Percentage of time CAM-ICU results documented every 8 hours by bedside nursed | ----- | 50 (33.3, 66.7) | |
| Mechanically ventilated (MV) patients, median (IQR) | ---- | 50 (33.3, 66.7) | |
| Non-MV patients, median (IQR) | ----- | 60 (33.3, 68.6) | |
| Delirium anytime per bedside nurse documentation, n (%) | 76 (51%) | ||
| Percentage of time RASS score documented every 8 hours by bedside nurse | 66.3% | 68% | 0.84 0.68 |
| Richmond Agitation-Sedation Scale Score by bedside nurses, mean (SD) | −0.64 (1.1) | −0.59 (1.1) | |
| Physical therapy consults anytime during ICU stay, n (%) | 105 (71.9%) | 113 (75.3%) | 0.50 |
| Mobilized out of bed (OOB) at least once during ICU stay, n (%) | 70 (48%) | 99 (66.0%) | 0.002 |
| Mechanically ventilated patients, n (%) | 44/93 (47.3%) | 57/94 (60.6%) | 0.07 |
| Non mechanically-ventilated patients, N (%) | 26/53 (49.1%) | 42/56 (75%) | 0.005 |
| OOB days/ICU length of stay, median % (IQR) | 33.3 (16.7, 50) | 33.3 (20, 53.9) | 0.64 |
CAM = confusion assessment method; ICU = intensive care unit; IQR = interquartile range; mg = milligram; MV = mechanically ventilated; n = number; OOB = out of bed; ug = microgram.
Expressed in lorazepam equivalents. Includes the following medications: lorazepam, midazolam, clonazepam, diazepam, temazepam. The total dose includes continuous infusions and bolus doses given intravenously, intramuscularly, and orally.
Average daily dose calculated by taking the total dose per subject and dividing by their total days in ICU.
Expressed in morphine equivalents. Includes the following medications: morphine, hydromorphone, and fentanyl. The total dose includes continuous infusions and bolus doses given intravenously, intramuscularly, and orally.
Per the institution’s ABCDE bundle policy, bedside nurses were required to document results of the CAM-ICU every 8 hours.
DISCUSSION
We explored the effectiveness and safety of implementing into everyday clinical care an interprofessional, multicomponent, bundle of evidence-based interventions directed at reducing the harmful effects of over-sedation, mechanical ventilation, and immobility. In this prospective before-after study, implementation of the ABCDE bundle resulted in patients spending an additional three days breathing without mechanical ventilator assistance compared to patients treated with usual care. After adjusting for important covariates, the ABCDE bundle was found to be an important independent predictor of reduced delirium rates and increased likelihood of mobilizing out of bed. Implementation of the ABCDE bundle was also found to be safe and well tolerated. These efficacy and safety findings were present despite a lower than anticipated compliance with the ABCDE bundle.
Our results are consistent with RCTs that studied the individual components of the ABCDE bundle. Girard and colleagues (43) found that a ventilator liberation strategy pairing daily SATs and SBTs resulted in three more VFDs and less time in coma compared to usual care consisting of daily SBTs and patient-targeted sedation. We found a similar reduction in VFDs but not in coma days. This may be due to deeper sedation levels at enrollment in the Girard study (RASS of −4 compared to RASS of −1). Our findings are also consistent with randomized trial evidence from Schweickert and colleagues (30) that found a rehabilitation strategy consisting of SATs and physical and occupational therapy resulted in more VFDs and shorter duration of delirium for mechanically ventilated patients who were functionally independent prior to hospitalization.
In the current and previous investigations (30, 43), improvements in outcomes occurred despite the fact that the overall number of patients treated with sedative and opioid medications did not significantly differ between groups. This suggests (but does not prove) the potential benefit from the “act” of awakening. This awakening strategy ensures a period of maximum wakefulness that may mitigate harm through a variety of potential mechanisms. For example, daily awakenings may reduce the risks of prolonged deep sedation (44), provide beneficial effects of higher peak stimulations (45), and/or allow for patients to engage in physical and cognitive activity (46) that may be independently protective. It is equally plausible that observed improvements were due to other factors such as active care coordination or more intense delirium monitoring.
Despite intense education regarding the hazards of continuously infused sedation, more than two-thirds of the patients in the current study were treated with this sedation strategy. While clinicians in this study were more likely to discontinue sedative drips for a SAT after ABCDE bundle implementation, there was no difference in SAT performance for patients receiving opioid infusions, suggesting that clinicians may not view opioids as potentially harmful, or alternatively, believe the need for pain medication outweighs the need to discontinue sedation.
Similarly, despite the known benefits of early mobilization in mechanically ventilated patients (28–30), patients spent more than 65% of their ICU days in bed. This finding may be due to a number of factors, including the method by which early mobilization was conducted (i.e., primarily by ICU RNs without additional staffing), the patients to which it was applied (i.e., pre-hospitalization functional status not considered), and the outcomes that were used to evaluate effectiveness. Patient outcomes, however, were significantly improved despite lower-than-desired bundle adherence. Therefore, the current study may under-represent the potential impact with ABCDE bundle implementation, considering that we did not reach the goal of “applying it to every patient every day.” This also highlights the need for rigorously designed research to understand best practices for applying evidence to the bedside.
A major strength of this study was the daily assessment of patients’ sedation/agitation level and delirium status by trained study staff using valid and reliable screening instruments. Enhancing the applicability and feasibility for other ICUs, this study also included the results of bedside RNs’ assessment of sedation levels and delirium status, demonstrating good agreement between clinician and research personnel. In contrast to previous RCTs, our study had few exclusion criteria. We included a diverse patient population (e.g., intubated and non-intubated) and relied on clinicians to implement the interventions and monitor the patients, suggesting that the ABCDE bundle could be applied widely across ICUs. We also followed adherence to the individual components of the ABCDE bundle, critical to understanding effectiveness trial results. Finally, study focus was on knowledge translation, or applying research findings into everyday practice, an important yet understudied subject area in critical care.
There are several important limitations to this investigation. Because of the study’s design, relatively small sample size, and the fact that there was a delay in study enrollment until consent was obtained, our results are susceptible to both temporal changes as well as the impact of important unbalanced (or missing) confounders not included in our multivariable adjustment. The fact that most ABCDE bundle-related educational efforts took place during the pre-implementation period may have also influenced study findings. While we attempted to track the number of patients who received individual components of the ABCDE bundle each day, we were unable to determine the cause of coma (i.e., structural or drug-induced) and relied on medical record reviews, which limited our ability to determine definitively the reasons for withholding specific interventions. This was particularly true in the pre-implementation period, when there were no SAT or SBT safety screen criteria. Finally, we also did not follow pain levels using a valid and reliable tool as suggested in the new PAD guidelines. These limitations, in balance with the observed intervention benefit, suggest the value of confirmative trials.
While we followed a number of important effectiveness and safety outcomes, some applied to only to a segment of the ICU population (e.g., VFDs to mechanically ventilated patients), while others could be interpreted as either an outcome or compliance measure (e.g., mobilized out of bed anytime). Future studies would be strengthened by the use of a validated functional outcome measure. We also did not explore the role that specific sedative medications had on patient outcomes, but this is an appropriate direction for future analysis. Finally, it is important to reiterate that nearly all the evidence supporting the ABCDE bundle (and new PAD guidelines) was derived from RCTs that included only mechanically ventilated patients. Our inclusion of non-intubated patients extrapolated evidence derived from one population (mechanically ventilated patients) to a population less well studied (non-mechanically ventilated patients). While we found benefits and no obvious harm applying the ABCDE bundle to non-intubated patients, we believe individual ICUs should explore their own epidemiology/patient mix, culture, and staffing levels before they decide whether to apply the ABCDE bundle to their entire ICU population. Our data also suggest that RCTs may be warranted in non-mechanically ventilated patients to strengthen the PAD evidence base.
CONCLUSIONS
This prospective study explored the effectiveness and safety of the ABCDE bundle, an evidence-based, interprofessional, multicomponent ICU management strategy that promotes early liberation and animation of critically ill patients. In a diverse group of critically ill patients, implementation of the ABCDE bundle resulted in reduced time on the ventilator, less delirium, and more time spent out of bed compared to patients not treated with the bundle. These improvements were achieved despite little difference in medication exposure and incomplete bundle adherence. The ABCDE bundle appears to be a valuable tool in the management of critically ill patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for all the support provided by nurses, respiratory therapists, pharmacists, physical therapists, physicians, and administrative leadership at the Nebraska Medical Center. We are also deeply appreciative of the patients, families, and research staff who made this study possible.
The study was supported by the Robert Wood Johnson Foundation (RWJF) Interdisciplinary Nursing Quality Research Initiative (INRQI). Research reported in this publication was also supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) under Award Number K23AG040157. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest and Source of Funding: Dr. Balas is currently a Co-investigator on a grant supported by the Alzheimer’s Association and has received honoraria from ProCe, the France Foundation, Hospira, and Hillrom. Dr. Vasilevskis is currently receiving a Career Development Award from the NIH-NIA (K23AG040157). Dr. Sisson is currently receiving a NIH Grant (AA008769-20A1). Dr. Schmid serves as a consultant on the data safety and monitoring board (DSMB) for Puma Biotechnology and, in the past, served as a DSMB consultant for Pfizer. Dr. Ely received support from NIA AG-027472 and AG-035117, and he has received honoraria from Hospira, Abbott, and Orion. Dr. Burke has received grant support for clinical studies from the National Institute of Mental Health, National Institute on Aging, RWJF, Alzheimer Disease Cooperative Studies (ADCS), Forest Laboratories Inc., Astra Zeneca, Vanda Pharmaceuticals, Neosync Inc., Elan/Wyeth/Janssen, Baxter Health Care Corporation, Pfizer Inc., Noven Pharmaceuticals, and Novartis. For the remaining authors, none were declared.
Copyright Form Disclosures:
Dr. Balas’ institution received grant support from the RWJF INQRI and the Alzheimer’s Association (Co-I on mobile monitoring study). Dr. Balas received support for article research from the RWJF INQRI.
Dr. Vasilevskis received grant support from NIH-NIA 5K23AG040157 (Career Development Award) and support for article research from NIH.
Dr. Olsen’s institution received grant support from the RWJF (supported in part, the research related to this manuscript). Dr. Olsen received support for article research from the RWJF.
Dr. Schmid’s institution received grant support from the RWJF INQRI. Dr. Schmid consulted for Puma Biotechnology and Pfizer (Data Safety Monitoring Committee member) and received support for article research from the RWJF.
Marlene Z. Cohen C/F (institution received grant support from the RWJF; received support for article research from the RWJF).
Dr. Sullivan received support for article research from the RWJF.
Dr. Jawa disclosed that this study was supported by a grant from the RWJF INQRI, but he did not receive any compensation from this grant.
Dr. Ely consulted for Cumberland and Masimo, received grant support from Lilly, and lectured for Hospira.
Dr. Burke’s institution received support for travel (funds to attend INQRI annual meeting) from the RWJF and grant support from the RWJF (for the research), NIMH (R01 funding), Alzheimer Disease Cooperative Study (ADCS) in collaboration with NIMH and Baxter (grant funds), Pfizer (clinical trial funding), Neosync (clinical trial funding), Vanda Pharmaceuticals (clinical trial funding), Novartis (clinical trial funding), Elan/Wyeth (clinical trial funding), Noven Pharmaceuticals(clinical trial funding), Astra Zeneca (clinical trial funding), and Elan (clinical trial funding).
Footnotes
The work was performed at The Nebraska Medical Center.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 3.Witt NJ, Zochodne DW, Bolton CF, et al. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99:176–184. doi: 10.1378/chest.99.1.176. [DOI] [PubMed] [Google Scholar]
- 4.Berek K, Margreiter J, Willeit J, Berek A, Schmutzhard E, Mutz NJ. Polyneuropathies in critically ill patients: A prospective evaluation. Intensive Care Med. 1996;22:849–855. doi: 10.1007/BF02044106. [DOI] [PubMed] [Google Scholar]
- 5.De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;306:1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 6.Mendez-Tellez PA, Needham DM. Early physical rehabilitation in the ICU and ventilator liberation. Respiratory Care. 2012;57(12):1663–1669. doi: 10.4187/respcare.01931. [DOI] [PubMed] [Google Scholar]
- 7.Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131(5):1541–1549. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 8.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 9.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness P. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shehabi Y, Riker RR, Bokesch PM, et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group: Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 11.Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, Critical illness, polyneuropathy: Risk factors, clinical consequences, et al. A cohort study in septic patients. Intensive Care Med. 2001;27(8):1288–1296. doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- 12.Lat I, McMillian W, Taylor S, et al. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med. 2009;37(6):1898–1905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 13.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: A prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9(4):375–381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micek ST, Anand NJ, Laible BR, Shannon WD, Kollef MH. Delirium as detected by the CAM-ICU predicts restraint use among mechanically ventilated medical patients. Crit Care Med. 2005;33(6):1260–1265. doi: 10.1097/01.ccm.0000164540.58515.bf. [DOI] [PubMed] [Google Scholar]
- 16.Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: A prospective cohort study. Crit Care. 2009;13(3):77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balas MC, Happ MB, Yang W, Chelluri L, Richmond T. Outcomes associated with delirium in older patients in surgical ICUs. Chest. 2009;135(1):18–25. doi: 10.1378/chest.08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care. 2008;12(3):S3–S3. doi: 10.1186/cc6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10(5):393–400. doi: 10.1159/000017177. [DOI] [PubMed] [Google Scholar]
- 21.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 22.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: The ‘ABCDE’ approach. Curr Opin Crit Care. 2011;17(1):43–49. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 23.Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness--Crossing the quality chasm. Chest. 2010;138(5):1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasilevskis EE, Pandharipande PP, Girard TD, Ely EW. A screening, prevention and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med. 2010;38(10 Suppl):683–691. doi: 10.1097/CCM.0b013e3181f245d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Crit Care Nurse. 2012;32(2):35–38. doi: 10.4037/ccn2012229. 40–47; quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kress JP, Pohlman AS, O’Connor M, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 27.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 28.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: A quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Needham DM. Mobilizing patients in the intensive care unit: Improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–1690. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 30.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf. 2005;31(5):243–248. doi: 10.1016/s1553-7250(05)31031-2. [DOI] [PubMed] [Google Scholar]
- 32.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 33.Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013 Aug 19; doi: 10.1097/CCM.0b013e318298a139. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balas M, Olsen K, Gannon D, et al. Safety and efficacy of the ABCDE bundle in critically-ill patients receiving mechanical ventilation. Crit Care Med. 2012;40(12S):1–328. [Google Scholar]
- 35.Olsen K, Burke W, Peitz G, et al. The ABCDE bundle reduces the incidence of delirium in non-mechanically ventilated patients. Crit Care Med. 2012;40(12S):1–328. [Google Scholar]
- 36.Jawa RS, Thorell W, Stothert J, Balas M. Paper presented at American Association for the Surgery of Trauma meeting. Kauai, HI: 2012. Delirium is prevalent in the ICU: A call for implementation of evidence-based guidelines. [Google Scholar]
- 37.Balas MC, Burke WJ, Gannon D, et al. Implementing the ABCDE bundle into everyday care: Opportunities, challenges and lessons learned for implementing the ICU pain, agitation and delirium guidelines. Crit Care Med. 2013;41(9):S116–S127. doi: 10.1097/CCM.0b013e3182a17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 41.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 42.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 44.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 45.Seymour CW, Pandharipande PP, Koestner T, et al. Diurnal sedative changes during intensive care: Impact on liberation from mechanical ventilation and delirium. Crit Care Med. 2012;40(10):2788–2796. doi: 10.1097/CCM.0b013e31825b8ade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brummel NE, Jackson JC, Girard TD, et al. A combined early cognitive and physical rehabilitation program for people who are critically ill: The activity and cognitive therapy in the intensive care unit (ACT-ICU) trial. Phys Ther. 2012;92(12):1580–1592. doi: 10.2522/ptj.20110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.