Abstract
Background
Data regarding autonomic function in restless legs syndrome (RLS) is limited to heart rate and blood pressure changes in cases having periodic limb movements (PLMS).
Methods
We compared autonomic symptoms of 49 subjects with RLS vs. 291 Controls using the SCOPA-Autonomic questionnaire (23 items in six domains scored 0–3). The total score and domain scores were transformed to 0 to 100 points. Subjects with neurodegenerative disorders (i.e. dementia, parkinsonism) were excluded.
Results
The RLS group was younger (mean±SD 77.9 ± 8.0 vs. 80.5 ± 7.9 yrs, p=.03) and had more women (84% vs. 69%, p=.04). The mean SCOPA-Aut Total score was higher in the RLS group compared with Controls (20 ± 11 vs. 16 ± 9, p= .005). Additionally the RLS group had abnormalities in GI, cardiovascular, and pupillomotor domains. When comparing the percentage of subjects with any complaint on individual questions (score of ≥ 1) the RLS group had a greater number of subjects with sialorrhea, constipation, early abdominal fullness, lightheadedness when standing, and heat intolerance.
Conclusions
Autonomic complaints, especially GI, cardiovascular, and oversensitivity to light, are significantly increased in subjects with RLS. Causes for autonomic dysfunction in RLS require further investigation.
Keywords: Restless Leg Syndrome, Autonomic symptoms
Introduction
Restless legs syndrome (RLS) is a relatively common neurological disorder with an estimated prevalence in the Western adult population of about 5–11%, is more common in women, and increases with age1,2. RLS is a clinical diagnosis with subjective criteria that include uncomfortable feelings in the legs that appear or worsen during periods of inactivity, occur at night, and are relieved by moving the legs3. Periodic limb movements of sleep (PLMS) and wakefulness (PLMW) are involuntary flexions of the legs that are present in about 90% of patients with RLS4,5.
While RLS is associated with sleep difficulties, there is little data regarding autonomic function in RLS. One study reviewed the evidence suggesting a relationship between RLS/PLMS and hypertension6, cardiovascular disease and cerebrovascular disease7. Another found a higher frequency of erectile dysfunction in men with RLS vs. controls8.
The objective of this study was to compare autonomic complaints in patients with RLS vs. controls using an autonomic questionnaire.
Methods
As part of the longitudinal study being conducted by the Arizona Parkinson’s Disease Consortium and the Banner Sun Health Research Institute Brain and Body Donation Program, RLS and control subjects undergo annual examinations including a comprehensive movement exam, full cognitive exam, and an assessment for autonomic function using the Scales for Outcome in Parkinson’s disease - Autonomic (SCOPA-AUT) questionnaire9. The SCOPA-AUT questionnaire is divided into subscores for the following domains: gastrointestinal, urinary, cardiovascular, thermoregulatory, pupillomotor, and sexual. An additional item assessed the use of medications. Each autonomic domain was scored by the frequency of occurrence with response options ranging from 0 (“never”) to 3 (“often”). All questions included symptoms within the past month, except for syncope (past 6 months)9.
This retrospective analysis included subjects with RLS compared to a non-RLS control population. Subjects with neurodegenerative disorders, including parkinsonism or dementia, were excluded. We selected each subject’s most recent autonomic examination with a valid SCOPA-Aut Total score. The SCOPA-Aut Total score and domain scores were considered to be valid if responses were provided for at least 75% of the items. The total score and domain scores were transformed to a scale from 0 to 100 points. Comparisons were made using the two-sample t test. Adjusted means were compared using a general linear model. The proportion of subjects with a score greater than zero was compared using the Pearson chi-square test.
Results
There were 49 subjects with RLS and 291 without RLS included in the analysis. The RLS group was younger and included more women (Table 1). The RLS rating scale score (0–40) at the time of autonomic testing was available for 48 of the RLS subjects. The mean RLS rating scale score was 8.8 (SD ±8.0, range 0–29). Scores were as follows (n): severe (5), moderate (15), mild (14), and none (14). Mean BMI did not differ substantially between the RLS and Control groups. Smoking, use of cholinesterase inhibitors, and use of antipsychotics also did not differ substantially between groups. Use of antidepressants, anxiolytics, and dopaminergic agents was more common in the RLS group than in the Control group. PLMS was also more common in the RLS group than in the Control group.
Table 1.
Demographics and Medications
| RLS | Control | Δ | P | |
|---|---|---|---|---|
| Age (y); mean (SD), N | 77.9 (8.0), 49 | 80.5 (7.9), 291 | −2.6 | .03 |
| Female | 41/49 (85%) | 201/291 (69%) | 0.15 | .04 |
| BMI (kg/m2); mean (SD), N | 25.5 (5.4), 40 | 25.8 (4.6), 242 | −0.3 | .71 |
| Ever Smoked | 6/15 (40%) | 55/109 (50%) | −0.10 | .45 |
| Current Smoker | 0/15 (0%) | 2/109 (2%) | −0.02 | >.99 |
| Cholinesterase Inhibitor | 2/36 (6%) | 8/186 (4%) | 0.01 | .67 |
| Antidepressant | 14/35 (40%) | 29/186 (16%) | 0.24 | .001 |
| Antipsychotic | 0/35 (0%) | 1/185 (1%) | −0.01 | >.99 |
| Anxiolytic/Sedative | 11/36 (31%) | 24/185 (13%) | 0.18 | .008 |
| Dopaminergic Agent | 6/36 (17%) | 4/186 (2%) | 0.15 | .002 |
| PLMS | 5/49 (10%) | 2/291 (1%) | 0.10 | .001 |
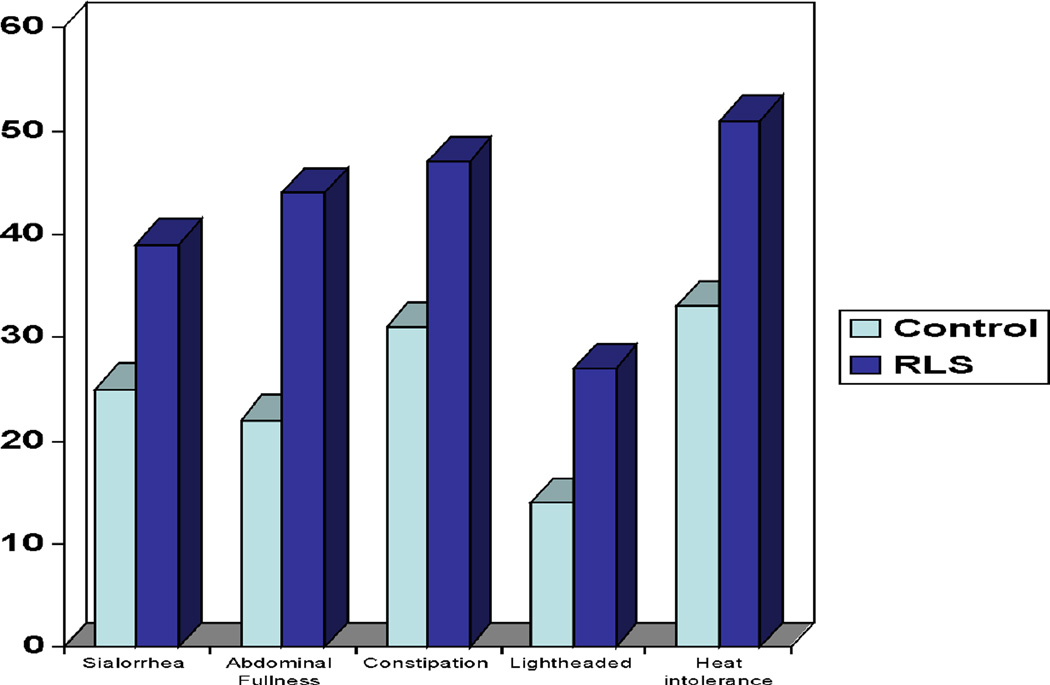
The mean SCOPA-Aut Total score was higher in the RLS group than Controls (Table 2). There were significant differences in GI, cardiovascular, and pupillomotor domains (Table 2). Urinary, thermoregulatory, and sexual function did not significantly differ. When comparing the percentage of subjects with any complaint on the individual questions (score of ≥ 1) RLS had a significantly greater number of subjects with sialorrhea (39% in RLS vs. 25% in control group, p=.046), constipation (47% vs. 31%, p=.03), early abdominal fullness (44% vs. 22%, p=.002), lightheadedness when standing (27% vs. 14%, p=.03), and heat intolerance (51% vs. 33%, p=.02) (Figure 1). Adjustment for age and sex, and adjustment for PLMS, did not substantially alter the mean difference in SCOPA-Aut scores between the RLS and Control groups (Table 3). Adjustment for medication use slightly reduced, but did not eliminate, the difference between groups.
Table 2.
SCOPA-Aut.
| RLS Mean (SD), N |
Control Mean (SD), N |
P | |
|---|---|---|---|
| Total (0–100) | 20 (11), 49 | 16.0 (9.1), 291 | .005 |
| Gastrointestinal (0–100) | 14 (11), 49 | 10.1 (9.2), 283 | .005 |
| Swallowing/Choking | 0.36 (0.60), 49 | 0.24 (0.50), 290 | .19 |
| Sialorrhea | 0.55 (0.84), 49 | 0.28 (0.51), 287 | .002 |
| Dysphagia | 0.31 (0.58), 49 | 0.21 (0.47), 289 | .23 |
| Early Abdominal Fullness | 0.46 (0.54), 48 | 0.25 (0.49), 284 | .007 |
| Constipation | 0.55 (0.65), 49 | 0.40 (0.66), 286 | .13 |
| Straining for Defecation | 0.63 (0.64), 49 | 0.61 (0.65), 284 | .79 |
| Fecal Incontinence | 0.16 (0.37), 49 | 0.18 (0.45), 285 | .86 |
| Urinary (0–100) | 31 (19), 49 | 28 (16), 289 | .12 |
| Urinary Urgency | 0.84 (0.87), 49 | 0.64 (0.71), 288 | .08 |
| Urinary Incontinence | 0.67 (0.81), 48 | 0.58 (0.68), 288 | .44 |
| Incomplete Emptying | 0.62 (0.67), 48 | 0.51 (0.70), 290 | .29 |
| Weak Stream of Urine | 0.71 (0.76), 49 | 0.59 (0.73), 291 | .29 |
| Frequency | 1.04 (0.76), 49 | 1.00 (0.78), 289 | .76 |
| Nocturia | 1.78 (0.82), 49 | 1.64 (0.75), 290 | .25 |
| Cardiovascular (0–100) | 9 (14), 48 | 4.6 (9.8), 286 | .02 |
| Lightheaded Standing Up | 0.38 (0.64), 48 | 0.23 (0.53), 287 | .10 |
| Lightheaded Standing for Some Time | 0.33 (0.62), 49 | 0.17 (0.45), 290 | .03 |
| Syncope | 0.06 (0.24), 49 | 0.02 (0.13), 290 | .06 |
| Thermoregulatory (0–100) | 16 (14), 49 | 12 (14), 289 | .06 |
| Hyperhidrosis During the Day | 0.29 (0.58), 49 | 0.27 (0.60), 287 | .87 |
| Hyperhidrosis During the Night | 0.33 (0.69), 49 | 0.27 (0.56), 291 | .54 |
| Cold Intolerance | 0.65 (0.90), 49 | 0.41 (0.65), 287 | .02 |
| Heat Intolerance | 0.61 (0.70), 49 | 0.44 (0.71), 285 | .12 |
| Pupillomotor (0–100) | 33 (34), 48 | 21 (26), 289 | .003 |
| Oversensitive to Bright Light | 1.0 (1.0), 48 | 0.62 (0.79), 289 | .003 |
| Sexual - Men (0–100) | 50 (17), 3 | 49 (33), 58 | .95 |
| Erection Problem | 1.67 (0.58), 3 | 1.6 (1.0), 70 | .97 |
| Ejaculation Problem | 1.33 (0.58), 3 | 1.4 (1.1), 58 | .86 |
| Sexual - Women (0–100) | 40 (32), 12 | 25 (27), 39 | .10 |
| Vaginal Lubrication | 1.2 (1.3), 12 | 0.84 (0.95), 45 | .23 |
| Problem with Orgasm | 1.1 (1.1), 15 | 0.62 (0.81), 40 | .11 |
Figure 1.
Percentage of subjects with a SCOA-Aut item score greater than zero.
Table 3.
Difference (P) between RLS and Control.
| Not Adjusted |
Adjusted Age and Sex |
Adjusted Antidepressants |
Adjusted Anxiolytics |
Adjusted Dopaminergics |
Adjusted PLMS |
|
|---|---|---|---|---|---|---|
| Total (0–100) | 4.1 (.005) | 4.3 (.004) | 3.0 (.10) | 3.5 (.05) | 3.7 (.05) | 4.3 (.004) |
| Gastrointestinal (0–100) | 4.2 (.005) | 4.1 (.006) | 3.2 (.09) | 3.6 (.05) | 3.2 (.09) | 4.6 (.003) |
| Swallowing/Choking | 0.11 (.19) | 0.08 (.30) | 0.02 (.84) | 0.06 (.47) | 0.05 (.59) | 0.09 (.26) |
| Sialorrhea | 0.28 (.002) | 0.32 (<.001) | 0.35 (.002) | 0.36 (.001) | 0.32 (.005) | 0.32 (.001) |
| Dysphagia | 0.09 (.23) | 0.09 (.26) | −0.01 (.91) | 0.03 (.73) | 0.02 (.78) | 0.10 (.22) |
| Early Abdominal Fullness | 0.21 (.007) | 0.21 (.009) | 0.19 (.06) | 0.22 (.03) | 0.17 (.08) | 0.21 (.008) |
| Constipation | 0.16 (.13) | 0.14 (.17) | 0.15 (.25) | 0.13 (.32) | 0.14 (.28) | 0.20 (.06) |
| Straining for Defecation | 0.03 (.79) | 0.03 (.76) | −0.07 (.61) | −0.06 (.64) | −0.08 (.53) | 0.03 (.75) |
| Fecal Incontinence | −0.01 (.86) | −0.02 (.73) | 0.01 (.93) | 0.00 (.99) | 0.01 (.90) | −0.01 (.85) |
| Urinary (0–100) | 3.9 (.12) | 4.7 (.06) | 4.1 (.19) | 4.0 (.19) | 4.4 (.16) | 4.2 (.10) |
| Urinary Urgency | 0.20 (.08) | 0.20 (.08) | 0.22 (.14) | 0.18 (.20) | 0.18 (.21) | 0.23 (.048) |
| Urinary Incontinence | 0.08 (.44) | 0.07 (.49) | 0.13 (.34) | 0.10 (.46) | 0.10 (.49) | 0.17 (.12) |
| Incomplete Emptying | 0.11 (.29) | 0.14 (.20) | 0.13 (.34) | 0.14 (.29) | 0.17 (.21) | 0.07 (.53) |
| Weak Stream of Urine | 0.12 (.29) | 0.20 (.06) | 0.15 (.31) | 0.15 (.29) | 0.12 (.39) | 0.05 (.65) |
| Frequency | 0.04 (.76) | 0.04 (.73) | −0.04 (.77) | −0.02 (.87) | 0.00 (.97) | 0.05 (.67) |
| Nocturia | 0.13 (.25) | 0.17 (.16) | 0.14 (.33) | 0.16 (.27) | 0.21 (.15) | 0.16 (.20) |
| Cardiovascular (0–100) | 3.9 (.02) | 3.7 (.03) | 1.0 (.62) | 2.4 (.23) | 2.9 (.14) | 3.9 (.02) |
| Lightheaded Standing Up | 0.14 (.10) | 0.13 (.13) | −0.02 (.88) | 0.06 (.59) | 0.09 (.43) | 0.13 (.13) |
| Lightheaded Standing for Some Time | 0.16 (.03) | 0.15 (.047) | 0.06 (.49) | 0.11 (.22) | 0.13 (.13) | 0.17 (.03) |
| Syncope | 0.04 (.06) | 0.04 (.07) | 0.04 (.20) | 0.03 (.22) | 0.03 (.27) | 0.05 (.05) |
| Thermoregulatory (0–100) | 4.1 (.06) | 2.5 (.24) | 2.6 (.32) | 3.1 (.22) | 4.1 (.11) | 3.1 (.16) |
| Hyperhidrosis During the Day | 0.01 (.88) | −0.04 (.64) | −0.03 (.82) | −0.03 (.77) | 0.01 (.96) | −0.06 (.50) |
| Hyperhidrosis During the Night | 0.06 (.54) | 0.02 (.79) | 0.02 (.87) | 0.01 (.89) | 0.02 (.88) | 0.04 (.67) |
| Cold Intolerance | 0.25 (.02) | 0.20 (.06) | 0.14 (.27) | 0.21 (.10) | 0.24 (.06) | 0.27 (.02) |
| Heat Intolerance | 0.17 (.12) | 0.12 (.29) | 0.17 (.22) | 0.18 (.19) | 0.22 (.11) | 0.13 (.25) |
| Pupillomotor (0–100) | 12.8 (.003) | 12.6 (.004) | 7.5 (.17) | 10.1 (.06) | 12.3 (.03) | 15.4 (.001) |
| Oversensitive to Bright Light | 0.38 (.003) | 0.38 (.004) | 0.22 (.17) | 0.30 (.06) | 0.37 (.03) | 0.46 (.001) |
Discussion
Given that data regarding autonomic dysfunction in patients with RLS is very limited this study establishes that subjects with RLS have subjective complaints in multiple autonomic areas that are greater than a similarly assessed control population. Previously, one study found a higher frequency of erectile dysfunction in men with RLS8. There were 23,119 men health professionals age 45–75 (22,175 controls, 549 with RLS 4–15 times/month, and 395 with RLS more than 15 times/month) who participated in the Health Professional Follow-up Study. According to study results 52.9% of RLS patients reported erectile dysfunction, vs 40.3% of men without RLS8. No other investigations of autonomic function in RLS have been reported to our knowledge. There is data showing an association between RLS and hypertension6 and between periodic limb movements of sleep and hypertension10. Patients with PLMS have increases of blood pressure, heart rate, and wakefulness suggestive of autonomic dysfunction in patients with PLMS11.
The pathophysiology of RLS is unclear. One study hypothesizes that hypofunction of the A11 diencephalospinal pathway (one of the dopaminergic pathways of the brain that innervates preganglionic sympathetic neurons and the dorsal horn in the spinal cord) leads to enhanced sympathetic outflow to the periphery7. At the same time hypofunction of the A11 diencephalospinal pathway results in enhancing sensory inputs, by inadequate suppression of sensory afferents leading to RLS7.
One study showed periodic limb movements of sleep, with and without EEG arousal, have a greater increase in blood pressure, than periodic limb movements while awake12. Several other studies identified no relationship between RLS and hypertension, however not all these studies used polysomnography to evaluate for PLMs13–17. About 80–90% of RLS patients have PLMS among their symptoms, but PLMS are also common in subjects without RLS, especially the elderly population18. Therefore it is difficult to conclude the impact of RLS alone or the combination of RLS and PLMs on raising blood pressure.
The limitations of this study include; small sample size, lack of polysomnogram data to evaluate for PLMs, detailed medication history at timing of the autonomic questionnaires, and contribution of co-existed conditions such as lower extremity venous insufficiency, neuropathy, or arthritis pain in this elderly population.
In conclusion, using a questionnaire methodology, this study found that subjects with RLS had more autonomic complaints than a similarly assessed control population. This study supports the need for objectively assessing autonomic function in RLS subjects, especially those with detected dysfunction by SCOPA-Aut questionnaires.
Acknowledgments
Funding
This study was funded by the Arizona Biomedical Research Commission (contracts 4001, 05-901, 0011, and 1001), the Michael J. Fox Foundation for Parkinson’s Research (Prescott Family Initiative), the Arizona Department of Health Services (contract 211002), the National Institute on Aging (P30 AG19610), the Mayo Clinic Foundation for Medical Education and Research, and the Sun Health Foundation.
References
- 1.Allen R, Walters A, Montplaisir J, et al. Restless Leg Syndrome Prevalence and Impact: REST General Population Study. Arch Intern Med. 2005;165:1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Hening W, Allen R, Chadhuri K, et al. Clinical Significance of RLS. Movement Disorders. 2007;22(Suppl. 18):S395–S400. doi: 10.1002/mds.21665. [DOI] [PubMed] [Google Scholar]
- 3.Allen R, Picchietti D, Hening W, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 4.Trotti L, Bliwise D, Greer S, et al. Correlates of PLMs variability over multiple nights and impact upon RLS diagnosis. Sleep Med. 2009;6:668–671. doi: 10.1016/j.sleep.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for the recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–183. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Batool-Anwar S, Malhoutra A, Forman J, et al. Restless legs syndrome and hypertenstion in middle-aged women. Hypertension. 2011;58:791–796. doi: 10.1161/HYPERTENSIONAHA.111.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walters A, Rye D. Review of the Relationship of Restless Legs Syndrome and Periodic Limb Movements in Sleep to Hypertension, Heart Disease, and Stroke. Sleep Med. 2009;32:589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao X, Schwarzschild M, O’Reilly E, et al. Restless Legs Syndrome and Erectile Dysfunction. Sleep. 2010;33:75–79. doi: 10.1093/sleep/33.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visser M, Marinus J, Stiggelbout A, et al. Assessment of Autonomic Dysfunction in Parkinson’s Disease: The SCOPA-AUT. Mov Disord. 2004;19:1306–1312. doi: 10.1002/mds.20153. [DOI] [PubMed] [Google Scholar]
- 10.Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51:103–107. doi: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui F, Strus J, et al. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–1930. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Pennestri MH, Montplaisir J, Colombo R, et al. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–1218. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 13.Phillips B, Hening W, Britz P, et al. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 14.Winkelman J, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7:545–552. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Winkelman J, Shahar E, Sharief I, et al. Associations of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 16.Hogl B, Kiechl S, Willeit J, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64:1920–1924. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 17.Rothdach A, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg elderly. Neurology. 2000;54:1064–1068. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 18.Ohayon M, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]