Introduction
Temporal processing deficits in schizophrenia have been reported since the early twentieth century and continue to interest investigators (Carroll et al., 2008; Davalos et al., 2003; Fisher, 1929; Rammsayer, 1990). Motivating this interest is the fact that temporal processing is central to many aspects of human cognition. The ability to form a time perspective helps us adapt to environmental events while also allowing us to plan for the future. Accurately judging elapsed time has been associated with relatively basic tasks such as planning and sequencing to higher order processes that are involved in driving, sports, music, or understanding cues that predict later events (Ferrandez et al. 2003; Macar, 2006; Mangels et al. 1998; Tracy et al. 1998). Navon (1978) argued that our perception of the world consists of a hierarchy of dimensions and that time occupies the highest level of that hierarchy.
The primacy of timing in human cognition has led investigators to suggest that its dysfunction may be at the root of deficits in planning and aspects of decision making that have long been observed in schizophrenia (Macar & Vidal, 2009; Volz, et al., 2001). Carroll and colleagues (2008) have suggested that timing deficits also can be conceptualized in the context of the temporal coordination of information processing. A disturbance in this process, referred to as cognitive dysmetria, may underlie many symptoms of schizophrenia, including hallucinations, delusions, and disorganized speech and behavior (Andreasen et al. 1998). As such, understanding the etiology of timing deficits in schizophrenia may provide important insights into disease pathology.
Previous studies suggest that temporal processing deficits in schizophrenia occur at multiple levels. A study evaluating pre-attentive event-related potentials (ERPs) demonstrated that timing-related responses at an “easy” level of difficulty in patients with schizophrenia did not differ from those in healthy comparison subjects (Davalos et al., 2005). These same evoked responses did differ, however, under more “difficult” conditions. Despite a task-difficulty-related difference in early evoked responses, behavioral judgments were impaired under both difficulty levels. The results suggest that the fidelity of timing information can be preserved at some level, possibly during initial processing, but is not appropriately processed at later stages.
Functional magnetic resonance imaging (fMRI), which provides information about the task-related responses of large populations of neurons across the brain, is well-suited to further investigate the nature of timing deficits in schizophrenia. To date, however, only one prior fMRI study has examined timing in the disorder (Volz et al. 2001). With performance level matched between groups, Volz and colleagues observed decreased prefrontal cortex and striatum timing-related responses in patients with schizophrenia, relative to comparison subjects. This finding was interpreted as evidence for deficits in both central timing mechanisms (striatum) and working memory and attention (prefrontal cortex).
The present study builds on the work of Volz and colleagues in three ways. First, we employ a task with varying levels of difficulty. Fletcher and colleagues (1999) have asserted that studies focusing on clinical populations may benefit from inclusion of a wide range of difficulty-levels to detect whether dysfunctional performance reflects a gross inability to perform a specific type of task or whether deficits are only observed on more difficult conditions (Fletcher et al. 1999). The inclusion of multiple levels of difficulty also improves our ability to interpret task-related responses. Second, the present study includes a larger cohort of patients and healthy comparison subjects. Third, greater sensitivity is afforded in the present study by the use of a 3 Tesla scanner, compared to 1.5T scanner used previously. The present study was designed to test the hypothesis that individuals with schizophrenia will exhibit impaired neuronal responses during temporal processing independently of whether there are significant differences in behavior between groups.
Experimental Materials and Methods
Subjects
Participants consisted of twenty individuals who met DSM-IV criteria for schizophrenia and twenty healthy comparison subjects matched for age (18–55 years), gender, and right-handedness. Patient diagnoses were made by two raters following interviews with the Diagnostic Instrument for Genetic Studies (DIGS). The DIGS includes the Structured Clinical Interview for DSM-IV in addition to other qualitative and quantitative assessment. Two patients were neuroleptic naive, five were treated with first-generation neuroleptics (haloperidol, prolixin, thiothixene, and fluphenazine), and thirteen with a variety of second-generation neuroleptics including olanzapine, risperidone, and ziprasidone. All patients were clinically stable with a minimum illness duration of three years. Healthy comparison subjects were recruited from the community. Subjects in the comparison group participated in a previous fMRI study of temporal processing (Tregellas et al., 2006). Exclusion criteria included a current diagnosis of major depression, substance abuse, neurological disorders, head trauma, or any personal or first-degree family history of psychosis. Four schizophrenia participants and two control participants were excluded due to excess head motion (>1 mm) during scanning. The final group mean age was 48.4 (SD=6.8) for the schizophrenia subjects and 41.2 (SD= 9.7) for the comparison subjects. There was no significant difference in age between groups. There were seven women in the final control group and eight in the final schizophrenia group.
Subjects who smoked refrained from smoking for at least 30 min prior to the study. All volunteers provided written, informed consent approved by the University of Colorado IRB.
Task Design
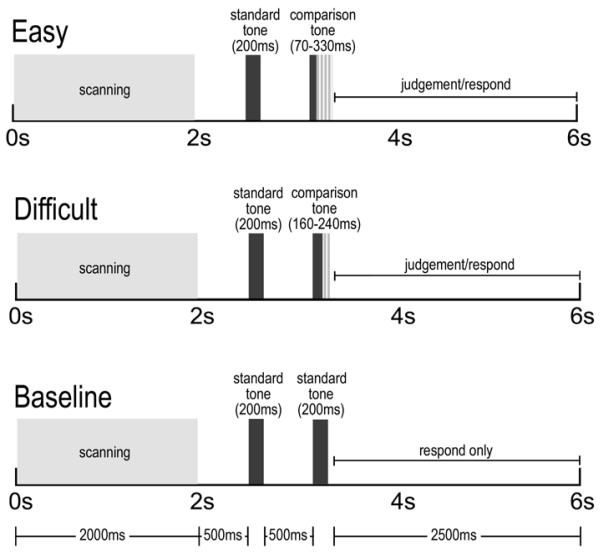
The methods used in this study were similar to those described previously (Tregellas, et al., 2006). Clustered volume fMR images were obtained while subjects performed an auditory time discrimination task. The task consisted of two discrimination conditions, “easy” and “difficult”, and a baseline condition (Fig. I). Categorizations of “easy” or “difficult” were based on behavioral accuracies in a previous study (Davalos et al., 2005). In the “easy” condition, participants were presented with two tones, the first 200 ms, the second either shorter (70 ms, 100 ms) or longer (300 ms, 330 ms) than the first, separated by 500 ms. Half of the comparison tones were shorter and half were longer and all were presented in a pseudo randomized order. Participants were asked to press one of two buttons on a handheld response pad to indicate whether the second tone was longer or shorter than the first tone. In the “difficult” condition, the duration of the second tone was more similar to the standard tone, either shorter (160 ms, 170 ms) or longer (230 ms, 240 ms). Finally, in the baseline condition, both tones were 200 ms. Participants were told the tones were identical and were instructed to simply press either button after they heard the pair of tones. Use of the clustered volume approach allowed stimuli to be presented and judgments made in the absence of scanner noise. Because of the hemodynamic response delay, neuronal activity can be measured by acquiring volumes several seconds following timing processes of interest. This technique substantially improves signal detection in auditory fMRI experiments (Edmister et al., 1999). Instructions to make a judgment or simply press a button (in the baseline condition) were presented via MR-compatible goggles (Resonance Technology, Inc.). The same screen was maintained throughout the run, with a change in text color indicating when subjects were to engage in each condition. MR-compatible headphones (Resonance Technology, Inc.) were used to present tones 500 ms after each echo-planar volume was acquired. Stimuli were complex tones (75 dB SPL) composed from 5 frequencies (0.125, 0.25, 0.50, 0.75 and 1 kHz), each with a 50 ms attack and 25 ms decay. The paradigm was written and presented in E-prime (Psychology Tools, Inc.). Timing for each discrimination/baseline event included 2-s scanning, 500 ms silence, 1-s stimulus presentation and 2.5 s to judge and/or respond, totaling 6 s (Fig. I). Each of two runs contained 10 blocks (40 presentations) of each condition, “easy”, “difficult” or baseline, presented in pseudo-randomized order across the run. There was a brief (approximately 30 seconds) pause between the runs. Subjects were not informed of the “easy” versus “difficult” block distinction during the run, but were told ahead of time that task difficulty varied throughout the experiment.
Figure I.
Schematic representation of experimental design. During the “easy” condition, the 200ms standard tone was followed by a comparison tone of 70, 100 300 or 330ms (+/- 50 or 65%). During the “difficult” condition, the comparison tone was 160,170, 230 or 240ms (+/− 15, 20%). During “baseline”, subjects pressed a button, but did not judge, following presentation of two 200ms tones.
MR Parameters
Studies were performed with on 3T GE MR system using a standard quadrature head coil. At the beginning of each scan, a high-resolution, T1-weighted anatomical scan was acquired. Functional images were acquired with a gradient-echo T2* Blood Oxygenation Level Dependant (BOLD) contrast technique, with TR=6000ms (as a clustered volume acquisition of 2000 ms, plus an additional 4000 ms silent interval), TE=30ms, FOV=220mm2, 642 matrix, 31 slices, 4mm thick, no gap, angled parallel to the planum sphenoidale. Additionally, one IR-EPI (TI=505ms) volume was acquired from each subject to improve coregistration between the functional and anatomical images.
Data analysis
Data were analyzed using SPM2 (Wellcome Dept. of Imaging Neuroscience, London). The first four image volumes were excluded for saturation effects. Data from each subject were realigned to the first volume, normalized to the Montreal Neurological Institute template, using a gray-matter-segmented IR-EPI as an intermediate to improve registration between the functional and anatomical images, and smoothed with an 8 mm FWHM Gaussian kernel. Data were modeled with a hemodynamic response function, using the general linear model in SPM2.
A whole-brain, random effects analysis was implemented by entering parameter estimates for each individual’s first level analysis (SPM contrast images) into second-level t-tests for each contrast of interest, “easy” – baseline, “difficult” – baseline, and “difficult” – “easy”. Group differences were assessed for the three contrasts. To improve statistical power, results were masked with a gray-matter mask.
Based on previous temporal processing research, a priori hypotheses about specific regions of interest (ROIs) were evaluated. ROIs included the supplementary motor area (Pastor, et al., 2004), insula/opercula (Bamiou, et al., 2006), striatum (Malapani, et al., 1998), thalamus (Stevens, et al., 2007), dorsolateral prefrontal cortex (DLPFC) (Rao, et al., 2001) and cerebellar vermis (Ivry, et al., 2002). Regions were defined anatomically based on the Wake Forest University Pickatlas (Maldjian, et al., 2003). The DLPFC ROI consisted of Brodmann Areas 9 and 46 combined, excluding the superior frontal gyrus. The thalamic ROIs included the entire anatomical structure. The mean response averaged across all voxels in each ROI was determined using the Marsbar toolbox (Brett, et al., 2002). Functional results are overlaid onto the group average T1-weighted anatomical images.
Results
Behavioral Results
Behavioral data collected during scanning were evaluated to assess accuracy and reaction times. No significant differences between groups were observed in terms of mean reaction times in the easy condition (controls = 589ms, SD= 163; schizophrenia group = 673ms, SD= 195) or the difficult condition (controls = 835ms, SD= 237; schizophrenia group = 841ms, SD= 210). In terms of the baseline condition, during which participants were simply asked to press either of the two buttons following presentation of identical stimuli, mean reaction time for the controls was 417ms (SD=204ms) compared to 524ms (SD=234ms) for the schizophrenia group (p=.09). The control group made significantly fewer errors than the schizophrenia group in both conditions. On the “easy” task, (based on 80 trials per participant), the control group’s mean percentage correct during easy trials was 98.89% (SD=1.48), versus 90.70% (SD=11.02) for the schizophrenia group (t=3.13, df =32, p<.005). For the “difficult” condition (based on 80 trials per participant), the control group (88.61%, SD=7.60) continued to perform significantly better than the schizophrenia group (75.31%, SD=13.40), t=3.61, df =32, p< .001.
fMRI Results
Hemodynamic responses during the temporal discrimination task in the healthy subjects were reported previously (Tregellas et al., 2006). In the current study, group differences in hemodynamic response (control >schizophrenia) were assessed for three contrasts. In the easy discrimination condition, individuals with schizophrenia showed reduced activation in the supplementary motor area (SMA), insular/opercular cortex, and dorsolateral prefrontal cortex (DLPFC) (Table I, Fig. II).
Table I.
MNI coordinates and statistics for brain regions with greater activation in controls compared to individuals with schizophrenia
| x | y | z | tvalue | P value | |
|---|---|---|---|---|---|
| Easy | |||||
| Operculum/Insula (L) | −36 | 24 | −3 | 1.79 | 0.042 |
| Pre-SMA (R) | 12 | 21 | 51 | 1.69 | 0.051 |
| DLPFC (L) | −48 | 9 | 36 | 1.88 | 0.035 |
| Difficult | |||||
| Operculum/Insula (R) | 51 | 24 | 0 | 2.06 | 0.03 |
| 30 | 24 | −6 | 2.25 | 0.016 | |
| Operculum/Insula (L) | 36 | 27 | −9 | 2.69 | 0.006 |
| Pre-SMA (R) | 12 | 24 | 51 | 2.8 | 0.004 |
| Putamen (R) | 18 | 12 | 6 | 1.85 | 0.37 |
| Putamen (L) | −24 | −3 | 3 | 2.24 | 0.016 |
| DLPFC (R) | 45 | 12 | 18 | 1.95 | 0.03 |
| DLPFC (L) | −45 | 12 | 36 | 1.77 | 0.043 |
| Thalamus (R) | 9 | −6 | 6 | 1.93 | .031 |
| Difficult-Easy | |||||
| Operculum/Insula (R) | 48 | 27 | 3 | 2.07 | 0.023 |
| Putamen (R) | 21 | 18 | 3 | 2.46 | >0.001 |
| Putamen (L) | −24 | 18 | −6 | 1.67 | 0.052 |
Figure II.
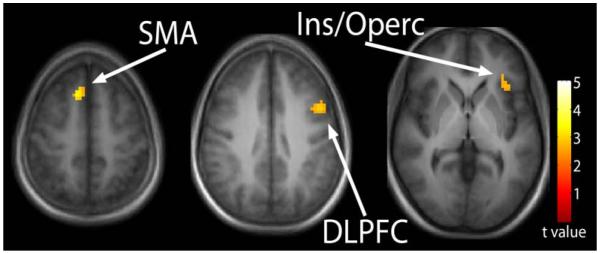
Temporal processing during “easy” temporal processing. Reduced activation in the schizophrenia group relative to controls was observed in the supplementary motor area (SMA), insula/opercular cortex, and the dorsolateral prefrontal cortex (DLPFC). Statistical parametric maps thresholded at p < 0.01, overlaid onto the average T1-weighted anatomy of all subjects.
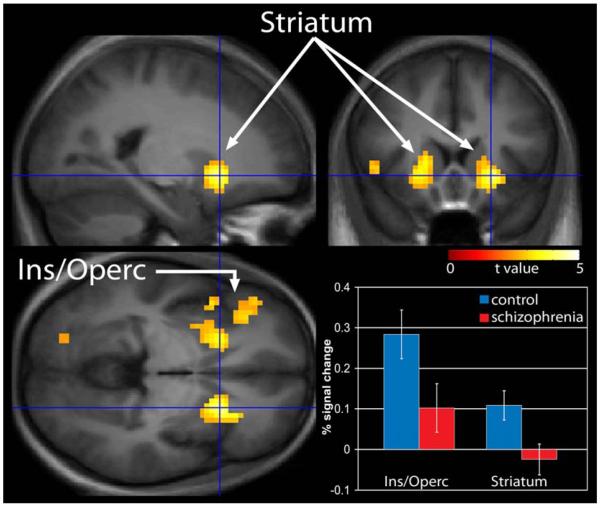
In the difficult discrimination condition, the schizophrenia group exhibited a more robust reduction in response in the SMA, insular/opercular cortex and DLPFC. In addition, reduced activation was observed in the striatum and thalamus (Fig. III). Results from the difficult–easy contrast indicate that the schizophrenia group exhibited reduced activation in the insular/opercular cortex and striatum (Fig. IV). Figure IV also shows the average percent signal change, relative the global mean, for the mean of all voxels in the insular/opercular cortex and striatum ROIs. Lastly, task related activation during the “difficult” condition compared to “easy” temporal processing condition in both healthy controls and patients with schizophrenia is presented (supplementary figure).
Figure III.
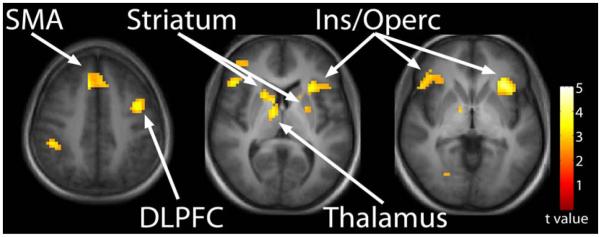
Temporal processing during “difficult” temporal processing. Reduced activation in the schizophrenia group relative to controls was observed in the SMA, insula/opercular cortex, and the DLPFC, as well as the striatum and thalamus. Statistical parametric maps thresholded at p < 0.01, overlaid onto the average T1-weighted anatomy of all subjects.
Figure IV.
“Difficult” compared to “easy” temporal processing. Reduced activation in the schizophrenia group relative to controls was observed in the insula/opercular cortex and the striatum. Statistical parametric maps thresholded at p < 0.01, overlaid onto the average T1- weighted anatomy of all subjects.
Two additional exploratory analyses of the difficult-easy contrast were performed to examine the possible effects of neuroleptics on results. Within the schizophrenia group, no differences were observed between the 14 neuroleptic-treated patients and the two neuroleptic-free patients, either in an ROA analysis, or a more liberal whole-brain analysis thresholded at a statistical threshold of p < 0.01, uncorrected. In addition, a comparison of the two unmedicated patients to healthy comparison subjects revealed insular/opercular and striatum deficits in the patients. This deficit was significant at p < 0.01, uncorrected, in an exploratory whole-brain analysis, and nearly significant (right insula/operculum t=1.61, p=0.062; left striatum t=1.42, p=0.086) in the more conservative ROI analysis.
To examine the possibility that activation differences between conditions were due to differences in processing time or accuracy, reaction times and response accuracies were evaluated as regressors in additional second level regression analyses. No significant results were observed, suggesting that activation differences were not related simply to differences in behavioral performance.
Discussion
The present study replicated previous findings of temporal processing deficits in schizophrenia (Carroll et al. 2008; Davalos, et al., 2002; Davalos, et al., 2003; Davalos, et al., 2005; Rammsayer, 1990; Tysk, 1990), and identified a network of brain regions that showed reduced temporal processing-related responses in patients with schizophrenia including the supplementary motor area (SMA), dorsolateral prefrontal cortex (DLPFC), striatum, thalamus and insula/operculum. A comparison of task-related responses at different difficulty levels suggests that deficits in striatum and insula/operculum function were especially pronounced in schizophrenia under conditions of high task difficulty.
Behaviorally, patients with schizophrenia performed less accurately during both conditions. This finding is consistent with results from a previous timing study that measured both ERPs and behavioral responses (Davalos, et al., 2005). In that study, pre-attentive ERPs were found to be sensitive to task-difficulty, but temporal judgments were impaired during all difficulty levels. These data are consistent with the notion that while early temporal processing in schizophrenia can be unimpaired at low difficulty levels, later stages of temporal processing, including temporal judgment, may be impaired more broadly in the disease. Some insight into the specificity of impaired temporal judgments in schizophrenia can be informed by the fMRI results described below.
The following sections focus on specific regions of the brain implicated in temporal processing deficits in schizophrenia, and the roles that each region is thought to play in timing. The (pre)SMA was found to be underactive in schizophrenia in both conditions. The SMA has repeatedly been shown to be a key structure involved; during temporal processing (Ferrandez et al. 2003; Macar et al., 2002; Rao et al., 2001; Tregellas et al., 2006), in the ‘pulse accumulation’ process (Macar et al., 2004), and in attending to an internal time-line against which timing comparisons can be made (Coull et al., 2004). Although our prior study in controls found recruitment of the SMA in temporal processing to be load-dependent (Tregellas et al., 2006), a group x difficultly interaction was not observed in the present study.
Decreased timing-related responses in patients with schizophrenia also were observed in the insular/opercular cortices across conditions, with more pronounced deficits in the difficult compared to the easy condition. The insular/opercular cortex is involved in many aspects of timing, including attention to time components (Coull et al. 2000; Coull et al. 2004), encoding interval sequence information (Schubotz et al. 2000), and duration perception (Maquet et al. 1996; Ferrandez et al. 2003; Lewis and Miall 2003; Hinton et al. 2004).
The dorsolateral prefrontal cortex (DLPFC) also showed reduced activity in patients with schizophrenia in both conditions. Involvement of the DLPFC in timing has been observed across a wide range of timing tasks, from duration perception (Rao et al. 2001; Lewis and Miall 2003; Smith et al. 2003) to interval time estimation (Macar et al. 2002; Basso et al. 2003) and motor timing (Rubia et al. 1998;Jancke et al. 2000). The specific role of the DLPFC in timing, however, is not clearly understood. In timing studies which involve longer-term durations, the DLPFC may be primarily involved in working memory, i.e. maintaining temporal information online for later comparisons. However, given that the DLPFC also is involved in paradigms which minimize working memory components (i.e. ISIs less than 500 ms used in the current study), we and others argue that reduced response in the region in schizophrenia is thought to reflect a timing-specific deficit (Smith et al. 2003; Tregellas et al., 2006).
Unlike in the DLPFC and SMA, timing-related responses in the thalamus were significantly diminished in schizophrenia only in the difficult condition, suggesting a load-dependent group difference. The thalamus previously has been identified as a key node of the fronto-striatal network that is involved in complex temporal processing tasks (e.g. motor sequencing and perceptual timing (in the range of hundreds of milliseconds) (Menon et al, 2000; Rubia & Smith, 2004). Rao and colleagues (2001) observed thalamic response during timing, but not pitch perception, and suggested a thalamic role in forming representations of time.
Finally, the striatum, specifically the putamen, was the region most impaired in schizophrenia, in terms of spatial extent of response differences. The striatum is thought to play a central role in temporal processing which has been demonstrated in studies using animal models, controls and populations with disorders affecting the striatum, such Parkinson’s disease (Maricq et al., 1981; Meck, 1996; Schubotz et al. 2000; Harrington et al., 1998; Ferrandez et al., 2003; Coull et al., 2004). Striatum involvement in temporal processing dysfunction in schizophrenia previously was observed in the one prior fMRI study of temporal processing deficits in schizophrenia (Volz et al., 2001).
As with the thalamus, timing-related responses in the striatum were reduced in the schizophrenia group only in the difficult condition, even at a substantially more liberal statistical threshold (whole brain p<0.05, uncorrected). This finding suggests that relatively easy temporal judgment deficits in schizophrenia may result from dysfunction of components of the temporal processing network other than the striatum.
Inferences about striatum dysfunction observed in the present study potentially are limited by neuroleptic use in patients with schizophrenia. Because animal studies have shown that dopamine agonists and antagonists can affect temporal processing, it is possible that neuroleptics, nearly all of which act on dopaminergic systems, may alter dopamine-dependent striatum function (Meck 1986. Maricq et al., 1981). Exploratory analyses evaluating two neuroleptic-free patients in the current study argue against this possibility, however. Striatum responses in the unmediated patients were not significantly different from mediated patients, and, like the neuroleptic-treated patients, were reduced when compared to healthy controls.
Another limitation of the current study is that because behavioral differences between the two groups were observed, we cannot rule out the possibility that some aspects of the differences in neuronal responses may have been affected by poor behavioral task performance. Past studies have suggested that when clinical populations (e.g. schizophrenia) are unable to behaviorally execute tasks, patterns of brain activation may represent compensatory mechanisms when they fail to successfully recruit brain regions necessary for cognitive tasks (Hugdahl et al., 2004).
Three conclusions can be drawn from this study. First, we conclude that individuals with schizophrenia exhibit timing deficits across easy and difficult conditions, a finding generally consistent with prior studies. Given previous work showing unimpaired early temporal processing in schizophrenia during relatively easy task difficulty, it is likely that the neuronal and behavioral deficits observed in this study involve later stages of temporal processing or duration judgment. Second, temporal processing deficits in schizophrenia involve a wide-spread network of brain regions, including the SMA, DLPFC, striatum, thalamus and insula/operculum. Lastly, response deficits in specific parts of this network, the striatum and insula/operculum, are highly load-dependent. This suggests that generalized timing deficits in schizophrenia may involve a broad network dysfunction, but that specific regions contribute to more demanding temporal judgment deficits. Future work is needed to determine the effect of neuroleptics on temporal processing deficits in schizophrenia, and whether timing deficits may be a type of cognitive dysfunction that can be ameliorated with rehabilitation and/or pharmacological therapy.
Supplementary Material
Supplementary Figure“Difficult” compared to “easy” temporal processing in healthy controls (top) and patients with schizophrenia (bottom). Statistical parametric maps thresholded at p < 0.001, overlaid onto the average T1-weighted anatomy of all subjects.
Acknowledgements
The authors thank Dr. Robert Freedman for critical comments during manuscript preparation, and Dr. Jody Tanabe for valuable methods contributions. This work was supported by the Office of National Drug Control Policy, NIH Silvio O. Conte Center grant 5 P50 MH068582 and NIH grant 5 R01 MH60214.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen N, Paradiso S, O’Leary D. “ Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Bamiou D, Musiek FE, Stow I, Stevens J, Cipolotti L, Brown MM, Luxon LM. Auditory temporal processing deficits in patients with insular stroke. Neurology. 2006;67:614–619. doi: 10.1212/01.wnl.0000230197.40410.db. [DOI] [PubMed] [Google Scholar]
- Barabasz A. Temporal orientation: A review of the literature. Child Study Journal. Mono. 1973;1-3:43–49. 1973. [Google Scholar]
- Basso G, Nichelli P, Wharton C, Peterson M, Grafman J. Distributed neural systems for temporal production: a functional MRI study. Brain Res Bull. 2003;59:405–411. doi: 10.1016/s0361-9230(02)00941-3. [DOI] [PubMed] [Google Scholar]
- Bauermeister JJ, Barkley RA, Martinez JV, Cumba E, Ramirez RR, Reina G, Matos M, Salas CC. Time estimation and performance on reproduction tasks in subtypes of children with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol. 2005;34:151–162. doi: 10.1207/s15374424jccp3401_14. [DOI] [PubMed] [Google Scholar]
- Bey CE, Zatorre RJ. Recognition of interleaved melodies. An fMRI study. Ann N Y Acad Sci. 2003;999:152–154. doi: 10.1196/annals.1284.017. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6, 2002.2002. [Google Scholar]; Available on CD-ROM in NeuroImage. 16:1032. [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Carroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick W. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condray R. Language disorder in schizophrenia as a developmental learning disorder. Schizophr Res. 2005;73:5–20. doi: 10.1016/j.schres.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat Neurosci. 2002;5:175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Buchel C, Nobre AC. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38:808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Coull JT, Walsh V, Frith CD, Nobre AC. Distinct neural substrates for visual search amongst spatial versus temporal distractors. Brain Res Cogn Brain Res. 2003;17:368–379. doi: 10.1016/s0926-6410(03)00138-1. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, Freedman R. Behavioral and electrophysiological indices of temporal processing dysfunction in schizophrenia. J Neuropsych and Clin Neurosci. 2005;17:517–525. doi: 10.1176/jnp.17.4.517. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, Ross RG. Deficits in auditory and visual temporal perception in schizophrenia. Cogn Neuropsychiatry. 2002;7:273–282. doi: 10.1080/13546800143000230. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, Ross R. Effects of interval duration on temporal processing in schizophrenia. Brain Cog. 2003;52:295–301. doi: 10.1016/s0278-2626(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Diener H. Clinical evidence for functional compartmentalization of the cerebellum. In: Bloedel J, Dichgans J, Precht W, editors. Cerebellar functions. Springer, Berlin, Heidelberg; New York: 1984. pp. 126–147. [Google Scholar]
- Eisler AD. The human sense of time: Biological, cognitive, and cultural considerations. In: Buccheri R, Saniga M, editors. The nature of time: Geometry, Physics and Perception. Kluwer Academic Publishers; Dordrecht: 2003. pp. 5–18. [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum. Brain Mapp. 1999;7:89–97. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandez AM, Hugueville L, Lehericy S, Poline JB, Marsault C, Pouthas V. Basal ganglia and supplementary motor area subtend duration perception: an fMRI study. Neuroimage. 2003;19:1532–1544. doi: 10.1016/s1053-8119(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Raichle ME, Miezin FM, Katz WF, Petersen SE. PET studies of auditory and phonological processing: effects of stimulus characteristics and task demands. J Cogn Neurosci. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- Fischer F. Zeitstruktur and Schizophrenie. Zeitschrift fur die gesamte Neurologie and Psychiatrie. 1929;121:544–574. [Google Scholar]
- Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel CR. Toward a neurobiology of temporal cognition: advances and challenges. Cur Opin Neurobio. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12:3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Harrington DL, Binder JR, Durgerian S, Rao SM. Neural systems supporting timing and chronometric counting: an FMRI study. Brain Res Cogn Brain Res. 2004;21:183–192. doi: 10.1016/j.cogbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Rishovd Rund B, Lund A, Asbjornsen A, Egeland J, Ersland L, Landro NI, Roness A, Stordal KI, Sundet K, Thomsen T. The American Journal of Psychiatry. 2004;161:286–293. doi: 10.1176/appi.ajp.161.2.286. [DOI] [PubMed] [Google Scholar]
- Ivry R, Hazeltine RE. Perception and production of temporal intervals across a range of durations: Evidence for a common timing mechanism. J of Exp Psychology: Human Perception and Performance. 1995;21:3–18. doi: 10.1037//0096-1523.21.1.3. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann. N. Y. Acad. Sci. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Jancke L, Loose R, Lutz K, Specht K, Shah NJ. Cortical activations during paced finger-tapping applying visual and auditory pacing stimuli. Brain Res Cogn Brain Res. 2000;10:51–66. doi: 10.1016/s0926-6410(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Kaminsky M, Eviatar Z, Norman J. The timing deficit hypothesis of dyslexia and its implications for Hebrew reading. Brain Cogn. 2002;48:394–398. [PubMed] [Google Scholar]
- Kraus N. Auditory pathway encoding and neural plasticity in children with learning problems. Audiol Neurootol. 2001;6:221–227. doi: 10.1159/000046837. [DOI] [PubMed] [Google Scholar]
- Lewis PA. Finding the timer. Trends Cogn Sci. 2002;6:195–196. doi: 10.1016/s1364-6613(02)01906-x. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003;41:1583–1592. doi: 10.1016/s0028-3932(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Livesey AC, Wall MB, Smith AT. Time perception: Manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45:321–331. doi: 10.1016/j.neuropsychologia.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Macar F, Anton JL, Bonnet M, Vidal F. Timing functions of the supplementary motor area: an event-related fMRI study. Brain Res Cogn Brain Res. 2004;21:206–215. doi: 10.1016/j.cogbrainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Macar F, Coull J, Vidal F. The supplementary area in motor and perceptual time processing: fMRI studies. Cog Processing. 2006;7:89–94. doi: 10.1007/s10339-005-0025-7. [DOI] [PubMed] [Google Scholar]
- Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, Maquet P. Activation of the supplementary motor area and of attentional networks during temporal processing. Exp Brain Res. 2002;142:475–485. doi: 10.1007/s00221-001-0953-0. [DOI] [PubMed] [Google Scholar]
- Macar F, Vidal F. Event related potentials as indices of time processing: A review. J of Psychophys. 2004;18:89–104. [Google Scholar]
- Macar F, Vidal F. Timing processes: an outline of behavioral and neural indices not systematically considered in timing models. Canadian Journal of Experimental Psychology. 2009;63:227–239. doi: 10.1037/a0014457. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitan B, Levy R, Meck W, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson’s disease: A dopamine-related dysfunction. J Cog Neuroscience. 1998;10:316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Brain Res Cogn Brain Res. 1998;7:15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, Timsit-Berthier M, Vidal F, Ferrara A, Degueldre C, Quaglia L, Delfiore G, Luxen A, Woods R, Mazziotta JC, Comar D. Brain activation induced by estimation of duration: a PET study. Neuroimage. 1996;3:119–126. doi: 10.1006/nimg.1996.0014. [DOI] [PubMed] [Google Scholar]
- McGee TJ, King C, Tremblay K, Nicol TG, Cunningham J, Kraus N. Long-term habituation of the speech-elicited mismatch negativity. Psychophysiology. 2001;38:653–658. [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol Biochem Behav. 1986;25:1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Navon D. On a conceptual hierarchy of time, space, and other dimensions. Cognition. 1978 Sep;6:228. [Google Scholar]
- Pastor MA, Day BL, Macaluso E, Friston KJ, Frackowiak RSJ. The functional neuroanatomy of temporal discrimination. J Neuroscience. 2004;24:2585–2591. doi: 10.1523/JNEUROSCI.4210-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel H, Price C, Baron JC, Wise R, Lambert J, Frackowiak RS, Lechevalier B, Eustache F. The structural components of music perception. A functional anatomical study. Brain. 1997;120(Pt 2):229–243. doi: 10.1093/brain/120.2.229. [DOI] [PubMed] [Google Scholar]
- Pouthas V, George N, Poline JB, Pfeuty M, Vandemoorteele PF, Hugueville L, Ferrandez AM, Lehericy S, Lebihan D, Renault B. Neural network involved in time perception: an fMRI study comparing long and short interval estimation. Hum Brain Mapp. 2005;25:433–441. doi: 10.1002/hbm.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammsayer T. Temporal discrimination in schizophrenic and affective disorders: Evidence for a dopamine-dependent internal clock. Int J of Neuroscience. 1990;53:111–120. doi: 10.3109/00207459008986593. [DOI] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, Bullmore E. Prefrontal involvement in “temporal bridging“ and timing movement. Neuropsychologia. 1998;36:1283–1293. doi: 10.1016/s0028-3932(98)00038-4. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp (Wars) 2004;64:329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Friederici AD, von Cramon DY. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. Neuroimage. 2000;11:1–12. doi: 10.1006/nimg.1999.0514. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Interval and ordinal properties of sequences are associated with distinct premotor areas. Cereb Cortex. 2001;11:210–222. doi: 10.1093/cercor/11.3.210. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Lidzba K, Rubia K. A right hemispheric frontocerebellar network for time discrimination of several hundreds of milliseconds. Neuroimage. 2003;20:344–350. doi: 10.1016/s1053-8119(03)00337-9. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits, for mental timekeeping. Hum Brain Mapp. 2007;28:394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JI, Monaco C, McMichael H, Tyson K, Chambliss C, Christensen HL, Celenza MA. Information-processing characteristics of explicit time estimation by patients with schizophrenia and normal controls. Percept Mot Skills. 1998;86:515–526. doi: 10.2466/pms.1998.86.2.515. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC. Effect of task difficulty on the functional neuroanatomy of temporal processing. Neuroimage. 2006;32:307–315. doi: 10.1016/j.neuroimage.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Treisman M, Faulkner A, Naish PL, Brogan D. The internal clock: evidence for a temporal oscillator underlying time perception with some estimates of its characteristic frequency. Perception. 1990;19:705–743. doi: 10.1068/p190705. [DOI] [PubMed] [Google Scholar]
- Tysk L. Estimation of time by patients with positive and negative schizophrenia. Percept Mot Skills. 1990;71:826. doi: 10.2466/pms.1990.71.3.826. [DOI] [PubMed] [Google Scholar]
- Volz H, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H. Time estimation in schizophrenia: an FMRI study at adjusted levels of difficulty. Neuroreport. 2001;313:316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Zakay D, Block RA. The role of attention in time estimation processes. In: Pastor MA, editor. Time, internal clocks, and movement. Elsevier; Amsterdam: 1996. pp. 143–164. [Google Scholar]
- Ziegler W, von Cramon D. Disturbed coarticulation in apraxia of speech: acoustic evidence. Brain Lang. 1986;29:34–47. doi: 10.1016/0093-934x(86)90032-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure“Difficult” compared to “easy” temporal processing in healthy controls (top) and patients with schizophrenia (bottom). Statistical parametric maps thresholded at p < 0.001, overlaid onto the average T1-weighted anatomy of all subjects.