Abstract
Sterile protection against malaria infection can be achieved through vaccination of mice and humans with whole Plasmodium spp. parasites. One such method, known as infection–treatment–vaccination (ITV), involves immunization with wild type sporozoites (spz) under drug coverage. In this work, we used the different effects of antimalarial drugs chloroquine (CQ) and artesunate (AS) on blood stage (BS) parasites to dissect the stage-specific immune responses in mice immunized with Plasmodium yoelii spz under either drug, as well as their ability to protect mice against challenge with spz or infected RBCs (iRBCs). Whereas CQ-ITV induced sterile protection against challenge with both spz and iRBCs, AS-ITV only induced sterile protection against spz challenge. Importantly, AS-ITV delayed the onset of BS infection, indicating that both regimens induced cross-stage immunity. Moreover, both CQ- and AS-ITV induced CD8+ T cells in the liver that eliminated malaria-infected hepatocytes in vitro, as well as Abs that recognized pre-erythrocytic parasites. Sera from both groups of mice inhibited spz invasion of hepatocytes in vitro, but only CQ-ITV induced high levels of anti-BS Abs. Finally, passive transfer of sera from CQ-ITV–treated mice delayed the onset of erythrocytic infection in the majority of mice challenged with P. yoelii iRBCs. Besides constituting the first characterization, to our knowledge, of AS-ITV as a vaccination strategy, our data show that ITV strategies that lead to subtle differences in the persistence of parasites in the blood enable the characterization of the resulting immune responses, which will contribute to future research in vaccine design and malaria interventions.
Introduction
Malaria remains one of the most prevalent global infectious diseases, causing a significant burden of morbidity and mortality in developing countries in tropical and subtropical areas (1). An effective and affordable vaccine and/or new drugs are required to save lives and to end this devastating disease. The eradication of malaria will require novel vaccination strategies that induce effective immune responses (2).
Protection of animals and humans against experimental challenge with Plasmodium sporozoites (spz) has been accomplished by immunization with whole parasites attenuated to prevent symptoms that associate with blood stage (BS) infection (reviewed in Ref. 3). For example, sterile protection in human volunteers can be achieved by exposure to ∼1,000 bites of irradiated (irr) mosquitoes infected with Plasmodium falciparum spz (4–6) or by i. v. administration of ∼450,000 irr-spz (7). In comparison, the alternative infection–treatment–vaccination (ITV) strategy, which involves administration of wild type (wt) malaria parasites under drug treatment, requires significantly less spz to elicit protection (8–10), suggesting that irr-spz are not as immunogenic as wt spz under drug coverage. For example, infection of naive individuals via 3 doses of 15 bites each of mosquitoes infected with wt P. falciparum under chloroquine (CQ) coverage induces long-term protection against challenge (11, 12). Despite these promising results, the severity of CQ resistance in the African continent precludes the use of CQ-ITV as an immunization strategy. The present malaria treatment strategy of choice consists of artemisinin-based combination therapy (1). Artemisinin derivatives such as artesunate (AS) are potent plant-derived antimalaria drugs that act rapidly against the intraerythrocytic BS of P. falciparum and P. vivax infection, and are effective for both CQ-sensitive and CQ-resistant parasites (13). Neither CQ nor AS affects the pre-erythrocytic stages of the malaria parasite; but whereas CQ kills Plasmodium parasites at the trophozoite stage, AS acts on all intraerythrocytic stages of BS development (14, 15).
Whole-parasite vaccination–mediated protection has been shown to involve both direct killing of infected hepatocytes by CD8+ T cells and Ab-mediated blocking of hepatocyte invasion and traversal by spz (5, 16–18). More recently, it was shown in a rodent malaria model of infection that CQ-ITV leads to Ab-mediated immunity against BS Ags resulting from transient exposure to BS parasites during the treatment regimen (19). In general, immunization with irr-spz or early-arresting genetically attenuated parasites (GAPs) exposes the host to a restricted range of spz and early liver stage (LS) parasite Ags, whereas immunization with ITV or late-arresting GAPs likely broadens the repertoire of Ags to include those expressed by late LS and early BS parasites (3, 20). This difference may explain why protection by CQ-ITV requires a much lower dose of wt spz as compared with irr-spz or early-arresting GAPs (21, 22).
To understand immune responses elicited by immunization with whole Plasmodium parasites in the presence and absence of BSs, we have used two drugs that have differential chemical effects on BS parasites. We identified a dose of AS-ITV that eliminates BSs, and compared it with a previously studied dose of CQ-ITV, which results in the persistence of BSs (8, 19, 23). This is the first time, to our knowledge, that these immunization strategies have been directly compared in terms of protection against different forms of the malaria parasite.
Materials and Methods
Mice and parasites
Female BALB/cJ or Swiss Webster mice (6–8 wk old) were purchased from Jackson Laboratory or Harlan, respectively. Animal handling was conducted following the protocol approved by Institutional Animal Care and Use Committee at Seattle Biomedical Research Institute. Both wt and GFP-luciferase Plasmodium yoelii 17XNL parasites were cycled between Swiss Webster mice and Anopheles stephensi mosquitoes. Infected mosquitoes were maintained on sugar water at 24°C and 70% humidity. P. yoelii spz were obtained by dissection of salivary glands from infected mosquitoes on day 14 postinfection (p.i.) with blood meal.
Vaccination
BALB/c mice were immunized 3 times by i.v. injection with 10,000 live P. yoelii spz at 4-wk intervals. Uninfected mosquito salivary gland debris was used to immunize mice in the mock control group. Mice were treated with AS or CQ for 10 consecutive days starting on the same day that mice received the spz vaccination. Chloroquine diphosphate salt (Sigma-Aldrich) was diluted in 1X PBS (Sigma-Aldrich), and artemisinin (Sigma-Aldrich) was dissolved in 5% sodium bicarbonate (Sigma-Aldrich) and then diluted in 1X PBS before use. Parasitemia was examined daily by thin blood smear and Giemsa staining, starting on day 3 after vaccination.
Quantitative PCR
RNA was extracted from 100-μl aliquots of blood collected from mice on days 1, 4, 8, and 14 after drug withdrawal (i.e., days 11, 14, 18, and 24 p.i.) using an RNeasy kit (Qiagen) following the manufacturer’s instructions. cDNA generation and quantitative PCR (qPCR) were performed as previously described (24). The P. yoelii 18S rRNA signal was normalized to that of mouse GAPDH.
Challenge
Before challenge, the absence of BS infection was confirmed by Giemsa staining of thin blood smears. Mice were challenged with 10,000 wt P. yoelii spz or with 20,000 BS parasite-infected RBCs (iRBCs) by i.v. injection 4 wk after the last immunization. BS infection was examined daily from days 3–24 postchallenge, and parasitemia was calculated as the percentage of iRBCs. Sterile protection was determined by the absence of BS infection on day 24 postchallenge.
Lymphocyte purification, staining, and flow cytometry analysis
Livers and spleens were collected 28 d after the first, second, and third vaccinations, and 6 days after challenge with spz. Lymphocytes were isolated as previously described with minor modifications (25). In brief, mice were euthanized by CO2, and the liver was perfused with 10 ml liver perfusion buffer (Life Technologies). After harvest, the liver was cut into small pieces in prewarmed liver digestion medium (Life Technologies) and incubated in a 37°C water bath for 45 min before passing through a 100-μm nylon cell strainer. The resulting cell pellet was resuspended in 44% Percoll solution (GE Healthcare) and underlaid with 67% Percoll buffer, followed by centrifugation at 500 × g for 20 min at room temperature. The lymphocyte-containing middle layer was harvested for downstream analysis.
Phenotypic analysis of lymphocytes was performed by flow cytometry using six-color mAb staining: Pacific Blue–conjugated anti-mouse CD3ε, eFluor605-conjugated anti-CD62L, PerCP anti-mouse CD8a, and PE/Cy5-conjugated anti-mouse CD127 Abs were purchased from Biolegend; FITC-conjugated anti-mouse CD4 Ab was ordered from BD Biosciences; and allophycocyanin-conjugated circumsporozoite protein (CSP; SYVPSAEQI) tetramer was obtained from the National Institutes of Health (NIH) Tetramer Core Facility. In brief, 106 cells were resuspended in FACS buffer (PBS supplemented with 2% heat shocked FBS; Gemini Bioproducts) and washed twice by centrifugation at 300 × g for 5 min Next, 25 μl diluted anti-mouse CD16/32 mAb (eBioscience) was added to each sample, which was then incubated on ice for 10 min. This was followed by the addition of 25 μl staining mixture containing the fluorophore-conjugated Abs described earlier and incubation on ice for 30 min. After two washes with FACS buffer, cells were fixed by incubating in 2% paraformaldehyde (Sigma-Aldrich) buffer for 20 min and resuspended in FACS buffer for measurement. Flow cytometry was performed using a BD LSR II Flow Cytometer (BD Biosciences), and data were analyzed using FlowJo software (Tree Star).
Inhibition of LS development assay
Lymphocytes obtained from mice livers on day 6 after the third dose of ITV were stained with PE/Cy7-conjugated anti-mouse CD3ε Ab, PerCP-Cy5.5–conjugated anti-mouse CD8a Ab, and FITC-conjugated anti-mouse CD4 Ab (BD Biosciences). After staining for 20 min and two washes as described earlier, the CD3+CD8+ T cells were sorted by flow cytometry. Purity of the CD8+ cell fraction was determined to be >95% by flow cytometry. Cells were diluted at a concentration of 50,000 per 100 μl in 10% FBS RPMI 1640 medium containing anti-CD28 Ab (5 μg/ml; Biolegend) and hIL-2 (10 ng/ml; Promega). Cells were then transferred into a 96-well plate and incubated at 37°C in 5% CO2 for 3 d. Next, cells were transferred into a 6-well plate with 2 ml 10% FBS RPMI 1640 containing additional hIL-2 (at 10 ng/ml), and cultured for a further 3–4 d.
Primary hepatocytes were harvested from BALB/c mice 2 d before performing the killing assay, as previously described (18). Isolated hepatocytes were diluted in William’s E medium (Sigma-Aldrich) supplemented with 10% FBS at a concentration of 400 cells/μl. Next, 100 μl of this cell suspension was added to each well of a 96-well tissue culture plate (Becton Dickinson Labware) and incubated overnight at 37°C in 5% CO2. The next day, hepatocyte cultures were infected with 30,000 GFP-luciferase P. yoelii wt spz. Three hours p.i., plates were shaken gently, the supernatant was aspirated, and fresh culture medium was added. Infected cultures were further incubated for 24 h at 37°C in 5% CO2. Various dilutions of expanded CD8+ T cells were added to the infected hepatocytes and incubated for an additional 16 h. Finally, luminescence units were measured using a Centro XS3 LB 960 Microplate Luminometer under the Bright-Glo Luciferase Assay System protocol (Promega). The percentage inhibition was calculated as 1 − OD of test sample/mean OD of medium.
Immunofluorescence assay and ELISA
Sera were collected from immunized mice on day 9 after the last immunization. Ab titers were determined by immunofluorescence assay (IFA) using paraformaldehyde-fixed spz, LS parasites, or iRBCs, respectively, as previously described (8). For the MSP1 and schizont lysate ELISAs, Immulon 4 HBX 96-well plates were coated with 100 μl of 0.5 μg/ml yP.y.MSP1-19(XL)/VQ1 (MRA-48, deposited by D.C. Kaslow) or with 50 μl schizont lysate (5 μg/ml in PBS) prepared as previously described (26). Plates were incubated in blocking buffer (5% milk in PBS with 0.05% Tween 20; Fisher) for 1 h at room temperature. For the MSP1 ELISA, serum samples were serially diluted in blocking buffer, and for the schizont lysate ELISA, serum samples were diluted at 1:10 in blocking buffer. After a 1-h incubation at room temperature, 100 μl rabbit anti-mouse IgG (Sigma) diluted 1:400 in blocking buffer was added to each well, and plates were incubated for an additional 30 min. For the CSP ELISA, maleimide-activated plates (Thermo Scientific Pierce) were coated with 2 ng/ml PyCSP peptide (QGPGAPQGPGAPQGPGAP) in 100 μl of 1× PBS, and the ELISA was performed as per the manufacturer’s instruction. All plates were read using a SpectraMax M2 Microplate reader.
Inhibition of spz invasion assays
Thirty thousand GFP-luciferase P. yoelii wt spz were coincubated for 20 min at room temperature with serum collected on day 9 after each vaccination and diluted 1:10 in RPMI. Next, the serum-incubated spz were transferred into 96-well plates that were preseeded with 30,000 HepG2 cells the previous day. Forty hours p.i., cells were lysed by incubation with 100 μl Glo lysis buffer (Promega) containing 0.5% Triton X. Luminescence units were measured as described earlier.
Passive transfer
Serum from AS-ITV or CQ-ITV mice immunized with three doses of wt P. yoelii spz (or mock immunized) was collected on day 9 after the last immunization and pooled. Naive mice were injected by i.v. with 300 μl pooled serum and challenged i.v. with 10,000 P. yoelii spz or 20,000 P. yoelii iRBCs 24 h postinjection, as described previously (26). Parasitemia was examined daily by thin blood smear and Giemsa staining, starting on day 3 postvaccination.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analysis was performed using Prism software version 5.0 (GraphPad), and means were compared by two-tailed Student t tests or one-way ANOVA. A p value <0.05 was considered statistically significant. Analysis of FACS data was performed using FlowJo software (Tree Star).
Results
AS-ITV, but not CQ-ITV, eliminates BS infection after vaccination with wt P. yoelii spz
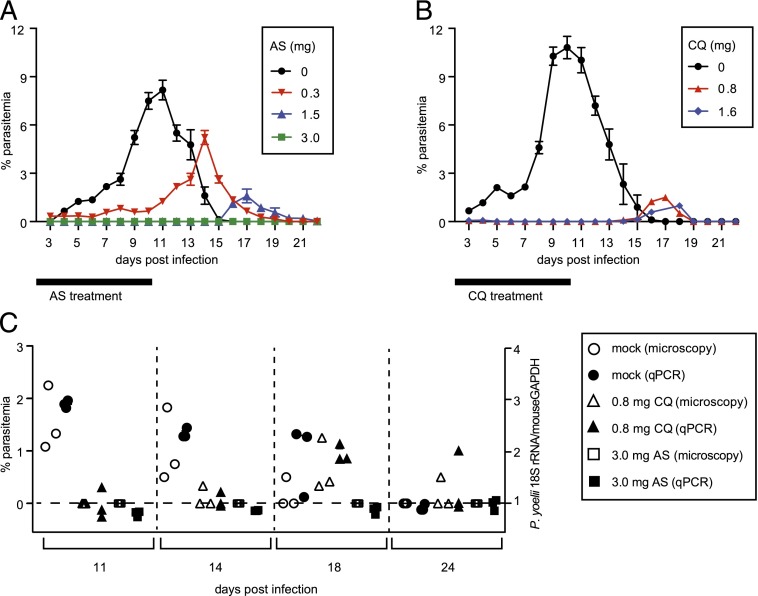
First, we sought to select a chemoprophylactic dose of AS that eliminates BS forms. To do this, we infected BALB/c mice with 10,000 wt P. yoelii spz isolated from the salivary glands of infected A. stephensi mosquitoes by i.v. injection and treated them with either 0.3, 1.5, or 3.0 mg AS daily from days 1–10 p.i. (Fig. 1A). BS infection was monitored daily for 3 wk by thin blood smear microscopy. Mock-treated mice developed BS infection at day 4 p.i., with parasitemia levels peaking on day 11. Similarly, mice treated with 0.3 mg AS became patent on day 3, although parasitemia levels were lower overall and the peak of parasitemia was delayed by 3–4 d as compared with that of mock-treated mice. Mice treated with 1.5 mg AS displayed a low-level burst of patent parasitemia 4–5 d after drug withdrawal. Mice treated with 3 mg AS displayed no evidence of BS infection, indicating that 3 mg AS constitutes an optimal dose to eliminate BS infection.
FIGURE 1.
AS-ITV can control BS parasites, whereas CQ-ITV allows the persistence of BS parasites. (A) Groups of mice infected with 10,000 wt P. yoelii spz by i.v. injection were mock treated (black circles, n = 3) or treated with either 0.3 (red inverted triangles, n = 5), 1.5 (blue triangles, n = 5), or 3.0 mg (green squares, n = 5) AS for 10 d after the infection (black bar). The y-axis shows the percentage of erythrocytes infected with parasites, as determined by microscopy of Giemsa-stained thin blood smears, and the x-axis corresponds to days p.i. (B) Groups of mice infected as described earlier were mock treated (black circles, n = 8) or treated with either 0.8 (red triangles, n = 10) or 1.6 mg (blue diamonds, n = 10) CQ for 10 d after the infection (black bar). (C) Blood samples taken on days 11, 14, 18, and 24 p.i. with P. yoelii spz as described in (A) were analyzed by thin blood smear microscopy and by qPCR quantitation of P. yoelii 18S rRNA versus the mouse GAPDH to determine the presence of parasites. The left y-axis shows percentage of erythrocytes infected with parasites as determined by microscopy of Giemsa-stained thin blood smears, the right y-axis shows the ratio of P. yoelii 18S rRNA versus mouse GAPDH detected by qPCR, and the x-axis indicates days p.i.
We also treated mice with 0.8 mg CQ, the previously established chemoprophylaxis dose for this drug in the rodent malaria model (8). Similarly to the results observed with 1.5 mg AS, these mice developed a low-level burst of patent parasitemia 4–5 d after drug withdrawal (Fig. 1B). Doubling the dose to 1.6 mg CQ did not abrogate this low-level burst of parasitemia, in agreement with a recent report (19). This result agrees with the known mechanism of action of CQ, which only affects late trophozoites and schizonts, allowing for the persistence of ring and early trophozoites in the iRBCs during the CQ treatment phase that become patent after drug withdrawal (15, 19).
We repeated this experiment in additional groups of P. yoelii–immunized mice treated for 10 d with 0.8 mg CQ, 3.0 mg AS (or mock treated), and observed a close correspondence between the parasitemia levels determined by blood smear and the detection of P. yoelii 18S rRNA by qPCR on blood samples taken on days 11, 14, 18, and 24 postimmunization, as compared with that of mouse GAPDH (Fig. 1C).
In conclusion, we identified a dose of AS (3.0 mg) that completely abolishes BS forms as determined both by Giemsa staining of thin blood smears and by parasite RNA qPCR, as expected from the mode of action of this drug on BS Plasmodium parasites (15). Thus, next we compared the protective immunity induced by AS-ITV with that of CQ-ITV, which results in the exposure of the host immune system to replicating BS forms.
AS-ITV induces protection only to pre-erythrocytic malaria infection, whereas CQ-ITV provides protection from both pre-erythrocytic and erythrocytic infections
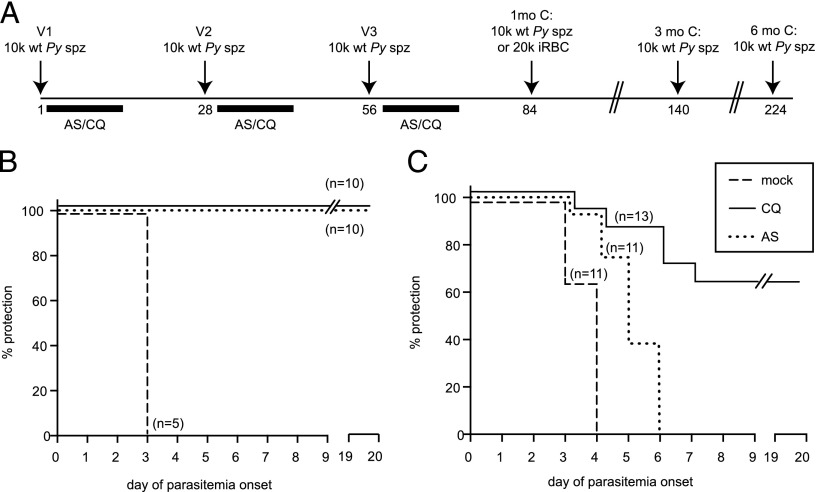
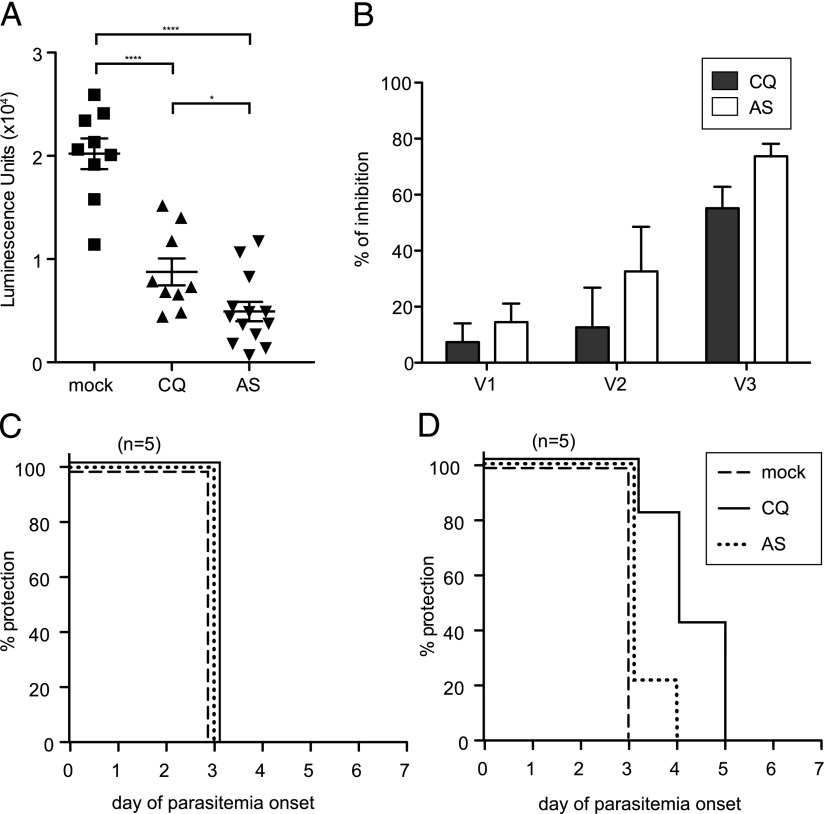
To characterize the protection resulting from immunization with wt P. yoelii spz under drug coverage, we vaccinated groups of 10 mice with 1–3 doses of 10,000 wt P. yoelii spz 1 mo apart and treated with either CQ or AS for 10 d starting on the day of vaccination (Fig. 2A). In agreement with the data presented in Fig. 1, 10 of 10 mice had BS parasitemia upon drug withdrawal after the first course of CQ-ITV, compared with 0 of 10 mice under AS coverage (Table I, Experiment 1). After subsequent courses of ITV, none of the mice had detectable BS parasitemia (Table I, Experiments 2 and 3). One month after each course of ITV, mice were challenged with 10,000 wt P. yoelii spz. As a control, a group of five mice were mock immunized with salivary gland extract from noninfected mosquitoes in the absence of drug treatment. None of the mice in either the CQ- or the AS-ITV groups displayed protection against challenge with wt P. yoelii spz 1 mo after the first course of ITV (Table I, Experiment 1, spz: 1 mo). However, 90% of the mice in each group were sterilely protected against spz challenge after the second course of ITV (Table I, Experiment 2, spz: 1 mo), and 100% of the mice in each group acquired sterile protection from spz challenge after the third course of ITV (Fig. 2B, Table I, Experiment 3, spz: 1 mo). In comparison, all mock-vaccinated mice had parasitemia on day 3 after challenge (Fig. 2B). To determine the length of the observed protection, we challenged additional groups of immunized mice with 10,000 wt P. yoelii spz 3 and 6 mo after the third course of CQ- or AS-ITV (Table I, Experiment 3, spz: 3 and 6 mo). Sterile protection was maintained for at least 6 mo after three courses of CQ- or AS-ITV, confirming that both treatments induce prolonged sterile protection against spz challenge (17).
FIGURE 2.
CQ- and AS-ITV induce differential protection against challenge with P. yoelii spz or iRBCs. (A) Experimental design. Mice received 10,000 wt P. yoelii spz (or mosquito salivary gland debris) on days 1 (V1), 28 (V2), and 56 (V3), followed in each case by 10 d of treatment with 0.8 mg CQ or 3.0 mg AS. One month after the last dose, they were challenged with 10,000 wt P. yoelii spz or 20,000 wt P. yoelii iRBCs. Separate groups of mice were challenged on days 140 or 224 (3 and 6 mo after V3) with 10,000 wt P. yoelii spz. (B and C) Patency curves of groups of mice immunized with three courses of CQ-ITV (solid line) or AS-ITV (dotted line), or untreated, mock-immunized mice (dashed line) challenged with P. yoelii spz (B) or infected erythrocytes (C) 1 mo later. The y-axis shows the percentage of mice protected from infection at each time point, and the x-axis corresponds to the day of parasitemia onset after challenge.
Table I. Experimental design and outcome of ITV and challenge phases.
| Vaccination Phase (No. Infected/No. Vaccinated = % Infection) |
Challenge Phase (No Protected/No. Challenged = % Protection) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Experiment | Treatment | V1 | V2 | V3 | spz, 1 mo | spz, 3 mo | spz, 6 mo | iRBC, 1 mo |
| 1 | None | ND | 0/5 (0%) | |||||
| CQ | 10/10 (100%) | 0/10 (0%) | ||||||
| AS | 0/10 (0%) | 0/10 (0%) | ||||||
| 2 | None | ND | ND | 0/5 (0%) | ||||
| CQ | 10/10 (100%) | 0/10 (0%) | 9/10 (90%) | |||||
| AS | 0/10 (0%) | 0/10 (0%) | 9/10 (90%) | |||||
| 3 | None | ND | ND | ND | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | |
| CQ | 10/10 (100%) | 0/10 (0%) | 0/10 (0%) | 10/10 (100%) | 5/5 (100%) | 5/5 (100%) | ||
| AS | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 10/10 (100%) | 5/5 (100%) | 5/5 (100%) | ||
| 4 | None | ND | ND | ND | 0/11 (0%) | |||
| CQ | 13/13 (100%) | 0/13 (0%) | 0/13 (0%) | 8/13 (61.5%) | ||||
| AS | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | ||||
For each experiment, the table indicates the drug used for the treatment (none, CQ, or AS) and the outcome of each course of ITV (V1, V2, and V3), both as the number and percentage of infected mice divided by the number of vaccinated mice, as well as the outcome of the challenge with spz or iRBCs 1, 3, or 6 mo, respectively, after the corresponding course of ITV, both as the number and the percentage of protected mice divided by the number of challenged mice.
Next, we analyzed whether AS- or CQ-ITV elicits immunity against BS forms of the malaria parasite by challenging mice i.v. with 20,000 malaria iRBCs 1 mo after three courses of CQ- or AS-ITV, as described earlier (Fig. 2A). We saw that the majority (8/13, or 62%) of the CQ-ITV treated mice were sterilely protected against challenge with iRBC, whereas most of the remaining mice experienced a 1- to 4-d delay in the onset of parasitemia, as compared with the mice in the control group (Fig. 2C, Table I, Experiment 4, iRBC: 1 mo). In contrast, none of the mice under AS-ITV treatment acquired sterile protection. Nevertheless, the majority (8/11, or 73%) of the mice in the AS-ITV group experienced a 1- to 3-d delay in the onset of parasitemia after iRBC challenge, as compared with untreated, mock-immunized mice (Fig. 2C, Table I, Experiment 4, iRBC: 1 mo). Taken together, our data demonstrate that both CQ- and AS-ITV induced long-term sterile protection against pre-erythrocytic stages of malaria parasites. Moreover, CQ-ITV treatment also resulted in sterile protection against BS parasites, in agreement with previous reports (19). Interestingly, although unable to confer sterile protection, AS-ITV delayed the onset of BS infection, suggesting that although we were unable to detect BS in mice treated with AS, this regimen also induces some level of cross-stage immunity.
CQ- and AS-ITV induce CSP-specific CD8+ T effector memory cells in the liver
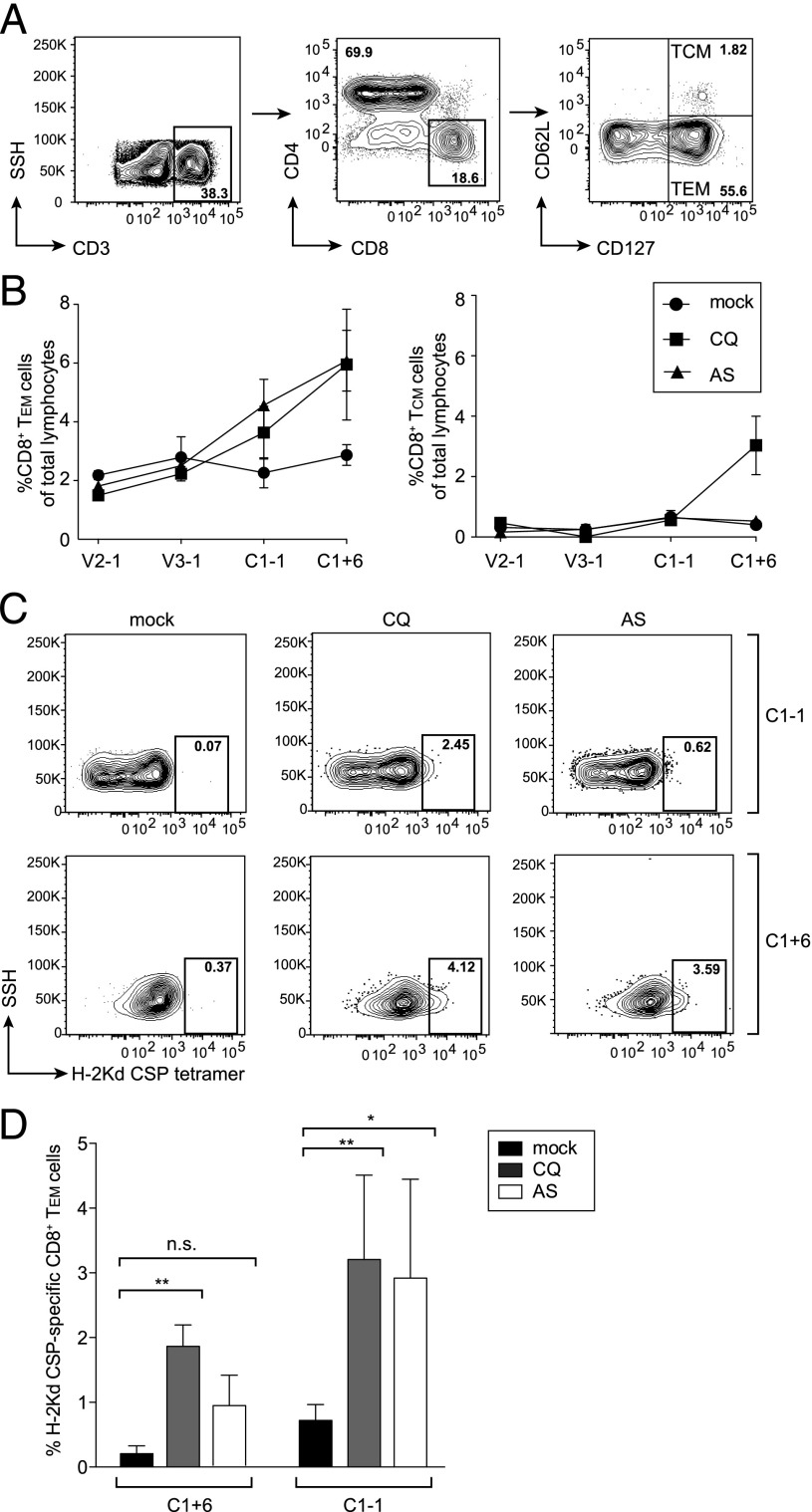
The long-lasting sterile protection against challenge with high doses of spz that we observed in mice subjected to three courses of CQ- or AS-ITV suggests that both treatments elicit strong immune responses against pre-erythrocytic stage parasites. Previously, it has been shown that CD8+ T cells play a major role in protection against pre-erythrocytic infection induced by vaccination with drug-attenuated spz (16, 17, 27). Moreover, protection against pre-erythrocytic infection has been recently demonstrated to correlate with the induction of malaria-specific CD8+ effector memory T cells (TEM) (28).
To assess the abundance of liver TEM and central memory T cells (TCM) in mice immunized three times with P. yoelii spz under CQ or AS coverage (or mock-immunized, untreated mice), we purified liver lymphocytes on days 27, 55, 83, and 90, which correspond to 1 d before the second and third immunizations, as well as the day before and 6 d after challenge with wt P. yoelii spz (Fig. 2A). We used the flow cytometry–based strategy shown in Fig. 3A to gate CD3+ CD8+ T cells and to quantify the abundance of TCM (CD127+ CD62L+) and TEM (CD127+ CD62L−) cells in the liver. We observed an increase in the abundance of TEMs only after the third course of AS- and CQ-ITV, as compared with mock-immunized mice (Fig. 3B). This increase was most pronounced after challenge with P. yoelii spz. In comparison, the abundance of TCMs remained low throughout the course of the immunization, and we only observed an increase in TCMs in CQ-ITV mice after challenge.
FIGURE 3.
CQ- and AS-ITV lead to an increase in total and CSP-specific CD8+ TEMs. (A) Gating strategy. Liver lymphocytes were gated based on CD3+ staining, followed by gating on CD3+CD8+ or CD3+CD4+ T staining. Memory T cells were gated into TEM (CD127+, CD62−) and TCM (CD127+, CD62+). (B) Percentage of total lymphocytes corresponding to CD8+ TEMs (left panel) or CD8+ TCMs (right panel) isolated on days 27 (V2-1), 55 (V3-1), 83 (C1-1), or 90 (C1+6) from the livers of mock-immunized, untreated mice (circles); CQ-ITV mice (squares); or AS-ITV mice (triangles). (C) Representative FACS plots of CSP-specific CD8+ TEMs isolated from untreated, mock-immunized mice (left panel) or from CQ-ITV (middle panel) or AS-ITV (right panel) mice on day 83 (C1-1; top panel) or 90 (C1+6; bottom panel). (D) Cumulative data of CSP-specific CD8+ TEMs as a percent of total CD8+ TEMs (C1-1: n = 4; C1+6: n = 5). Significance was analyzed using two-tailed Student t tests. Error bars represent SEM. n.s., not significant. *p < 0.05, **p < 0.01.
We then determined whether the increase in the abundance of CD8+ TEMs observed on days 83 and 90 in the AS-ITV and CQ-ITV mice was specific for malaria pre-erythrocytic Ags by quantifying the abundance of CSP-specific CD8+ TEMs. CSP-specific CD8+ TEMs were identified using an allophycocyanin-labeled MHC H2-Kd tetramer epitope (SYIPSAEKI) that recognizes P. yoelii CSP, as previously described (29). Three courses of CQ-ITV resulted in a significant increase (p = 0.0087) in the percentage of CSP-specific CD8+ TEMs as compared with mock-immunized, untreated mice measured 1 mo after the last ITV course, that is, 1 d before spz challenge (Fig. 3C, 3D). AS-ITV also resulted in an increase in the percentage of CD8+ TEMs, although this increase was not statistically significant. We also observed a significant expansion of CSP-specific CD8+ TEMs in mice treated with either CQ- or AS-ITV 6 d after spz challenge (p = 0.0037 and p = 0.02, respectively). No significant difference in the levels of CSP-specific CD8+ TEMs was observed between the mice under CQ or AS coverage either before or after spz challenge. In summary, both CQ- and AS-ITV induce pre-erythrocytic stage-specific CD8+ TEM responses, which are long lasting and can be boosted significantly by re-exposure to wt spz infection, in agreement with previous studies using similar immunization strategies (16, 28).
ITV-induced CD8+ T cell responses are cytotoxic to LS parasite-infected hepatocytes in vitro
It has been demonstrated that CD8+ T cells are cytotoxic to Plasmodium-infected hepatocytes in vitro. For example, we have previously shown that contact-dependent killing of infected hepatocytes by CD8+ T cells is a major effector mechanism in BALB/c mice vaccinated with genetically attenuated spz (18). More recently, it has been proposed that CD8+ T cells that interact with infected hepatocytes can recruit both parasite-specific and bystander CD8+ T cells to the proximity of infected hepatocytes, although only parasite-specific CD8+ T cells appear to be involved in killing the infected hepatocytes (30, 31).
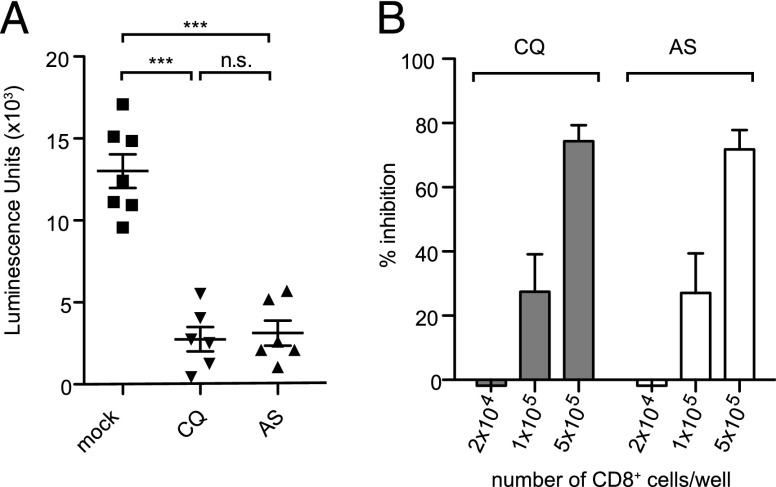
Thus, we evaluated whether CD8+ T cells induced by ITV have the ability to inhibit the development of intrahepatocytic parasites. CD8+ T cells obtained from the livers of mice 6 d after the third course of CQ- or AS-ITV, which corresponds to the peak of CD8+ T response (19), were sorted by flow cytometry, and their purity was determined to be >95% (Supplemental Fig. 1). These cells were cocultured with primary hepatocytes infected with luciferase-expressing P. yoelii spz (24), and parasite development was quantified as previously described (29). We observed that addition of 5 × 105 liver CD8+ T cells from CQ- or AS-ITV–treated mice significantly inhibited parasite development (81.2 and 78.5%, respectively) as compared with those from mock-immunized, untreated mice (Fig. 4A). The observed reduction in LS parasite development was specific, because inhibition was proportional to the number of CD8+ T cells added to the coincubation assay (Fig. 4B). These data confirm that CD8+ T cells induced by ITV have the ability to affect the development of Plasmodium-infected hepatocytes (Fig. 4B), although the mechanism of killing remains to be fully elucidated.
FIGURE 4.
CQ- and AS-ITV induce CD8+ T cells that inhibit the development of P. yoelii–infected hepatocytes in vitro in a concentration-dependent manner. (A) Quantification of P. yoelii GFP-luciferase intrahepatocytic development in the presence of 500,000 pooled liver CD8+ T cells, isolated from CQ-ITV (CQ), AS-ITV (AS), or mock-immunized, untreated mice (mock) 6 d after the last dose. The y-axis shows luminescence units. Data correspond to two independent experiments. Significance was analyzed using two-tailed Student t tests. Error bars represent SEM. ***p < 0.001, n.s., not significant. (B) Percent inhibition of P. yoelii GFP-luciferase intrahepatocytic development in the presence of increasing number of CD8+ T cells isolated from CQ- or AS-ITV–treated mice, calculated as the percentage reduction in luciferase signal compared with that of the mock-immunized, untreated control.
CQ- and AS-ITV induce similar patterns of anti–pre-erythrocytic Abs, but display different reactivity to BS Ags
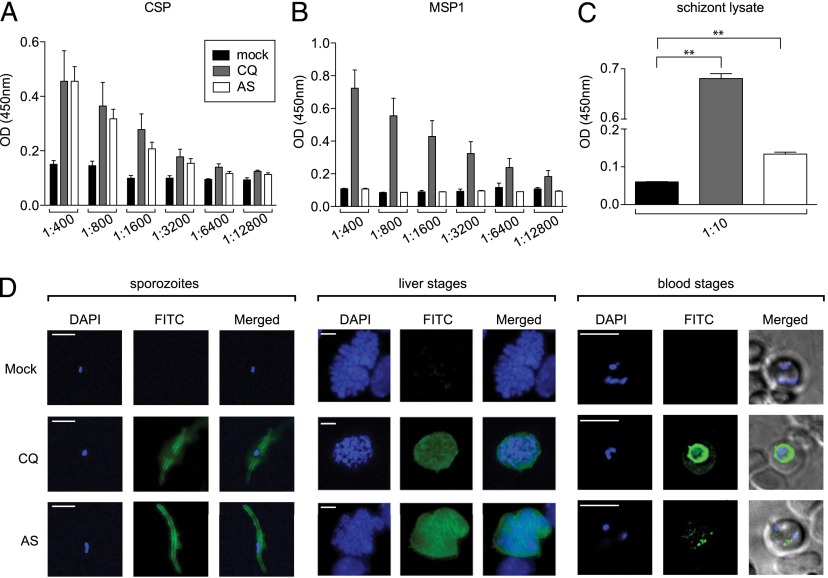
Abs against malaria parasites have been shown to play a critical role in the inhibition of hepatocyte invasion by spz, as well as in BS parasite clearance (7, 19). We next assessed the humoral responses elicited by CQ-ITV and AS-ITV by analyzing sera from mice immunized under each of the regimens described earlier. First, we measured the specificity of these sera against the dominant spz surface Ag CSP, the prevalent BS Ag MSP1, and total schizont lysate by ELISA using sera obtained from mice subjected to three courses of CQ-ITV or AS-ITV 9 d after the third immunization. We observed that both CQ-ITV and AS-ITV induced similar levels of anti-CSP Abs (Fig. 5A). However, only CQ-ITV–treated mice developed MSP1 reactivity (Fig. 5B). Finally, although CQ-ITV induced high levels of reactivity against a schizont lysate, AS-ITV induced low but significant reactivity (Fig. 5C), suggesting that AS-ITV results in the induction of Abs against BS Ags other than MSP1.
FIGURE 5.
Sera from mice treated with CQ- or AS-ITV recognize pre-erythrocytic and erythrocytic Ags and parasites to different degrees. (A–C) Reactivity of serum collected from mock-immunized, untreated mice (mock, black bars), CQ-ITV–treated (CQ, gray bars), or AS-ITV–treated (AS, white bars) mice 9 d after the third immunization against pre-erythrocytic protein CSP B cell epitope QGPGAP (A), erythrocytic MSP 1 protein (B), or schizont lysate (C) by ELISA. The y-axis shows OD at 450 nm. The x-axis indicates the dilution factor of the sera used for CSP (A) and MSP1 (B); a 1:10 dilution of sera was used for the schizont lysate (C). Significance was analyzed using two-tailed Mann–Whitney tests. Error bars represent SEM. **p < 0.01. (D) Immunofluorescence assays with serum collected from mock-immunized, untreated mice (top panel), CQ-ITV–treated mice (middle panel), or AS-ITV–treated mice (bottom panel), on P. yoelii spz (left panel); LSs (middle panel); or BSs (right panel). Serum reactivity is shown in green (FITC), and DNA staining is shown in blue (DAPI). Merge images show a merge of the green and blue channels. Scale bar, 10 μm.
We also determined whether these sera could recognize spz, LS, and BS of P. yoelii parasites by IFA. IFAs were performed using a series of 2-fold titrations of sera obtained from mice treated with CQ-ITV or AS-ITV. We observed that sera from both CQ-ITV– and AS-ITV–treated mice recognized spz and LS parasites with dilutions of up to 1:16,640 ± 3,840 and 7,040 ± 1,568, respectively (Fig. 5D, left and middle panels). Sera from CQ-ITV–treated mice also recognized iRBCs with a similar dilution (1:10,240 ± 1,568; Fig. 5D, right panel), whereas sera purified from AS-ITV–treated mice showed very low reactivity to iRBCs, consistent with the ELISA results presented earlier. These data demonstrate that mice immunized with P. yoelii spz under CQ coverage develop Abs that can recognize spz, LS, and BS forms of the malaria parasite, whereas sera from mice under AS-ITV treatment predominantly recognize spz and LS but display low reactivity to BS Ags.
Sera from both CQ- and AS-ITV–treated mice inhibits invasion of hepatocytes by P. yoelii spz in vitro
To determine the functionality of the Ab response induced by CQ- and AS-ITV, we performed inhibition of spz invasion assays in vitro. Sera collected from mice immunized with P. yoelii spz under CQ or AS coverage on day 9 after the third course of ITV significantly inhibited (p < 0.0001) the invasion of HepG2:CD81 cells by luciferase-expressing P. yoelii spz (Fig. 6A). Furthermore, sera obtained from AS-ITV–treated mice showed significantly higher inhibition efficiency than sera from CQ-ITV mice (p = 0.024). Sera collected after each course of CQ-ITV inhibited the invasion of hepatocytes by spz with increasing efficiency (i.e., 7, 13, and 55% for vaccination doses 1, 2, and 3, respectively; Fig. 6B). Similarly, sera obtained from mice under AS coverage inhibited spz invasion at 15, 33, and 74% after vaccination doses 1, 2, and 3, respectively. In summary, both CQ- and AS-ITV elicit Abs that are capable of blocking spz invasion in vitro, and furthermore, successive vaccinations increase this functional Ab response.
FIGURE 6.
Sera from mice treated with CQ- or AS-ITV inhibits spz invasion of hepatocytes in vitro. (A) Quantification of HepG2 cell invasion and development after incubation of P. yoelii GFP-luciferase spz with serum collected from mock-immunized, untreated (n = 9), ITV-CQ–treated (n = 9), or ITV-AS–treated (n = 13) mice 9 d after the third dose. The y-axis shows luminescence units. Significance was determined by two-tailed Student t tests. Results are from two independent experiments. Error bars represent SEM. *p < 0.05, ****p < 0.001. (B) Percent inhibition of HepG2 cell invasion and development upon incubation of P. yoelii GFP-luciferase spz with sera obtained after the first (V1), second (V2), or third (V3) course of CQ- or AS-ITV (AS), calculated as the percentage reduction in luciferase signal compared with that of the mock-immunized, untreated control. (C and D) Patency curves of naive mice challenged with wt spz (C) or iRBC (D) after adoptive transfer of immune sera from CQ-ITV–treated (solid line) or AS-ITV–treated (dotted line), or untreated, mock-immunized mice (dashed line). The y-axis shows the percentage of mice protected from infection at each time point, and the x-axis corresponds to the day of parasitemia onset after challenge.
Adoptive transfer of sera from mice treated with CQ-ITV, but not with AS-ITV, significantly delayed BS infection in naive mice challenged with P. yoelii iRBCs
Finally, to determine whether Abs from vaccinated mice could protect in vivo against spz or iRBC infection, we adoptively transferred sera from CQ-ITV– and AS-ITV–treated mice to naive mice. Twenty-four hours after receiving 300 μl donor sera by i.v. injection (26), mice were challenged with either 10,000 P. yoelii spz or 20,000 iRBC by i.v. injection. We observed that none of the mice that received sera from donor mice immunized under either CQ- or AS-ITV treatment acquired sterile protection against spz challenge (Fig. 6C). Interestingly, four of five mice (80%) that received sera from donor mice from the CQ-ITV treatment group showed a 1- to 2-day delay of patency, as compared with control mice upon challenge with iRBCs (Fig. 6D). However, only one of five mice (20%) that received sera from AS-ITV–treated mice displayed delayed patency after iRBC challenge (Fig. 6D).
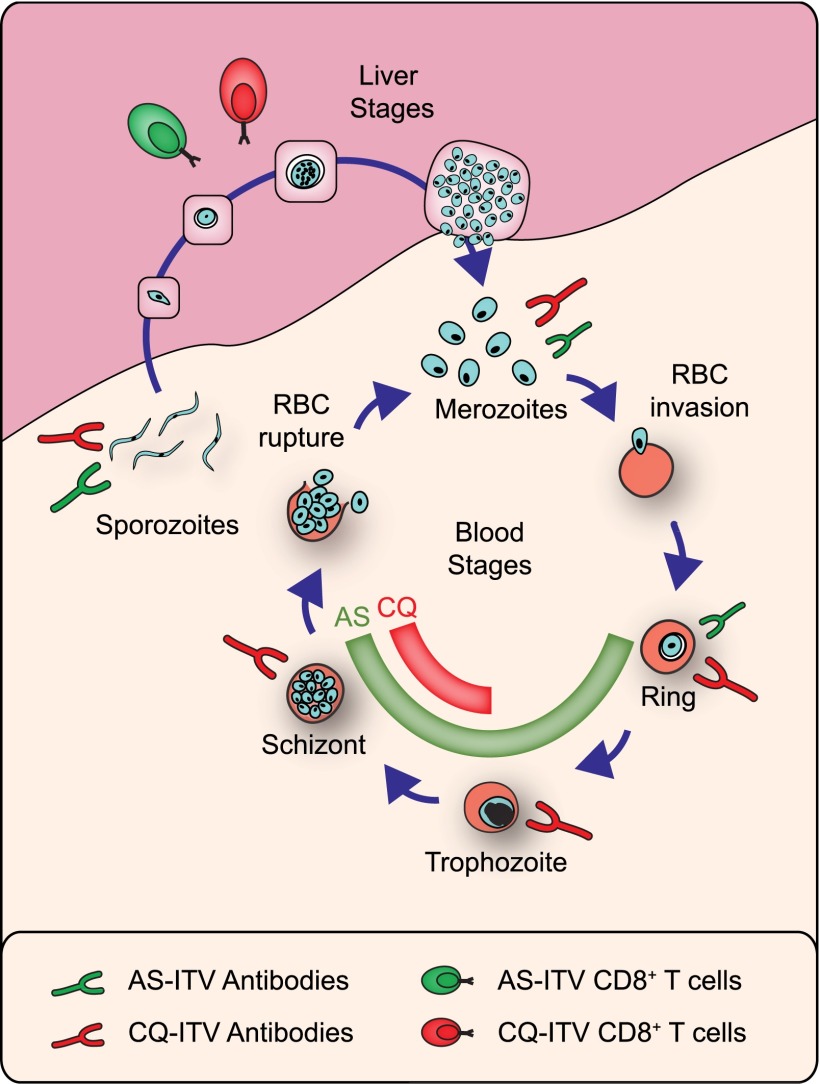
Overall, the in vivo experiments described earlier demonstrate that Abs generated under CQ- or AS-ITV are not sufficient to confer sterile protection against either an i.v. spz or an i.v. BS challenge in a passive transfer experiment. Taken together, our data suggest that the protective immunity induced by CQ- and AS-ITV associates with the different effects of these drugs during the Plasmodium life cycle in the mammalian host (Fig.7).
FIGURE 7.
Protective immunity induced by CQ- and AS-ITV associates with the different effects of these drugs during the Plasmodium life cycle in the mammalian host. The diagram depicts the main phases of P. yoelii development in mice, the stage-specific parasite forms affected by CQ (red) or AS (green), and the induction of cellular and humoral immune responses elicited by CQ-ITV and AS-ITV, as indicated. The relative size of the Abs and T cells represents the magnitude of their effect against each stage-specific parasite form.
Discussion
After decades of malaria vaccination studies in both animal models and malaria-naive humans, whole-parasite immunization is by far the most effective strategy in generating sterile protection, whether through irr spz, ITV, or GAP (reviewed in Refs. 22, 32). Although sterile protection can be achieved by immunization with a low number of bites of malaria-infected mosquitoes under CQ coverage, widespread resistance to this drug limits the applicability of the CQ-ITV approach in malaria control. Artemisinin-based combination therapies are currently the treatment of choice for uncomplicated P. falciparum and P. vivax malaria in adults and children in all endemic areas (33), although there have been several reports of drug resistance in the same geographic area where CQ resistance originated in the 1950s, where artemisinin and its derivatives have been used as a monotherapy for more than three decades at subtherapeutic doses (34, 35). Like CQ, AS prevents BS infection by blocking the ability of the parasite to complete the intraerythrocytic cycle. A major difference between CQ and AS is that CQ acts mainly on late BS parasites, whereas AS acts throughout the BSs, including early ring stages (reviewed in Ref. 15). In this work, we exploited the difference between the effects of AS and CQ on malaria erythrocytic parasites as a tool to define correlates of protection.
We first identified a dose of AS that completely blocks parasite development before the formation of pathogenic BSs (Fig. 1). By comparing the immune responses elicited by this AS-ITV strategy with those induced by CQ-ITV, we were able to characterize liver-stage immunity in the absence and presence of BS infection, respectively.
Consistent with the fact that neither CQ nor AS affect the pre-erythrocytic stages of malaria parasites (15), we observed that although a single course of either CQ- or AS-ITV was insufficient to induce sterile immunity against challenge with 10,000 wt P. yoelii spz, three courses resulted in sterile protection of all mice that lasted for at least 6 mo (Fig. 2, Table I). Our results agree with a recent study demonstrating that three courses of CQ-ITV induced long-lasting protective immunity against P. falciparum in malaria-naive volunteers (36). In contrast, two previous mouse studies reported that a single course of CQ-ITV is sufficient to generate 78–100% protection against spz challenge (19, 23). This apparent contradiction can be explained by the number of spz used in the challenge phase of each of these studies: Doll et al. (19), who report 100% sterile protection after a single course of CQ-ITV, used only 1000 spz for the challenge; Belnoue et al. (23), who report partial protection, used only 4000 spz; whereas our study used 10,000 spz. Therefore, these data suggest that the level of protection observed is inversely correlated to the number of spz used for the challenge. In addition to this, the level of protection from a particular immunization regimen has been shown to depend on the strain of mouse used for experimentation (22, 37). Therefore, the differences discussed earlier could also be attributed in part to the use of different mouse strains: whereas both Belnoue et al. (23) and this study used BALB/c mice, Doll et al. (19) used C57BL/6 mice.
Sterile protection induced by CQ-ITV in the mouse model has been shown to depend on immune responses against both pre-erythrocytic and BS Ags that involve CD8+, CD4+ T cells, and Abs (17, 19, 23). CD8+ T cells have been shown to be required for protection against malaria LSs (16, 18, 20, 38, 39). In agreement with this, we observed that repeated exposure of mice to malaria parasites under either CQ or AS coverage resulted in the induction of total and malaria-specific liver CD8+ TEMs, whereas CD8+ TCM levels remained low (Fig. 3). Challenge with spz further induced the levels of CD8+ TEMs in CQ- and AS-ITV mice, as well as slightly increasing CD8+ TCMs in CQ-ITV mice. These data agree with previous reports suggesting that CD8+ TEM levels correlate with protection (16, 40), and that passive transfer of CD8+ TEMs into naive mice results in sterile protection against spz challenge (28).
Previous studies from our group and others have shown that CD8+ T cells induced by whole parasites are capable of killing Plasmodium-infected hepatocytes in vitro and in vivo (18, 30, 31). In this study, we determined that ITV-induced liver CD8+ T cells are also capable of interfering with the development of malaria-infected hepatocytes in vitro (Fig. 4). Several mechanisms have been implicated in the killing of infected hepatocytes by CD8+ T cells, including the production of proinflammatory cytokines such as IFN-γ and TNF-α, the production of cytotoxic molecules such as perforin and granzyme, and the clustering of CD8+ T cells around infected hepatocytes (18, 31, 39).
Although the role of CD8+ T cells in immunity against whole parasites is well established, humoral immunity also plays a crucial role in controlling malaria infection (7, 19, 40–42). Thus, we investigated the Ab response mounted against the predominant pre-erythrocytic protein, CSP, by mice immunized with wt P. yoelii spz under CQ and AS coverage. Both CQ-ITV and AS-ITV induced similar levels of Abs against pre-erythrocytic Abs (Fig. 5). Furthermore, whereas both CQ- and AS-ITV induced functional Abs that were capable of blocking invasion of hepatocytes by P. yoelii spz in vitro, passive transfer of sera from mice immunized under CQ-ITV or AS-ITV did not have any protective effect against challenge with wt P. yoelii spz by i.v. injection (Fig. 6). The difference observed between in vitro and in vivo assays could be because of the route of challenge used in our experiments, as it was recently shown that challenge by mosquito bite instead of i.v. not only allows Abs to act against the skin traversal phase of parasite infection, but also gives them sufficient time to block hepatocyte invasion (26).
Previous studies using CQ-ITV and late LS arresting GAP parasites have suggested that cross-stage immunity plays a role in protection against malaria challenge (19, 20, 23). This immunity has been attributed to expression of shared Ags by LS and BS parasites, in addition to exposure to low levels of BS parasites in the case of CQ-ITV. Similarly to what was recently reported by Doll et al. (19), we saw that CQ-ITV resulted in sterile protection against BS challenge in >60% of mice. This protection was shown to be dependent on the presence of transient BS parasitemia after drug withdrawal, because a prolonged treatment that prevented the reoccurrence of these parasites abolished cross-stage protection (19). In contrast, we observed that a dose of AS that completely blocks the development of BSs did not result in sterile protection against BS challenge, although the majority of the AS-ITV–treated mice displayed a delayed patency, suggesting that AS-ITV induces some level of cross-stage immunity (Fig. 2). This could be caused by an immune response to Ags expressed by late LS parasites (20, 43), or alternatively by transient exposure to BS Ags expressed by merozoites as they exit the liver and briefly exist in the circulation before invading RBCs and being cleared by AS. Similarly to AS-ITV, late-arresting GAPs also result in cross-stage protection that is likely mediated by exposure to BS Ags during late LS (20).
Although some reports have suggested that CD8+ and CD4+ cells are involved in BS immunity (44, 45), most studies indicate that Abs are the main mediators of protection (reviewed in Ref. 41). Consistent with the mode of action of CQ on late BS parasites, we saw a high level of reactivity of sera from CQ-ITV–treated mice against BS Ags (including MSP1 and schizont lysate) and iRBCs (Fig. 5). In addition, passive transfer of Abs from CQ-ITV–treated mice into naive mice delayed the onset of parasitemia after BS challenge. These results suggest that Abs could contribute to the sterile protection against BS challenge observed in CQ-ITV mice (Fig. 2). In contrast, sera from AS-ITV–treated mice did not react against MSP1 (Fig. 5B), although it showed low levels of reactivity against schizont lysate and iRBCs. Although this low level of cross-stage Ab response elicited by AS-ITV did not result in protection against challenge with iRBC, it could partially explain the delay in patency observed. Further studies are required to establish whether BS-specific CD8+ and CD4+ cells are involved in cross-stage protection in the AS-ITV model, as well as to determine the identity of shared liver and BS Ags that may associate with cross-stage immunity.
In summary, we showed that the ability of ITV to induce protection against LS and BS parasites depends on the stages of the malaria parasite affected by the drug used to cover the spz infection (Fig. 7). Furthermore, although both CQ- and AS-ITV induce cross-stage immune responses against BS, only CQ-ITV results in sterile protection. This suggests that exposure to a significant load of BS Ags during immunization is essential to confer sterile protection. Therefore, although pre-erythrocytic vaccines have the advantage that they act during a bottleneck in the parasite life cycle and before the large LS and BS expansions in parasite numbers, to be fully protective they might need to also elicit cross-stage protection that is able to block any break-through infection. Thus, vaccines that rely on ITV or on late-LS arresting parasites and allow expression of LS and BS Ags might constitute the most efficient means to confer anti-infection and antidisease immunity.
Supplementary Material
Acknowledgments
The following reagent was obtained through the MR4 as part of the Biodefense and Emerging Infections Research Resources Repository, National Institute of Allergy and Infectious Diseases, NIH: P. yoelii yP.y.MSP1-19(XL)/VQ1, MRA-48, deposited by D.C. Kaslow. The allophycocyanin-conjugated CSP (SYVPSAEQI) tetramer was obtained from the NIH Tetramer Core Facility. We thank Drs. Brandon Sack and Ashley Vaughan for critical review of the manuscript.
This work was supported by the Bill & Melinda Gates Foundation (Grant OPP1016829) and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (Grant 5R01AI76498).
The online version of this article contains supplemental material.
- AS
- artesunate
- BS
- blood stage
- CQ
- chloroquine
- CSP
- circumsporozoite protein
- GAP
- genetically attenuated parasite
- IFA
- immunofluorescence assay
- iRBC
- infected red blood cell
- irr
- irradiated
- ITV
- infection–treatment–vaccination
- LS
- liver stage
- NIH
- National Institutes of Health
- p.i.
- postinfection
- qPCR
- quantitative PCR
- spz
- sporozoite
- TCM
- central memory T cell
- TEM
- effector memory T cell
- wt
- wild type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1. World Health Organization. 2013. World Malaria Report 2013. Geneva, Switzerland: WHO. Available at: http://www.who.int/malaria/publications/world_malaria_report_2013/en/. Accessed January 22, 2014.
- 2.Wang R., Smith J. D., Kappe S. H.. 2009. Advances and challenges in malaria vaccine development. Expert Rev. Mol. Med. 11: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan A. M., Wang R., Kappe S. H.. 2010. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum. Vaccin. 6: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clyde D. F., McCarthy V. C., Miller R. M., Hornick R. B.. 1973. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am. J. Med. Sci. 266: 398–403 [DOI] [PubMed] [Google Scholar]
- 5.Hoffman S. L., Goh L. M., Luke T. C., Schneider I., Le T. P., Doolan D. L., Sacci J., de la Vega P., Dowler M., Paul C., et al. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185: 1155–1164 [DOI] [PubMed] [Google Scholar]
- 6.Rieckmann K. H., Beaudoin R. L., Cassells J. S., Sell K. W.. 1979. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull. World Health Organ. 57(Suppl. 1): 261–265 [PMC free article] [PubMed] [Google Scholar]
- 7.Seder R. A., Chang L. J., Enama M. E., Zephir K. L., Sarwar U. N., Gordon I. J., Holman L. A., James E. R., Billingsley P. F., Gunasekera A., et al. VRC 312 Study Team . 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341: 1359–1365 [DOI] [PubMed] [Google Scholar]
- 8.Belnoue E., Costa F. T., Frankenberg T., Vigário A. M., Voza T., Leroy N., Rodrigues M. M., Landau I., Snounou G., Rénia L.. 2004. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172: 2487–2495 [DOI] [PubMed] [Google Scholar]
- 9.Friesen J., Silvie O., Putrianti E. D., Hafalla J. C., Matuschewski K., Borrmann S.. 2010. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci. Transl. Med. 2: 40ra49. [DOI] [PubMed] [Google Scholar]
- 10.Putrianti E. D., Silvie O., Kordes M., Borrmann S., Matuschewski K.. 2009. Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. J. Infect. Dis. 199: 899–903 [DOI] [PubMed] [Google Scholar]
- 11.Roestenberg M., McCall M., Hopman J., Wiersma J., Luty A. J., van Gemert G. J., van de Vegte-Bolmer M., van Schaijk B., Teelen K., Arens T., et al. 2009. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361: 468–477 [DOI] [PubMed] [Google Scholar]
- 12.Roestenberg M., Bijker E. M., Sim B. K., Billingsley P. F., James E. R., Bastiaens G. J., Teirlinck A. C., Scholzen A., Teelen K., Arens T., et al. 2013. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am. J. Trop. Med. Hyg. 88: 5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray P. G., Ward S. A., O’Neill P. M.. 2005. Quinolines and artemisinin: chemistry, biology and history. Curr. Top. Microbiol. Immunol. 295: 3–38 [DOI] [PubMed] [Google Scholar]
- 14.Klonis N., Xie S. C., McCaw J. M., Crespo-Ortiz M. P., Zaloumis S. G., Simpson J. A., Tilley L.. 2013. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc. Natl. Acad. Sci. USA 110: 5157–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White N. J. 2008. Qinghaosu (artemisinin): the price of success. Science 320: 330–334 [DOI] [PubMed] [Google Scholar]
- 16.Nganou-Makamdop K., van Gemert G. J., Arens T., Hermsen C. C., Sauerwein R. W.. 2012. Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNγ responses of hepatic CD8+ memory T cells. PLoS ONE 7: e36508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bijker E. M., Bastiaens G. J., Teirlinck A. C., van Gemert G. J., Graumans W., van de Vegte-Bolmer M., Siebelink-Stoter R., Arens T., Teelen K., Nahrendorf W., et al. 2013. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc. Natl. Acad. Sci. USA 110: 7862–7867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trimnell A., Takagi A., Gupta M., Richie T. L., Kappe S. H., Wang R.. 2009. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J. Immunol. 183: 5870–5878 [DOI] [PubMed] [Google Scholar]
- 19.Doll K. L., Butler N. S., Harty J. T.. 2014. CD8 T cell independent immunity after single dose infection-treatment-vaccination (ITV) against Plasmodium yoelii. Vaccine 32: 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler N. S., Schmidt N. W., Vaughan A. M., Aly A. S., Kappe S. H., Harty J. T.. 2011. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9: 451–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rénia L. 2008. Protective immunity against malaria liver stage after vaccination with live parasites. Parasite 15: 379–383 [DOI] [PubMed] [Google Scholar]
- 22.Nganou-Makamdop K., Sauerwein R. W.. 2013. Liver or blood-stage arrest during malaria sporozoite immunization: the later the better? Trends Parasitol. 29: 304–310 [DOI] [PubMed] [Google Scholar]
- 23.Belnoue E., Voza T., Costa F. T., Grüner A. C., Mauduit M., Rosa D. S., Depinay N., Kayibanda M., Vigário A. M., Mazier D., et al. 2008. Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage. J. Immunol. 181: 8552–8558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J. L., Murray S., Vaughan A. M., Harupa A., Sack B., Baldwin M., Crispe I. N., Kappe S. H.. 2013. Quantitative bioluminescent imaging of pre-erythrocytic malaria parasite infection using luciferase-expressing Plasmodium yoelii. PLoS ONE 8: e60820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Z. J., Wei H. M., Sun R., Tian Z. G., Gao B.. 2004. Isolation of murine hepatic lymphocytes using mechanical dissection for phenotypic and functional analysis of NK1.1+ cells. World J. Gastroenterol. 10: 1928–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sack B. K., Miller J. L., Vaughan A. M., Douglass A., Kaushansky A., Mikolajczak S., Coppi A., Gonzalez-Aseguinolaza G., Tsuji M., Zavala F., et al. 2014. Model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infect. Immun. 82: 808–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy S. C., Kas A., Stone B. C., Bevan M. J.. 2013. A T-cell response to a liver-stage Plasmodium antigen is not boosted by repeated sporozoite immunizations. Proc. Natl. Acad. Sci. USA 110: 6055–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes-Sandoval A., Wyllie D. H., Bauza K., Milicic A., Forbes E. K., Rollier C. S., Hill A. V.. 2011. CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 187: 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooney L. A., Gupta M., Thomas S., Mikolajczak S., Choi K. Y., Gibson C., Jang I. K., Danziger S., Aitchison J., Gardner M. J., et al. 2013. Short-lived effector CD8 T cells induced by genetically attenuated malaria parasite vaccination express CD11c. Infect. Immun. 81: 4171–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cockburn I. A., Amino R., Kelemen R. K., Kuo S. C., Tse S. W., Radtke A., Mac-Daniel L., Ganusov V. V., Zavala F., Ménard R.. 2013. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc. Natl. Acad. Sci. USA 110: 9090–9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cockburn I. A., Tse S. W., Zavala F.. 2014. CD8+ T cells eliminate liver-stage Plasmodium berghei parasites without detectable bystander effect. Infect. Immun. 82: 1460–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler N. S., Vaughan A. M., Harty J. T., Kappe S. H.. 2012. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 33: 247–254 [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization. 2001. Antimalarial drug combination therapy: report of a WHO technical consultation. Available at: http://whqlibdoc.who.int/hq/2001/WHO_CDS_RBM_2001.35.pdf. Accessed: January 22, 2014.
- 34.Dondorp A. M., Nosten F., Yi P., Das D., Phyo A. P., Tarning J., Lwin K. M., Ariey F., Hanpithakpong W., Lee S. J., et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361: 455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Na-Bangchang K., Karbwang J.. 2013. Emerging artemisinin resistance in the border areas of Thailand. Expert. Rev. Clin. Pharmacol. 6: 307–322 [DOI] [PubMed] [Google Scholar]
- 36.Roestenberg M., Teirlinck A. C., McCall M. B., Teelen K., Makamdop K. N., Wiersma J., Arens T., Beckers P., van Gemert G., van de Vegte-Bolmer M., et al. 2011. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377: 1770–1776 [DOI] [PubMed] [Google Scholar]
- 37.Rénia L., Grüner A. C., Mauduit M., Snounou G.. 2013. Vaccination using normal live sporozoites under drug treatment. Methods Mol. Biol. 923: 567–576 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt N. W., Butler N. S., Badovinac V. P., Harty J. T.. 2010. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 6: e1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doolan D. L., Hoffman S. L.. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165: 1453–1462 [DOI] [PubMed] [Google Scholar]
- 40.Berenzon D., Schwenk R. J., Letellier L., Guebre-Xabier M., Williams J., Krzych U.. 2003. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J. Immunol. 171: 2024–2034 [DOI] [PubMed] [Google Scholar]
- 41.Beeson J. G., Osier F. H., Engwerda C. R.. 2008. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol. 24: 578–584 [DOI] [PubMed] [Google Scholar]
- 42.Good M. F., Stanisic D., Xu H., Elliott S., Wykes M.. 2004. The immunological challenge to developing a vaccine to the blood stages of malaria parasites. Immunol. Rev. 201: 254–267 [DOI] [PubMed] [Google Scholar]
- 43.Vaughan A. M., Mikolajczak S. A., Wilson E. M., Grompe M., Kaushansky A., Camargo N., Bial J., Ploss A., Kappe S. H.. 2012. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Invest. 122: 3618–3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai T., Shen J., Chou B., Duan X., Tu L., Tetsutani K., Moriya C., Ishida H., Hamano S., Shimokawa C., et al. 2010. Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur. J. Immunol. 40: 1053–1061 [DOI] [PubMed] [Google Scholar]
- 45.Lau L. S., Fernandez Ruiz D., Davey G. M., de Koning-Ward T. F., Papenfuss A. T., Carbone F. R., Brooks A. G., Crabb B. S., Heath W. R.. 2011. Blood-stage Plasmodium berghei infection generates a potent, specific CD8+ T-cell response despite residence largely in cells lacking MHC I processing machinery. J. Infect. Dis. 204: 1989–1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.