Abstract
Objective:
To determine functional and structural neuroimaging correlates of cognitive dysfunction associated with cannabis use in multiple sclerosis (MS).
Methods:
In a cross-sectional study, 20 subjects with MS who smoked cannabis and 19 noncannabis users with MS, matched on demographic and neurologic variables, underwent fMRI while completing a test of working memory, the N-Back. Resting-state fMRI and structural MRI data (lesion and normal-appearing brain tissue volumes, diffusion tensor imaging metrics) were also collected. Neuropsychological data pertaining to verbal (Selective Reminding Test Revised) and visual (10/36 Spatial Recall Test) memory, information processing speed (Paced Auditory Serial Addition Test [2- and 3-second versions] and Symbol Digit Modalities Test), and attention (Word List Generation) were obtained.
Results:
The cannabis group performed more poorly on the more demanding of the Paced Auditory Serial Addition Test tasks (i.e., 2-second version) (p < 0.02) and the 10/36 Spatial Recall Test (p < 0.03). Cannabis users had more diffuse cerebral activation across all N-Back trials and made more errors on the 2-Back task (p < 0.006), during which they displayed increased activation relative to nonusers in parietal (p < 0.007) and anterior cingulate (p < 0.001) regions implicated in working memory. No group differences in resting-state networks or structural MRI variables were found.
Conclusions:
Patients with MS who smoke cannabis are more cognitively impaired than nonusers. Cannabis further compromises cerebral compensatory mechanisms, already faulty in MS. These imaging data boost the construct validity of the neuropsychological findings and act as a cautionary note to cannabis users and prescribers.
Cognitive dysfunction affects 40% to 60% of patients with multiple sclerosis (MS).1 Data suggest that patients with MS who smoke cannabis have more extensive cognitive difficulties than those who do not. In particular, problems with working memory,2 information processing speed, and executive dysfunction3 appear to be exacerbated by the use of cannabis. Given that cognitive deficits in patients with MS are known to adversely affect the ability to sustain employment, maintain relationships, continue recreational pursuits, and attend to activities of daily living,4 a better understanding of how smoking cannabis may impair brain function in MS is needed.
To date, there have been no imaging-cognition studies of patients with MS who smoke cannabis. This contrasts with an extensive MRI literature of patients with MS in general in which cognitive deficits have been linked to numerous structural and functional brain metrics.5 The present study addresses this gap by looking at the association between neuropsychological deficits on the one hand and brain MRI abnormalities on the other. In light of our earlier findings of greater cognitive problems in patients with MS who smoke cannabis2,3 and in keeping with what we know of cognition-MRI correlates from the MS literature in general,5 we hypothesized that these deficits would be associated with the following: (1) greater cerebral atrophy; (2) more subtle indices of brain pathology derived from diffusion tensor imaging (DTI); and (3) a more dysfunctional pattern of cerebral activation during an fMRI-based working memory task, namely the N-Back. Our choice of the latter as a marker of working memory was based on the fact that there is an available N-Back fMRI literature in subjects with MS,6–8 albeit small, and a much larger one in healthy controls.9
METHODS
Sample selection.
Thirty-nine patients (aged 18–60 years) with a confirmed diagnosis of MS according to modified McDonald criteria were recruited from MS clinics. Exclusion criteria included a history of brain injury, illicit drug use other than cannabis, alcohol abuse, concurrent neurologic diseases, treatment with steroids in the past 3 months, neuropsychological testing in the past year, claustrophobia, mental handicap, and psychosis. All subjects had normal or corrected-to-normal vision.
Cannabis group.
Subjects (n = 20) who regularly used cannabis and whose urine tested positive for cannabis metabolites only were enrolled. The duration, method, and frequency of use of cannabis were obtained. The reasons for using cannabis were divided into medical, recreational, or both. To avoid assessing subjects who were acutely intoxicated, participants were asked to refrain from using cannabis for 12 hours before testing. Before proceeding with the protocol, saliva samples were screened for Δ9-tetrahydrocannabinol using NarcoCheck, which detects cannabis use within the last 4 to 6 hours. If positive, subjects were withdrawn from the study. Finally, cannabis subjects completed the Cannabis Withdrawal Scale,10 which quantifies symptoms of cannabis withdrawal according to these scores: <51 none; 52–66 mild to moderate; and >66 severe.
Control sample.
Subjects with MS (n = 19) who had never used cannabis were group-matched to the cannabis group on demographic and disease-related variables. All control subjects had negative urine and saliva tests.
Urinalysis for cannabis metabolites.
A broad-spectrum urine analysis was conducted by a toxicology laboratory to determine the presence of cannabis, cocaine, opiates, amphetamines, barbiturates, and phencyclidine. The cannabinoid assay detected levels of THC-COOH glucuronide and THC-COOH, which were reported as a composite score.
Demographic and neurologic data.
Demographic and disease-related variables were obtained at the time of testing. Visual acuity (Snellen Chart), handedness (all right-handed), and Expanded Disability Status Scale scores were recorded.
Neuropsychological testing.
All participants were given the Brief Repeatable Battery of Neuropsychological Tests in MS,11 which includes measures of verbal (Selective Reminding Test Revised) and visual (10/36 Spatial Recall Test) memory, information processing speed (Paced Auditory Serial Addition Test [PASAT; 2 and 3 seconds] and Symbol Digit Modalities Test), and attention (Word List Generation). Subjects were deemed to have failed a test if their scores were more than 1.5 SDs below age- and education-corrected published normative data. Failure on 2 or more tests, by convention, signaled global impairment.1
In addition, manual dexterity was assessed with the Purdue Pegboard Test,12 premorbid IQ with the Wechsler Test of Adult Reading,13 anxiety and depression with the Hospital Anxiety and Depression Scale in which scores ≥8 denote clinically significant anxiety and depression, respectively,14 and fatigue with the modified Fatigue Impact Scale.15
The N-Back,16 a test of working memory, was modified for fMRI presentation to avoid verbal responses. Subjects responded to each visual stimulus via a 2-button response pad (Current Designs, Inc., Philadelphia, PA) with accuracy and response times recorded. A 3-letter version (0, 1, and 2 Backs) was used in which test stimuli are pseudo-randomized alphabetic letters presented one at a time for 500 milliseconds with an interstimulus interval of 1,500 milliseconds. In the 0-Back condition, subjects press a “target” button when a designated letter appears and the “nontarget” button when presented with any other letter. In the 1- and 2-Back conditions, participants press the target button when the letter presented matches to the letter 1 or 2 letters preceding it, respectively. Otherwise, they press the nontarget button. The N-Back was presented in 30-second blocks.
MRI scanning parameters.
MRIs were collected on a 3-tesla MRI scanner (GE Healthcare, Milwaukee, WI) using a standard birdcage head coil. Before the functional scans, high-resolution anatomical scans were acquired for each subject (repetition time [TR] = 8.1 milliseconds, echo time [TE] = 3.2, flip angle = 8°, field of view [FOV] = 22 cm, 190 slices, slice thickness = 1 mm) for later coregistration with functional maps. PD/T2 (TR = 2,500 milliseconds, TE = 11.1/90, flip angle = 90°, FOV = 22 cm, 48 slices, slice thickness = 3 mm) and fluid-attenuated inversion recovery (TR = 9,700 milliseconds, TE = 140, FOV = 22 cm, 48 slices, slice thickness = 3 mm) images were also collected.
The fMRI acquisitions utilized T2-weighted, gradient-echo imaging to obtain blood oxygenation level–dependent images, from which maps of inferred neuronal activation were derived. The current protocole1 involves single-shot spiral k-space acquisitions with in-out readout, as developed at Stanford University (flip angle/TE/TR = 70°/30 milliseconds/2,000 milliseconds, 20 cm FOV, 5-mm thick, 26 slices, effective matrix size 90 × 90). The durations of the resting-state and N-Back fMRI scans were 7 minutes and 10 minutes, 30 seconds, respectively.
An axial cardiac-gated DTI sequence (TR = 8,800 milliseconds, TE = 80 milliseconds, FOV = 38 cm, slice thickness = 3 mm, 48 slices with 23 gradient orientations) was acquired with a b value = 1,000 with 2 non-diffusion-weighted baseline images (b = 0).
fMRI analysis.
First, the resting-state analysis was performed using Probabilistic Independent Component Analysise2 in MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components) version 3.13, part of FSL (FMRIB's Software Library).e3 The fMRI data were processed with FEAT (FMRI Expert Analysis Tool) version 6.00 and applied to the input data as follows: high-pass filter cutoff of 100 (seconds), MCFLIRT motion correction with a slice timing correction, spatial smoothing with a full-width half-maximum value of 5 mm and high-pass temporal filtering. The resulting voxel size of the resting-state data was 4 × 4 × 4 mm. Single-session MELODIC analyses were inspected visually to exclude structured noise components of noninterest. Cumulative head motion accrued during the resting-state fMRI was calculated as a single displacement metric for each subject and used as a covariate in the group analysis. Resting-state fMRIs were temporal concatenated for multisession group Independent Component Analysis on variance-normalized data. Registration was performed using the standard Montreal Neurological Institute (MNI)152 2-mm brain template. Registration to high-resolution structural and standard-space images was performed using FLIRT (FMRIB's Linear Image Registration Tool).e4,e5 The functional data were registered first to each subject's T1 structural images and then into the MNI 2-mm standard space.
Independent components were reviewed and selected from the group MELODIC analysis. The default mode network, frontoparietal networks, and the executive control network were chosen because of their potential relevance to cannabis effects. As primary sensory regions, the medial visual and sensory-motor networks were also chosen. An expanded list of additional resting-state networks (RSNs) would entail more conservative correction for multiple comparisons (i.e., Bonferroni correction for every RSN). Therefore, the within-RSN voxel-wise group comparison was restricted to 6 RSNs. The RSNs were back-projected into individual subject datasets using dual regression to facilitate a voxel-wise analysis.e6 An unpaired group analysis comparing cannabis and noncannabis users was performed with 5,000 permutations using an established neuroimaging group analysis tool.e7 Significant voxels were corrected for multiple comparisons at p < 0.05 and incorporated default settings for threshold-free cluster enhancement.e8
Second, for the N-Back task, image preprocessing and statistical analyses were performed using BrainVoyager QX 2.6 (Brain Innovation, Maastricht, the Netherlands). Before coregistration, the task-fMRI data were preprocessed by linear trend removal, gaussian spatial smoothing with a full-width half-maximum value of 6 mm, and a 3-dimensional motion correction using trilinear interpolation to detect and correct for small head movements during the scan by spatially realigning all subsequent volumes to the fifth volume. Functional datasets were transformed into Talairach space by coregistering the functional data with the anatomical data for each participant. Subsequent analyses were performed within individual participants and across the groups. The first 5 of 320 volumes of each time series were deleted to remove transient signal changes related to the steady magnetization.
To statistically evaluate the relative differences across the 3 N-Back conditions within groups (0, 1, 2 Back), a multiple regression approach was employed using 3 predictors with the rest periods (10 seconds before each block and 30 seconds between N-Back conditions) serving as a baseline. The stimulation protocol was convolved with a boxcar hemodynamic response functione9 to account for the expected shape and temporal delays of the physiologic response and was used in the general linear model. A random-effects analysis was used within groups to generate activation maps for each N-Back task individually. A random-effects analysis was also used to compare activations across the groups. Contrast maps were created using a voxel-based approach to show relative changes between tasks or across groups. Activated voxels were considered significant if the threshold exceeded p < 0.001 uncorrected and formed a cluster of 33 contiguous voxels, based on a cluster size threshold estimator simulation (BrainVoyager QX 2.6 software, Brain Innovation), corresponding to a corrected threshold of p < 0.05.e10 The center of gravity and t statistics were extracted for each significant cluster. All functional imaging analyses were conducted blinded to the cognitive results.
Structural imaging.
For lesion analysis, total hyper- and hypointense lesion volumes were obtained using FLEX, an in-house–derived software program, details of which have been published elsewhere.e11
The T1-weighted image was segmented into gray matter, white matter, and CSF using an in-house automatic segmentation algorithm.e12,e13 Lesion volumes were subtracted from the white and gray matter tissue thereby leaving regions of normal-appearing white matter (NAWM) and gray matter (NAGM). Atrophy of NAWM and NAGM was measured as the proportion of the total intracranial volume including CSF.
DTI analysis provided fractional anisotropy (FA) and mean diffusivity (MD) of NAWM and NAGM according to a previously published in-house methodology summarized here.e14 The DTI data were corrected for motion and eddy-current distortions. FA and MD maps were created from the corrected data using DTI studio (version 2.4.01). To generate whole-brain FA and MD data, the tissue segmentations were moved into DTI space by nonlinear registration of the T1-weighted images with their first baseline DTI volume. For this dataset, the optimal pipeline consisted of a multistage affine registration of T1 to DTI and T2 to DTI using the spin-echo and fast spin-echo images. Once image alignment had been achieved, the T2 image and DTI were fully registered using nonlinear registration. Finally, rotation matrices were combined to generate the nonlinear rotation required to move the T1-weighted image and subsequent segmentation image into DTI space, which were resliced using nearest neighbor interpolation. The tissue segmentation masks were combined with the FA and MD maps to obtain average values for NAWM and NAGM.
Standard protocol approvals, registrations, and patient consents.
The study received ethical approval and informed consent was obtained from all subjects.
RESULTS
Demographic and disease-related variables.
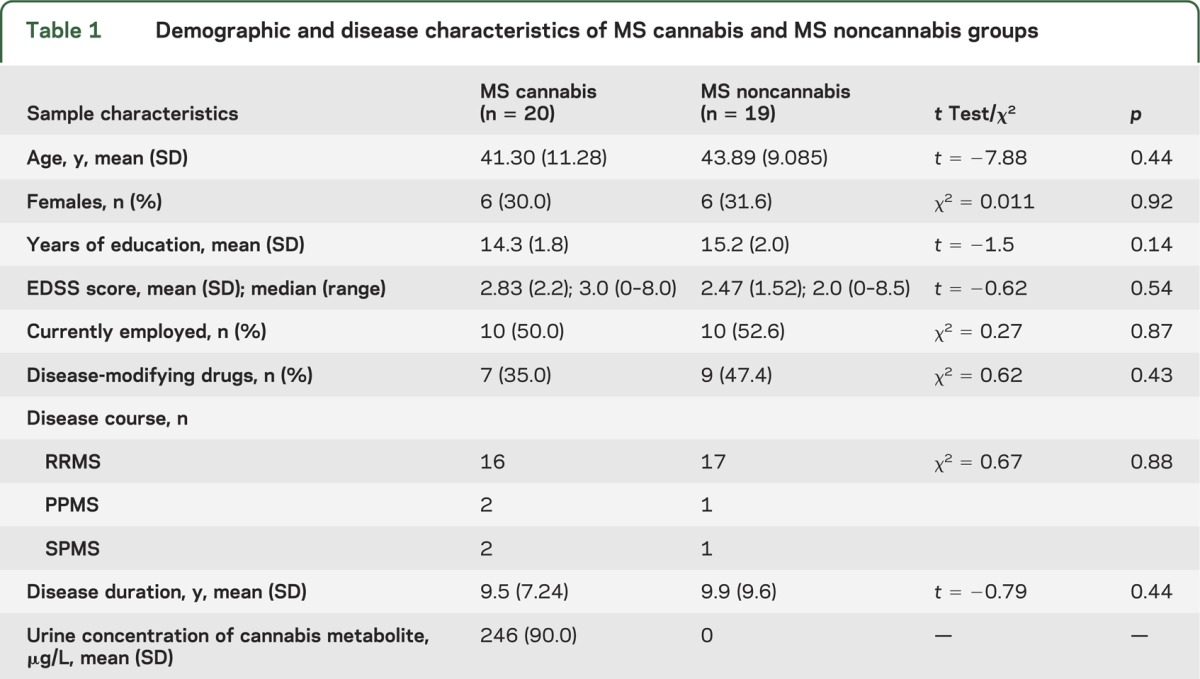
There were no significant differences in demographic and disease characteristics between the 2 groups (see table 1).
Table 1.
Demographic and disease characteristics of MS cannabis and MS noncannabis groups
Cannabis data.
Smoking was the only method of cannabis use, with frequency varying as follows: daily (n = 17), 4 to 5 times a week (n = 2), and 2 to 3 times a week (n = 1). Fourteen subjects reported cannabis use for medical reasons (pain, spasticity, insomnia, anxiety, a combination of factors), 2 for recreation, and 4 for both. The mean score on the Cannabis Withdrawal Scale was 15 (SD = 18.4). Two subjects endorsed withdrawal symptoms at the very mildest end of the range (scores of 52 and 56, respectively).
Psychometric data.
The cannabis group performed statistically more poorly on the 2-second PASAT and the 10/36 test (see table 2). The 2 groups had similar scores on the modified Fatigue Impact Scale and Hospital Anxiety and Depression Scale. Global cognitive impairment did not correlate with frequency of cannabis use (r = 0.32; p = 0.17) or urine concentration of metabolites (r = 0.32; p = 0.17).
Table 2.
Premorbid IQ and cognitive test scores for cannabis and noncannabis groups
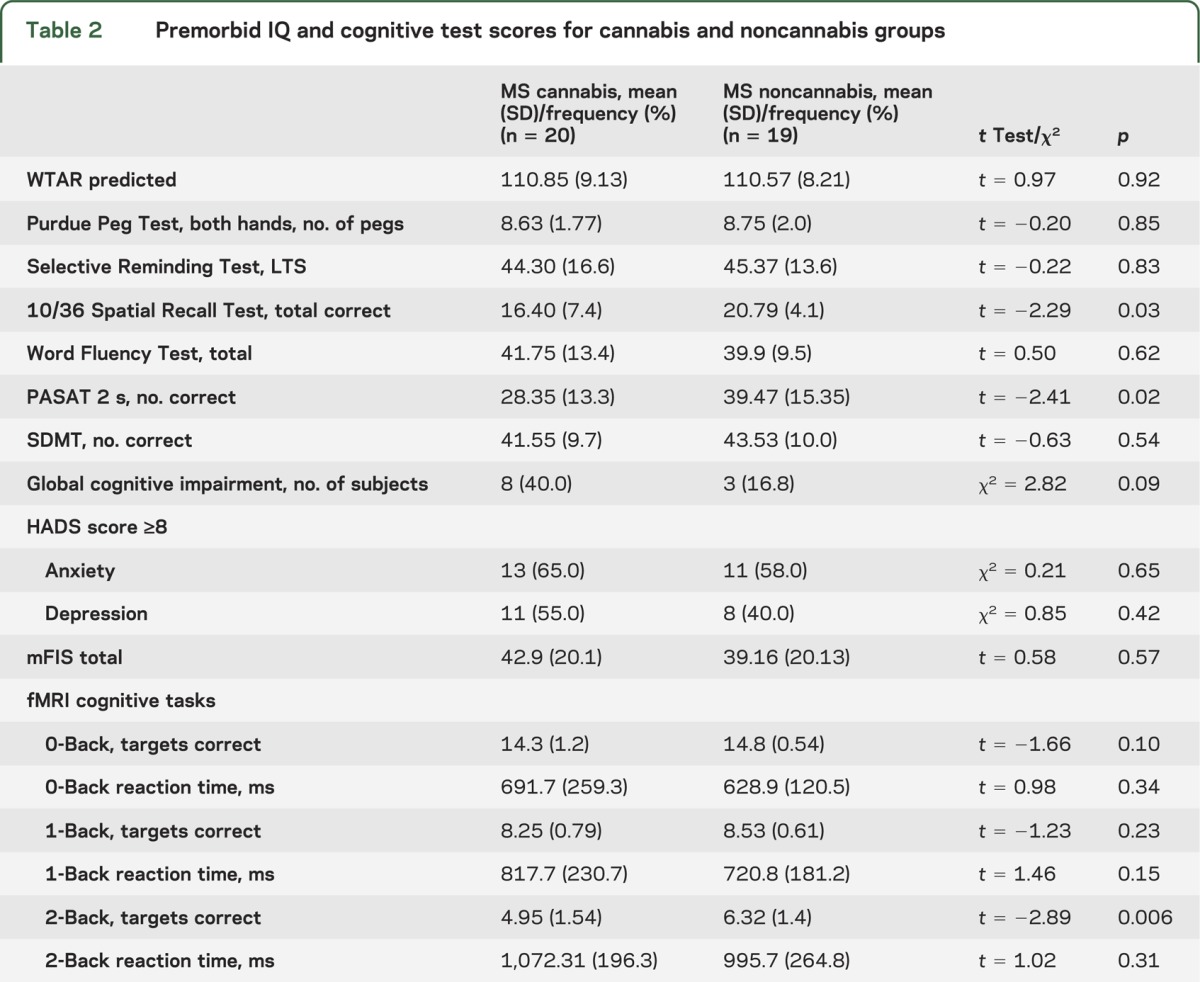
Regarding the fMRI N-Back task, there were no statistically significant between-group differences for the 0- and 1-Back trials. However, on the 2-Back trial, the cannabis group obtained fewer correct responses but showed no difference in reaction times (see table 2). A repeated-measures analysis of variance revealed a significant group (cannabis vs noncannabis) × trial interaction for the number of correct responses (F = 3.83; p = 0.03) but not for reaction times (F = 0.41; p = 0.67).
fMRI data.
The key areas of coactivation identified by the RSN group analysis are summarized in table e-1 on the Neurology® Web site at Neurology.org. The default mode, right frontoparietal, left frontoparietal, executive control, sensory-motor, and visual networks were clearly identified in both groups. No between-group contrasts of each network proved to be significant (pcorrected > 0.05). The noncannabis groups showed a small but significantly larger amount of cumulative head motion during the resting-state fMRI (p = 0.045). Between-group analyses in RSNs were performed using cumulative head motion as a covariate, which again showed no significant RSN differences (pcorrected > 0.05).
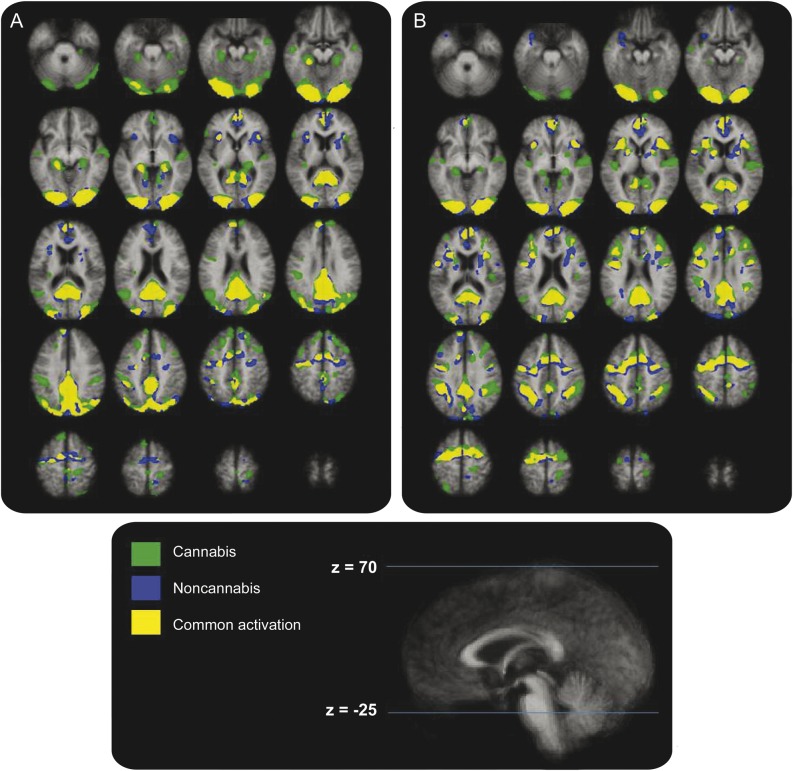
Within-group regions of activation on the N-Back task are shown in table e-2. Both groups activated prefrontal, anterior cingulate, and posterior parietal circuits that are known to characterize the functional neuroanatomy of the N-Back task.9 Focusing on the 0-Back and 2-Back trials, numerous ancillary regions were seen to activate as well with an even more diffuse pattern observed in the cannabis group (see figure 1).
Figure 1. Within-group activation maps for the cannabis and noncannabis groups.
Within-group activation maps for the cannabis and noncannabis groups during the 0-Back (A) and 2-Back (B) tasks.
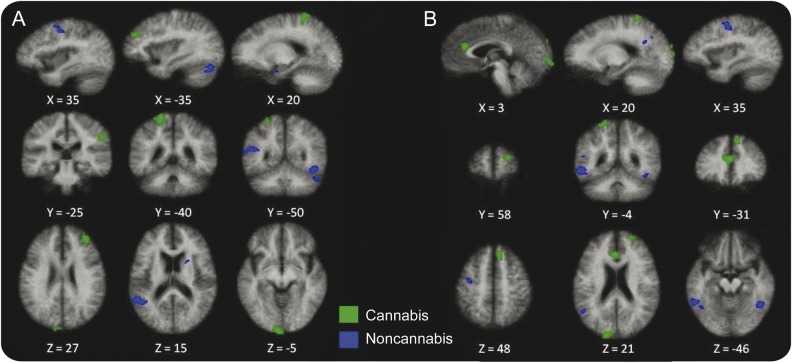
Between-group comparisons (cannabis > noncannabis) for the 0-Back and 2-Back task are shown in table 3. Of note is that parietal and anterior cingulate activations were only present in the cannabis group for both tasks. Similarly, frontal activation appeared more prominently in the cannabis group across tasks (see figure 2).
Table 3.
Activation for the 0-Back and 2-Back contrasts
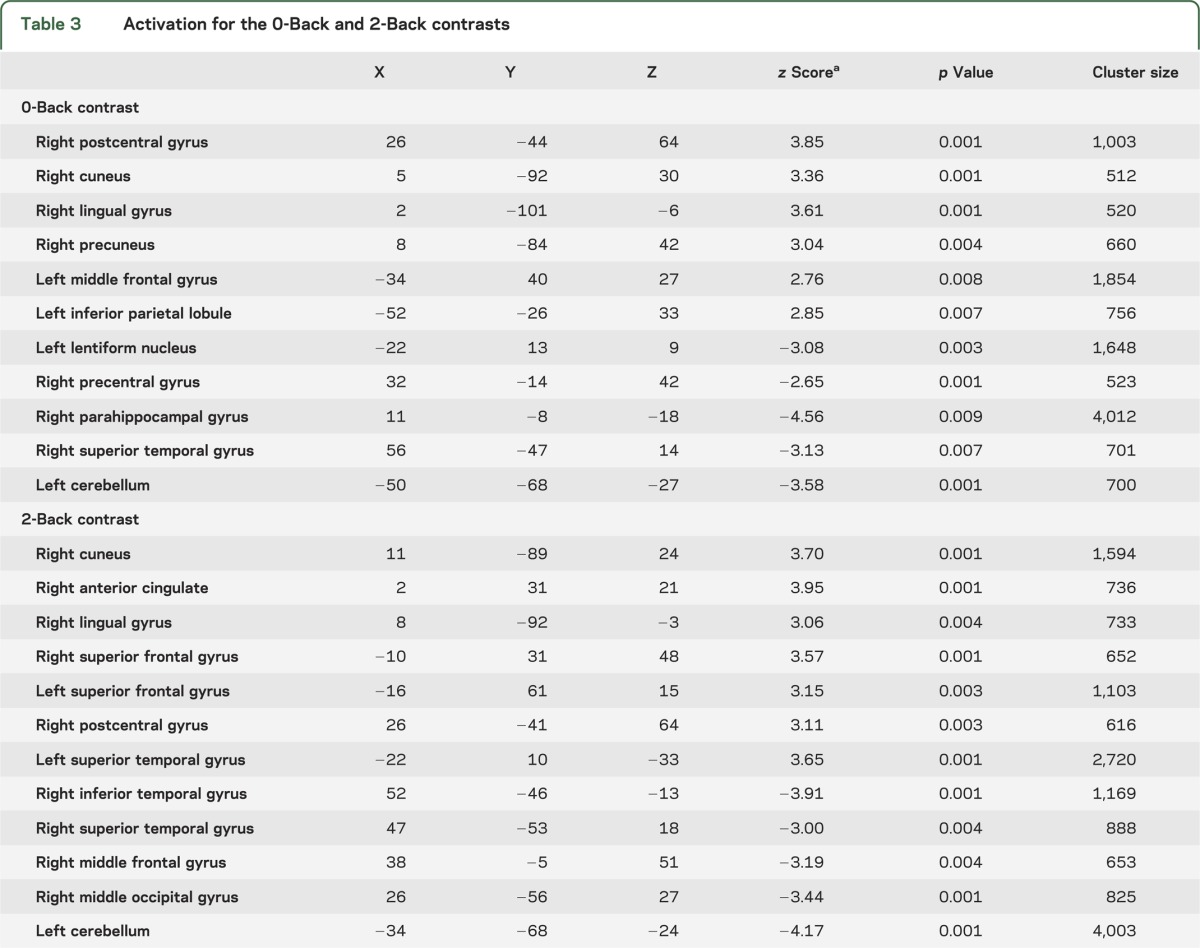
Figure 2. Between-group activation maps for the cannabis and noncannabis groups.
Between-group activation maps for the cannabis and noncannabis groups during the 0-Back (A) and 2-Back (B) tasks.
Structural MRI.
The 2 MS groups did not differ regarding total T2 (t = 0.56; p = 0.58) and T1 (t = 0.25; p = 0.81) lesion volumes and whole-brain gray (t = −0.29; p = 0.78) and white (t = 0.51; p= 0.62) matter volumes. No differences in FA in NAWM (t = −1.5; p = 0.13) and NAGM (t = 1.2; p = 2.4) and MD in NAWM (t = 1.5; p = 0.15) and NAGM (t = 1.1; p = 0.27) were found.
DISCUSSION
We have shown that patients with MS who smoke cannabis on a regular basis have more cognitive deficits than a matched group of patients with MS who are drug-free. Our cognitive findings therefore replicate the results of previous studies in samples of similar composition.3 Furthermore, we have shown that more extensive working memory problems in the cannabis group were associated with a different pattern of cerebral activation observed on fMRI. Significantly, no structural differences, be they lesion volume, global atrophy, or DTI metrics, were discernible between the groups, a finding that overlaps with results from numerous cannabis imaging studies in healthy subjects (see Martin-Santos et al.17 for a review).
When interpreting our resting-state data, some clinical points need emphasis. First, we achieved a strong group matching regarding demographic and standard neurologic variables, such as Expanded Disability Status Scale scores and disease type. Moreover, the cannabis group was no more anxious, depressed, or fatigued than their noncannabis counterparts. Each one of these symptoms could have influenced the RSN data.18 Second, we excluded subjects using cannabis who were acutely intoxicated or going through cannabis withdrawal symptoms, variables that may influence cognition in healthy subjects.19 Acute cannabis intoxication is more clearly associated with cognitive dysfunction,20 emotional changes,21 and an altered physiology22 unlike the nonacute state under investigation, while cannabis withdrawal is associated with a plethora of symptoms (e.g., irritability, sleeplessness, restlessness) all of which, in theory, could have influenced RSN, including default mode network, findings. Third, our cannabis and noncannabis groups did not differ in structural measures of brain integrity (lesions and atrophy volumes, indices of normal-appearing brain tissue), indices known to influence RSN activity.23 This multidimensional overlap between the 2 groups may therefore explain the absence of RSN differences, notwithstanding the presence of cannabis metabolites in one of the groups only.
There are no antecedent published results that address resting-state activation or deactivation in patients with MS who have smoked cannabis. Similarly, looking beyond MS, the resting-state functional imaging literature gives few clues as well. Of the 6 resting-state studies that explored the nonacute effects of cannabis, only one used fMRI24 and here the emphasis was on cerebral blood volume as elicited by dynamic susceptibility contrast MRI. Serial data were collected during an enforced period of abstinence from cannabis users and healthy, nondrug-using control subjects. Initial measures (days 0 and 7) revealed the former to have increased cerebral blood volume in the right frontal region, bilateral temporal regions, and the cerebellum, a finding that was only applicable to the left temporal region and cerebellum by day 28. The remaining 5 imaging studies that compared cannabis users and nonusers comprise 3 with SPECT and 2 with PET and showed a mixed picture of regional or global cerebral blood flow: no differences,25 reduced,26–28 or both reduced and increased depending on anatomical location29 in the cannabis users.
An additional aim of the study was to assess cerebral activation in cannabis-positive and -negative subjects during a working memory task. The most notable psychometric finding was that differences between groups only became apparent on the 2-Back task. Functional brain imaging differences were, however, evident before this cognitive fall-off became apparent and were present even in the 0-Back trial.
The N-Back has been thoroughly studied in healthy subjects. A meta-analysis of fMRI studies with a methodology similar to ours has consistently revealed a network that comprises prefrontal, premotor, anterior cingulate, and posterior parietal activation.9 This pattern of activation differs from that seen in identity monitoring of verbal stimuli where activity in the ventrolateral prefrontal cortex, in particular, features more prominently.30 A different picture emerges in subjects with MS.6–8 Here, the findings can broadly be summarized as patterns of underactivity and overactivity in regions identified as functionally relevant in the healthy control data plus overactivity in ancillary regions, i.e., those not typically observed in the healthy control subjects. In addition, subjects with MS show increased activity relative to healthy controls as cognitive tasks become more complex. This shifting pattern of activation intensity and location has been interpreted as evidence of neural plasticity.31 To a degree, these compensatory efforts can boost psychometric performance giving rise to similar N-Back results between subjects with MS and healthy controls.7,8 However, plasticity in MS has a threshold beyond which greater cognitive deficits become apparent, particularly as task complexity increases. This was revealed in our study, albeit in the context of cannabis use, where cognitive differences on the N-Back only emerged in the 2-back trial.
Both MS groups (cannabis and noncannabis) activated medial frontal, posterior parietal, and anterior cingulate regions when performing the N-Back task across all 3 trials. These regions are known to underpin the performance of this paradigm in healthy control subjects as well.9 Of note, however, is that the anterior cingulate region was more ventral (BA24) than the dorsal activation found in healthy subjects. This less anatomically focused activation, which matches findings from the MS N-Back literature in general appears to disperse even further in cannabis users. Not only is brain activation more scattered, it is increased in prefrontal, anterior cingulate, postparietal regions relative to noncannabis users. This is most telling revealed in between-group comparisons on the 2-Back task where heightened activation is associated with more errors made. In interpreting this cannabis-related finding, the possibility must be considered that certain regional activations owe more to the direct effects of regular cannabis use on brain function, for example, changes in vigilance or cerebral perfusion, than a cerebral response to the cognitive demands of the N-Back task.32 If this was indeed the case, one could expect to find differences in RSNs between the cannabis and noncannabis groups. No such differences were, however, noted.
It is unclear to what degree these findings in the cannabis group replicate or differ from those seen in healthy subjects who smoke cannabis. Comparisons are hindered by few studies and samples limited to adolescents33 or young adults where the focus has been on visuospatial rather than verbal memory.34 That said, the few studies of working memory suggest a mixed pattern of increased activity both in regions implicated in verbal memory, i.e., the parietal cortex,35 coupled with hypoactivity in other regions, most notably frontal.36 A similar pattern of increased activation, thought to reflect compensatory “work harder” mechanisms has been observed in tests of visuospatial memory in cannabis users compared with drug-free healthy subjects.34
In 2 previous MS studies, we reported that smoking cannabis added to the cognitive burden associated with the neurologic disease. The present functional imaging study provides confirmation of our earlier psychometric results and adds to it by demonstrating a more disorderly pattern of cerebral activation in cannabis users indicative of compensatory attempts that cannot overcome increasing task complexity. These latest findings need replication before more definitive conclusions can be reached. In addition, the failure to find more extensive structural brain abnormalities in cannabis smokers needs to be revisited with the emphasis not only on global brain metrics, as in this study, but also on focal regions associated with the kinds of cognitive deficits, i.e., working memory and information processing speed, elicited in our study. Such an approach, currently under way, holds out the promise of linking functional abnormalities more closely to underlying structural deficits.
Supplementary Material
GLOSSARY
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FOV
field of view
- MD
mean diffusivity
- MNI
Montreal Neurological Institute
- MS
multiple sclerosis
- NAGM
normal-appearing gray matter
- NAWM
normal-appearing white matter
- RSN
resting-state network
- TE
echo time
- TR
repetition time
- PASAT
Paced Auditory Serial Addition Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Bennis Pavisian: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis. Bradley MacIntosh: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. Greg Szilagyi: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Richard Staines: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis, study supervision. Paul O'Connor: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision. Anthony Feinstein: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis, study supervision, obtaining funding.
STUDY FUNDING
Supported by the Multiple Sclerosis Society of Canada.
DISCLOSURE
B. Pavisian reports no disclosures relevant to the manuscript. B. MacIntosh received an honorarium from Philips Healthcare. G. Szilagy and R. Staines report no disclosures relevant to the manuscript. P. O'Connor serves on scientific advisory boards for Novartis, Sanofi-Aventis, Bayer Schering Pharma, Genentech, Inc., and Roche; has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., Novartis, and Sanofi-Aventis; has served as a consultant for Biogen Idec, Actelion Pharmaceuticals Ltd., Bayer Schering Pharma, EMD Serono, Inc., Teva Pharmaceutical Industries Ltd., Genentech Inc., and Warburg Pincus; has received research support from Abbott, Bayer Schering Pharma, Novartis, BioMS Medical, Sanofi-Aventis, CIS Pharma, Genmab A/S, Cognosci, Inc., Wyeth, Daiichi Sankyo, and Roche; and serves as the National Scientific and Clinical Advisor to the Multiple Sclerosis Society of Canada. A. Feinstein has served on scientific advisory boards for Merck Serono and Avanir Pharmaceuticals; has received speaker honoraria from Merck Serono, Teva Pharmaceutical Industries Ltd., Bayer Schering Pharma, and Biogen Idec; serves on the editorial boards of Multiple Sclerosis and the African Journal of Psychiatry; receives publishing royalties for The Clinical Neuropsychiatry of Multiple Sclerosis (Cambridge University Press, 2007); chairs the Medical Advisory Committee for the Multiple Sclerosis Society of Canada; conducts neuropsychiatric evaluation, cognitive testing, and brain imaging in neuropsychiatry in his clinical practice; and receives research support from the Canadian Institute of Health Research, the Multiple Sclerosis Society of Canada and Teva Pharmaceutical Industries Ltd. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis: I: frequency, patterns, and prediction. Neurology 1991;41:685–691 [DOI] [PubMed] [Google Scholar]
- 2.Ghaffar O, Feinstein A. Multiple sclerosis and cannabis: a cognitive and psychiatric study. Neurology 2008;71:164–169 [DOI] [PubMed] [Google Scholar]
- 3.Honarmand K, Tierney MC, O'Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology 2011;76:1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis: II: impact on employment and social functioning. Neurology 1991;41:692–696 [DOI] [PubMed] [Google Scholar]
- 5.Filippi M, Rocca MA, Benedict RHB, et al. The contribution of MRI in assessing cognitive impairment in multiple sclerosis. Neurology 2010;75:2121–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumowski J, Wylie G, DeLuca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain 2010;133:362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweet L, Rao S, Primeau M, Mayer A, Cohen R. Functional magnetic resonance imaging of working memory among multiple sclerosis patients. J Neuroimaging 2004;14:150–157 [PubMed] [Google Scholar]
- 8.Wishart HA, Saykin AJ, McDonald BC, et al. Brain activation patterns associated with working memory in relapsing-remitting MS. Neurology 2004;62:234–238 [DOI] [PubMed] [Google Scholar]
- 9.Owen AM, McMillan KM, Laird AR, Bullmore E. N-Back working memory paradigm: a meta analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend 2011;119:123–129 [DOI] [PubMed] [Google Scholar]
- 11.Rao SM. A Manual for the Brief, Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. New York: National Multiple Sclerosis Society; 1990:121–123 [Google Scholar]
- 12.Tiffin J, Asher EJ. The Purdue Pegboard: norms and studies of reliability and validity. J Appl Psychol 1948;32:234–247 [DOI] [PubMed] [Google Scholar]
- 13.Wechsler D. Wechsler Test of Adult Reading: WTAR. San Antonio: Psychological Corporation; 2001 [Google Scholar]
- 14.Honarmand K, Feinstein A. Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler 2009;15:1518–1524 [DOI] [PubMed] [Google Scholar]
- 15.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the Fatigue Impact Scale. Clin Infect Dis 1994;18(suppl 1):S79–S83 [DOI] [PubMed] [Google Scholar]
- 16.Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci 2003;4:829–839 [DOI] [PubMed] [Google Scholar]
- 17.Martin-Santos R, Fagundo AB, Crippa JA, et al. Neuroimaging in cannabis: a systematic review. Psychol Med 2010;40:383–398 [DOI] [PubMed] [Google Scholar]
- 18.Bonavita S, Gallo A, Sacco R, et al. Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler 2011;17:411–422 [DOI] [PubMed] [Google Scholar]
- 19.Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002;287:1123–1131 [DOI] [PubMed] [Google Scholar]
- 20.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med 2011;5:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tunving K. Psychiatric effects of cannabis use. Acta Psychiatr Scand 1985;72:209–217 [DOI] [PubMed] [Google Scholar]
- 22.Mathew RJ, Wilson WH, Tant SR. Acute changes in cerebral blood flow associated with marijuana smoking. Acta Psychiatr Scand 1989;79:118–128 [DOI] [PubMed] [Google Scholar]
- 23.Rocca MA, Valsasina P, Absinta M, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 2010;74:1252–1259 [DOI] [PubMed] [Google Scholar]
- 24.Sneider JT, Pope HG, Jr, Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA. Differences in regional blood volume during a 28 day abstinence in chronic cannabis smokers. Eur Neuropsychopharmacol 2008;18:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathew RJ, Tant S, Burger C. Regional cerebral blood flow in marijuana smokers. Br J Addict 1986;81:567–571 [DOI] [PubMed] [Google Scholar]
- 26.Sevy S, Smith GS, Ma Y, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology 2008;197:549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tunving K, Thulin SO, Risberg J, Warkentin S. Regional cerebral blood flow in long-term heavy cannabis use. Pyschiatry Res 1986;17:15–21 [DOI] [PubMed] [Google Scholar]
- 28.Lundqvist T, Jönsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol 2001;23:437–443 [DOI] [PubMed] [Google Scholar]
- 29.Block RI, O'Leary D, Hichwa RD, et al. Cerebellar hypoactivity in frequent marijuana users. Neuroreport 2000;11:749–753 [DOI] [PubMed] [Google Scholar]
- 30.Crottaz-Herbette S, Anagnoson RT, Menon V. Modality effects in verbal working memory: differential prefrontal and parietal responses to auditory and visual stimuli. Neuroimage 2004;21:340–351 [DOI] [PubMed] [Google Scholar]
- 31.Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp 2006;27:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jager G, Van Hell HH, De Win MM, et al. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol 2007;17:289–297 [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen LK, Mencl WE, Westerveld M, Pudge KR. Impact of cannabis use on brain function in adolescents. Ann NY Acad Sci 2004;1021:384–390 [DOI] [PubMed] [Google Scholar]
- 34.Smith AM, Longo CA, Fried PA, Hogan MJ, Cameron I. Effects of marijuana on visuospatial working memory: an fMRI study in young adults. Psychopharmacology 2010;210:429–438 [DOI] [PubMed] [Google Scholar]
- 35.Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:837–845 [DOI] [PubMed] [Google Scholar]
- 36.Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage 2008;40:1328–1339 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.