Abstract
Objectives:
Our aim was to assess cortical thickness in a large multicenter cohort of drug-naive patients with early Parkinson disease (PD), with and without mild cognitive impairment (MCI), and explore the cognitive correlates of regional cortical thinning.
Methods:
One hundred twenty-three newly diagnosed patients with PD and 56 healthy controls with 3-tesla structural MRI scans and complete neuropsychological assessment from the Parkinson's Progression Markers Initiative were included. Modified Movement Disorders Society Task Force level II criteria were applied to diagnose MCI in PD. FreeSurfer image processing and analysis software was used to measure cortical thickness across groups and the association with cognitive domains and tests.
Results:
In patients with MCI, atrophy was found in temporal, parietal, frontal, and occipital areas compared with controls. Specific regional thinning in the right inferior temporal cortex was also found in cognitively normal patients. Memory, executive, and visuospatial performance was associated with temporoparietal and superior frontal thinning, suggesting a relationship between cognitive impairment and both anterior and posterior cortical atrophy in the whole patient sample.
Conclusions:
These findings confirm that MCI is associated with widespread cortical atrophy. In addition, they suggest that regional cortical thinning is already present at the time of diagnosis in patients with early, untreated PD who do not meet the criteria for MCI. Together, the results indicate that cortical thinning can serve as a marker for initial cognitive decline in early PD.
The pattern of initial cognitive impairment is gaining increased clinical significance in Parkinson disease (PD).1 There is preliminary evidence that deficits in specific cognitive domains pose a differential risk of developing dementia.2 According to this hypothesis, while patients with early frontal executive deficits remain stable over time, patients with early posterior cortical deficits seem to have a higher risk of developing subsequent dementia. In addition, specific cognitive domains may not only have different regional underpinnings but also differ in their pathologic substrates, with frontal deficits being related to variations in dopaminergic activity in frontal regions whereas posterior cognitive deficits are nondopaminergic and associated with structural changes in temporoparietal areas.3,4
Understanding the brain mechanisms underlying early mild cognitive impairment (MCI) in PD is important because it could help in identifying a target group for treatment.5 In this study, we explored the association between cortical thickness and cognition in patients with newly diagnosed, untreated PD. We hypothesized that (1) patients with PD-MCI would have more cortical atrophy compared to patients with normal cognition (PD-CN); (2) cognitive domains subserved by the temporoparietal cortex would be associated with cortical thinning in these regions, whereas executive and attentional impairment would be associated with frontal thinning or no structural changes; and (3) semantic fluency and verbal encoding would correlate with posterior cortical thinning, consistent with research showing that semantic fluency deficits predict dementia3,4 and memory deficits are due to an encoding rather than retrieval impairment in early PD.6
METHODS
Participants.
All subjects included in this study were enrolled in the Parkinson’s Progression Markers Initiative (PPMI) (2011)7 (accessed in March 2013), an observational, multicenter study designed to identify PD progression biomarkers. Only subjects with an MRI volume acquired on a 3-tesla Siemens scanner with similar acquisition parameters, which passed quality control before and after image preprocessing, were included in this study.8
At baseline, patients were required to meet standard diagnostic criteria for PD, have been diagnosed within 2 years, and to be untreated for PD. In addition, all subjects underwent dopamine transporter imaging, and a significant dopamine transporter deficit was required for neurobiological confirmation of a PD diagnosis. Inclusion criteria for healthy controls consisted of not having neurologic dysfunction, no first-degree family member with PD, and a Montreal Cognitive Assessment (MoCA) score >26. Psychiatric assessment included the 15-item Geriatric Depression Scale.9 Motor severity assessed by part III of the Movement Disorders Society (MDS) Unified Parkinson's Disease Rating Scale (UPDRS),10 disease stage by Hoehn and Yahr scale (1967),11 and disability by Schwab and England scale12 were obtained for patients.
Standard protocol approvals, registrations, and patient consents.
Each participating PPMI site (1) received approval from an ethical standards committee on human experimentation before study initiation, and (2) obtained written informed consent for research from all individuals participating in the study.
Neuropsychological assessment.
All subjects underwent a comprehensive neuropsychological battery. Visuospatial functions were evaluated using the 15-item version of the Benton Judgment of Line Orientation test and the clock and cube items of the MoCA. Verbal memory was assessed using the Hopkins Verbal Learning Test–Revised. Total immediate recall (encoding) and delayed recall (retrieval) scores were included in this study. Executive functions were evaluated using 3 semantic fluency tests (names of animals, fruits, and vegetables), the MoCA phonemic fluency subtest, and the MoCA trail making item. Attention was assessed by means of the Letter Number Sequencing (LNS) test, the Symbol Digit Modalities Test (SDMT), the MoCA backward digit span item, the MoCA vigilance item, and the MoCA serial 7s item. Because of the lack of specific language tests in the PPMI assessments, language abilities were evaluated using the items of MoCA including sentence repetition, abstraction, and naming. For cognitive tests yielding continuous scores, the individual raw scores of patients with PD were transformed to z scores using the means and SDs of the control group.
Diagnosis of MCI.
The classification of MCI was performed by an approximation to the guidelines of the MDS Task Force for the level II diagnosis of PD-MCI.13 Because the PPMI study was launched before the publication of these guidelines and does not include at least 2 comprehensive neuropsychological tests for all 5 domains or a validated measure to address subjective cognitive impairment, some adjustments had to be made. In line with this, the following MoCA items were included for assessment of the cognitive domains: backward digit span, vigilance and serial 7s (attention and working memory), phonemic fluency and trail making (executive), sentence repetition, abstraction and naming (language), clock, and cube (visuospatial). Patients were classified as having MCI if they showed impairment in 2 or more tests or items within the same cognitive domain or in 2 or more domains. Impairment was defined as a score below 2.0 SDs for the individual continuous tests, or a score below the maximum for the ordinal and categorical items, based on previous recommendations made by the MDS Task Force criteria for PD dementia.14
To enable correlation analyses between cognition and cortical thickness as well as comparisons with previous literature, we included a second approach to diagnose PD-MCI. This approach consisted of generating cognitive domain scores by calculating the average of the z scores of continuous tests pertaining to the same domain, as previously described.15,16 Two cognitive domains were built using the continuous cognitive tests: memory (z scores of immediate and delayed recall tests) and executive/attention (z scores of semantic fluency, phonemic fluency, SDMT, and LNS test). A third visuospatial domain was based solely on the Judgment of Line Orientation test. Impairment in these cognitive domains was established if patients scored below 2.0 SD compared with the means of the control group. We used the modified MDS criteria13 as our primary classification (PD-MCI-MDS), but we also performed the same analyses using the second domain-based approach (PD-MCI-Domains).
MRI acquisition.
T1-weighted MRI scans were acquired in the sagittal plane on 3T Siemens (TIM Trio and Verio) scanners (Erlangen, Germany) using a magnetization-prepared rapid-acquisition gradient echo sequence. The MRI parameters were as follows: repetition time = 2,300/1,900 milliseconds; echo time = 2.98/2.96/2.27/2.48/2.52 milliseconds; inversion time = 900 milliseconds; flip angle: 9°; 256 × 256 matrix; and 1 × 1 × 1 mm3 isotropic voxel.
MRI preprocessing.
The FreeSurfer image processing (version 5.3, http://surfer.nmr.mgh.harvard.edu/fswiki) was used to generate a cortical surface model providing a measure of cortical thickness at each vertex,17–20 as published elsewhere.21 The cortical maps were smoothed using a 15-mm full width at half maximum kernel.
Statistical analyses.
Differences between groups in sociodemographic and neuropsychological variables were analyzed using Mann-Whitney U tests for non-normally distributed data (as indicated by the Kolmogorov-Smirnov test), Student t test for normally distributed data, and χ2 for categorical data in SPSS 20.0 (IBM Corp., Armonk, NY).
Differences in cortical thickness between controls, PD-CN, and PD-MCI were examined on the cortical maps using FreeSurfer. Age, sex, education, MRI software version, and scanner were included as covariates. For the PD-CN and PD-MCI group comparisons, UPDRS III score was included as an additional covariate.
Correlation analyses between cortical surface maps and cognition were performed in the whole patient sample, adjusting for age, sex, education, scanner/software versions, and UPDRS scores. First, we performed correlations between cortical thickness and the z scores of the 3 cognitive domains. In addition, because some specific information might be lost by combining tests into domains, we also correlated cortical thickness with z scores of the individual continuous cognitive tests. In all imaging analyses, cluster-wise correction using Monte Carlo simulation with 10,000 iterations (vertex-wise threshold of p < 0.05) was applied. The plots of significant correlations between thickness and cognition were created using Spearman ρ.
RESULTS
Frequency and profile of MCI.
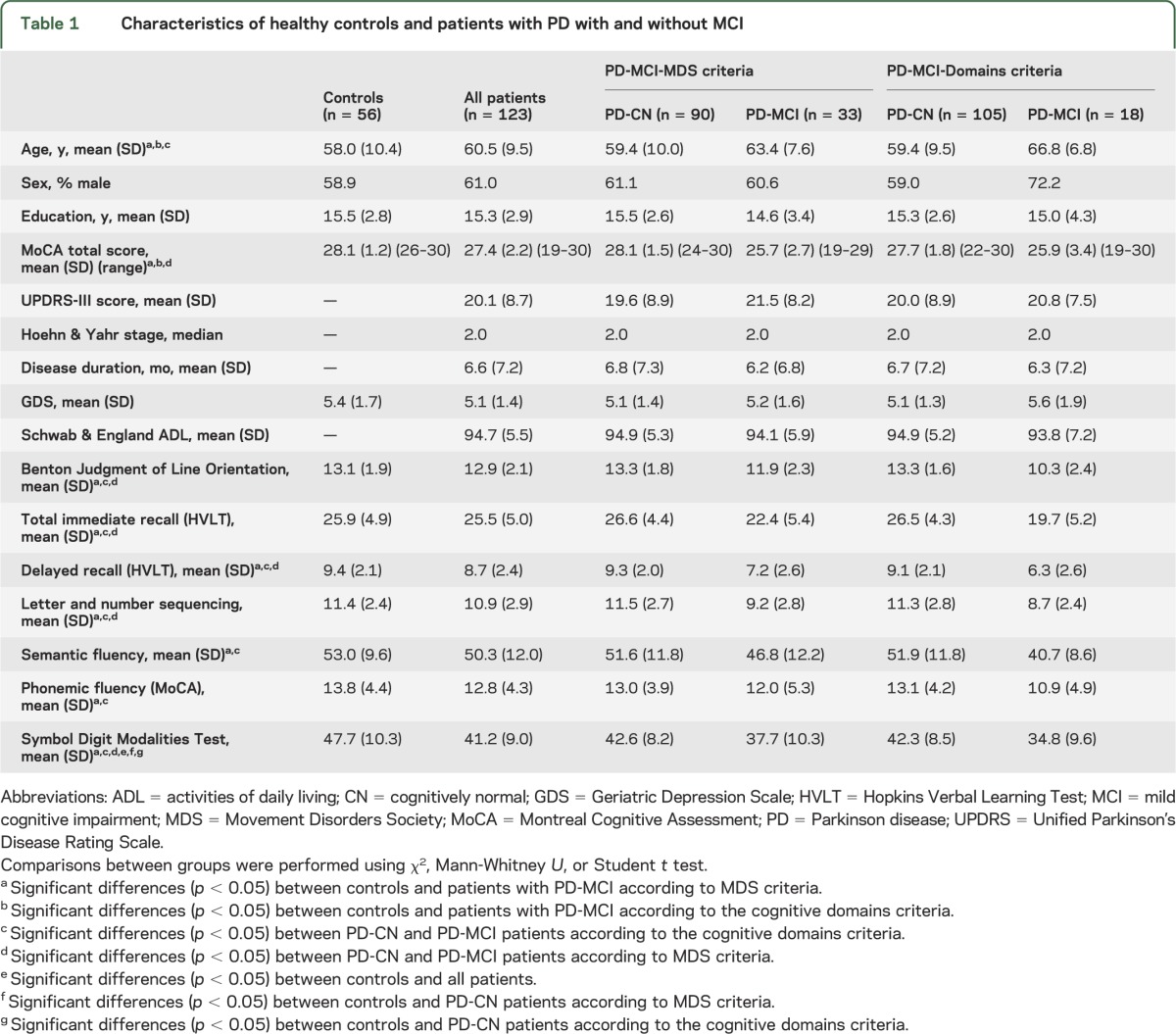
The characteristics of the sample can be found in table 1. Using the modified MDS Task Force definition, 33 patients with PD (28.5% [95% confidence interval 20.7%–37.7%]) were classified as having MCI. The comparisons between groups showed that patients with PD-MCI were significantly older than controls (table 1). MoCA scores differed between controls and patients with PD-MCI according to both classifications and between PD-CN and PD-MCI based on the PD-MCI-MDS classification. Only 18 patients (15.5% [95% confidence interval 9.7%–23.7%]) were classified with MCI based on the PD-MCI-Domain approach. Both classification methods coincided in the classification of 16 patients (88.9%) with MCI. Following the PD-MCI-MDS criteria, memory and executive impairment were the most common deficits in patients with PD-MCI (33.3% each), followed by visuospatial (27.3%) and attention (21.2%) impairment. There were no significant language deficits. Further results can be found in appendix e-1 on the Neurology® Web site at Neurology.org.
Table 1.
Characteristics of healthy controls and patients with PD with and without MCI
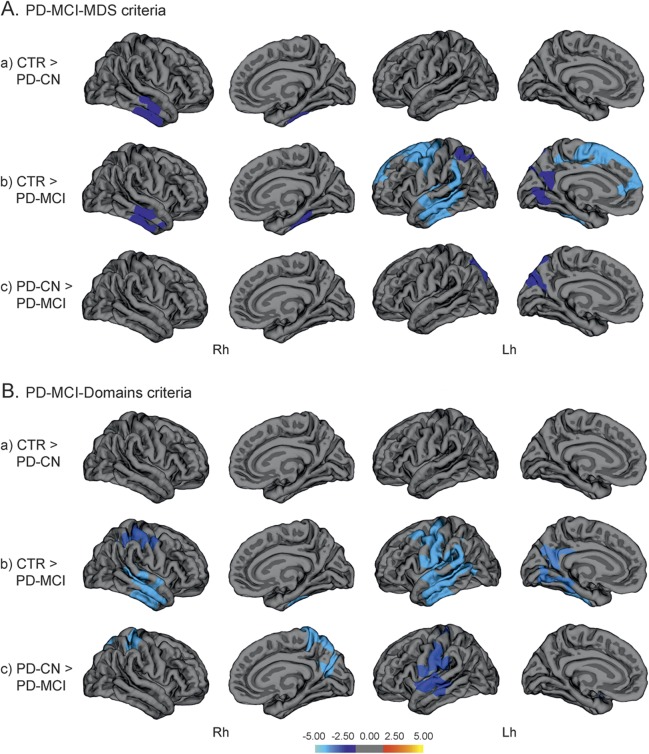
Cortical thickness in PD with and without MCI.
Based on the PD-MCI-MDS classification, cortical thinning in the right inferior temporal gyrus was found in PD-CN patients compared with controls, while the left hemisphere did not show significant thinning, suggesting an asymmetric pattern of atrophy (figure 1, table 2). To further explore this asymmetry, we performed a secondary correlation analysis between cortical thickness in the region that showed significant group differences and neuropsychological performance in the PD-CN group. The results of this analysis showed a significant correlation between that region’s thickness values and verbal learning scores (r = 0.25, p < 0.019).
Figure 1. Vertex-wise comparisons of cortical thickness.
Vertex-wise comparisons of cortical thickness between (a) controls (CTR) and cognitively normal patients with Parkinson disease (PD-CN), (b) controls and patients with PD and mild cognitive impairment (PD-MCI), and (c) PD-CN and PD-MCI, following the modified PD-MCI-MDS criteria (A) and the PD-MCI-Domains criteria (B). The color scale bar shows the logarithmic scale of p values (−log10). Lh = left hemisphere; MDS = Movement Disorders Society; Rh = right hemisphere.
Table 2.
Significant cortical thickness differences across groups following the PD-MCI-MDS criteria
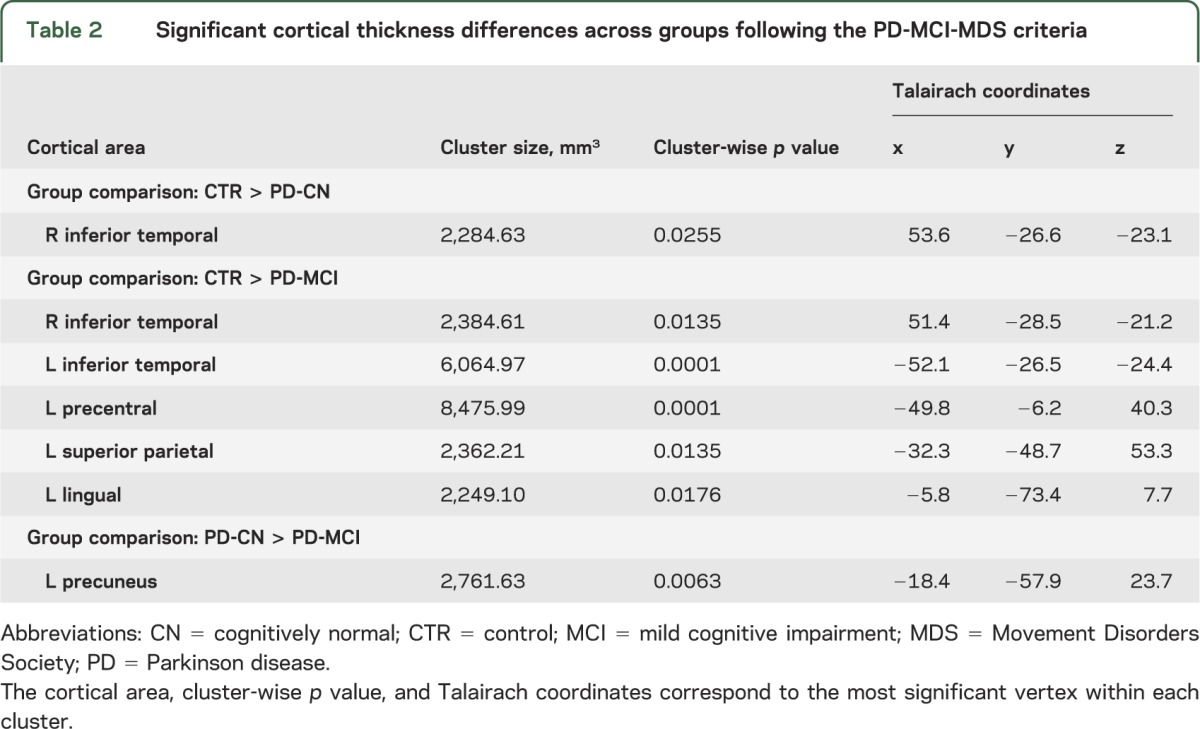
In patients with PD-MCI, atrophy was present in the right inferior temporal region as seen in the PD-CN group, but, in addition, regional thinning was more prominent in the left than the right side, compared with controls. Moreover, in patients with PD-MCI, changes were also observed in the left superior parietal cortex, precuneus, lateral occipital, temporal, anterior cingulate, and superior frontal gyri compared with controls (figure 1, table 2). The comparison between PD-CN and PD-MCI patient groups showed regional thinning in the left precuneus in patients with PD-MCI (figure 1, table 2).
When MCI was defined according to the PD-MCI-Domains strategy, similar findings emerged in the group comparisons. However, according to this classification approach, no significant cortical thinning was identified in PD-CN patients compared with controls. In patients with PD-MCI, there was a bilateral pattern of thinning involving temporal, superior parietal, and precentral areas, as well as the precuneus and occipital area in the right medial surface, compared with controls. The comparison between the PD patient groups showed more widespread changes involving temporal, precentral, and superior parietal regions in PD-MCI (figure 1, table e-1).
We did not find any regions showing increased cortical thickness in patients with PD with or without MCI compared to controls.
Associations between regional cortical thickness and cognition.
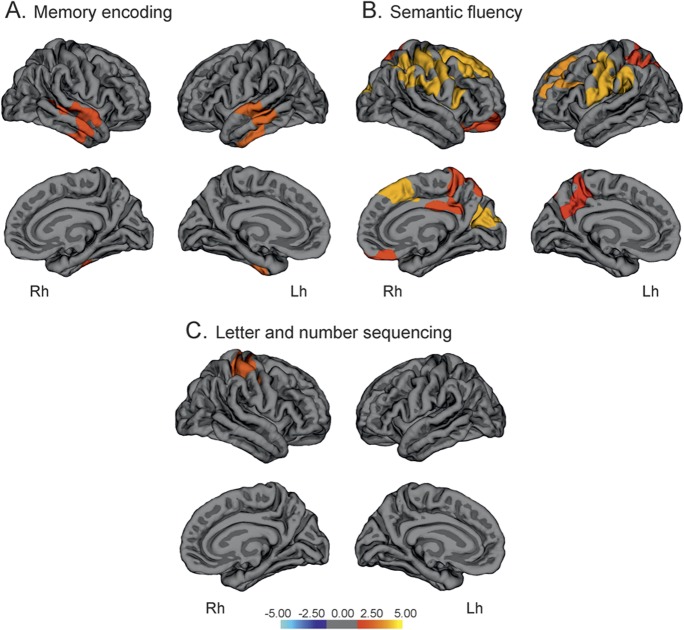
The visuospatial domain was associated with superior parietal thickness bilaterally, which in the right hemisphere extended to the right superior frontal gyrus and precuneus, while adjusting for potentially confounding covariates. Visuospatial scores also correlated with temporal and precentral thickness in the left hemisphere (ρ = 0.422, p < 0.001). The executive-attention domain scores correlated with cortical thickness in several areas, including the superior frontal, precentral, temporal, and parietal regions (ρ = 0.511, p < 0.001) (figure 2, table e-2). Finally, scores in the memory domain did not correlate with thickness in any cortical area.
Figure 2. Vertex-wise correlations between cortical thickness and mean scores in the visuospatial domain (A) and executive domain (B) in patients with Parkinson disease.
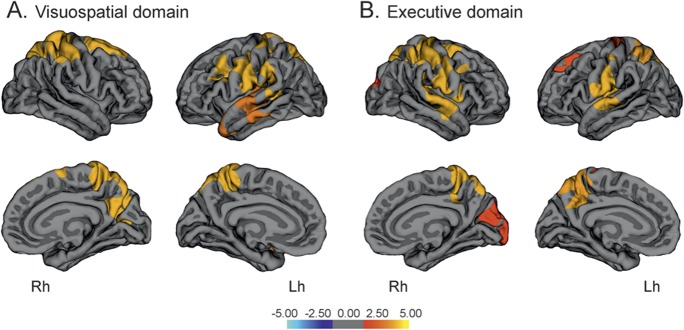
The color scale bar shows the logarithmic scale of p values (−log10). Lh = left hemisphere; Rh = right hemisphere.
Regarding the memory subtests, in contrast to the cognitive memory domain, higher performance in verbal encoding was associated with increasing thickness in middle, superior, and inferior temporal areas in both hemispheres (ρ = 0.457, p < 0.001) (figure 3, table e-2). In contrast, delayed recall scores (retrieval) did not show any significant correlations with cortical thickness. We observed a dissociation in the neuroanatomical correlates of the 2 verbal fluency tests. Higher scores in semantic fluency were related to increased thickness in the superior temporal, supramarginal, occipital, medial parietal, and frontal regions (ρ = 0.388, p < 0.001) (figure 3, table e-2). By contrast, phonemic fluency performance was not associated with thickness in any area. The LNS test scores correlated with thickness in a restricted area comprising the postcentral, precentral, and superior parietal gyri (ρ = 0.367, p < 0.001). The plots of all significant correlations are shown in figure e-1. There were no significant correlations between thickness and the SDMT scores, UPDRS motor scores, or disease duration in patients with PD or controls.
Figure 3. Vertex-wise correlations between cortical thickness and test performance.
Vertex-wise correlations between cortical thickness and performance on tests of memory encoding of Hopkins Verbal Learning Test–revised (A), semantic fluency (B), and letter and number sequencing (C) in patients with Parkinson disease. The color scale bar shows the logarithmic scale of p values (−log10). Lh = left hemisphere; Rh = right hemisphere.
DISCUSSION
The current study is the largest to date to assess the relationship between cognition and cortical thickness using 3T MRI in an early, drug-naive, multicenter PD cohort. We found that MCI in PD was associated with temporal, parietal, and frontal cortical thinning. Contrary to previous assumptions that atrophy does not accompany PD per se or in the absence of cognitive impairment,22,23 temporal thinning was also observed in patients with PD who did not meet criteria for MCI. Memory, visuospatial, and executive cognitive tests were mainly related to temporoparietal and superior frontal thinning, suggesting a relationship between cognitive impairment and both anterior and posterior cortical atrophy in PD.
The nature of cognitive impairment in early PD is still a matter of debate. Recent evidence suggests that both Lewy24 and Alzheimer disease pathologies25,26 could have an important role in the cortical changes underlying the development of cognitive decline, which could serve as a preclinical biomarker of dementia.27 However, it remains unclear how cortical thinning is related to the earliest cognitive changes in PD. In addition, dopaminergic treatment of early PD often ameliorates some cognitive functions at the expense of compromising others,1 masking the true effects of PD on cognition and its relationship with brain structure.
The discrimination of PD-CN from PD-MCI patients has shown promise in detecting the underlying cortical changes that precede cognitive impairment. While some studies have identified extensive gray matter volume loss in temporoparietal and prefrontal areas in PD-MCI compared with controls,28 others detected small density reductions in the precuneus, primary motor, parietal cortex,29 in addition to occipital areas,30 or cortical thinning in focal frontal regions.31 However, the small sample sizes, lack of consistent criteria for defining MCI, and the use of different measures of atrophy are among the parameters that could account for inconsistent findings among previous studies. In a recent study using rigorous methodology and the MDS criteria for diagnosing MCI,23 atrophy in the precentral and postcentral gyri, precuneus, frontal gyri, and temporal regions was found in patients with PD-MCI compared with controls, in line with our findings. However, in contrast to our study, no significant cortical changes were found in patients with PD that did not meet the criteria for MCI. Several reasons may account for this inconsistency. For instance, our study included a larger and more homogeneous sample of patients with PD, which might be more representative of earlier disease stages. The fact that all patients in our study had neurobiological confirmation of PD diagnosis and none were receiving dopaminergic treatment likely influenced the MCI classifications, resulting in a more reliable identification of PD-MCI cases. Indeed, compared with previous studies assessing cortical atrophy in PD-MCI, the main novelty of our study is the assessment of de novo, drug-naive PD patients at the earliest stages of cognitive impairment. The regional cortical thinning in PD-CN patients we observed suggests that cortical changes are already present at the time of diagnosis, before meeting criteria for MCI. The finding that cortical changes were detected specifically in temporal areas is in line with a recent study,32 in which a temporoparietal pattern of atrophy, similar to that observed in Alzheimer disease, was found in patients with PD-MCI. This pattern was suggested to be a preclinical biomarker of cognitive decline even in patients with normal cognition at baseline. The asymmetry we found in the cortical thinning patterns of PD-CN patients affecting the right hemisphere is not without precedent33 and suggests that cortical changes could start initially in one hemisphere and later on, as the disease progresses, extend to the other, as observed in the PD-MCI group. In addition, this asymmetric pattern significantly correlated with immediate recall performance in PD-CN group, in line with the thickness-memory correlation we found in the entire PD sample and the known association between memory and temporal areas.34
Consistent with our predictions, the memory and visuospatial test scores were significantly associated with cortical thickness in temporal and parietal regions. However, contrary to our expectations, the executive domain scores also correlated with these areas and visuospatial scores also showed a correlation with frontal regions. These results are in agreement with the notion that high-level cognitive functions rely on networks of brain areas that carry out different attentional functions35 as well as previous evidence showing that executive impairment is associated with gray matter loss in frontal, temporal, and parietal areas,36 while visuospatial impairment is associated with frontal gray matter reductions in PD.37
In contrast, these findings do not comply with the CamPaIGN study hypotheses,4 on which we based some of our predictions. It should be noted that these hypotheses were established in the context of a longitudinal design using neuropsychological data to predict cognitive decline and dementia in PD. Hence, they have not been corroborated using neuroimaging structural analyses.
Our correlation analyses showed that the immediate recall scores (encoding) correlated with temporal thickness. The fact that no significant correlations were found between thickness and delayed recall, by contrast to total immediate recall, is in agreement with the hypothesis that memory impairment in drug-naive, early PD is to a large degree a deficit of immediate recall (encoding) and not of long-term recall (retrieval).6
We also observed that semantic fluency performance was associated with widespread thickness in posterior and frontal areas, while phonemic fluency did not show any correlation. This is in line with previous reports suggesting that semantic fluency impairment is associated with a higher risk of developing dementia4 and more widespread gray matter atrophy in frontal, temporal, and parietal areas in PD38,39 compared with phonemic fluency.
The current study has several strengths including the large sample size and comprehensive neuropsychological and 3T MRI assessment. The neurobiological confirmation of all PD diagnoses with DaTscan, the fact that they were drug-naive, and the use of modified MDS-MCI criteria for classifying MCI are also relevant strengths. Some limitations should also be recognized, such as the potential bias of including super-normal controls in the PPMI cohort with scores >26 in MoCA and the lack of 2 standardized cognitive tests for each of the 5 cognitive domains. Finally, although representative of early stages of PD, the PPMI sample is a research-based cohort and might not be truly representative of a population-based cohort. In addition, the disease duration of the patients with PD was very short, so it is possible that patients with other diseases could have been included in our study.
Supplementary Material
ACKNOWLEDGMENT
Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. The authors thank the Strategic Research Programme in Neuroscience at Karolinska Institutet (StratNeuro) and Swedish Brain Power.
GLOSSARY
- CN
cognitively normal
- LNS
Letter Number Sequencing
- MCI
mild cognitive impairment
- MDS
Movement Disorders Society
- MoCA
Montreal Cognitive Assessment
- PD
Parkinson disease
- PPMI
Parkinson's Progression Markers Initiative
- SDMT
Symbol Digit Modalities Test
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
J.B.P., K.B., A.L., E.W., and D.A. were involved in the conception and design of the study, analysis and interpretation of the data, and drafting/revising the manuscript for content. P.S. and D.W. were involved in the interpretation of the data, and drafting/revising the manuscript for content.
STUDY FUNDING
Parkinson's Progression Markers Initiative, a public-private partnership, is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abbott, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, Élan, GE Healthcare, Genentech, GSK-GlaxoSmithKline, Lilly, Merck, MSD-Meso Scale Discovery, Pfizer, Roche, and UCB (www.ppmi-info.org/fundingpartners). J.B.P. was funded by a Marie Curie fellowship for postdoctoral researchers (grant no. FP7-PEOPLE-2012-IEF-328758).
DISCLOSURE
J. Pereira and P. Svenningsson report no disclosures relevant to the manuscript. D. Weintraub is part of the following scientific advisory boards: commercial—Pfizer, CHDI, Teva Pharmaceuticals, Avanir Pharmaceuticals, Merck, Eli Lilly and Company, Biogen, and Lundbeck Inc. He is part of the editorial board of Movement Disorders and receives funding for travel or speaker honoraria: commercial—Teva Pharmaceuticals; research support, commercial entities: Novartis Pharmaceuticals, principal investigator (PI), 2011–; research support from government entities: National Institute of Neurological Disorders and Stroke, P50 NS053488, investigator, 2007–, National Institute of Neurological Disorders and Stroke, R01NS065087, investigator, 2009–, and NIA, RO1AG031348, investigator, 2008–; research support, foundations, and societies: Michael J. Fox Foundation for Parkinson's Research, PI, 2009–2011, Michael J. Fox Foundation for Parkinson's Research, PI, 2011–, Michael J. Fox Foundation for Parkinson's Research, investigator, 2011–; license fee payments, technology or inventions: licensing fees from the University of Pennsylvania for licensing of Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease. K. Brønnick has received funding for travel and speaker honoraria from Solvay Pharmaceuticals, Inc. A. Lebedev and E. Westman report no disclosures relevant to the manuscript. D. Aarsland serves on scientific advisory boards for Lundbeck Inc. and Merck Serono; has received funding for travel and speaker honoraria from Lundbeck Inc., Novartis, GE Healthcare, and GlaxoSmithKline; serves on the editorial boards of International Psychogeriatrics, Movement Disorders, and the Journal of Neurology, Neurosurgery, and Psychiatry; and receives research support from Lundbeck Inc. and Merck Serono. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010;9:1200–1213 [DOI] [PubMed] [Google Scholar]
- 2.Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson's disease. Neurobiol Dis 2012;46:590–596 [DOI] [PubMed] [Google Scholar]
- 3.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 2007;130:1787–1798 [DOI] [PubMed] [Google Scholar]
- 4.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain 2009;132:2958–2969 [DOI] [PubMed] [Google Scholar]
- 5.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol 2012;11:697–707 [DOI] [PubMed] [Google Scholar]
- 6.Bronnick K, Alves G, Aarsland D, Tysnes OB, Larsen JP. Verbal memory in drug-naive, newly diagnosed Parkinson's disease: the retrieval deficit hypothesis revisited. Neuropsychology 2011;25:114–124 [DOI] [PubMed] [Google Scholar]
- 7.Parkinson Progression Marker Initiative. The Parkinson progression marker initiative (PPMI). Prog Neurobiol 2011;95:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons A, Westman E, Muehlboeck S, et al. The AddNeuroMed framework for multi-centre MRI assessment of longitudinal changes in Alzheimer's disease: experience from the first 24 months. Int J Geriatr Psychiatry 2011;26:75–82 [DOI] [PubMed] [Google Scholar]
- 9.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986:165–173 [Google Scholar]
- 10.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22:41–47 [DOI] [PubMed] [Google Scholar]
- 11.Fahn S, Elton RL; the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information; 1987:153–163 [Google Scholar]
- 12.Hoehn MH, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 13.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord 2007;22:2314–2324 [DOI] [PubMed] [Google Scholar]
- 15.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G; Norwegian ParkWest Study Group. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest Study. Neurology 2009;72:1121–1126 [DOI] [PubMed] [Google Scholar]
- 16.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010;75:1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I: segmentation and surface reconstruction. Neuroimage 1999;9:179–194 [DOI] [PubMed] [Google Scholar]
- 18.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207 [DOI] [PubMed] [Google Scholar]
- 20.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11–22 [DOI] [PubMed] [Google Scholar]
- 21.Westman E, Muehlboeck JS, Simmons A. Combining MRI and CSF measures for classification of Alzheimer's disease and prediction of mild cognitive impairment conversion. Neuroimage 2012;62:229–238 [DOI] [PubMed] [Google Scholar]
- 22.Weintraub D, Doshi J, Koka D, et al. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol 2011;68:1562–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melzer TR, Watts R, MacAskill MR, et al. Grey matter atrophy in cognitively impaired Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:188–194 [DOI] [PubMed] [Google Scholar]
- 24.Compta Y, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain 2011;134:1439–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compta Y, Ibarretxe-Bilbao N, Pereira JB, et al. Grey matter volume correlates of cerebrospinal markers of Alzheimer-pathology in Parkinson's disease and related dementia. Parkinsonism Relat Disord 2012;18:941–947 [DOI] [PubMed] [Google Scholar]
- 26.Compta Y, Pereira JB, Ríos J, et al. Combined dementia-risk biomarkers in Parkinson's disease: a prospective longitudinal study. Parkinsonism Relat Disord 2013;19:717–724 [DOI] [PubMed] [Google Scholar]
- 27.Duncan GW, Firbank MJ, O'Brien JT, Burn DJ. Magnetic resonance imaging: a biomarker for cognitive impairment in Parkinson's disease? Mov Disord 2013;28:425–438 [DOI] [PubMed] [Google Scholar]
- 28.Nishio Y, Hirayama K, Takeda A, et al. Corticolimbic gray matter loss in Parkinson's disease without dementia. Eur J Neurol 2010;17:1090–1097 [DOI] [PubMed] [Google Scholar]
- 29.Lee JE, Park HJ, Song SK, Sohn YH, Lee JD, Lee PH. Neuroanatomic basis of amnestic MCI differs in patients with and without Parkinson disease. Neurology 2010;75:2009–2016 [DOI] [PubMed] [Google Scholar]
- 30.Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord 2011;26:289–296 [DOI] [PubMed] [Google Scholar]
- 31.Pagonabarraga J, Corcuera-Solano I, Vives-Gilabert Y, et al. Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson's disease. PLoS One 2013;8:e54980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weintraub D, Dietz N, Duda JE, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain 2012;135:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarei M, Ibarretxe-Bilbao N, Compta Y, et al. Cortical thinning is associated with disease stages and dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry 2013;84:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol 2001;11:194–201 [DOI] [PubMed] [Google Scholar]
- 35.Corbetta M, Shulman GL. Control of goal-directed and stimulus driven-attention in the brain. Nat Rev Neurosci 2002;3:201–215 [DOI] [PubMed] [Google Scholar]
- 36.Camicioli R, Gee M, Bouchard TP, et al. Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord 2009;15:187–195 [DOI] [PubMed] [Google Scholar]
- 37.Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargalló N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson's disease. Mov Disord 2009;24:1193–1199 [DOI] [PubMed] [Google Scholar]
- 38.Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bartres-Faz D, Tolosa E. Structural brain correlates of verbal fluency in Parkinson's disease. Neuroreport 2009;20:741–744 [DOI] [PubMed] [Google Scholar]
- 39.Ibarretxe-Bilbao N, Ramirez-Ruiz B, Junque C, et al. Differential progression of brain atrophy in Parkinson's disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry 2010;81:650–657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.