Abstract
Retrospective studies indicate 2 major classes of autism spectrum disorder (ASD) onset: early and later, after a period of relatively healthy development. This prospective, longitudinal study examined social, language, and motor trajectories in 235 children with and without a sibling with autism, ages 6–36 months. Children were grouped as: ASD identified by 14 months, ASD identified after 14 months, and no ASD. Despite groups’ initial similar developmental level at 6 months, ASD groups exhibited atypical trajectories thereafter. Impairment from 14 to 24 months was greater in the Early-ASD than the Later-ASD group, but comparable at 36 months. Developmental plateau and regression occurred in some children with ASD, regardless of timing of ASD diagnosis. Findings indicate a preclinical phase of varying duration for ASD.
Autism spectrum disorders (ASD) are a group of neurodevelopmental disorders distinguished by impairment in social reciprocity and communication, along with repetitive and stereotyped patterns of behavior and interests (DSM–IV–TR; American Psychiatric Association, 2000). Autism was originally defined as a congenital disorder (Kanner, 1943) and there are indications that the neurobiological origins or predisposing causes of ASD are present prenatally (Bauman & Kemper, 2003; Pardo & Eberhart, 2007). However, little is known about the onset and early behavioral developmental trajectory of children with ASD. Defining patterns of developmental trajectory within and across different developmental systems in ASD is important for advancing theories of neurobiology and plasticity in ASD (Pardo & Eberhart, 2007), developing screening tools and guidelines, and detecting ASD early for access to early intervention. In the absence of medical tests or treatments for ASD, early behaviorally based interventions are the best means to improve outcomes (Harris & Handelman, 2000; Landa, Holman, O’Neill, & Stuart, 2011; Rogers & Vismara, 2008). This prospective, longitudinal study compares developmental characteristics and trajectories of children with early versus later manifestation of ASD, and compares those groups to children without ASD from 6 through 36 months of age.
A commonly held notion is that children with ASD fall into one of two major symptom onset patterns. One pattern involves an early course of rather typical development or mild delays followed by a loss of language or social skills (or both) paired with the emergence of ASD-related atypical behaviors (e.g., repetitive and stereotyped behaviors and interests; Wilson, Djukic, Shinnar, Dharmani, & Rapin, 2003). The other involves early onset of impairment and signs of ASD without regression (Luyster et al., 2005). There is mixed evidence regarding the prognostic implication of regression. Some reports indicate that regression is associated with worse outcomes (Kalb, Law, Landa, & Law, 2010; Luyster et al., 2005), whereas others report no differences in outcomes for children with ASD with or without regression (Werner & Dawson, 2005; Werner, Dawson, Munson, & Osterling, 2005).
Two primary approaches have been taken to understand the emergence of abnormal development in children with ASD: retrospective and prospective. Retrospective studies are principally based on information from medical record reviews, parent recall, or systematic observational coding of home videotapes taken during the 1st or 2nd year of life and prior to the diagnosis of ASD. Retrospective studies have generated conflicting information about the developmental status of children with ASD prior to the onset of regression, which generally occurs between 18 and 24 months (Davidovitch, Glick, Holtzman, Tirosh, & Safir, 2000; Luyster et al., 2005). Werner and Dawson (2005) reported that, at 12 months, development of complex babbling and first words was accelerated in children with regressive autism compared to typical development, and social development was comparable to that of 12-month-olds with typical development. In contrast, Luyster et al. (2005) reported that the age of first words was later in children with regressive autism than in those with typical development. Although the core deficits of ASD have been considered to involve social functioning (Pelphrey & Carter, 2008), retrospective studies indicate that children with ASD exhibit disruption in other developmental domains in the 1st year of life, including motor, attention, and temperament (Baranek, 1999; Maestro, Casella, Milone, Muratori, & Palacio-Espasa, 1999; Werner et al., 2005). To investigate the nature and timing of the emergence of ASD, prospective, longitudinal studies would be optimal.
Prospective, longitudinal studies of ASD from infancy provide a potent means of defining patterns of development in infants later diagnosed with ASD, avoiding confounds such as recall bias. To date, five published prospective studies have provided longitudinal data prior to the third birthday in children with and without ASD (Landa & Garrett-Mayer, 2006; Landa, Holman, & Garrett-Mayer, 2007; Ozonoff et al., 2010; Sullivan et al., 2007; Zwaigenbaum et al., 2005). These studies indicate that development in motor, cognitive (Landa & Garrett-Mayer, 2006), language (Landa & Garrett-Mayer, 2006; Ozonoff et al., 2010), and social (Ozonoff et al., 2010; Zwaigenbaum et al., 2005) domains appear to be grossly intact at 6 months of age, followed by a slowing in development that is observable on objective developmental measures around the first birthday (Landa & Garrett-Mayer, 2006; Ozonoff et al., 2010; but see Flanagan, Landa, Bhat, & Bauman, 2012, documenting abnormal postural control at age 6 months). By preschool age, children with ASD often exhibit motor delays (reviewed by Bhat, Landa, & Galloway, 2011). Further insight into onset patterns was provided by Landa et al. (2007) who distinguished two ASD major onset groups: (a) ASD manifestation around the time of the first birthday and (b) ASD manifestation later, but still prior to the 3rd birthday. In the present study, we further investigate trajectories and short-term outcomes of early and later ASD manifesting groups with increased sample sizes and contemporaneous assessments of language, social, motor, and cognitive functioning in the first 3 years of life. In doing so, we address questions pertaining to the timing of departure from typical development within and across developmental systems, pattern of altered trajectory (e.g., slowing, plateauing, and skill loss), and short-term prognosis.
Study of the developmental trajectory of multiple systems (motor, cognitive, social, and language) in the first 3 years of life in children with and without ASD could shed light on the susceptibility of the developing brain to the impact of genetic, epigenetic, and environmental factors in children with ASD. For typical development to unfold, active processes of neuronal synaptic plasticity, involving addition and pruning of synapses and alterations in strength or efficacy of synapses, and connectivity must be established (Huttenlocher, 1979; Johnston et al., 2009; Knudsen, 2004). The timing of ASD manifestation may provide information about the preparedness of developmental brain mechanisms to support the demands of such active neuronal processes. Disruptions of normal synaptic plasticity mechanisms by either genetic or environmental influences (or both) may perturb brain readiness for the coupling of neurobiological processes with other mechanisms of adaptation and learning that will have implications for susceptibility to regression, comprehensiveness, or severity of impairment, and for prognosis.
We compared trajectories of three groups of children studied prospectively: (a) those without ASD, (b) those with early (by 14 months), and (c) later ASD classification. We hypothesized that children with later ASD classification would be protected by greater brain maturity during an age of active processes of neuronal synaptic plasticity and exhibit less severe and global developmental disruption, with better short-term prognosis, across the first 3 years of life than children with very early manifestation of ASD. Similarly, we hypothesized that language regression would occur less often in children with later-manifesting ASD than in those with early-manifesting ASD. Furthermore, we hypothesized that, on measures not used in the ASD classification process, the early-manifesting ASD group would exhibit less matured social and communication development compared to the later-manifesting ASD and non-ASD groups by 14 months. Finally, we hypothesized that children with later classification of ASD would manifest a preclinical phase of the disorder characterized by mild and focal differences from the non-ASD group by 14 months of age. Specifically, we anticipated that the later-manifesting ASD group would lag behind the non-ASD group in communication development by 14 months.
Method
This study was approved by the Johns Hopkins Institutional Review Board before data collection; all families gave written informed consent for their child’s participation.
Participants
Participants included infant siblings of children with autism (sibs-A; n = 204; 119 boys) and infants with no family history of autism (low risk for ASD; n = 31; 19 boys). The sibs-A group included 21 minority and 179 Caucasian children; 4 children’s ethnicities were unknown. The low-risk group included 1 minority and 26 Caucasian children; 4 children’s ethnicities were unknown. Fifty-one percent of the sample was included in different analyses by Landa et al. (2007). The present study builds on that study by extending the age range of observation from 14–24 months to 6–36 months, and by including additional measures of language and motor functioning. Details about recruitment sources and diagnosis of probands with autism through which younger siblings were ascertained are provided in Landa et al. (2007).
Exclusion criteria were as follows: family’s first language being other than English (language measures are normed on English speakers), low birth weight (< 1500 g), severe birth trauma, head injury, prenatal illicit drug or excessive alcohol exposure, and severe birth defects.
Participants were tested at 6, 14, 18, 24, 30, and 36 months of age. Table 1 shows the numbers of participants tested at each age. Sample sizes at 6, 18, and 30 months of age are smaller by design because funding for data collection at these ages was not available until later in the study. At all other ages, follow-up rates were high. Confirmatory outcome ASD classification was made at 30 (n = 7) or 36 months for all participants because diagnosis at this age is reliable (Chawarska, Klin, Paul, & Volkmar, 2007). One child was diagnosed at 4 years due to missing the 36-month visit. All children in this sample had an outcome assessment and confirmatory diagnostic classification of ASD or non-ASD (see below).
Table 1.
Group Means and Sample Sizes on the Autism Diagnostic Observation Scale (ADOS) and Mullen Early Learning Composite (ELC; n = 235)
| Target age | Sample size N | Age range Months | ADOS communication M (SD) | ADOS social M (SD) | ADOS SBRI M (SD) | Mullen ELC M (SD) |
|---|---|---|---|---|---|---|
| Non-ASD (n = 181; months) | ||||||
| 6 | 140 | 5.6, 10.2 | — | — | — | 97.7 (13.2) |
| 14 | 177 | 13.4, 15.4 | 2.3 (1.7) | 3.9 (3.0) | 1.1 (1.1) | 101.3 (16.7) |
| 18 | 97 | 16.1, 19.3 | 1.3 (1.2) | 2.5 (2.4) | 1.2 (1.2) | 99.8 (16.9) |
| 24 | 175 | 23.6, 26.3 | 1.5 (1.3) | 3.1 (2.6) | 1.2 (1.3) | 107.9 (16.0) |
| 30 | 81 | 29.5, 31.7 | 1.3 (1.1) | 2.4 (1.9) | 1.2 (1.2) | 106.6 (16.1) |
| 36 | 173 | 35.3, 39.0 | 1.7 (1.2) | 2.9 (2.1) | 1.2 (1.3) | 101.1 (17.3) |
| Early-ASD (n = 28; months) | ||||||
| 6 | 14 | 5.8, 8.3 | — | — | — | 89.8 (6.8) |
| 14 | 27 | 13.7, 15.8 | 5.1 (1.1) | 9.9 (2.6) | 2.5 (1.7) | 81.7 (12.7) |
| 18 | 19 | 17.7, 20.9 | 3.8 (1.8) | 8.8 (3.5) | 2.2 (1.9) | 80.0 (14.2) |
| 24 | 27 | 23.6, 25.1 | 4.3 (1.6) | 9.7 (3.1) | 3.4 (1.5) | 78.2 (20.2) |
| 30 | 14 | 29.7, 31.9 | 4.2 (1.6) | 8.3 (3.4) | 2.9 (1.5) | 79.4 (21.5) |
| 36 | 26 | 35.3, 40.5 | 4.2 (1.9) | 8.9 (3.6) | 3.5 (1.7) | 75.0 (22.7) |
| Later-ASD (n = 26; months) | ||||||
| 6 | 17 | 5.5, 10.6 | — | — | — | 100.4 (13.5) |
| 14 | 26 | 12.8, 15.8 | 2.9 (1.9) | 4.6 (2.3) | 2.3 (1.6) | 94.6 (11.3) |
| 18 | 14 | 16.6, 19.9 | 1.9 (1.3) | 3.0 (2.9) | 1.5 (1.4) | 91.9 (16.4) |
| 24 | 26 | 21.7, 26.3 | 3.6 (1.6) | 7.0 (3.2) | 2.5 (1.5) | 88.6 (18.5) |
| 30 | 15 | 29.5, 31.4 | 3.1 (1.9) | 6.6 (2.9) | 3.1 (1.9) | 88.4 (19.6) |
| 36 | 26 | 35.0, 39.9 | 4.5 (2.3) | 7.1 (3.4) | 3.6 (1.8) | 83.6 (24.8) |
Note. SBRI = stereotyped patterns of behavior and interests. At 6 months of age, 140 Non-ASD children, 14 Early-ASD children, and 17 Later-ASD children were assessed, but the ADOS was not given until age 14 months.
Measures
The Mullen Scales of Early Learning (Mullen, 1995) provided measures of motor and language functioning. The Communication and Symbolic Behavior Scales Developmental Profile (Wetherby & Prizant, 2002) provided measures of two social functions that are related to diagnostic criteria for ASD (initiation of joint attention [IJA], shared positive affect) and a measure of communication that enabled us to document change over time in children who may have expressive language delays (diversity of consonants produced communicatively in nonlinguistic vocalizations and spoken words). We selected these variables a priori to determine which ones distinguish the groups because we wished to understand the timing of derailment of different developmental systems, and to understand which, if any, developmental systems remain unimpaired in the early stages of development of ASD. We did not attempt data reduction (e.g., factor analysis) because preliminary analyses revealed that the selected measures did not contribute to common variation to similar degrees at different ages. Furthermore, composite scores would have limited the interpretability of the findings.
The Mullen Scales of Early Learning (Mullen, 1995; given at all ages) is a standardized developmental test for children age 3–69 months that was normed on a national sample. Raw scores from three scales served as dependent variables: Fine Motor, Receptive Language, and Expressive Language. A fourth scale from the Mullen, Visual Reception, assesses visual processing, visual spatial, and memory skills, and was used to adjust for non-verbal cognitive level. All four Mullen scales contribute to the Early Learning Composite (ELC), a norm-referenced, standardized score analogous to a developmental quotient (M = 100, SD = 15). The mean ELC for each of the groups, at each age, is presented as a descriptive variable in Table 1. Internal consistency over time was high for each Mullen scale (all Cronbach’s αs > .74).
The Communication and Symbolic Behavior Scales Developmental Profile (CSBS DP; Wetherby & Prizant, 2002; given 14–24 months) evaluates communicative, social-affective, and symbolic abilities. Children are presented with play-based communication temptations and a standardized set of toys for use in a free play session. Dependent variables coded from videotapes were as follows: (a) inventory of consonants used in communicative vocal or verbal utterances (hereafter, consonant inventory) and frequency of (b) IJA and (c) shared positive affect (smiling paired with eye contact). Internal consistency over time was moderate for CSBS DP scores because this measure was administered at fewer visits (Cronbach’s α > .50). Interrater reliability was calculated for 20% of the coded videotapes. The absolute intraclass correlation coefficient was .886.
Diagnostic Classification Procedures
The Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999) is a play-based assessment with standardized administration and scoring schema without age norms (given at every age from 14 through 36 months). It provides systematic probes for autism symptoms in social interaction, communication, play, and stereotyped behaviors and repetitive interests. Higher scores represent greater levels of autism symptomatology. Module 1 (minimal to no language; given to all 14- and 18-month-olds and most 24-month-olds) or 2 (nonechoed phrase speech) was given. The ADOS provides algorithm criteria for classification of “ASD” or “autism.” As the ADOS was not designed for children younger than 18 months of age, its use at 14 months was experimental. ADOS scores were used in conjunction with clinical judgment to classify children with ASD. Meeting algorithm criteria for ASD or autism did not automatically result in an ASD diagnosis. ADOS scores for each group are displayed in Table 1. The ADOS was administered by clinical research staff who had achieved and maintained research reliability (≥ 80% interrater reliability).
Children with a confirmed outcome diagnosis of ASD were divided into two groups based on the age at which they first received a clinical impression of ASD by master’s or doctoral level clinical research examiners having extensive experience with autism and with testing infants and toddlers. Most were licensed clinicians who also provided assessments of young children in a clinical setting. Clinical impressions of ASD versus non-ASD were assigned from age 14 months onward. In the absence of published guidelines for diagnosing ASD at 14 months, we followed DSM–IV criteria for Autism and Pervasive Developmental Disorder-Not Otherwise Specified (DSM–IV–TR; American Psychiatric Association, 2000) as closely as possible. Some DSM–IV–TR criteria were not applicable at 14 months (e.g., reciprocal friendships), so diagnostic impressions were based on impairments in social interaction and consideration of the presence of stereotyped behaviors and repetitive interests. Clinical judgment is a valid basis on which to determine diagnostic classification (Klin, Lang, Cicchetti, & Volkmar, 2000). The proportion of sibs-A with ASD (23%) is aligned with ASD recurrence rates in infant siblings of children with ASD as reported by the Autism Speaks Baby Sibs Research Consortium (Ozonoff et al., 2011). Children were categorized into one of the three groups: early identified ASD (Early-ASD), later identified ASD (Later-ASD), and a Non-ASD group.
The Early-ASD group (n = 28; 22 boys, 6 girls) received a diagnostic impression of ASD at 14 months and confirmatory diagnostic classification of ASD at outcome. ADOS criteria for ASD or autism were met at both points. As we did not generate clinical impressions of ASD at 6 months of age, 14 months was the earliest that a diagnostic impression of ASD was possible in this study. Concern was expressed by 42.8% and 70.4% of the Early-ASD children’s parents at 6 and 14 months, respectively. Twelve children (43%) in this group entered intervention by 18 months; the mean total intervention hours received between 6 and 18 months of age was 33.
The Later-ASD group (n = 26; 22 boys, 4 girls) did not have a diagnostic impression of ASD until after 14 months (the next earliest age of possible ASD classification was 18 months). Concern was expressed by 29.4% and 65.4% of the Later-ASD children’s parents at 6 and 14 months of age, respectively. All received clinical impressions of mild to moderate non-ASD delay at 14 months. Three (12%) of these children entered into intervention prior to 18 months, and the mean total intervention hours received between 6 and 18 months of age was 8.
The Non-ASD group (n = 181; 94 boys, 87 girls) did not meet outcome criteria for ASD. We combined children without ASD who were low-risk controls or sibs-A because these groups did not differ systematically from each other on any variable at any age. Concern was expressed by 30% and 55.9% of the Non-ASD children’s parents at 6 and 14 months of age, respectively. Eighteen of the children in the Non-ASD group had clinically significant language or fine motor delays (or both) at 36 months based on Mullen nationally normed standardized test scores of at least 1.5 SD below the Mullen test mean.
Analysis
Differences between the three diagnostic groups were examined for all dependent variables. Generalized estimating equations (GEE) were fit to the data to describe group-specific trajectories and identify differences between diagnostic groups at each age and in their growth over time (Zeger & Liang, 1986). GEE accounts for correlated outcomes that arise from repeated measures of the same children over time and are valid for data missing completely at random. Not accounting for correlations among outcomes can lead to biased standard errors, which affect inferences in regular regression (Diggle, Heagerty, Liang, & Zeger, 2002). These analyses were replicated using random effects models that better accommodate data that are missing at random conditional on covariates in the model and results were similar. Because some random effects models did not converge, we report findings from GEE models.
For each dependent variable, a flexible GEE model was fit including indicator variables for each time point, main effects for groups (Non-ASD, Early-ASD, and Later-ASD), and interactions between the time and group variables, allowing the growth between each time point to differ for each group. Models were adjusted for nonverbal cognitive functioning (Mullen Visual Reception raw score) and hours spent in any type of treatment. This allowed us to control for effects of nonverbal cognitive delay on communication and social behavior, and also to control for benefits that intervention may be having on functioning in all areas examined herein. All GEE analyses were specified with an exchangeable correlation structure and an identity link function after reviewing autocorrelation plots. Inferences did not change when unstructured or autoregressive correlation matrices were specified instead. Residual plots and other graphical and numerical tools were used to assess linearity and other model assumptions.
Coefficient estimates and standard errors from these models were used to compare the growth and levels of each measure between groups using Stata Version 1.0 (StataCorp, 2007). To be conservative and to partially account for multiple comparisons, a two-sided α level of .01 was used as a cutoff for statistical significance, and trends toward significance were indicated for p values between .011 and .05.
Results
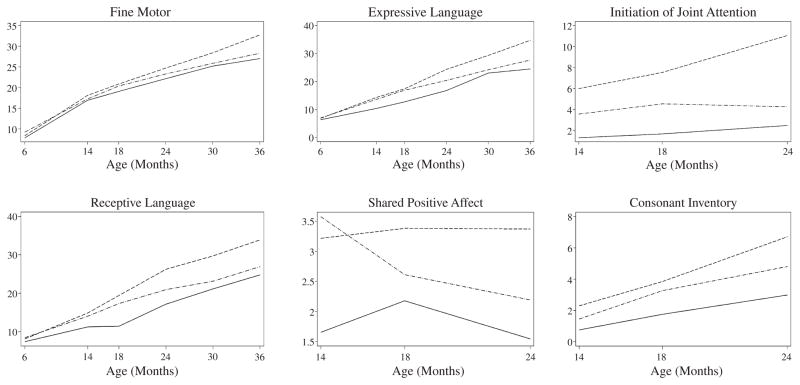
Results of the GEE analyses comparing the groups at specific points in time, summarized in Table 2, are presented in raw coefficients, representing differences between diagnostic groups on the scale of the original tests, and standardized coefficients, representing diagnostic group differences in standard deviation units. Results of GEE trajectory analyses for the dependent measures from the Mullen and CSBS DP, showing group differences in growth over time, are illustrated in Figure 1. A figure is used, rather than a table, for ease and efficiency of summarizing the data that result from the numerous age intervals examined.
Table 2.
Mean Differences Between Groups in Levels of Dependent Variables: Results From Parametric GEE Models (n = 235)
| 6 months b (β) | 14 months b (β) | 18 months b (β) | 24 months b (β) | 30 months b (β) | 36 months b (β) | |
|---|---|---|---|---|---|---|
| Early-ASD versus Non-ASD | ||||||
| Mullen Scales of Early Learning | ||||||
| Fine motor | −.4 (−.2) | −.6 (−.2) | −.8 (−.3) | −.8 (−.3) | −.6 (−.2) | −2.8 (−1.1)* |
| Receptive language | −.6 (−.4) | −2.6 (−1.7)* | −6.4 (−4.2)* | −6.4 (−4.3)* | −4.6 (−3.1)* | −5.4 (−3.6)* |
| Expressive language | −.3 (−.2) | −3.1 (−1.8)* | −3.4 (−2.0)* | −5.3 (−3.1)* | −2.9 (−1.7) | −6.1 (−3.6)* |
| Communication and Symbolic Behavior Scales | ||||||
| Shared positive affect | — | −1.5 (−.6)* | −1.2 (−.4)* | −1.6 (−.6)* | — | — |
| Initiation of joint attention | — | −4.2 (−.9)* | −4.9 (−1.0)* | −7.1 (−1.5)* | — | — |
| Consonant inventory | — | −1.4 (−.8)* | −1.5 (−.9)* | −3.3 (−1.9)* | — | — |
| Later-ASD versus Non-ASD | ||||||
| Mullen Scales of Early Learning | ||||||
| Fine motor | .7 (.3) | −.9 (−.4)* | −.1 (.0) | −.4 (−.2) | −.8 (−.3) | −2.2 (−.8)* |
| Receptive language | .0 (.0) | −.9 (−.6) | −1.4 (−.9) | −3.7 (−2.4)* | −3.8 (−2.5) | −3.6 (−2.4)* |
| Expressive language | −.1 (.0) | −.8 (−.4) | .1 (.0) | −2.7 (−1.6)* | −2.8 (−1.6) | −4.1 (−2.4)* |
| Communication and Symbolic Behavior Scales | ||||||
| Shared positive affect | — | .3 (.1) | −.8 (−.3) | −1.1 (−.4)* | — | — |
| Initiation of joint attention | — | −2.4 (−.5) | −2.4 (−.5) | −6.1 (−1.3)* | — | — |
| Consonant inventory | — | −.9 (−.5)* | −.1 (−.1) | −1.6 (−.9)* | — | — |
| Later-ASD versus Early-ASD | ||||||
| Mullen Scales of Early Learning | ||||||
| Fine motor | 1.1 (.4) | −.3 (−.1) | .7 (.3) | .4 (.2) | −.1 (.0) | .6 (.2) |
| Receptive language | .6 (.4) | 1.7 (1.1) | 5.0 (3.3)* | 2.8 (1.8) | .8 (.5) | 1.8 (1.2) |
| Expressive language | .3 (.2) | 2.4 (1.4)* | 3.4 (2.0)* | 2.6 (1.5) | .1 (.1) | 2.0 (1.2) |
| Communication and Symbolic Behavior Scales | ||||||
| Shared positive affect | — | 1.9 (.7)* | .4 (.1) | .5 (.2) | — | — |
| Initiation of joint attention | — | 1.8 (.4) | 2.6 (.5) | 1.0 (.2) | — | — |
| Consonant inventory | — | .5 (.3) | 1.3 (.8) | 1.7 (1.0) | — | — |
Note. Communication and Symbolic Behavior Scales DP = Communication and Symbolic Behavior Scales Developmental Profile. Raw coefficients are mean differences in a score between groups at each age. Standardized coefficients in parentheses are standardized effect sizes. Models are adjusted for nonverbal cognitive function (Mullen Visual Reception raw score) and cumulative hours spent in treatment.
p ≤ .01 for mean differences between groups.
Figure 1.
Trajectories between 6 and 36 months for Mullen Fine Motor, Receptive Language, and Expressive Language scale raw scores, and between 14 and 24 months for frequency of shared positive affect and initiation of joint attention, and consonant inventory coded from the behavior sampled elicited using the CSBS DP.
Note. Dashed lines: Non-ASD group; solid lines: Early-ASD group; dash-dotted lines: Later-ASD group. CSBS DP = Communication and Symbolic Behavior Scales Developmental Profile.
Later-ASD Versus Early-ASD Groups
First we present data related to our hypothesis that, in the first 3 years of life, children with very early manifestation of ASD would have earlier and more global developmental disruption (beyond social impairment of ASD) on measures not used for ASD classification than children with later ASD manifestation. At 6 months, development within the Early- and Later-ASD groups was comparable with each other and with the Non-ASD group (ps > .1; Table 2). By 14 months, the Early-ASD group exhibited significantly lower expressive language (p < .001) and shared positive affect scores than the Later-ASD group (p < .001). By 18 months, the Early-ASD group exhibited greater delays in receptive (p < .001) and expressive language development (p = .001) compared to the Later-ASD group. At 24 months, the gap between the Early- and Later-ASD groups had closed, and no differences from the Later-ASD group were detected at subsequent ages. These findings indicate that the Early-ASD group was indeed manifesting earlier developmental disruption, particularly affecting language and social functioning, than the Later-ASD group, but was not more severely affected at 30 or 36 months.
Comparison of the ASD groups’ trajectories (slope of change) from 6 to 36 months revealed a trend for slower growth in expressive language between 6 and 14 months in the Early-ASD compared to the Later-ASD group (p = .02; Figure 1).
Early-ASD Versus Non-ASD Groups
The Early-ASD and Non-ASD groups exhibited comparable development at 6 months (ps > .1), but the Early-ASD group diverged from Non-ASD development by 14 months in all aspects of development measured here (ps < .01; see Table 2) except for fine motor functioning as measured by the Mullen. With the exception of age 30 months (where there were fewer children with ASD tested and thus lower statistical power), these differences were sustained through 36 months (ps < .01). At 36 months, fine motor functioning in the Early-ASD group had fallen significantly below that of the Non-ASD group. This reveals a nontransient difference from non-ASD development within the Early-ASD group that was evident by 14 months.
GEE analyses comparing average growth trajectories of the Early-ASD and Non-ASD groups from 6 to 36 months revealed significant differences in language, motor, and cognitive domains (ps < .01; see Figure 1). Between 14 and 24 months, the Non-ASD group exhibited surges in expressive and receptive language development of magnitudes not observed in the Early-ASD group (ps < .002 for change in language scores from 14 to 24 months in the Non-ASD group and for differences between groups’ trajectories). An atypical plateau in receptive language development was observed in the Early-ASD group between 14 and 18 months and in expressive language development between 30 and 36 months. The Early-ASD group’s attenuated rate of growth, per the observed trajectories, was initially most noteworthy in receptive language development. During one age interval only, receptive language raw score slopes in the Early-ASD and Non-ASD groups did not differ (p = .42) when, from 18 to 24 months, the Early-ASD group was resuming gain in receptive language development after a plateau. The difference between the Early-ASD and Non-ASD groups’ social communication (IJA) growth patterns between 14 and 24 months was significant (p = .001), with the Early-ASD group’s rate of growth being significantly slower.
Later-ASD and Non-ASD Groups
The Later-ASD group scored within 1 SD of the Mullen nationally normed mean for all scales at every age except 36 months. However, from 14 to 36 months, Mullen raw scores gradually departed from typical development in the Later-ASD group. At age 14 months, the Later-ASD group’s scores were significantly lower than the Non-ASD group’s for fine motor (p = .008) and consonant inventory (p = .005) variables. At 24 and 36 months, the Later-ASD group performed below the Non-ASD group on all variables examined (ps < .01) except fine motor functioning at 24 months.
GEE analyses comparing average growth trajectories of the Later-ASD and Non-ASD groups from 6 to 36 months revealed abnormal trajectories in the ASD group, with variation in the timing of altered development across developmental domains. The Later-ASD group’s average rate of growth in receptive and expressive language development slowed relative to the Non-ASD group from 14 to 24 months (ps < .001). In social development, the Later-ASD group’s development of IJA slowed relative to the Non-ASD group between 18 and 24 months (p = .005), characterized by a plateau, visible in Figure 1.
These findings support the hypothesis that the Later-ASD group’s development, particularly language development, would show signs of alteration by 14 months of age. Although not classified as having ASD at 14 months, the Later-ASD group did show reduced frequency of social communicative initiation at that age, an early sign of ASD.
Diminishing Language Skills
We hypothesized that developmental regression would occur less often in the Later-ASD than the Early-ASD group. Examination of percent of children in each group losing raw score points on both the Mullen Receptive and Expressive Language scales revealed that this occurred in eight (29%), five (19%), and four (2%) children in the Early-ASD, Later-ASD, and Non-ASD groups, respectively (Fisher exact test p = .02). Among these children, loss of Receptive Language scale raw score points persisted across two or more age intervals in four and two children from the Early- and Later-ASD groups, respectively. For expressive language, persistent loss across two consecutive age intervals occurred in two children each from the Early- and Later-ASD groups. Four of the five children in the Non-ASD group who exhibited decreasing raw scores on the Mullen language scales were sibs-A, and four had language or social impairment (or both) at 36 months.
Discussion
In the largest prospective, longitudinal study of children with early and later diagnosis of ASD compared with children without ASD to date, we examined language and motor development from 6 to 36 months of age, and social development from 14 to 24 months, the time during which ASD regression usually occurs (Luyster et al., 2005). As hypothesized, children with earlier ASD classification exhibited earlier and more widespread developmental disruption than children with later ASD classification, but this distinction between the two ASD groups vanished by age 24 months. Also as hypothesized, a preclinical phase preceded the manifestation of ASD symptoms in the Later-ASD diagnosis group, affecting communication development by 14 months of age. Although not part of the diagnostic criteria for ASD, fine motor development was also less mature in the Later-ASD than in the Non-ASD group by 14 months. Atypical trajectories were observed in the ASD groups.
These findings replicate and extend Landa et al.’s (2007) findings in a much larger sample, and across a broader range of developmental functioning. Our findings confirm the presence of generally intact development at 6 months of age, also documented by Ozonoff and colleagues (Ozonoff et al., 2010; Young, Merin, Rogers, & Ozonoff, 2009). The period of early, reasonably healthy, behavioral development is followed by progressive divergence from typical development continuing throughout the first 3 years of life. This period of developmental divergence is particularly robust between 14 and 24 months, and particularly affects language and social development.
In the Early-ASD group, rapid developmental deceleration was observed between 6 and 14 months, with evident delays across all measured developmental domains by 36 months. In the Later-ASD group, a preclinical phase of the disorder was characterized by less mature motor and speech development compared to the Non-ASD group. Gradual developmental deceleration was observed, and by 36 months, the Later-ASD and Non-ASD groups differed on all variables examined herein.
Commonalities between the ASD groups include: grossly typical development at 6 months, increasing divergence from the Non-ASD group beginning by 14 months that was detected in multiple developmental systems, plateauing in one or more aspects of communication development, reduction in frequency of shared positive affect by 24 months, and comparable level of impairment by 24 months (which could possibly be related to the higher intensity of early intervention received by the Early-ASD group). Of interest is the presence of fine motor delay by 14 months in the Later-ASD group, and by 36 months in the Early-ASD group. Although not a diagnostic indicator of autism, motor delays were also noted at age 3 months in younger siblings of children with autism who later exhibited communication and motor delays (Bhat, Galloway, & Landa, in press), at 6 months in younger siblings of children with autism who were later diagnosed with ASD (Bryson et al., 2007; Flanagan et al., 2012), in 18-month-olds at risk for autism (Brian et al., 2008), and in older children and adults with autism, even in the absence of comorbid intellectual disabilities (Bhat et al., 2011). More research is needed to understand the persistence of early motor delays into later childhood and beyond, and the degree to which poor motor performance on standardized measures is related to impaired language comprehension and imitation in older children with ASD. Bhat et al. (2011) provide a more complete discussion of these issues. Differences in the ASD groups primarily involved less pronounced developmental deceleration.
Growth
On average, all three groups showed gain in raw score over time on the Mullen scales. However, the ASD groups’ developmental trajectories for all variables were significantly slower than those of the Non-ASD group. Only the Non-ASD group exhibited a large growth spurt in receptive and expressive language development in the 2nd year of life. The presence of a spurt in vocabulary development has been documented in children with typical development (e.g., Goldfield & Reznick, 1990). The absence of this spurt in the ASD groups is atypical.
The ASD groups’ divergence from non-ASD development supports the notion of ASD as a progressive disorder, with development failing to advance at the expected pace and gradual appearance of atypical behavioral features. Some aspects of development, particularly involving symbolic and social communication appeared to be arrested in the ASD groups in the 2nd year of life. This early disruption in symbolic development may be a forerunner of the abnormal abstract representation abilities observed in adults with ASD, even those without intellectual disability (Minshew, Siegel, Goldstein, & Weldy, 1994). Decreases in social–emotional engagement were noted between 18 and 24 months in both ASD groups. Decreases in Mullen Receptive and Expressive Language scale raw scores occurred within some children with ASD, regardless of whether they were in the Early- or Later-ASD group. Such decreases were rare in the Non-ASD group, occurring mainly in sibs-A with other delays. Parents of the children meeting our criteria for language regression had denied the occurrence of this phenomenon on the Autism Diagnostic Interview–Revised (Lord, Rutter, & Le-Couteur, 1994) regression probes, possibly because the changes in their children were gradual rather than abrupt. The atypical trajectories observed in both ASD groups included developmental deceleration, plateauing, and regression, reflecting a continuum of developmental derailment.
The timing of the ASD-related alteration in development reported here occurs “after neurogenesis, neuronal migration, and maturation” (Zoghbi, 2003, p. 827), suggesting that ASD “might affect synaptic maturation, connectivity, or stabilization” (Zoghbi, 2003, p. 827). The first clear in vivo anatomic manifestations of abnormal brain development in ASD appear at around 7 months, prior to the appearance of overtly abnormal behavioral patterns (Schumann et al., 2010; Webb et al., 2010). Abnormal brain growth has been documented in children with ASD, beginning after 6 months of age (Courchesne, Carper, & Akshoomoff, 2003) and before the second birthday (Hazlett et al., 2011; Schumann et al., 2010), suggesting abnormal proliferation or death of neurons, dendrites, or neuroglia (DiCicco-Bloom et al., 2006; Herbert, 2011; Pardo, 2008; Schumann et al., 2010). Abnormality in neural connectivity also occurs during this time (Müller et al., 2011), likely contributing to the ASD behavioral phenotype. It is possible that children with ASD display rather typical development (preclinical or prodromal phase) until the time at which healthy behavioral development becomes dependent on long-range (e.g., fronto-posterior) connectivity. Such long-range connectivity is abnormal in ASD (Damarla et al., 2010; Jou et al., 2011). The atypical trajectories observed in our ASD groups are temporally aligned with these abnormal neurobiological processes.
We did not find full support for our prediction that early-manifesting ASD would be associated with less optimal short-term prognosis, based on the notion that early symptom expression may reflect more substantial abnormalities in developmental synaptic plasticity. Although the Early-ASD group had a higher percentage of children with cognitive impairment, and showed greater impairment compared to the Later-ASD group on at least some measures through 18 months, no statistically significant differences were detected between the ASD groups at 36 months of age.
There are numerous possible explanations for the different patterns of ASD onset in the two ASD groups studied here. Perhaps children with later manifestation of ASD have greater vulnerability to environmental influences (Pardo & Eberhart, 2007). Perhaps children with earlier ASD manifestation would have remained more severely affected, with more guarded short-term prognosis compared to those with later manifestation of ASD, but early exposure to intervention was protective and thwarted the cascading effects of early atypical developmental processes in the Early-ASD group. More longitudinal research is needed in which children with early and later manifestation of ASD are followed from infancy into later childhood to determine whether differences in longer term outcomes exist. More research on the long-term effects of early intervention is also needed.
Clinical Implications
Our findings indicate that ASD symptoms may appear as early as the first birthday, even in the absence of cognitive delay. About half of children with ASD in this study had a protracted period of about 2 years in which quantitative aspects of development were within normal limits as measured on standardized tests, but during which time development decelerated, subtle differences from typical development were detectable (e.g., reduced language comprehension, sparse inventory of speech sounds used communicatively; Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011), typical forms of social engagement (IJA, shared positive affect) diminished in frequency, and autism symptoms emerged. These findings indicate that not all children with ASD may be detected at the same age. Indeed, the earliest signs of developmental disruption during the ASD preclinical phase are likely to be nonspecific to ASD, such as communication or motor delay. Implications for screening practices are as follows. First, a general developmental screener should be administered to all children at well visits by the time of the first birthday, with screening at regular intervals through the preschool years. This practice may increase early detection of motor, babbling, and communication delays that may be associated with preclinical ASD or that are associated with non-ASD delays. Scores from such a screener should be archived, allowing health professionals to document developmental deceleration or qualitative divergence from typical development, as is the practice with physical measures such as head circumference. Any sign of atypical developmental trajectory should result in referral for further developmental assessment. The addition of an ASD-specific screener should be standard of care in pediatric practices by 18 months of age, with repetition of the screening through the preschool years so that later-manifesting ASD may be identified. Given the known high risk for infant siblings of children with autism to develop autism and milder impairments (Landa et al., 2007; Landa et al., 2012; Ozonoff et al., 2011), and the general absence of clear symptomatology in mid-infancy, a public health approach to developmental stimulation via parent education is recommended.
The patterns of slowing, plateauing, and decline in development noted in our participants with ASD occurred within the timeframe when parents report first concerns about children with ASD (Howlin & Moore, 1997). The subtlety of early ASD signs and a gradual shift from typical developmental trajectory, particularly in children with later manifestation of ASD, may not be detected by ASD screeners or by health professionals in a brief office visit. Thus, parents’ expression of concern about their toddler’s development or behavior requires careful consideration, with referral for Stage II screening or developmental assessment if concerns persist for any length of time. Also, public awareness efforts focused on early detection of developmental delays and ASD, such as the Centers for Disease Control (2010) Learn the Signs, Act Early campaign, should be widely promoted to acquaint the public with developmental milestones and signs of delay that extend beyond the common benchmarks of healthy development involving walking and talking. This will increase recognition of early signs of ASD, such as infrequent initiation of social communication and engagement, reduced frequency and quality of affective reciprocity, poor eye contact, socially engaged imitation of others, and diminished responsiveness to social others’ cues (e.g., response to name, gestural, and gaze cues within response to joint attention bids).
In addition, more sensitive measures of early social and communication development are needed; these measures should offer semistructured opportunities for child initiation, particularly of social engagement, and provide for rating the presence of positive symptoms (e.g., echolalia and qualitatively atypical social and communicative behavior). Standardized measures of quantitative behavior, such as the Mullen, are not likely to be sensitive to ASD-related abnormality in communicative reciprocity and reduced social communicative initiative in toddlers. In the absence of such measures, parents and primary health care providers may implement simple probes for shared attention, shared emotion, playful reciprocal engagement (as in imitation games), language comprehension, and communicative initiation that should be conducted beginning at the first birthday (for examples and guidelines, see Landa, 2011a). A new ASD screening tool (Landa, 2011b) that provides parents with video examples of early typical and ASD development involving social, communication, play, and behavior is being validated and may be available for widespread use in the near future. Tools such as this will help parents develop a keener eye for qualitative differences between early typical and ASD-related developmental features, empowering them to more aptly discern when they should seek a professional’s opinion about their child’s display of ASD indicators. Our finding that multiple aspects of development are disrupted in ASD indicates that a single behavioral difference from typical development, particularly if it is transient in nature, is not likely to be a red flag for ASD. However, as our findings highlight, ASD may occur in the absence of intellectual delay, so delays in major milestones like walking, play, or babbling should not be required for concerns about ASD to exist. Early detection of ASD is vital, as early intervention improves language and cognitive outcomes (Dawson et al., 2010; Landa et al., 2011) and core social deficits of ASD (Landa et al., 2011).
Limitations
There are several limitations to be recognized. First, our findings may not generalize to the population at large, as most participants with ASD were at high genetic risk for ASD and most were Caucasian. It is possible that children with a family history of autism may have different phenotypes than children with ASD who have denovo genetic variations (Gauthier, 2009; Sebat et al., 2007). In addition, more research is needed with larger ASD samples and using more sensitive measures of development (Bhat, Galloway, & Landa, 2010; Iverson & Wozniak, 2007; Pierce, Conant, Hazin, Stoner, & Desmond, 2011) to explore patterns of early and later identification of ASD, and establish early behavioral and biological ASD markers. With larger prospectively obtained longitudinal samples of children with early- and later-manifesting ASD, more fine-grained analyses may be conducted to determine whether there are additional defining features within or across these groups of children. This is necessary because our data show increasing heterogeneity within the Early- and Later-ASD groups with age as indicated by increasing standard deviations associated with mean test results. Heterogeneity within children with ASD has been documented (Charman et al., 2005). With larger sample sizes, differences between the Early- and Later-ASD groups may become apparent at 36 months of age. Future studies on ASD onset patterns should carefully document early intervention experiences, which may affect outcomes and prognosis. Another limitation is the possibility that change in certain variables is related to change in other variables over time in certain diagnostic groups. We had insufficient sample size to reliably correlate change in all the variables using sound psychometric approaches.
Acknowledgments
We thank the participants and staff at Kennedy Krieger Institute and The Lurie Center, LADDERS of Massachusetts General Hospital for their help in data acquisition, coding, and/or processing. Special appreciation is expressed to Dr. Margaret Bauman for her oversight of the data collection at the Lurie Center-MGH, Massachusetts General Hospital, Harvard Medical School. All authors had full access to all of the data in the study; Dr. Landa and Ms. Faherty take responsibility for the integrity of the data; Dr. Gross and Dr. Stuart take responsibility for the accuracy of data analysis. We extend our gratitude to Drs. Mary Blue, Carlos Pardo, and Michael V. Johnston for their comments on the neurobiological theories of ASD we discussed herein.
Funding from the National Institute of Mental Health, awarded to Rebecca Landa (PI): MH59630 (design, study conduct, data collection, management, analysis, interpretation; preparation, review, and approval of manuscript), Autism Speaks (data collection), and U54 MH066417-04 (data collection).
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Rebecca J. Landa, Kennedy Krieger Institute and The Johns Hopkins University School of Medicine
Elizabeth A. Stuart, The Johns Hopkins Bloomberg School of Public Health
Alden L. Gross, The Johns Hopkins Bloomberg School of Public Health and Aging Brain Center, Institute for Aging Research Hebrew SeniorLife, Harvard Medical School
Ashley Faherty, Kennedy Krieger Institute.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29:213–224. doi: 10.1023/a:1023080005650. doi:1.1023/A:1023080005650. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. The neuropathology of the autism spectrum disorders: What have we learned? Novartis Foundation Symposium. 2003;251:281–297. doi:1.1002/047086938.ch8. [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ. Social and non-social visual attention patterns and associative learning in infants at risk for autism. Journal of Child Psychology and Psychiatry. 2010;51:989–997. doi: 10.1111/j.1469-7610.2010.02262.x. doi:1.1111/j.1469-761.201.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Development and Behavior. doi: 10.1016/j.infbeh.2012.07.019. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy Journal. 2011;9:1116–1129. doi: 10.2522/ptj.20100294. doi:1.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, et al. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12:433–456. doi: 10.1177/1362361308094500. doi:1.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, et al. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. doi:1.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Learn the signs, act early. 2010 Retrieved January 9, 2012, from http://www.cdc.gov/ncbddd/actearly/index.html.
- Charman T, Taylor E, Drew A, Cockerill H, Brown J, Baird G. Outcome at 7 years of children diagnosed with assessments conducted at 2 and 3 years of age and pattern of symptom change over time. Journal of Child Psychology and Psychiatry. 2005;5:500–513. doi: 10.1111/j.1469-7610.2004.00377.x. doi:1.1111/j.1469-761.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48:128–138. doi: 10.1111/j.1469-7610.2006.01685.x. doi:1.1111/j.1469-761.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Journal of the American Medical Association. 2003;290:337–344. doi: 10.1001/jama.290.3.337. doi:1.1001/jama.29.3.337. [DOI] [PubMed] [Google Scholar]
- Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ, et al. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Research. 2010;3:273–279. doi: 10.1002/aur.153. doi:1.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovitch M, Glick L, Holtzman G, Tirosh E, Safir MP. Developmental regression in autism: Maternal perception. Journal of Autism and Developmental Disorders. 2000;30:113–119. doi: 10.1023/a:1005403421141. doi:1.1023/A:1005403421141. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. doi:1.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. Journal of Neuroscience. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. doi:1.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang KY, Zeger SL. The analysis of longitudinal data. 2. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- Flanagan J, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: A preliminary study. American Journal of Occupational Therapy. 2012;66:577– 585. doi: 10.5014/ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- Gauthier J. Novel de novo SHANK3 mutation in autistic patients. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B:421–424. doi: 10.1002/ajmg.b.30822. doi:1.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- Goldfield BA, Reznick JS. Early lexical acquisition: Rate, content, and the vocabulary spurt. Journal of Child Language. 1990;17:171–183. doi: 10.1017/s0305000900013167. doi:1.1017/S0305000900013167. [DOI] [PubMed] [Google Scholar]
- Harris SL, Handelman JS. Age and IQ at intake as predictors of placement for young children with autism: A four to six year follow-up. Journal of Autism and Developmental Disorders. 2000;30:137–142. doi: 10.1023/a:1005459606120. doi:1.1023/A:1005459606120. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Archives of General Psychiatry. 2011;68:467–476. doi: 10.1001/archgenpsychiatry.2011.39. doi:1.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR. SHANK3, the synapse, and autism. New England Journal of Medicine. 2011;365:173–175. doi: 10.1056/NEJMcibr1104261. doi:1.1056/NEJMcibr1104261. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moore A. Diagnosis in autism: A survey of over 1200 patients in the UK. Autism. 1997;1:135–162. doi:1.1177/1362361397012003. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. doi:1.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:158–170. doi: 10.1007/s10803-006-0339-z. doi:1.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M. Plasticity and injury in the developing brain. Brain Development. 2009;31:1–10. doi: 10.1016/j.braindev.2008.03.014. doi:1.1016/j.braindev.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, Pelphrey KA. Structural neural phenotype of autism: Preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. American Journal of Neuroradiology. 2011;32:1607–1613. doi: 10.3174/ajnr.A2558. doi:1.3174/ajnr.A2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb LG, Law JK, Landa R, Law PA. Onset patterns prior to 36 months in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40:1389–1402. doi: 10.1007/s10803-010-0998-7. doi:1.1007/s10803-010-0998-7. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Klin A, Lang J, Cicchetti DV, Volkmar FR. Brief report: Interrater reliability of clinical diagnosis and DSM–IV criteria for autistic disorder: Results of the DSM–IV autism field trial. Journal of Autism and Developmental Disorders. 2000;30:163–167. doi: 10.1023/a:1005415823867. doi:1.1023/A:1005415823867. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16:1412–1425. doi: 10.1162/0898929042304796. doi:1.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Landa R. Developmental features and trajectories associated with autism spectrum disorders in infants and toddlers. In: Amaral D, Geschwind D, Dawson G, editors. Autism spectrum disorders. New York: Oxford Press; 2011a. pp. 213–228. [Google Scholar]
- Landa R. National Children’s Study Formative Research Project. Kennedy Krieger Institute; Baltimore, MD: 2011b. Video-guided parent report of ASD symptoms. [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. doi:1.1111/j.1469-761.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. doi:1.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, O’Neill A, Stuart EA. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: A randomized controlled trial. Journal of Child Psychology and Psychiatry. 2011;52:13–21. doi: 10.1111/j.1469-7610.2010.02288.x. doi:1.1111/j.1469-761.201.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. doi:1.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luyster R, Richler J, Risi S, Hsu WL, Dawson G, Bernier R, et al. Early regression in social communication in autism spectrum disorders: A CPEA study. Developmental Neuropsychology. 2005;27:311–336. doi: 10.1207/s15326942dn2703_2. doi:1.1207/s15326942dn2703_2. [DOI] [PubMed] [Google Scholar]
- Maestro S, Casella C, Milone A, Muratori F, Palacio-Espasa F. Study of the onset of autism through home movies. Psychopathology. 1999;32:292–300. doi: 10.1159/000029102. doi:1.1159/000029102. [DOI] [PubMed] [Google Scholar]
- Minshew N, Siegel DJ, Goldstein G, Weldy S. Verbal problem solving in high functioning autistic individuals. Archives of Clinical Neuropsychology. 1994;9:31–40. doi:1.1093/arclin/9.1.31. [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. (AGS Edition) [Google Scholar]
- Müller R, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cerebral Cortex. 2011;21:2233–2243. doi: 10.1093/cercor/bhq296. doi:1.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:256–266. doi:1.1016/j.jaac.2009.11.009. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. doi:1.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo CA. Can neuroinflammation influence the development of autism spectrum disorders? In: Zimmerman AW, editor. Autism: Current theories and evidence. Totowa, NJ: Humana Press; 2008. pp. 329–346. [Google Scholar]
- Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathology. 2007;17:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. doi:1.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry. 2011;5:588–598. doi: 10.1111/j.1469-7610.2010.02332.x. doi:1.1111/j.1469-761.201.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Charting the typical and atypical development of the social brain. Development and Psychopathology. 2008;20:1081–1102. doi: 10.1017/S0954579408000515. doi:1.1017/S0954579408000515. [DOI] [PubMed] [Google Scholar]
- Pierce K, Conant D, Hazin R, Stoner R, Desmond J. A preference for geometric patterns early in life is a risk factor for autism. Archives of General Psychiatry. 2011;68:101–109. doi: 10.1001/archgenpsychiatry.2010.113. doi:1.1001/archgenpsychiatry.201.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. Journal of Clinical Child and Adolescent Psychology. 2008;37:8–38. doi: 10.1080/15374410701817808. doi:1.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. Journal of Neuroscience. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. doi:1.1523/JNEU-ROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations in autism. Science. 2007;316:445–459. doi: 10.1126/science.1138659. doi:1.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. Journal of Autism and Developmental Disorders. 2007;37:37–48. doi: 10.1007/s10803-006-0335-3. doi:1.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Nalty T, Munson J, Brock C, Abbott R, Dawson G. Rate of head circumference growth as a function of autism diagnosis and history of autistic regression. Journal of Child Neurology. 2010;10:1182–1190. doi: 10.1177/0883073807306263. doi:1.1177/0883073807306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E, Dawson G. Validation of the phenomenon of autistic regression using home videotapes. Archives of General Psychiatry. 2005;62:889–895. doi: 10.1001/archpsyc.62.8.889. doi:1.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G, Munson J, Osterling J. Variation in early developmental course in autism and its relation with behavioral outcome at 3–4 years of age. Journal of Autism and Developmental Disorders. 2005;35:337–350. doi: 10.1007/s10803-005-3301-6. doi:1.1007/s10803-005-3301-6. [DOI] [PubMed] [Google Scholar]
- Wetherby A, Prizant B. CSBS DP manual: Communication and Symbolic Behavior Scales Developmental Profile. Baltimore: Paul H Brookes; 2002. [Google Scholar]
- Wilson S, Djukic A, Shinnar S, Dharmani C, Rapin I. Clinical characteristics of language regression in children. Developmental Medicine and Child Neurology. 2003;45:508–514. doi: 10.1017/s0012162203000951. doi:1.1111/j.1469-8749.2003.tb0095.x. [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6-months: Predicting clinical outcomes and language development in typically developing infants and infants at-risk for autism. Developmental Science. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. doi:1.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. doi:1.2307/2531248. [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: Meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. doi:1.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. doi:1.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]