Abstract
Objective
To compare gross and histologic patterns of age-related degeneration within the intervertebral disc and adjacent vertebra between rhesus monkeys and humans.
Materials and methods
We examined age-related patterns of disc degeneration from mid-sagittal sections of the intervertebral disc and adjacent vertebral bodies among six rhesus monkey thoracolumbar and seven human lumbar spines. Gross morphology and histopathology were assessed via the Thompson grading scheme and other degenerative features of the disc and adjacent bone.
Results
Thompson grades ranged from 3 through 5 for rhesus monkey discs (T9-L1) and 2 through 5 for the human discs (T12-S1). In both rhesus monkey and human discs, presence of distinct lesions were positively associated with Thompson grade of the overall segment. Degenerative patterns differed for radial tears, which were more prevalent with advanced disc degeneration in humans only. Additionally, compared to the more uniform anteroposterior disc degeneration patterns of humans, rhesus monkeys showed more severe osteophytosis and degeneration on the anterior border of the vertebral column.
Conclusions
Rhesus monkey spines evaluated in the present study appear to develop age-related patterns of disc degeneration similar to humans. One exception is the absence of an association between radial tears and disc degeneration, which could reflect species-specific differences in posture and spinal curvature. Considering rhesus monkeys demonstrate similar patterns of disc degeneration, and age at a faster rate than humans, these findings suggest longitudinal studies of rhesus monkeys may be a valuable model for better understanding the progression of human age-related spinal osteoarthritis and disc degeneration.
Keywords: spinal osteoarthritis, intervertebral disc degeneration, histopathology, rhesus monkey
INTRODUCTION
Intervertebral disc degeneration and spinal osteoarthritis (OA) are age-related processes that underlie several painful disorders of the spine in humans. The prevalence of age-related spinal OA has been shown to be as high as 85%1 and some degree of disc degeneration appears to be present in all adults2. Considering the high economic impact (from both health care services and absence from the workplace) associated with disc degeneration and its complications3,4, strong interest in improving the understanding of the etiology of disc degeneration and in developing new therapies exists.
A major challenge in investigating the etiology of disc degeneration and in evaluating new therapies is selecting a suitable animal model that mimics the morphology and progression of age-related disc degeneration of humans5,6. In most animal models, for example, disc degeneration does not occur naturally and must be artificially induced. Even in animal models that do develop disc degeneration naturally (e.g. sand rats7 and various canines8), differences in spine morphology and biomechanics can make it difficult to extrapolate the findings to humans.
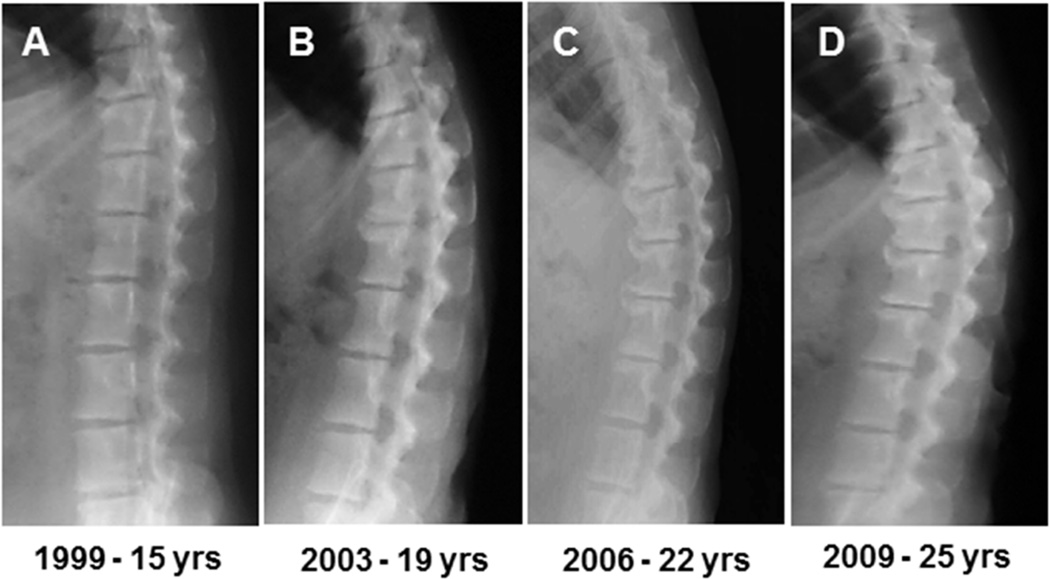
The rhesus monkey (Macaca mulatta) is a non-human primate that shares genetic, anatomical, and biomechanical similarities with humans9,10. Although rhesus monkeys are technically quadrupedal and ambulate on four legs, they load their spines similar to humans when sitting11 Captive rhesus monkeys have an average lifespan of 27 years and maximal lifespan of 40 years12. Interestingly, the rhesus monkey naturally develops polyarticular OA with age13,14 (Figure 1). The aging spines of rhesus monkeys are afflicted with disc degeneration, osteophytosis, and kyphosis.. As in humans, these degenerative changes are most severe in the thoracolumbar and lumbosacral zones2,15,16. Although this suggests that rhesus monkeys exhibit similar anatomic patterns of disc degeneration as humans, it remains unclear whether rhesus monkeys exhibit similar histologic features of disc degeneration as well. If the histologic progression of spinal OA and disc degeneration in rhesus monkeys is comparable to that of humans, utilizing monkeys as an animal model for disc degeneration in basic science and preclinical studies is potentially appropriate. The objective of this study was, therefore, to compare the process of age-related degeneration within the intervertebral disc and adjacent vertebra between rhesus monkeys and humans.
Fig. 1.
A through D are lateral radiographs of the thoracolumbar spine from an individual rhesus monkey used in this study. Radiographs span 10 years and depict increasing osteophytosis and disc space narrowing with age. Note, rhesus monkeys typically have seven lumbar vertebrae.
MATERIALS AND METHODS
Study design
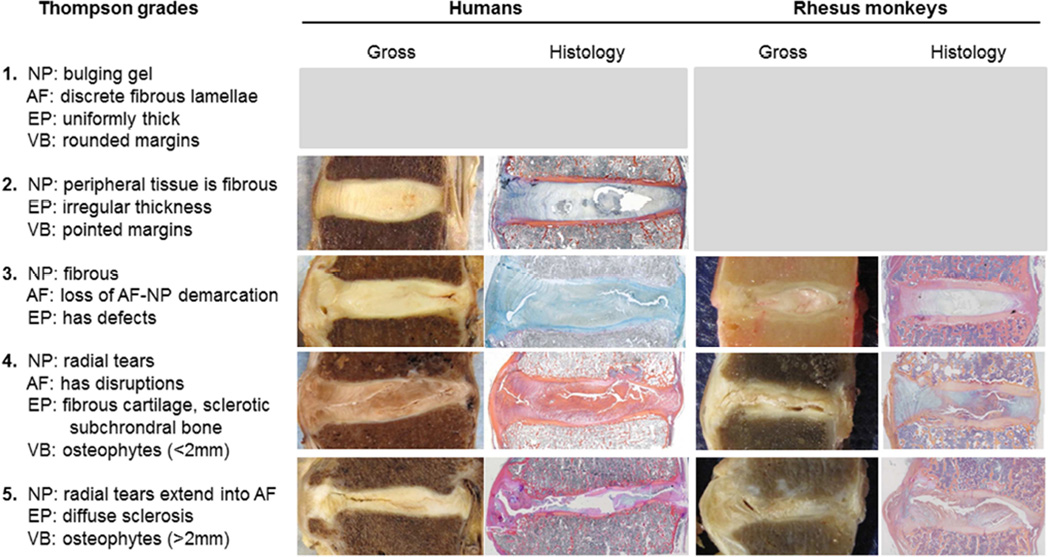
We used the Thompson grading scheme to create scores to compare the progression of disc degeneration between humans and rhesus monkeys. Thompson grades are a gross measure of disc degeneration across five categories based on the accumulation of tissue pathologies in and adjacent to the disc17. See methods from Thompson et al. 1990 for thorough description of grading. Consequently, the Thompson grade provides an overall measure of gross degeneration of the disc segment that is independent of chronological age, which is necessary for our study because humans and rhesus monkeys age at different rates. Also, both the rhesus monkey and human samples are samples of convenience and, therefore, are not matched for age or degree of degeneration. Figure 2 indicates Thompson grading scheme in relation to the rhesus monkeys and human disc segments in this study.
Fig. 2.
In the left column, Thompson grades are defined based on anatomical changes in the nucleus pulposus (NP), annulus fibrosus (AF), endplate (EP; mainly cartilage, but includes changes to adjacent subchondral bone), and adjacent vertebral bodies (VB). Examples of various grades from specimens analyzed in this study are depicted via gross anatomy images and histology sections. Histology sections in figure are stained with Mallory-Heidenhain.
Monkey spines
The rhesus monkeys used in this study were part of a longitudinal study of the effect of caloric restriction on mortality, the details of this study have been described elsewhere18–20. Monkeys were housed indoors in single cages in a temperature-controlled environment and fed two meals a day (0700 and 1400 hours). Following natural death, thoracolumbar spines were harvested and stored at −80° C. Six spines, spanning mid-thoracic to upper lumbar (T9-L2) from 3 female and 3 male rhesus monkeys (aged 19.4 – 36.1 years with a mean age of 28.0 years), were randomly selected from this collection for evaluation in the current study. The NIH Animal Center in Poolesville, MD, is fully accredited by the American Association for Accreditation of Laboratory Animal Care, and all procedures were approved by the Animal Care and Use Committee of the NIA Intramural Program.
Human spines
Lumbar spines (T11-S1) were obtained from seven human cadavers (UCSF Willed Body Program), including two females and five males with ages ranging from 51 to 67 years with a mean age of 60.3. Tissue was then stored at −80° C and did not thaw until further dissection and preparation in the Orthopaedic Research Laboratory at the University of California, San Francisco.
Tissue processing and histology
Spines from the human cadavers and rhesus monkeys were processed for histology using similar procedures. Paraspinal soft tissues and posterior elements were removed from the spinal column. The spinal column was then sectioned into parasagittal slabs (1cm thick). One midsagittal slab from each spine was further separated into individual motion segments (rhesus monkeys, n=18; humans, n=44), which comprised whole discs with 3–4 cm of bone from the adjacent vertebrae. Each motion segment was then fixed using 10% neutral buffered formalin (Thermo Fisher Scientific). Segments were photographed and then decalcified with Ion Exchange Decal, a mild but rapid ion exchange decalcifier (BioCare Medical), until a radiographic endpoint test confirmed the specimens devoid of calcium. Segments were dehydrated in an ascending series of ethanol and cleared with Clearite 3 (Richard Allen), then infiltrated and embedded in paraffin wax. Once sectioned and mounted on slides, serial sections were stained with Mallory-Heidenhain21 and Safranin-O.
Thompson grading
The extent of spinal degeneration in the motion segments was assessed with Thompson grading. The Thompson grading scheme is a five-category scheme used for assessing the gross morphology of the human lumbar intervertebral disc. Grades range from 1 to 5, where 1 is healthy and 5 represents end-stage disc degeneration. Numerous degenerative features are used to determine the Thompson grade of a disc and Figure 2 describes the criteria for each grade and depicts examples of grades from both humans and rhesus monkeys observed in this study. All discs, both human and rhesus monkey, were Thompson graded by two raters (JFB & EL; 82.5% agreement with an inter-rater reliability of κ = 0.76).
Evaluating individual histopathologic features
Thompson grade represents the accrual of degenerative features in and the around the disc and does not indicate which features contributed to the grade. Two specimens with identical Thompson grade could, therefore, have different manifestations of degenerative features. In order to assess if the same individual degenerative features are present in humans and rhesus monkeys and contribute to Thompson grade similarly, we evaluated the presence and location of specific histopathologic features within the disc, endplates, and adjacent vertebral bodies. Regions of interest were defined by anterior (within the boundary of the anterior annulus fibrosus and anterior portion of nucleus pulposus) and posterior (within the boundary of the posterior annulus fibrosus and posterior portion of nucleus pulposus). Histopathologic features of the disc and adjacent vertebrae included osteophytosis (OST), annular tears (concentric (CT) and radial (RT)), inner annulus morphology (inward versus outward bulging, IAM), nucleus-annulus ratio (relevant to loss of nucleus-annulus demarcation, NP/AF), cartilage endplate (CEP), and endplate rim lesions (RL). Histopathologic features are demonstrated in Figure 3 and formally defined in Table 1.
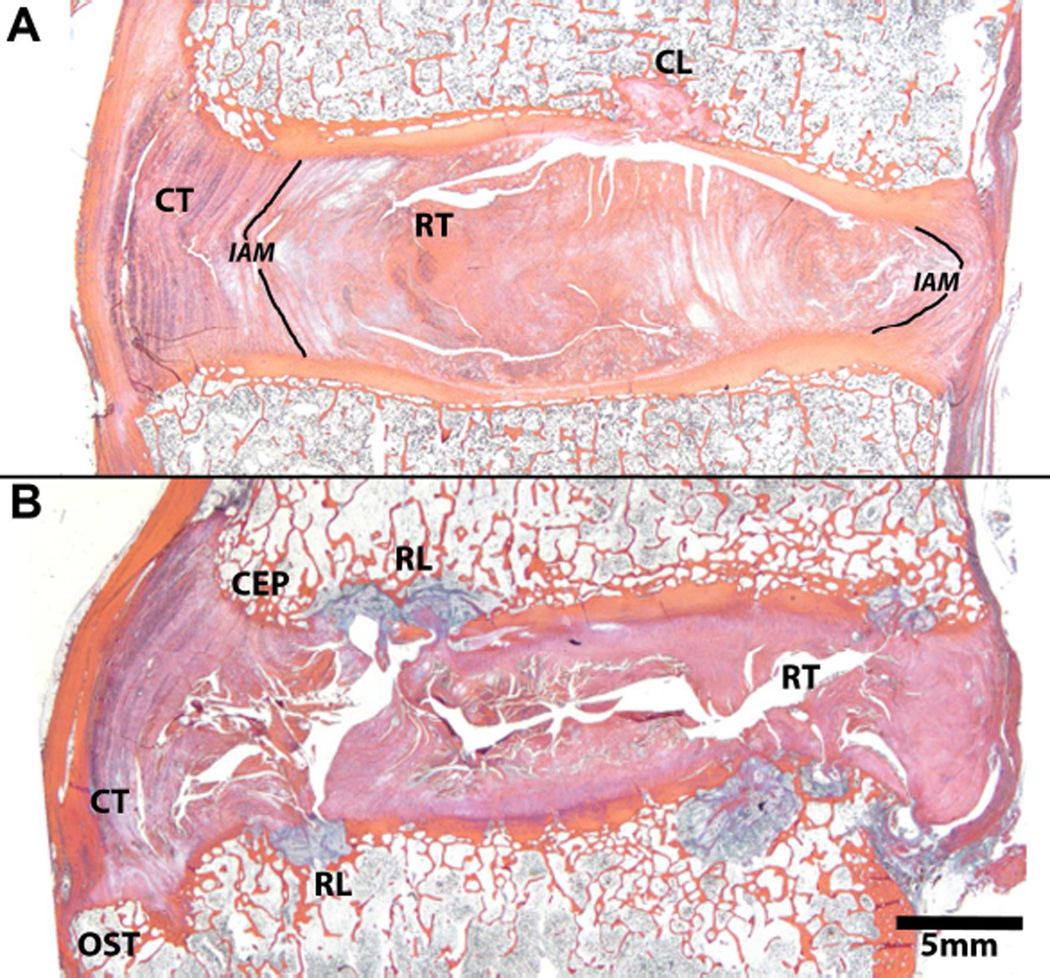
Fig. 3.
Mid-sagittal histological sections of human discs depicting histopathologies that coincide with disc degeneration. Both discs are Thompson grade 4 and from the same spine, the top image (A) is L4–L5 and the bottom image (B) is L5–S1. See Table 1 for definitions of labeled histopathologies. Note, the centrally-located lesion through the upper endplate in 3A labeled CL is a general endplate defect and is not by definition, a rim lesion. It differs from a rim lesion in its central location, adjacent to the nucleus pulposus. Histology sections in figure are stained with Mallory-Heidenhain.
Table 1.
Definitions of histopathologies associated with disc degeneration. The provided definition regards the pathological state of anatomical features within the disc and vertebral body.
| Osteophytosis OST |
Abnormal bone growth or bony spurs that occur around degenerating joints |
| Concentric tears CT |
Vertically oriented tears that result in separations between annular fibers |
| Radial tears RT |
Transversally oriented tears that perpendicularly disrupt annular fibers |
| Cartilage endplate CEP |
Degradation of the hyaline cartilage between the disc and bone resulting in disruptions and absence |
| Endplate rim lesions RL |
Broad term for disruptions through both the cartilage and bony endplate of the vertebral rim |
| Inner AF Morphology IAM |
Inward bulging of the annulus that occurs with disc degeneration |
Abbreviations given are used in other figures.
Statistical analysis
Scores of the specific degenerative features within anterior and posterior regions of interest were based on binary grading, with 0 representing “not-present” and 1 representing “present.” The correlation of individual degenerative feature to Thompson grade was determined using a Fisher’s Exact test with significance based on p < 0.05. Due to our relatively small sample size, we also describe trends in relationships based on p < 0.10. All statistical analyses were conducted using STATA (StataCorp LP, College Station, TX).
RESULTS
Thompson grades ranged from 3 through 5 for the rhesus monkeys (3=17%, 4=44%, 5=39%) and 2 through 5 for the humans (2=22%, 3=40%, 4=31%, 5=7%). Examples of gross morphology and histology for both rhesus monkeys and humans can be found in Figure 2. The following subcategories address both the overall (disc as a whole) and regional (anterior and posterior regions) prevalence of specific histopathologic features with Thompson grade. (All p-values for categorical associations are provided in Table 2).
Table 2.
P-values represent the significance of association between the presence of specific histopathology features and their inclusion in the Thompson grade of disc degeneration.
| Rhesus monkeys | Humans | |||||
|---|---|---|---|---|---|---|
| Whole | Anterior | Posterior | Whole | Anterior | Posterior | |
| Osteophyosis | 0.015* | <0.001* | 0.051† | <0.001* | 0.001* | 0.039* |
| Concentric tears | 0.002* | 0.051† | 0.065† | 0.003* | 0.060† | 0.080† |
| Radial tears | 0.335 | 0.990 | 0.344 | <0.001* | <0.001* | 0.002* |
| EP rim lesions | <0.001* | <0.001* | 0.765 | <0.001* | 0.034* | <0.001* |
| Cartilage EP | 0.022* | 0.002* | 0.672 | 0.227 | 0.411 | 0.019* |
| Inner AF morph. | 0.004* | <0.001* | 0.032* | <0.001* | <0.001* | <0.001* |
Osteophytosis
Among both rhesus monkeys and humans, the overall prevalence of osteophytosis was significantly greater with increasing Thompson grade (monkeys, p=0.015; humans, p<0.001). In human discs, osteophytosis in both the anterior (p<0.001) and posterior (p=0.039) regions of the discs was more prevalent with increasing Thompson grade. In rhesus monkeys, the prevalence of osteophytosis in the anterior (p<0.001) and posterior (p=0.051) regions also was associated with increasing Thompson grade.
Concentric and radial disc tears
Among both rhesus monkeys and humans, the overall prevalence of concentric disc tears was significantly greater with increasing Thompson grade (monkeys, p=0.002; humans, p=0.003). Radial tears, however, were associated with Thompson grade in humans (p<0.001), but not in rhesus monkeys (p=0.335). Concentric tears in both rhesus monkeys and humans showed a trend with Thompson grade in both the anterior (monkeys, p=0.054; humans, p=0.060) and posterior (monkeys, p=0.065; humans, p=0.080) regions.
Inner annulus morphology
The contour of the inner annulus morphology (IAM) bulged inward toward the nucleus pulposus with more severe Thompson grade for both rhesus monkeys (p=0.004) and humans (p<0.001), and this was true for both anterior and posterior regions of the disc (monkey: anterior p<0.001, posterior p=0.032; human: anterior p<0.001, posterior <0.001).
Endplate rim lesions
Among both rhesus monkeys and humans, the overall prevalence of endplate rim lesions was significantly greater with increasing Thompson grade (p<0.001 for both). The prevalence of endplate rim lesions increased with Thompson grade in both the anterior (p=0.034) and posterior (p<0.001) regions of the human disc segments, while in the rhesus monkey discs, endplate rim lesions were associated with increasing Thompson grade in the anterior region only (p<0.001; posterior p=0.765).
Cartilage endplate
Damage and degradation of the cartilaginous endplate were prevalent with increasing Thompson grade for the rhesus monkey disc segments (p=0.022), but not the human disc segments (p=0.227). The prevalence of cartilage endplate damage or degradation in either the anterior or posterior regions of the disc segment showed a significant association with Thompson grade in the anterior region in rhesus monkey (p=0.002) and posterior region in human (p=0.020) disc segments. Conversely, cartilage damage and degradation in the posterior region in rhesus monkey and anterior region in human disc segments did not corresponded to increasing Thompson grade.
DISCUSSION
Rhesus monkeys (Macaca mulatta) have been proposed as a potential animal model for studying the etiology of disc degeneration and spinal OA, because they are known to have naturally-occurring degenerating intervertebral discs with advancing age13,14,22. In the present study, moreover, we found that spinal OA and disc degeneration among aged rhesus monkeys demonstrate comparable histologic pathologies as those that occur with advancing disc degeneration in humans as reported by Thompson17. Although it remains to be seen whether or not the mechanisms responsible for disc degeneration and spinal OA in the rhesus monkey are similar to those in humans, the present study suggests that monkeys demonstrate similar morphologic progression of disc degeneration and spinal OA, which suggests that the rhesus monkey is a potentially valuable animal model for the human condition.
We noted several differences between rhesus monkey and human disc degeneration, which may be attributable to species differences in thoracolumbar spine curvature and posture. On the one hand, humans exhibit a sigmoid curvature with substantial lumbar lordosis due to habitual bipedalism23,24. This curvature serves to center the weight of the upper body above the pelvis and legs when standing and moving. The lumbar vertebral bodies are loaded in compression across the vertebral body. On the other hand, although rhesus monkeys compressively load their spines by habitually sitting upright for long durations, particularly in cages, they are quadrupedal. The rhesus monkey lumbar spine shows only slight lordosis23 and is kyphotic from the upper lumbar to upper thoracic. The shape of the rhesus monkey thoracolumbar spine acts to passively support approximately half of the weight of the upper body while sitting upright11 and the anterior margin of the vertebral body is loaded in compression.
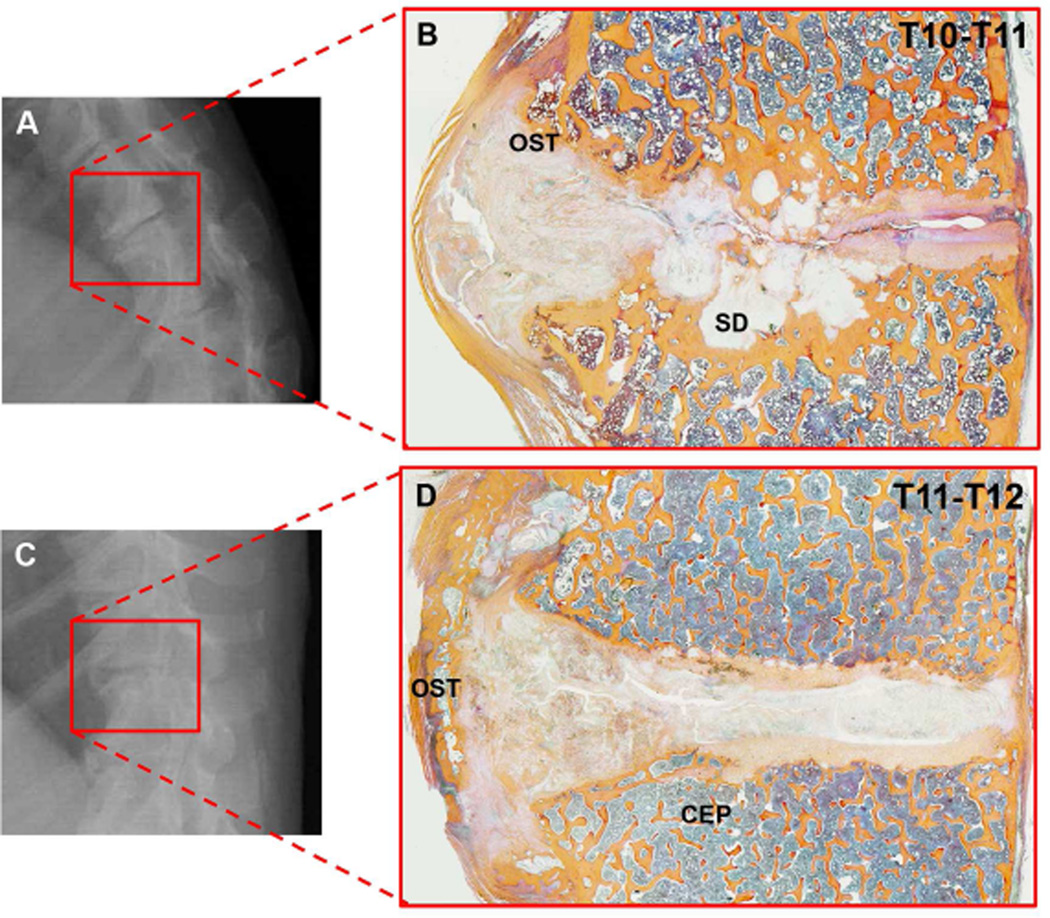
Most of the histopathologic differences between rhesus monkeys and humans that we demonstrate relate to the differential prevalence of damage in the anterior and posterior regions of the discs and vertebral bodies. For instance, osteophytosis is severe and mainly present on the anterior side of the vertebral column in rhesus monkeys – some Thompson grade 5 monkey discs even displayed bridged osteophytes (Figure 4). By contrast, osteophytosis patterns in humans appear to be more distributed between the anterior and posterior sides of the discs and vertebral bodies. A possible reason for these differences is the different spinal curvature in the two species.
Figure 4.
A & C are examples of disc spaces on radiographs of separate rhesus monkeys and B & D show the same disc spaces via histology. B shows a cluster of sclerotic depressions (SD) at the center of the endplate. D on the other hand, shows a bridged osteophyte (OST) that is visible via radiograph, but could promote a “healthier” radiographic assessment of disc space narrowing because it holds the disc space apart – while via histology we see that the disc is degenerated. Histology sections in figure are stained with Mallory-Heidenhain.
The relatively kyphotic shape of the rhesus monkey thoracolumbar spine may be responsible for the higher degree of osteophytosis we observed on the anterior border of the monkey segments, because the anterior border is loaded in compression. Vertebral osteophytosis usually appears on the concave borders of spinal curves25, where compressive loads are relatively greatest, and has been suggested to be an adaptive response, providing stability and motion resistance to an otherwise degenerating disc segment26. Figure 4 includes examples of severely degenerated rhesus monkey discs that originate from a curved region of the lower thoracic spine and have increased degeneration on the anterior border. The etiology of vertebral osteophytosis remains unclear, but we believe that the differences in osteophytosis patterns between humans and rhesus monkey are attributable to the differences in spinal curvature.
We compared the rhesus monkey thoracolumbar transitional zone to the human lumbar region, because these are the areas that have been previously described as the region of peak spinal OA in the spines of the two species27, 28 (demonstrated in Figure 1). Futures studies should investigate degeneration patterns in the mid to lower lumbar rhesus monkey spine, as there is a slight lordotic curve that may render degeneration trends similar to human lumbar discs.
A limitation of this study is that the range of Thompson grades gathered from rhesus monkey and human discs did not fully span the spectrum of degenerative grades (1 through 5). Naturally, cadaveric tissue is difficult to obtain from young people who tend to have less-degenerated discs. Given that the study from which we drew the animals is principally concerned with evaluating the effect of caloric restriction on longevity, we did not have younger spines available to sample. Rhesus monkey discs with less degeneration are, however, obtainable from national primate centers and future work should include younger animals.
Naturally-occurring spinal OA and similarities in disc degeneration biomechanical properties27 and pathological patterns makes rhesus monkeys an excellent model of age-related spinal OA and disc degeneration in humans. The average life span of captive rhesus monkeys is 27 years and they age at a rate roughly three times that of humans13,29,30. Additionally, national primate research centers, which have been established and maintained in order to provide animals for biomedical research, can provide longitudinal diagnostic imaging and tissue-sharing from animals of known pedigree. Monkeys are expensive to house and maintain in ethically appropriate facilities, so collaborations between longitudinal studies and ones requiring samples obtained at necropsy, such as the one described herein, that leverage resources provide unparalleled opportunities for furthering our understanding of spinal OA.
ACKNOWLEDGMENTS
The authors thank the department of Orthopaedic Surgery at the University of California, San Francisco and especially Dezba Coughlin for organizing the collaboration logistics and Robin Parmentier for his assistance with histology and image processing. The Biodemography Lab of the University of Washington and especially Kathleen O’Connor for freezer space. Raymond Huey and Tracy Larson for their review of previous version of this manuscript. Jerome Debs Chair in Orthopaedic Research for support of PAK and JFB. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Aging.
ROLE OF FUNDING SOURCE
This study was funded by NIH grants AG021379 and AR052811, the Debs Chair in Orthopaedic Research at the University of Washington, and Relievant Medsystems, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
JFB and PAK provided rhesus monkey spines and the original idea. AJP, EL, and JCL provided the human data, additional spine expertise, and facilities where JFB collected data on the rhesus monkey tissue. JAM played a significant role regarding the housing of and early data collection from rhesus monkey subjects. JFB drafted the manuscript and all authors contributed critical revision toward and approval of the final draft.
CONFLICT OF INTERESTS
The authors report no conflicts of interest.
REFERENCES
- 1.Goode AP, Carey TS, Jordan JM. Low back pain and lumbar spine osteoarthritis: how are they related? Curr Rheumatol Rep. 2013;15(2):305. doi: 10.1007/s11926-012-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(Suppl 2):3–9. doi: 10.2106/JBJS.E.01313. [DOI] [PubMed] [Google Scholar]
- 3.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2):353–371. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.An HS, Masuda K. Relevance of in vitro and in vivo models for intervertebral disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):88–94. doi: 10.2106/JBJS.E.01272. [DOI] [PubMed] [Google Scholar]
- 6.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruber HE, Johnson T, Norton HJ, Hanley EN. The sand rat model for disc degeneration: radiologic characterization of age-related changes: cross-sectional and prospective analyses. Spine. 2002;27(3):230–234. doi: 10.1097/00007632-200202010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Bergknut N, Rutges JPHJ, Kranenburg H-JC, Smolders LA, Hagman R, Smidt H-J, et al. The dog as an animal model for intervertebral disc degeneration? Spine. 2012;37(5):351–358. doi: 10.1097/BRS.0b013e31821e5665. [DOI] [PubMed] [Google Scholar]
- 9.Carlson CS, Loeser RF, Jayo MJ, Weaver DS, Adams MR, Jerome CP. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994;12(3):331–339. doi: 10.1002/jor.1100120305. [DOI] [PubMed] [Google Scholar]
- 10.Kramer PA, Newell-Morris LL, Simkin PA. Spinal degenerative disk disease (DDD) in female macaque monkeys: epidemiology and comparison with women. J Orthop Res. 2006;20(3):399–408. doi: 10.1016/S0736-0266(01)00122-X. [DOI] [PubMed] [Google Scholar]
- 11.Gal J. Mammalian spinal biomechanics: postural support in seated macaques. J Exp Biol. 2002;205(Pt 12):1703–1707. doi: 10.1242/jeb.205.12.1703. [DOI] [PubMed] [Google Scholar]
- 12.Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Aging (Milano) 1998;10(2):83–92. doi: 10.1007/BF03339642. [DOI] [PubMed] [Google Scholar]
- 13.DeRousseau CJ. Aging in the musculoskeletal system of rhesus monkeys: II. Degenerative joint disease. Am J Phys Anthropol. 1985;67(3):177–184. doi: 10.1002/ajpa.1330670303. [DOI] [PubMed] [Google Scholar]
- 14.Pritzker KP. Animal models for osteoarthritis: processes, problems and prospects. Ann Rheum Dis. 1994;53(6):406–420. doi: 10.1136/ard.53.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan AE, Colman RJ, Kramer PA. Sex Differences in Spinal Osteoarthritis in Humans and Rhesus Monkeys (Macaca mulatta) Spine. 2012;37(11):915–922. doi: 10.1097/BRS.0b013e31823ab7fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Videman T, Battié MC, Gill K, Manninen H, Gibbons LE, Fisher LD. Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine. 1995;20(8):928–935. doi: 10.1097/00007632-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15(5):411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Ingram D, Lane M, Cutler R, Roth G. Longitudinal study of aging in monkeys: effects of diet restriction. Neurobiol Aging. 1993;14:687–688. doi: 10.1016/0197-4580(93)90072-j. [DOI] [PubMed] [Google Scholar]
- 19.Lane M, Ingram D, Cutler R, Knapka J, Barnard D, Roth G. Dietary restriction in nonhuman primates: progress report on the NIA study. In: Fabis N, Harman D, Knook D, Steinhagen-Thiessen E, Zs-nagy I, editors. Physiological Processes of Aging: Towards a Multicausal Interpretation. Vol. 673. New York: NY Academy of Science; 1992. pp. 36–45. [DOI] [PubMed] [Google Scholar]
- 20.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38(1–2):35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 21.Cason JE. A rapid one-step Mallory-Heidenhain stain for connective tissue. Stain Technol. 1950;25(4):225–226. doi: 10.3109/10520295009110996. [DOI] [PubMed] [Google Scholar]
- 22.Duncan AE, Colman RJ, Kramer PA. Longitudinal study of radiographic spinal osteoarthritis in a macaque model. J Orthop Res. 2011;29(8):1152–1160. doi: 10.1002/jor.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Been E, Barash A, Marom A, Kramer PA. Vertebral bodies or discs: which contributes more to human-like lumbar lordosis? Clin Orthop Relat Res. 2010;468(7):1822–1829. doi: 10.1007/s11999-009-1153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005;87(2):260–267. doi: 10.2106/JBJS.D.02043. [DOI] [PubMed] [Google Scholar]
- 25.Nathan H. Osteophytes of the Vertebral Column An Anatomical Study of Their Development According to Age, Race, and Sex with Considerations as to Their Etiology and Significance. J Bone Joint Surg Am. 1962;44(2):243–268. [Google Scholar]
- 26.Al-Rawahi M, Luo J, Pollintine P, Dolan P, Adams MA. Mechanical function of vertebral body osteophytes, as revealed by experiments on cadaveric spines. Spine. 2011;36(10):770–777. doi: 10.1097/BRS.0b013e3181df1a70. [DOI] [PubMed] [Google Scholar]
- 27.Nuckley DJ, Kramer PA, Del Rosario A, Fabro N, Baran S, Ching RP. Intervertebral disc degeneration in a naturally occurring primate model: radiographic and biomechanical evidence. J Orthop Res. 2008;26(9):1283–1288. doi: 10.1002/jor.20526. [DOI] [PubMed] [Google Scholar]
- 28.Kramer PA, Newell-Morris LL, Simkin PA. Spinal degenerative disk disease (DDD) in female macaque monkeys: epidemiology and comparison with women. J Orthop Res. 2002;20(3):399–408. doi: 10.1016/S0736-0266(01)00122-X. [DOI] [PubMed] [Google Scholar]
- 29.Colman RJ, Kemnitz JW, Lane MA, Abbott DH, Binkley N. Skeletal effects of aging and menopausal status in female rhesus macaques. J Clin Endocrinol Metab. 1999;84(11):4144–4148. doi: 10.1210/jcem.84.11.6151. [DOI] [PubMed] [Google Scholar]
- 30.Colman RJ, Lane MA, Binkley N, Wegner FH, Kemnitz JW. Skeletal effects of aging in male rhesus monkeys. Bone. 1999;24(1):17–23. doi: 10.1016/s8756-3282(98)00147-1. [DOI] [PubMed] [Google Scholar]