Abstract
BACKGROUND
Human red blood cells (RBCs) can be stored for up to 42 days under controlled conditions. Physical and chemical changes occur during RBC storage, altering their function. This study links stored cells mechanical changes with hemodynamic functional alterations upon transfusion.
STUDY DESIGN AND METHODS
Mechanical properties of fresh and stored RBCs were evaluated in vitro. Their transfusion effects were evaluated in vivo using intravital microscopy of the rat's cremaster muscle preparation. Rats were hemodiluted to 30% hematocrit, to mimic an anemic state pre-transfusion, then exchange transfused with fresh or stored cells.
RESULTS
In vitro studies on rheology and oxygen affinity of stored cells confirmed previously published results. Storage was found to modify static and dynamic red cell mechanic behavior. Post transfusion, systemic hemodynamics were similar for fresh and stored cells; however, microvascular hemodynamics were drastically affected by stored cells. Stored cells reduced blood flow and oxygen delivery. Additionally, the presence of stored cells in circulation affected cell-to-cell and cell-to-wall interactions, affected cell hydrodynamics. Stored cells disrupted the erythrocyte cell free layer (CFL) and wall shear stress (WSS) signals.
CONCLUSION
The reduced cell deformability due to RBC “storage lesions” caused pathological changes in microvascular hemodynamics, endothelial cell mechanotransduction, and RBC dynamics. Thus, the mechanical changes of blood banked cells can limit transfusion ability to achieve its intended goal.
Keywords: Oxygenation, blood flow, anemia, cell free layer, wall shear rate, wall shear stress, cell dynamics, erythrocyte deformability
INTRODUCTION
Blood banks are responsible for the collection, testing, and storage of blood to be used by the nearly 5 million Americans who receive blood transfusions annually.1 According to the 2009 National Blood Collection and Utilization Survey (NBCUS) Report, 15 million units of blood were administered in the U.S., with a total of 140,000 transfusion-related adverse reactions during the year.1 The ability to store blood to be transfused when needed is a key element for medical care and public health. Human red blood cells (RBCs) are typically stored for as long as 42 days at 2° to 6°C with the appropriate additives. Despite transfusion widespread use, blood products regulation only covers the procedures for collection, processing, and storage.2 Concerns have recently been raised about the safety and efficacy of transfusing stored RBCs, since certain populations including trauma, critical care, and cardiac surgery patience often present negative clinical outcomes post transfusion.3
Stored RBCs undergo time dependent changes, so called “storage lesions” reducing post-transfusion RBCs survival.4-5 These changes include depletion of adenosine triphosphate (ATP) and 2,3-diphosphoglycerate acid (2,3 DPG), decreased in pH, released of potassium, reduced nitric oxide (NO), increased cell volume, and reduce deformability.4 The RBC motion in small blood vessels is an important factor in blood rheology, since blood apparent viscosity depends on the ability of RBC to deform and rearrange according to the flow regime. In blood vessels, RBCs are continuously forced to create an RBC core and to separate away from the vessel wall generating a cell free layer (CFL) or RBC depleted zone next to the vessel wall.6 The CFL acts as a lubricating layer reducing blood resistance to flow.7 The CFL thickness depends primarily on hematocrit (Hct) and cell hydrodynamic migration away from the wall. RBCs mechanical properties determine cell deformation under shear forces, cell hydrodynamic responses, CFL thickness and vascular wall shear stress (WSS).7 Reduced vascular WSS compromises NO production via vascular endothelial NO synthase (eNOS) and leads to vasoconstriction, inflammation and tissue hypoperfusion.8
Previous studies have reported changes on blood rheology and oxygen (O2) affinity after storage.9-10 Our hypothesis is that the RBC storage affects cell mechanical properties and blood rheology compromising blood hemodynamics, O2 delivery and the interaction between flowing blood and the vasculature. This experimental study aims to evaluate the in vivo effects of stored RBCs transfusion in the microcirculation using intravital microscopy combined with high-speed video recording of the rat cremaster muscle preparation. In vivo observations were correlated with in vitro characterization of the RBCs mechanical and O2 transport properties. Efforts to translate results during storage obtained with rat cells to human cells are already reported, in terms of cellular biochemical and functional alterations.11 When rat RBCs are stored in CPDA-1, they have reduced survival after 15 days of storage; therefore, we selected 14 days of storage for our study. Our results confirmed previous reported in vitro changes of RBCs rheology and O affinity upon storage.9-11 2 Microvascular hemodynamics, including: volumetric flow rate, velocity profiles analysis and cell hydrodynamic behavior; and systemic parameters were monitored before and after transfusion with stored or fresh red blood cells in anemic (30% Hct) rats.
MATERIALS AND METHODS
Please see supplemental material for expanded methods. Blood collection and storage
Experiments were approved by the University of California San Diego, Institutional Animal Care and Use Committee, and conducted accordingly to the Guide for the Care and Use of Laboratory Animals (US National Research Council, 2010). Blood was collected from rats into syringes containing citrate, phosphate, dextrose adenine solution (CPDA-1). Blood was leukoreduced, using leukocyte reduction filter Purecell NEO (Pall Company, East Hills, NY). Packed cell were kept at 4°C for 14 days until use. Fresh blood was collected with the same procedure and used within 1 hr after leukoreduction.
Animal model and intravital microscopy
Studies were performed in Sprague-Dawley rats. Experiments were approved by the University of California San Diego, Institutional Animal Care and Use Committee, and conducted accordingly to the Guide for the Care and Use of Laboratory Animals (US National Research Council, 2010). Microcirculation observations were completed using the cremaster muscle model. The complete surgical preparation is described in detail elsewhere.12 The intravital microscope (Olympus-BX51WI) equipped with a 40X objective (LUMPFL, NA 0.8; Olympus) and matching custom long working distance condenser (Thorlabs, Newton, NJ) was used to visualize the rat's cremaster.
Experimental Protocol
An isovolemic hemodilution to 30% Hct induced anemic conditions. Anemic animals were divided into two experimental groups, and exchange-transfused by 50% of their estimated blood volume with fresh or stored blood adjusted to 30% Hct. Systemic and microhemodynamic measurements were obtained at baseline (BL), after hemodilution (HD) and at 5 and 60 min after the exchange transfusion.
RESULTS
RBCs mechanical properties and O2 affinity
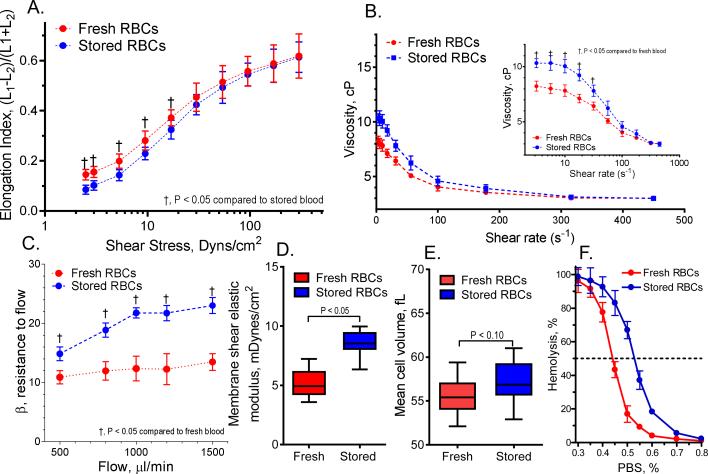
Fresh and stored cell presented normal biconcave shape. Deformability of cells stored for 14 days decreased compared to fresh cells. Summary of the mechanical and morphological changes are presented in Figure 1. Elongation index (EI): Ektacytometry results show reduced cell deformability for stored cells at low shear stress (<20 dyn/cm2) but no significant differences were found for high shear stresses (Figure 1A). Viscosity: For 30% Hct blood, viscosity at low shear rates (<30 s-1) increased after storage compared with fresh cells (Figure 1B). However, the relationship between viscosity and shear rate was similar for stored and fresh cells (shear thinning-like). Resistance to filtration: Filtration pressure drop increased at all flow rates for stored cells compared with fresh cells (Figure 1C), however, it did not increase proportional to the flow rate. Hemolysis was observed for stored cells during filtration test, and the amount of hemolysis (acellular Hb in the suspending fluid) increased with the flow rate applied. Fresh cell presented limited to undetectable hemolysis, and only slightly pink suspending media was observed at high flow rate. Membrane elastic modulus: Membrane mechanics were studied using the micropipette aspiration. After storage a large population of cells increased their membrane elastic modulus compared with fresh cells (Figure 1D). Fresh cells remained intact during the aspiration measurement, whereas some (less than 20%) of the stored cells could not be measured due to membrane rupturing upon aspiration. Static cell elastic modulus increased by 70% for stored compared to fresh cells. Poorly deformable stored cells had different stress-strain relaxation times compared with fresh cells. Osmotic fragility: Stored cells were less resistant to osmotic stress compared with fresh cells. While 50% of the stored cells lysed at 0.52±0.06% PBS concentration, fresh cells were more resilient, lysing at 0.44±0.05% (Figure 1E). The higher osmotic fragility of the stored cell is likely due to their higher surface to volume ratio. Mean corpuscular volume (MCV): Stored cells increased their MCV by 5% compared with fresh cells. A small population of cells increased their MCV by 15% and a third population of cells had no differences compared with fresh cells (Figure 1F). Oxygen affinity: Oxygen tension to attain 50% oxygen saturation of Hb (P50) for stored cells decreased to 24.1±2.7 mmHg compared with fresh cells, which P50 was 39.4±1.3 mmHg. Normal P50 from rat RBCs without handling or manipulation is (40.4±1.6 mmHg). Aggregability: Cell aggregability increased by 32% for stored cells compared with fresh cells.
Figure 1. Mechanical, morphological, and fragility changes of stored cells.
A. Changes in red cell elongation index (EI) between fresh and stored cells, measured based on the length and width of the diffraction pattern, as (L1−L2)/(L1+L2), where L1 and L2 are the length and width of the diffraction pattern. B. Viscosity of red cells suspension at 30% Hct. C. Cell deformability measured using the resistance of red cells to pass by pore, normalized by the cell concentration and cell/pore volume, called β factor.38 D. Red cell membrane shear elastic modulus based on the relationship pressure and aspiration length.16 E. Cell volume measure using the cell aspiration technique. F. Red cells osmotic fragility and osmotic fragility index.
In vivo Experiments
Exchange transfusion
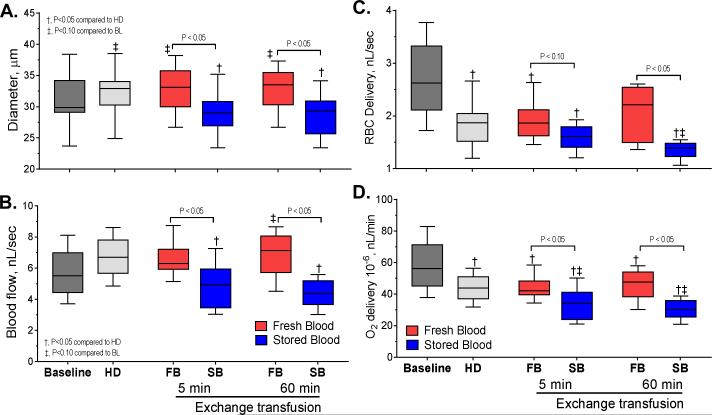
Fourteen animals were exchange transfused with fresh cells (n=7) or stored cells (n=7). Similarities between groups at baseline and hemodilution was statistically verified between groups (P>0.30). Systemic hemodynamics and blood gas parameters: MAP, HR, Hct, Hb and blood gas parameters are summarized in Table 1. Hemodilution decreased Hct to 30% and the exchange transfusion with fresh and stored RBCs preserved the Hct. However, to preserve such Hct in the stored RBC group over one hour, small infusions of packed stored cells (3.5±1.2% of the animal's BV) were needed. The MAP and HR were not significantly affected by the hemodilution or the exchange transfusion. Exchange transfusion with stored cells increased arterial PO2 and decreased arterial PCO2 compared to baseline, hemodilution and exchange transfusion (60 min) with fresh cells. The arterial blood pH was higher in animals exchanged with stored cells compared with fresh cells reflecting the respiratory changes.
Table 1.
Blood pressure and blood gas parameters
| Exchange | |||||
|---|---|---|---|---|---|
| Parameter | Baseline | HD | Group | 5 min | 60 min |
| Hct, % | 47.0 ± 0.5 | 30.0 ± 0.2† | Fresh | 30 ± 1† | 30 ± 1† |
| Stored | 30 ± 1† | 30 ± 1† | |||
| Hb, g/dL | 15 ± 0.2 | 9.5 ± 0.1† | Fresh | 9.6 ± 0.1† | |
| Stored | 9.6 ± 0.1† | ||||
| MAP, mmHg | 104 ± 12 | 98 ± 7 | Fresh | 103 ± 3 | 104 ± 4 |
| Stored | 102 ± 3 | 103 ± 3 | |||
| HR, bpm | 369 ± 7 | 359 ± 10 | Fresh | 357 ± 12 | 352 ± 15 |
| Stored | 380 ± 6 | 373 ± 5 | |||
| pO2, mmHg | 82 ± 3 | 85 ± 3 | Fresh | 89 ± 3 | |
| Stored | 97 ± 4†‡ | ||||
| pCO2, mmHg | 38 ± 1 | 37 ± 2 | Fresh | 39 ± 2 | |
| Stored | 34 ± 2†‡ | ||||
| pH | 7.43 ± 0.01 | 7.46 ± 0.01 | Fresh | 7.44 ± 0.02‡ | |
| Stored | 7.47 ± 0.01 | ||||
Values are means ± SD. Baseline and hemodilution (HD) included all the animals in the study. No significant differences were detected at baseline and HD between groups. Fresh, animals exchange transfused with fresh cells; Stored, animals exchange transfused with stored cells (14 days). Hct, systemic hematocrit; Hb, hemoglobin; MAP, mean arterial blood pressure; HR, heart rate; PO2, arterial partial O2 pressure; PCO2, arterial partial pressure of CO2.
P<0.05 compared to baseline
P<0.05 compared to HD.
Hemodynamics
Arterioles ranging between 24 and 38 μm were studied. Diameter and mean blood flow are presented in Figure 2A and 2B. Diameter increased after hemodilution compared with baseline. Exchange with fresh cells preserved the vasodilatation, while exchange with stored cells produced significant vasoconstriction compared with baseline, hemodilution and fresh cells. Mean blood flow increased after hemodilution and exchange transfusion with fresh cells preserved the increased blood flow. However, animals exchanged with stored cells had significant lower flows compared with baseline, hemodilution and exchange with fresh cells. The changes in blood flow affected RBC delivery; results are presented in Figure 2C. RBC delivery decreased after hemodilution, and was not affected by exchange with fresh cells. On the other hand, exchange with stored cells decreased RBC delivery compared with hemodilution and fresh cells. Oxygen delivery at the microcirculation is presented in Figure 2D. Oxygen delivery decreased after hemodilution compared to baseline. Exchange with fresh cells preserved the oxygen delivery attained after hemodilution, while stored cells decreased it. Pseudo-WSR was not affected by the experimental procedure. Pseudo-WSS decreased after hemodilution, it was not different between animals exchanged with fresh or stored cells.
Figure 2. Hemodynamics, oxygenation, and hemorheological changes produced by transfusion of stored cells.
A. changes in arteriolar diameter at baseline (n=72), hemodilution (n=72) and after exchange transfusion (n=36) with fresh and stored red cells (n=36). B. Changes in blood flow at baseline, hemodilution and after exchange transfusion with fresh and stored red cells. C. Changes in red cell delivery at baseline, hemodilution and after exchange transfusion with fresh and stored red cells. D. Changes in oxygen delivery at baseline, hemodilution and after exchange transfusion with fresh and stored red cells.
RBC-endothelium interaction
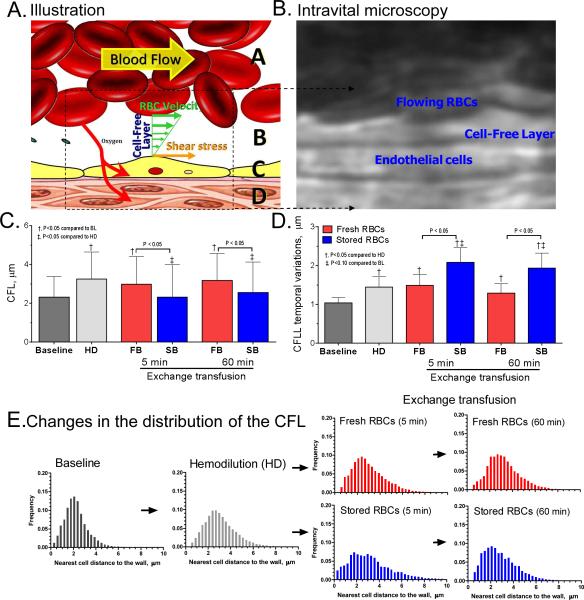
The changes in the CFL thickness are presented in Figure 3. Schematic representation of the CFL and microphotographs example of the images used to measured the CFL thickness are shown in Figure 3A and 3B. Mean CFL thickness increased after hemodilution (Figure 3C), resulting from the increased plasma to cells volume ration, which also increased the CFL temporal variations (Figure 3D). Transfusion of stored cells decreased the mean thickness of the CFL and increased the CFL temporal variations compared to fresh cells and hemodilution at the same Hct. The Hct, the cell migration away from the wall and the cell-to-cell interaction determine the distribution of the CFL thickness, as presented in Figure 3E. A decrease in Hct increases CFL thickness by reducing the volume occupied by red cells, allowing for greater axial migration and cell mobility. However, the mean thickness of the CFL after exchange with stored cells was not different from baseline, even though the Hct after the exchange with stored cells was only 64% of baseline. Exchange with stored cells increased the frequency of observation of RBCs near the wall compared to exchange with fresh cells. Stored cells appear to be unevenly distributed across the blood vessel, thus they reduce the mean CFL thickness. Over time, the frequency distribution of the CFL measurements for animals transfused with stored cells resembled the distribution measured for hemodilution and exchange with fresh cells. It is possible that some of the cells invading the CFL after the exchange with stored cells readapted to the physiological environment, or were trapped and eliminated post transfusion.
Figure 3. Cell interaction with the vessel wall changes after transfusion of stored cells.
A. Schematic illustration of the cell free layer (CFL). The yellow arrow indicates the direction of blood flow, green arrows velocity and the wall shear stress on endothelial cell. Zones marked as A is RBC column, B is the CFL, C is the endothelial cells and D is the tissue space. B. Intravital image example of the video sequences used to measure the CFL dimension. C. Changes in the magnitude of the CFL at baseline, hemodilution and after exchange transfusion with fresh and stored red cells. D. Changes in the temporal variation of the CFL (defined as the standard deviation of the CFL measurements in over 0.8 sec) at baseline, hemodilution and after exchange transfusion with fresh and stored red cells. E. Frequency distribution of the magnitude of the CFL at baseline (n= 115200), hemodilution (n= 115200) and after exchange transfusion (n=57600) with fresh and stored red cells (n=57600).
Microhemodynamics
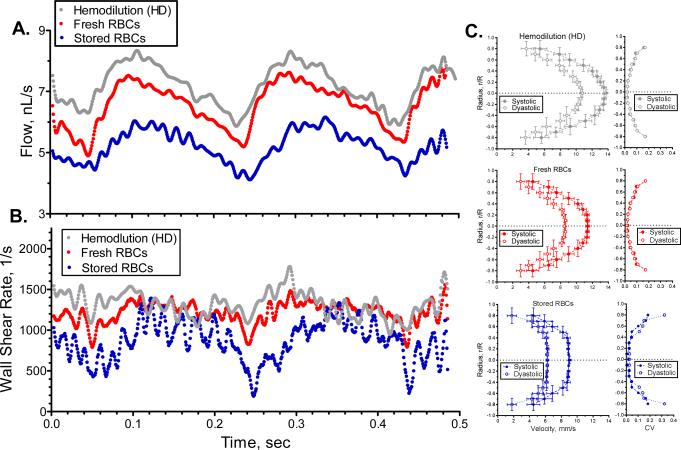
Instantaneous flow and wall shear rate are presented in Figure 4A and 4B. At baseline and after hemodilution, blood flow showed a normal pulsatile behavior, which was preserved after exchange with fresh or stored cells. Frequency analysis of the pulsatile flow shows the principal frequency component to be a function of the heart rate (~6 Hz) and its harmonics up to 30 Hz. Additional higher frequency components (50 to 120 Hz) were found in both groups after exchange with stored cells. Analysis at this temporal an special resolution allowed for the measurement of blood velocity, blood flow and shear rate over time, in addition to velocity profiles during systolic and diastolic phases Figure 4C. Temporal changes in WSR indicated that the high frequency variability in blood flow was due to rapid hemodynamic fluctuation near the vessels wall, as the variation in mean flow observed after transfusion with stored cells translated into large variability of WSR. Hemodilution and transfusion with fresh cells also showed variability on WSR, however it was significantly lower. Hemodilution showed a parabolic velocity profile (systolic, k = 2.1 ± 0.1; diastolic, k = 2.2 ± 0.1). Transfusion of fresh cells showed a velocity profile similar to hemodilution, (systolic, k=2.0 ± 0.1; diastolic, k = 2.2 ± 0.2). Transfusion of stored cells showed significantly blunted velocity profiles (systolic, k=2.8 ± 0.2; diastolic, k = 3.1 ± 0.3) compared to hemodilution or transfusion of fresh cells. Coefficient of variation (CV, SD/mean) for the velocity profiles are presented in Figure 4C. After hemodilution, the CV of the velocity profile increased near the walls to a max of 0.23 ± 0.06 and after transfusion of fresh cells the max CV was 0.25 ± 0.08. However, after transfusion of stored cell the max CV increased to 0.38 ± 0.10.
Figure 4. Microhemodynamics perturbation produced by transfusion of stored cells.
A. Changes in blood flow over time for hemodilution and after exchange transfusion with fresh and stored red cells. Low frequency filter (<125 Hz) was applied to reduce data variability. B. Changes in wall shear rate over time for hemodilution and after exchange transfusion with fresh and stored red cells. C. Velocity profiles during systolic and diastolic periods for hemodilution and after exchange transfusion with fresh and stored red cells.
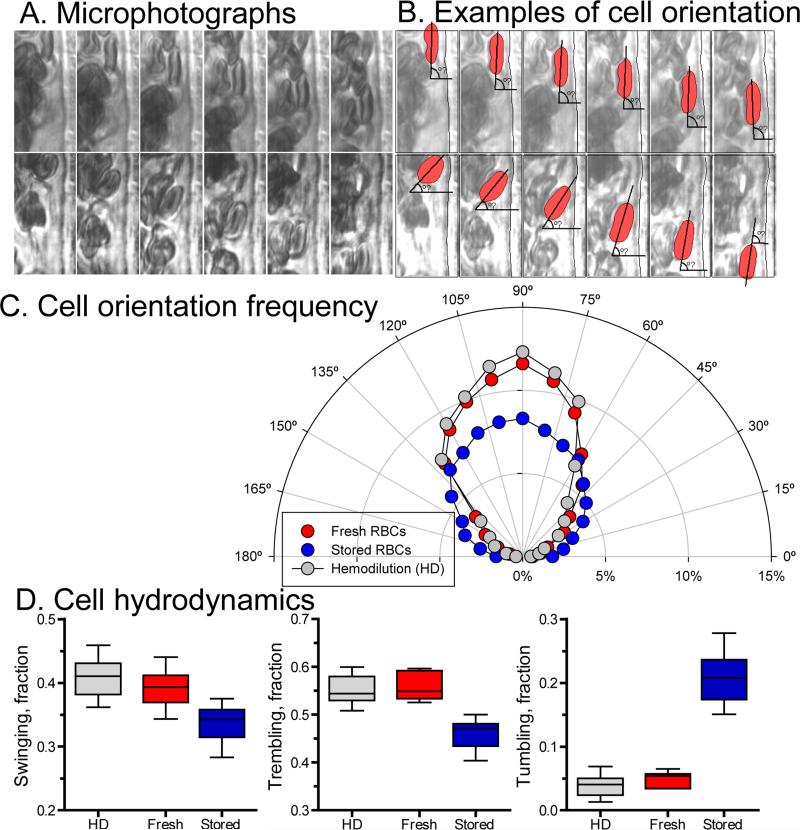
Cell orientation and hydrodynamics
Analysis of the cell major axis angle to vessel wall and cell dynamics are presented in Figure 5. Sample microphotographs and a schematic representation of the cells orientation are shown in Figure 5A and 5B. Orientation of cells near the wall shows a preferential orientation parallel to the vessel wall (90°, corresponding to trembling behavior of the cells) after hemodilution and exchange with fresh cells (Figure 5C). However, after exchange with stored cells fewer cells are flowing parallel to the wall. In addition, frequency of observation of cells flowing near perpendicularly to the vessel wall (corresponding to tumbling behavior) increased compared with hemodilution and transfusion with fresh cells. During hemodilution the majority of the cells were trembling and swinging, with very few cells tumbling near the vessel wall (Figure 5C and D). Similar distribution of cell dynamics was observed after transfusion of fresh cells. However, transfusion with stored cells the numbers of cells trembling and swinging decreased relative to hemodilution and transfusion with fresh cells, as well as the number of tumbling cells near the vessel wall increased. The abnormal variability in velocity, WSR and CFL thickness after transfusion with stored cells appears to be related to abnormal red cell hydrodynamics behavior near the vessel wall. Thus, the implications of stored cells mechanical properties alterations are manifested in vivo in terms of microhemodyanmic aberrations due to abnormal cell hydrodynamic behavior.
Figure 5. In vivo cell hydrodynamic changes after transfusion of stored cells.
A. Microphotographs examples obtained from video recordings used to analyzed cell orientation relative to the vessel wall. B. Overlap schematic illustration of the cells analyzed for cell hydrodynamic near the vessels wall and the relative angle of the major axis to the wall. C. Cell orientation frequency distribution for hemodilution (n = 12672 frames) and after exchange transfusion with fresh (n = 5760) and stored red cells (n = 7080). D. Cell hydrodynamics based on changes in orientation relative to the wall over the segment analyzed for hemodilution (n = 132 cells) and after exchange transfusion with fresh (n = 108) and stored red cells (n = 126). Cell hydrodynamics was defined based on major axis angle changes, where for tumbling cells major axis angle covered the range between 0° to 40°, 150° to 180°, or crosses the plane across the vessel; for trembling cells, their major axis angle changed within the range 50° to 130°; and for swinging cells, their major axis angle changed within the range 80° to 100° only.
DISCUSSION
Experimental studies related to transfusion medicine may have limited clinical implications, however they allow for exploring variables impossible to be tested in a clinical setting. The principal finding of this study is that exchange transfusion with stored RBCs produced microcirculatory vasoconstriction resulting in decreased blood flow and oxygen delivery, and affected microcirculation homeostasis compared to anemia alone or transfusion with fresh cells. Concomitant essential regulators of microvascular function, including CFL thickness, WSR and WSS were also affected by stored cells transfusion. The disruption of microcirculatory homeostasis by the presence of stored cells appears to be physically related to the changes in the mechanical properties the cells, which affect cell deformability and hydrodynamics under shear flow. These results are essential to understand the underlying mechanisms which originate acute problems associated with transfusion of stored RBCs and to define strategies to improve transfusion success. Fresh cells were intrinsically beneficial than stored cells at the microcirculatory level, although ex vivo manipulation of the cells may have introduced minor functional changes to the fresh cells.
Blood transfusions are called to preserve or restore systemic oxygen carrying capacity; however, the real goal is to preserve oxygen deliver to tissues, which requires the correct operation of the microcirculation. Therefore, transfusions major limitation is to achieve its intended objective without compromising microvascular function. Our results challenge whether transfusion of stored cells may create a secondary problem by generating nonuniform and irregular distribution of WSR and WSS and possibly leading to vascular inflammation and thrombosis.13 Although transfusion of stored cells may increase blood viscosity, the reduced and abnormal WSR prevented WSS from inducing vasodilation via endothelial mechanotransduction. Moreover, our results show that stored RBCs have a shorter circulating lifetime, as they appear to be trapped and removed from flowing blood due to their impaired elastic and hydrodynamic behavior. Understanding of the effects of disturbed flow by stored cells can provide insights into the pathogenesis of vascular diseases, and help to elucidate the phenotypic and functional differences between quiescent and activated vascular endothelium post transfusion.
Blood is a mixture of cells and plasma, RBCs comprising the largest fraction by volume. Thus, the mechanical properties of these small semisolid particles determine macro and micro-rheology behavior of blood. RBC mechanical changes are tightly related to the behavior of circulating blood, as the non-Newtonian behavior of blood becomes most evident at low shear rates, in arterioles were CFL reduces blood effective viscosity, and in capillaries where the cells squeeze through.14 Stored cells aggregability and rheology results are consistent with previous studies.15, 24 Ektacytometry showed shear dependent changes in elongation indexes between stored and fresh cells, with decreased elongation index for stored cells at low shear rates.16-17 Stress strain characteristic of red cells (EI Vs. shear stress) measured by laser diffraction ektacytometry patterns resembles a linear function when plotted in a semi-logarithmic plot for shear stress between 10 and 100 dyns/cm2. Previous studies showed that ektacytometry using a high viscosity suspending solution prevents artifacts in the laser diffraction patterns at low shear rate, and allows for identification of small deformations and cell orientation at low shear.18 Our ektacytometry system uses cell suspending solution with a viscosity of 19.6 cP (at 10 s-1) to prevent artifacts at low shear rate, thus stored cell ektacytometry data suggests differences in cell orientation and small deformation compared to fresh cells. Additionally, blood rheology at low shear rates also reflected changes in cell orientations and cell aggregation for stored cell relative to fresh cells. Cell storage increased filtration resistance, resulting from reduced deformability. Ektacytometry, rheology and filterability confirm a diminished cell deformability after storage independent of the moderate increase in cell volume (macrocytosis).19
Extrinsic factors like cell volume appear to be secondary compared to inherent factors like membrane elastic properties (relationship between membrane tension and aspirated length) after storage17,20. Stored cell mechanical changes appear to be from membrane and cytoskeleton alteration, since cytoplasm has negligible effects in membrane elastic properties.17,20 The cell aspiration results suggest changes on lipid-protein interactions after storage.21 As the cytoskeleton framework is regulated based on spectrin heads sliding on actin fibers, intracellular calcium, magnesium, ATP, pH and water content may be responsible for cytoskeleton and membrane composite structural changes.22 A second option to explain the difference in rigidity between fresh and stored cells is skeletal protein cross-linking as suggested by others before.21 Our previous studies using rigid RBCs (glutaraldehyde cross-linked whole cells) showed that reduced cell deformability limits blood flow and tissue oxygenation.23 Glutaraldehyde cell modification increased both cytoplasmic viscosity and membrane elastic modulus, although the net effect may be similar to storage induced changes.24 Future studies need to should investigate the nature of the membrane mechanical properties due to storage.
Systemic parameters showed no significant differences between transfusions with fresh or stored cells, while at the microvascular levels stored cell produced vasoconstriction, reduced blood flow and oxygen maldistribution. Our previous studies with stored cells focused on the relation between systemic and microvascular oxygen delivery, showing that 28 days stored cells compromised oxygen delivery and reduced functional capillary density.25 Other studies have presented similar results in septic and nonseptic rats, where stored cells failed to improve oxygenation.26-27 Stored cells had problems, transporting and delivering oxygen, arising from modified oxygen uptake (2,3 DPG depletion), reduced flexibility and vasoconstriction.
The present study focused on microhemodynamic changes, examining the underlying reason for the microvascular malfunction induced by stored cells. Stored cells decreased the CFL thickness and increased its temporal variations, which corresponds to changes in the zone of where flowing blood interacts with the endothelium. Changes in CFL thickness affect blood rheology, gas transfer at the microcirculation, and transport of chemical transmitters present in the blood stream.28 The CFL dynamics and the cell hydrodynamics near the vessel wall determine the WSR and WSS. In microvessels, WSS tightly is regulated to prevent strong variations, as vascular endothelial cell mechanotransduction mediates the production of autacoids, including NO via endothelial NO synthase.8 Transfusion of stored cells reduced the CFL thickness and WSS, negatively affecting the balance between WSS mediated NO production and NO consumption by the Hb in the RBC, which can explain the observed vasoconstriction. Erythrocytes manipulation and storage increase hemolysis and the formation of microparticles, both increase the NO scavenging by circulating blood when stored cells are infused, impairing vascular function.29 Our laboratory have shown low levels of acellular Hb disrupt perivascular NO levels, via NO dioxygenation reactions (NOD).30 Circlating microparticles form stored cells have an increased NO uptake compared to RBC, compromised NO bioavailability, and produce vasoconstriction.29,31 Therefore, transfusion of stored cell disrupts the balance between NO formation and NO scavenging, via: 1) altering the mechanical signal (WSS) on the vascular endothelium that determine NO production; 2) decreasing the thickness of the CFL increasing NO uptake by RBC, and increasing NOD reactions with acellular Hb and microparticles. NO unbalance can increase the risk of multiorgan dysfunction or hemostatic activation after transfusion of stored cell.
Image velocimetry provided continuous assessment of blood flow and its pulsatile nature. The small variations at higher frequencies resulted from pressure wave propagation, red cell axial migrations and red cell dynamics. Using an empirical power law model to interpret the measured velocity profile, transfusion of fresh cells redistributed viscous and inertial forces to the periphery of the vessel core producing near parabolic velocity profiles.32 Thus, the velocity gradient between the vessel core and the vessel wall drags and deform the cells. However, when stored cells are present in the circulation, their rigid nature makes them unable to deform according to the shear flow regime, and cells at the border of the core invade the CFL creating a pseudo-turbulent flow near the wall. Cells from the faster moving region move to the slower zone transferring momentum to the plasma and other cells, thus maintaining the cells near the wall in motion. The coefficient of variation (CV) of the velocity profiles indicates an increase in the scatter of longitudinal velocities near the wall after transfusion of stored cells. The irregular cell motion near the wall and the longitudinal velocity fluctuation produced radial variations in velocity and higher energy dissipation.
Two primarily actions were observed for fresh red cells near the wall, swing and tremble, as their membrane and interior undergo fluctuations to maintain an orientation within the flow; whereas stored cells tumble, as they flip like rigid bodies. Computational studies have shown the importance of cell deformability in the transition from cells oriented with flow to cells tumbling.33 This observation is consistent with the hydrodynamic theory of a rigid particle motion in shear flow, where the rate of tumbling is proportional to the shear rate.34 Cell size can also be a factor, as increased cell volume and a transformation to a more spherical shape decreases the resistance to change orientation with flow. At higher Hct, the tumbling motion may not be as prominent as during anemia due to cell-to-cell interactions. The tumbling motion of stored cells near the vessel wall increased the temporal variations of the CFL and the longitudinal velocity, which perturbed the WSR, the plasma flow within the CFL, and the transport of gasses and autacoids carried by the blood to the tissues. Plasma flow alterations near the wall promote the deposition of lipids and proteins on the vessel wall and activate the expression of adhesive molecules on endothelial, platelets and leukocytes.13,35-36 At the endothelial cell level, alterations in fluid shear flow lead to significant changes in signal transduction and consequently to functional and morphological changes like the formation of lesions.13,35 Once developed, these lesions can further disrupt blood flow, establishing a potential hazardous cycle that accelerates atherogenesis, and platelet activation in organism with an already compromised cardiovascular system13,40.
Limitations
All the cell deformability assays used in this study differ in sensitivity and specificity; they also provide insight into various aspects of the biomechanics of cells. Laser diffraction ektacytometry does not provide information about single cell behavior; it reflects the response of the population of cells in response to applied laminar fluid shear stress.18 The transfusion protocol used in this study does not mimic clinical conditions; we aimed to preserve systemic Hct during the observation period, as Hct is the principal determinant of blood macro and micro rheology. Our experimental design provided a more mechanistic analysis of the effects of stored cells, without excessively depleting oxygen carrying capacity and preserving normovolemia via an isovolemic exchange transfusion. The only factors creating the difference between fresh and stored cells resulted from the storage lesions. Previous studies have described biochemical and functional alterations for rat cells, in an attempt to translate the results obtained with rat cells to human cells during storage.11 Rat RBCs stored in CPDA-1 were more fragile after 29 days of storage compared to human cells. Although, rat cells stored rat red cells have reduced survival after 15 days of storage and their viability was reduced compared to human cells, rat cells were able to regenerate ATP, but not 2,3 DPG.11 Future studies should aim to consider species differences in cell structure and metabolism, and the physiological and adaptive differences between species.
In conclusion, exchange transfusion with fresh and stored cells in an anemic state provides a model to compare changes induced by cell mechanical alteration. Results from the fresh cells suggest that cell manipulation has minor effects in hemodynamic parameters insignificant compared to the alterations induced stored cells. Stored RBC mechanical properties were found to be greatly affected by storage, a finding consistent with those reported earlier.4,7,11 Decreased deformability correlates with reduced cell survival upon transfusion, as stored cells supplementation was required to preserve the Hct. In real clinical scenarios, patient with impaired cardiovascular function may worsen the situation that first triggered the need for a transfusion. Moreover, changes in RBC fragility suggest that hemolysis is likely to occur with stored cells, which can scavenge NO and increase the formation free radicals.37 Changes in WSS and NO depletion may impair cardiovascular homeostasis post transfusion of stored cells. The consequences of stored blood transfusion may be more sensitive in individuals with cardiovascular preexistent conditions, since stored blood can disrupts the already narrowed homeostasis, acting as a secondary injury. We hope our results motivate more mechanistic investigations to understand the underlying process that prevent transfusions form achieving its intended goal.
Supplementary Material
ACKNOWLEDGMENTS
This investigation was supported by the following grants: NIH R01 HL52684, R01 HL064395 and R01 HL062318; and ARMY: W81XWH-11-2-0012. Authors thank to Dr. Qiang Zhu (Structural engineering, UCSD) for his expertice and support in analysis of red cell mechanics. Authors also thank Tyler Watson, Brandon Senne and Constance Ardila for their help analysis the video recordings.
Footnotes
There is not potential conflict of interest, real or perceived by the authors.
Authors Contribution
O.Y. performed the experiments, analyzed the data and wrote manuscript. D.O-V analyzed the data. A.G.T. analyzed the data and wrote the manuscript. P.C.J. analyzed the data and wrote the manuscript; and P.C. performed the experiments, analyzed the data and wrote manuscript.
Disclosures
The authors state no conflict of interest to disclose.
LITERATURE CITED
- 1.2009 National Blood Collection and Utilization Survey Report . Services RotUDoHaH. US Department of Health and Human Services, the Assistant Secretary for Health; Washington, DC: 2011. [Google Scholar]
- 2.Sullivan MT, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47:385–94. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 3.Hayes MA, Timmins AC, Yau E, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. New England Journal of Medicine. 1994;330:1717–22. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 4.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM. Evolution of adverse changes in stored RBCs. Proceedings of the National Academy of Sciences. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, Sireci AN, Stephens HL, Stotler BA, Wojczyk BS, Zimring JC, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–82. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cokelet GR, Goldsmith HL. Decreased hydrodynamic resistance in the two-phase flow of blood through small vertical tubes at low flow rates. Circ Res. 1991;68:1–17. doi: 10.1161/01.res.68.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Johnson PC, Popel AS. Effects of erythrocyte deformability and aggregation on the cell free layer and apparent viscosity of microscopic blood flows. Microvascular research. 2009;77:265–72. doi: 10.1016/j.mvr.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai AG, Acero C, Nance PR, Cabrales P, Frangos JA, Buerk DG, Intaglietta M. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005;288:H1730–9. doi: 10.1152/ajpheart.00998.2004. [DOI] [PubMed] [Google Scholar]
- 9.Berezina TL, Zaets SB, Morgan C, Spillert CR, Kamiyama M, Spolarics Z, Deitch EA, Machiedo GW. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 10.Huruta RR, Barjas-Castro ML, Saad ST, Costa FF, Fontes A, Barbosa LC, Cesar CL. Mechanical properties of stored red blood cells using optical tweezers. Blood. 1998;92:2975–7. [PubMed] [Google Scholar]
- 11.d'Almeida MS, Jagger J, Duggan M, White M, Ellis C, Chin-Yee IH. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfus Med. 2000;10:291–303. doi: 10.1046/j.1365-3148.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- 12.Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 1973;5:384–94. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- 13.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiological Reviews. 2011;91:327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baskurt OgK, Neu B, Meiselman HJ. Red blood cell aggregation. CRC Press; Boca Raton: 2012. [Google Scholar]
- 15.Henkelman S, Dijkstra Tiekstra MJ, Wildt Eggen D, Graaff R, Rakhorst G, Van Oeveren W. Is red blood cell rheology preserved during routine blood bank storage? Transfusion. 2009;50:941–8. doi: 10.1111/j.1537-2995.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- 16.Evans EA. Bending elastic modulus of red blood cell membrane derived from buckling instability in micropipet aspiration tests. Biophys J. 1983;43:27–30. doi: 10.1016/S0006-3495(83)84319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochmuth R, Waugh R. Erythrocyte membrane elasticity and viscosity. Annual review of physiology. 1987;49:209–19. doi: 10.1146/annurev.ph.49.030187.001233. [DOI] [PubMed] [Google Scholar]
- 18.Mohandas N, Clark MR, Jacobs MS, Shohet SB. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980;66:563–73. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco RS. The measurement and importance of red cell survival. American journal of hematology. 2009;84:109–14. doi: 10.1002/ajh.21298. [DOI] [PubMed] [Google Scholar]
- 20.Waugh R, Evans EA. Thermoelasticity of red blood cell membrane. Biophys J. 1979;26:115–31. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park YK, Best CA, Auth T, Gov NS, Safran SA, Popescu G, Suresh S, Feld MS. Metabolic remodeling of the human red blood cell membrane. Proceedings of the National Academy of Sciences. 2010;107:1289–94. doi: 10.1073/pnas.0910785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu H, Puchulu-Campanella E, Galan JA, Tao WA, Low PS, Hoffman JF. Identification of cytoskeletal elements enclosing the ATP pools that fuel human red blood cell membrane cation pumps. Proceedings of the National Academy of Sciences. 2012;109:12794–9. doi: 10.1073/pnas.1209014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabrales P. Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293:H1206–H15. doi: 10.1152/ajpheart.00109.2007. [DOI] [PubMed] [Google Scholar]
- 24.Mohandas N, Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annual review of biophysics and biomolecular structure. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- 25.Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626–34. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald RD, Martin CM, Dietz GE, Doig GS, Potter RF, Sibbald WJ. Transfusing red blood cells stored in citrate phosphate dextrose adenine-1 for 28 days fails to improve tissue oxygenation in rats. Critical care medicine. 1997;25:726–32. doi: 10.1097/00003246-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Raat NJ, Verhoeven AJ, Mik EG, Gouwerok CW, Verhaar R, Goedhart PT, De Korte D, Ince C. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model*. Critical care medicine. 2005;33:39–45. doi: 10.1097/01.ccm.0000150655.75519.02. [DOI] [PubMed] [Google Scholar]
- 28.Vandrangi P, Sosa M, Shyy JYJ, Rodgers VGJ. Flow-Dependent Mass Transfer May Trigger Endothelial Signaling Cascades. PloS one. 2012;7:e35260. doi: 10.1371/journal.pone.0035260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108:3603–10. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azarov I, Liu C, Reynolds H, Tsekouras Z, Lee JS, Gladwin MT, Kim-Shapiro DB. Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vesicles. J Biol Chem. 2011;286:33567–79. doi: 10.1074/jbc.M111.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pries A, Secomb T, Gaehtgens P. Biophysical aspects of blood flow in the microvasculature. Cardiovascular research. 1996;32:654–67. [PubMed] [Google Scholar]
- 33.Sui Y, Chew Y, Roy P, Cheng Y, Low H. Dynamic motion of red blood cells in simple shear flow. Physics of Fluids. 2008;20:112106. [Google Scholar]
- 34.Maxey MR, Riley JJ. Equation of motion for a small rigid sphere in a nonuniform flow. Physics of Fluids. 1983;26:883. [Google Scholar]
- 35.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61. [PubMed] [Google Scholar]
- 36.Li YSJ, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. Journal of biomechanics. 2005;38:1949–71. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skalak R, Impelluso T, Schmalzer EA, Chien S. Theoretical modeling of filtration of blood cell suspensions. Biorheology. 1983;20:41–56. doi: 10.3233/bir-1983-20104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.