Abstract
Background
Men with germline breast cancer 1, early onset (BRCA1) or breast cancer 2, early onset (BRCA2) gene mutations have a higher risk of developing prostate cancer (PCa) than noncarriers. IMPACT (Identification of Men with a genetic predisposition to ProstAte Cancer: Targeted screening in BRCA1/2 mutation carriers and controls) is an international consortium of 62 centres in 20 countries evaluating the use of targeted PCa screening in men with BRCA1/2 mutations.
Objective
To report the first year's screening results for all men at enrolment in the study.
Design, setting and participants
We recruited men aged 40–69 yr with germline BRCA1/2 mutations and a control group of men who have tested negative for a pathogenic BRCA1 or BRCA2 mutation known to be present in their families. All men underwent prostate-specific antigen (PSA) testing at enrolment, and those men with PSA >3 ng/ml were offered prostate biopsy.
Outcome measurements and statistical analysis
PSA levels, PCa incidence, and tumour characteristics were evaluated. The Fisher exact test was used to compare the number of PCa cases among groups and the differences among disease types.
Results and limitations
We recruited 2481 men (791 BRCA1 carriers, 531 BRCA1 controls; 731 BRCA2 carriers, 428 BRCA2 controls). A total of 199 men (8%) presented with PSA >3.0 ng/ml, 162 biopsies were performed, and 59 PCas were diagnosed (18 BRCA1 carriers, 10 BRCA1 controls; 24 BRCA2 carriers, 7 BRCA2 controls); 66% of the tumours were classified as intermediate- or high-risk disease. The positive predictive value (PPV) for biopsy using a PSA threshold of 3.0 ng/ml in BRCA2 mutation carriers was 48%—double the PPV reported in population screening studies. A significant difference in detecting intermediate- or high-risk disease was observed in BRCA2 carriers. Ninety-five percent of the men were white, thus the results cannot be generalised to all ethnic groups.
Conclusions
The IMPACT screening network will be useful for targeted PCa screening studies in men with germline genetic risk variants as they are discovered. These preliminary results support the use of targeted PSA screening based on BRCA genotype and show that this screening yields a high proportion of aggressive disease.
Patient summary
In this report, we demonstrate that germline genetic markers can be used to identify men at higher risk of prostate cancer. Targeting screening at these men resulted in the identification of tumours that were more likely to require treatment.
Keywords: BRCA1, BRCA2, Prostate cancer, Prostate-specific antigen, Targeted screening
Take Home Message
This report demonstrates that germline genetic markers can be used to identify men at higher risk of prostate cancer. Targeting screening at these higher-risk men resulted in the identification of tumours that were more likely to require treatment.
1. Introduction
Prostate cancer (PCa) is the second most common cancer in men worldwide and the sixth most common cause of death [1]. There is a large degree of variation worldwide in both incidence and mortality because of differences in genetic background, lifestyle, the availability of screening programmes, and treatments.
Men with germline mutations in breast cancer 1, early onset (BRCA1) or breast cancer 2, early onset (BRCA2) genes have an increased risk of PCa. The relative risk of PCa by ≤65 yr is estimated at 1.8-fold to 4.5-fold for BRCA1 carriers [2], [3] and at 2.5-fold to 8.6-fold for BRCA2 carriers [4], [5], [6]. A number of retrospective studies consistently report that BRCA2 carriers present at a younger age with aggressive disease, higher rates of lymph node involvement, distant metastasis at diagnosis, and a higher mortality rate compared with noncarriers [7], [8], [9], [10], [11], [12]. While there is debate about whether there is an increased risk of PCa for BRCA1 carriers, there is increasing evidence that these men also present with more aggressive disease [7], [9], [13]. In addition, BRCA2 mutation status has been confirmed as an independent prognostic factor for poorer outcome [7]. Therefore, targeted screening of BRCA1/2 carriers for earlier detection may be beneficial.
The prostate-specific antigen (PSA) test is the most effective PCa biomarker currently available; however, its limitations are well documented. Expert groups have concluded that data from existing clinical trials—notably the Prostate, Lung, Colorectal and Ovary screening study (PLCO) [14] and the European Randomised Study of Screening for Prostate Cancer (ERSPC) [15]—are insufficient to recommend routine general population PSA screening. The main scientific challenge is to differentiate between men who will benefit from screening and men who will not, reducing overdiagnosis and overtreatment while maintaining benefits (ie, lower mortality).
There is no international consensus on targeting screening at men at higher risk. There have been a limited number of studies of screening in men with a family history of PCa [16], [17], [18]. Most of the studies support the use of targeted screening; however, methodological differences make it difficult to draw conclusions from these data [16], [17], [19], [20], [21], [22], [23], [24], [25], [26]. The IMPACT study (Identification of Men with a genetic predisposition to ProstAte Cancer: Targeted screening in BRCA1/2 mutation carriers and controls; www.impact-study.co.uk) is an international, multicentre study evaluating the role of targeted PSA screening in men with BRCA1/2 mutations. The aims of IMPACT are to evaluate the utility of PSA screening, to determine PCa incidence, to assess the positive predictive value (PPV) of biopsy using a PSA threshold of 3.0 ng/ml, to determine biopsy rates, and to evaluate the characteristics of the tumours to establish whether PSA screening detects clinically significant disease in this population compared with the control group. This analysis reports the results of the first screening round for all men enrolled in IMPACT from October 2005 to February 2013.
2. Materials and methods
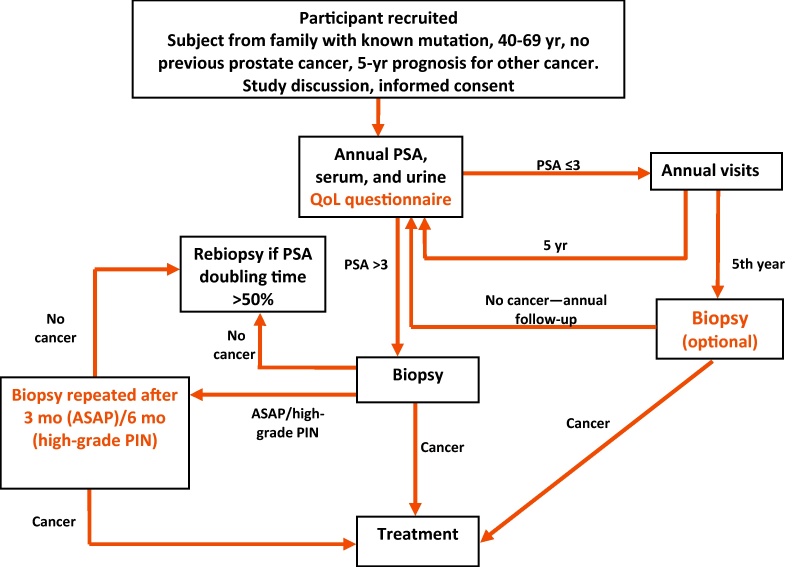
The IMPACT study design and methods have previously been reported elsewhere [27], [28] and are summarised below (Fig. 1). The protocol was approved by the West-Midlands Research and Ethics Committee in the United Kingdom (reference 05/MRE07/25) and subsequently by each participating institution's local committee. All participants provide written consent, and interim analyses are presented to the Independent Data and Safety Monitoring Committee biannually.
Fig. 1.
Study design.
ASAP = atypical small acinar proliferation; PIN = prostate intraepithelial neoplasia; PSA = prostate-specific antigen; QoL = quality-of-life.
The target sample is 500 BRCA1 mutation carriers and 350 BRCA2 mutation carriers and a control group of 850 men who tested negative for a pathogenic BRCA mutation in their family. IMPACT has been powered to detect a twofold increased risk over 5 yr of screening, with 80% power at p < 0.01.
We recruited men aged 40–69 from families with a BRCA mutation between October 2005 and February 2013. Men were recruited from cancer genetics clinics from families with known pathogenic BRCA1 or BRCA2 mutations. Men from these families could enter the study if they had tested positive or negative for the mutation, or if they were at 50% risk of inheriting a mutation but had not yet undergone testing. Men in the latter group were tested within the study to be allocated to the appropriate group for analysis, but this result was not fed back to participants. Men were excluded if they were known to have PCa or if they had a prior cancer diagnosis with a prognosis of <5 yr. In the Dutch cohort, men were also excluded if they had prior PSA screening.
Participants underwent PSA testing at enrolment, and if their PSA value was >3.0 ng/ml, a 10-core transrectal ultrasound–guided prostate biopsy was recommended. PSA quality assurance was measured on a concurrent serum sample. All available samples were tested using the ProStatus PSA Free/Total DELFIA assay at SUS (Malmö, Sweden). In addition, in men undergoing biopsy, serum samples were tested for microseminoprotein (MSP) and four kallikrein markers (free PSA, intact PSA, total PSA, and human kallikrein-related peptidase 2 [hK2]). The methods have been described previously [29], [30]. The results from the four kallikrein markers were combined to create a risk score (Rotterdam score) using a previously described model [30].
Participants with PSA ≤3.0 ng/ml will undergo annual PSA screening for ≥5 yr, except participants in the Dutch cohort, who are screened biennially (because of the constraints of the ministerial approval). Participants with PSA >3.0 ng/ml and a negative biopsy will undergo annual PSA testing, repeating the biopsy if PSA increases by >50%. All participants will be followed up for ≥5 yr to evaluate the cancer incidence and PCa-specific mortality and morbidity [27], [28].
The local histopathologist at each centre reported the biopsy results to guide treatment in accordance with local guidelines. The Gleason score, clinical stage, and classification of disease into low, intermediate, or high risk of metastasis [31] were reported for each case. Central pathology review was performed by the study pathologist (C.S.F.) to ensure consistency and standardisation. Prostate core biopsies were assessed in accordance with International Society of Urological Pathology guidelines [32] (described previously [10], [33]). Whenever high-grade prostate intraepithelial neoplasia (HG PIN) or atypical small acinar proliferation (ASAP) was detected, the biopsy was repeated within 3–6 mo.
2.1. Statistical analysis
Statistical analysis was undertaken using SPSS v.21 and Stata 12.0. The Fisher exact test was used to compare the number of PCa cases detected among groups and differences among disease types. The PPV of the biopsy using PSA >3.0 ng/ml in the different groups was compared using the chi-square test for independence. To compare the mean ages of men with high PSA levels, t tests were used; p < 0.05 was considered statistically significant. The Wald test was used to test the association between evidence of PCa at biopsy and the Rotterdam score, and the Spearman correlation was used to determine the relationship between PSA measurements taken in the clinical and laboratory settings.
3. Results
3.1. Study population
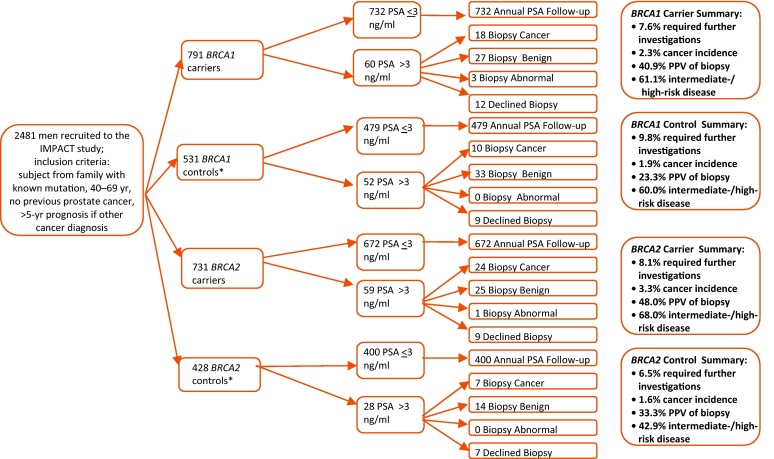
A total of 2481 participants from 62 centres in 20 countries were recruited over 90 mo (Supplemental Table 1); there were 791 BRCA1 carriers and 531 BRCA1 controls, as well as 731 BRCA2 carriers and 428 BRCA2 controls) (Fig. 2).
Fig. 2.
Consort diagram for the first round of screening.
BRCA1 = breast cancer 1, early onset; BRCA2 = breast cancer 2, early onset; PSA = prostate-specific antigen; PPV = positive predictive value.
* Controls were men who had a negative predictive genetic test for the BRCA mutation in their family.
The majority of participants were white (95%) and highly educated (measured using self-reported qualifications), and the mean age at enrolment was 54 yr (Table 1). Twenty-one percent of the men reported urinary symptoms, and 37% had previously had at least one PSA test. No statistically significant differences were observed among groups; 27% reported a family history of PCa in at least one blood relative.
Table 1.
Sociodemographic characteristics
| BRCA1+ (n = 791) | BRCA1− (n = 531) | BRCA2+ (n = 731) | BRCA2− (n = 428) | Total cohort | |
|---|---|---|---|---|---|
| Age group, yr, no. (%) | |||||
| 40–49 | 264 (33) | 148 (28) | 298 (41) | 118 (28) | 828 (33) |
| 50–59 | 294 (37) | 224 (42) | 254 (35) | 169 (40) | 941 (38) |
| 60–69 | 233 (30) | 159 (30) | 179 (25) | 141 (33) | 712 (29) |
| Qualifications, no. (%) | |||||
| No qualifications | 33 (4) | 13 (2) | 39 (5) | 24 (6) | 109 (4) |
| School to 16 | 105 (13) | 59 (11) | 116 (16) | 43 (10) | 323 (13) |
| School to 18/college degree | 133 (17) | 117 (22) | 89 (12) | 86 (20) | 425 (17) |
| Technical/vocational qualifications | 191 (24) | 134 (25) | 143 (20) | 81 (19) | 549 (22) |
| University graduate | 273 (35) | 179 (34) | 267 (37) | 145 (34) | 864 (35) |
| Other | 15 (2) | 16 (3) | 26 (4) | 17 (4) | 74 (3) |
| Unknown | 41 (5) | 13 (2) | 51 (7) | 32 (7) | 137 (6) |
| Family history of prostate cancer, no. (%) | |||||
| Yes | 177 (22) | 142 (27) | 234 (32) | 129 (30) | 682 (27) |
| No | 528 (67) | 307 (58) | 453 (62) | 249 (58) | 1537 (62) |
| Unknown | 86 (11) | 82 (15) | 44 (6) | 50 (12) | 262 (11) |
| Ethnicity, no. (%) | |||||
| Caucasian | 750 (95) | 514 (97) | 695 (95) | 410 (96) | 2369 (95.5) |
| East Asian | 3 (0.4) | 1 (0.2) | 3 (0.4) | 1 (0.2) | 8 (0.3) |
| North Asian | 8 (1,0) | 6 (1.1) | 0 | 2 (0.5) | 16 (0.6) |
| Caribbean | 1 (0.1) | 0 | 0 | 0 | 1 (0.0) |
| Aboriginal/Torres Strait Islander | 1 (0.1) | 0 | 1 (0.1) | 0 | 2 (0.1) |
| Mixed white and Caribbean | 5 (0.6) | 2 (0.4) | 2 (0.3) | 1 (0.2) | 10 (0.4) |
| Mixed white and Asian | 3 (0.4) | 0 | 0 | 0 | 3 (0.1) |
| Any other Asian background | 0 | 0 | 1 (0.1) | 0 | 1 (0.04) |
| Any other mixed background | 3 (0.4) | 1 (0.2) | 1 (0.1) | 0 | 5 (0.2) |
| Any other | 15 (2) | 7 (1) | 22 (3) | 5 (1) | 49 (2) |
| Not given | 2 (0.3) | 0 | 6 (0.8) | 9 (2) | 17 (0.7) |
BRCA1 = breast cancer 1, early onset; BRCA2 = breast cancer 2, early onset.
3.2. Prostate cancer detection rates at initial screening and positive predictive value of biopsy
Of the 2481 men, 199 (8.0%) had PSA >3.0 ng/ml (range: 3.0–27.0; median: 4.3) and were referred to a urologist to discuss prostate biopsy (Fig. 2). Of these men, 162 (81.4%) underwent biopsy. Biopsies were declined because of concurrent health conditions (n = 7), the urologist's choosing to repeat the PSA test prior to biopsy resulting in a reading ≤3.0 ng/ml (n = 17), men changing their minds (n = 8), or reason missing (n = 5). Fifty-nine of 162 biopsies (36.4%) contained cancer. There was no significant difference in cancer detection rates between men who had or had not undergone PSA screening prior to study entry. No significant differences were seen with the Dutch cohort, in which men with prior PSA screening were excluded. Other than in the Dutch cohort, the prior screening levels were similar in all countries.
The PCa detection rate was 2.4% (59 of 2481 men) (Table 2). The detection rate for BRCA1 carriers was 2.3% (18 of 791); it was 1.9% (10 of 531) for BRCA1 controls, 3.3% (24 of 731) for BRCA2 carriers, and 1.6% (7 of 428) for BRCA2 controls, with no significant difference among groups. The number of cores taken at biopsy ranged from 6 to 20; however, there were no differences in the median or mean number of cores taken among groups (Table 2). Four men had either ASAP or HG PIN (all mutation carriers) (Table 2). Two men underwent repeat biopsy with no cancers detected. Taking potential geographical variation in cancer incidence into consideration, the data were analysed by region (North America; Australia; Asia; and Western, Central, and Southern Europe), and no statistically significant differences were observed.
Table 2.
Summary of outcomes for men with prostate-specific antigen level >3.0 ng/ml
| BRCA1+ (n = 791) | BRCA1− (n = 531) | BRCA2+ (n = 731) | BRCA2− (n = 428) | Total cohort (n = 2481) | |
|---|---|---|---|---|---|
| Men PSA >3.0 ng/ml, no. | 60 | 52 | 59 | 28 | 199 |
| Mean age, yr | 60.1 | 59.8 | 58.1 | 62.2 | 59.7 |
| Biopsy rate, % | 7.6 | 9.8 | 8.1 | 6.5 | 8.0 |
| Biopsies performed, no. | 48 | 43 | 50 | 21 | 162 |
| Biopsy–benign, no. | 27 | 33 | 25 | 14 | 99 |
| Biopsy–cancer, no. | 18 | 10 | 24 | 7 | 59 |
| Biopsy–ASAP/HG PIN, no. | 3 | 0 | 1 | 0 | 4 |
| No biopsy, no. | 12 | 9 | 9 | 7 | 37 |
| PPV of biopsy, % | 37.5 | 23.3 | 48.0 | 33.3 | 36.4 |
| Biopsy cores, no., median; mean (range) | 10; 9.4 (6–13) | 11; 10.3 (6–20) | 10; 10.1 (5–12) | 10; 10.1 (6–13) | 10; 9.9 (5–20) |
BRCA1 = breast cancer 1, early onset; BRCA2 = breast cancer 2, early onset; PSA = prostate-specific antigen; ASAP/HG PIN = atypical small acinar proliferation/high-grade prostate intraepithelial neoplasia; PPV = positive predictive value.
The PPV of biopsy using a PSA threshold of 3.0 ng/ml (ie, the number of cancers detected divided by the number of biopsies performed) was 36% (59 of 162) (Table 2). Broken down by genetic status, the PPV in BRCA1 carriers was 37.5% (18 of 48); in BRCA1 controls, 23.3% (10 of 43); in BRCA2 carriers, 48.0% (24 of 50); and in BRCA2 controls, 33.3% (7 of 21). There was no statistically significant difference among groups (Pearson chi-square test for BRCA1, p = 0.14; for BRCA2, p = 0.26).
There was no significant difference between either mean age at PCa diagnosis or PSA level among groups. Twelve men (20%) reported urinary symptoms prior to diagnosis, and 20 men (34%) had a PSA test prior to study entry (29% BRCA2 carriers, 50% BRCA2 controls; 44% BRCA1 carriers, 33% BRCA1 controls). There was no difference observed in levels of PSA screening prior to study entry among groups.
Using the NICE classification [31], [34], intermediate- or high-risk tumours were diagnosed in 11 of 18 BRCA1 carriers (61%) compared with 8 of 10 BRCA1 controls (80%) and in 17 of 24 BRCA2 carriers (71%) compared with 3 of 7 BRCA2 controls (43%) (Table 3). There was no significant difference observed between genetic status and disease risk status. The PPV of biopsy using a PSA threshold of 3.0 ng/ml for detecting intermediate- and high-risk PCa for BRCA2 carriers and controls was 2.38% (17 of 714) and 0.71% (3 of 425), respectively; this difference is significant (Pearson chi-square, p = 0.04). No significant difference was observed in BRCA1 carriers compared with controls (1.41% [11 of 780] compared with 1.33% [8 of 524]; Pearson chi-square test, p = 0.86). No cases had nodal involvement or metastatic disease at diagnosis.
Table 3.
Clinical features of the prostate cancers at diagnosis
| Patient | Status | Age, yr | Disease risk classification | PSA test prior to study entry | PSA, ng/ml | Gleason score | Clinical stage | Treatment | Family history of prostate cancer | Urinary symptoms |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BRCA1+ | 55 | High | Yes | 5.9 | 4 + 4 | pT2c | Prostatectomy | No | No |
| 2 | BRCA1+ | 69 | High | Yes | 6.3 | 3 + 3 | pT3b | Prostatectomy | No | Yes |
| 3 | BRCA1+ | 60 | High | Yes | 3.3 | 3 + 3 | pT3a | Prostatectomy | Yes | No |
| 4 | BRCA1+ | 59 | High | No | 3.8 | 3 + 5 | T3a | * | Yes | No |
| 5 | BRCA1+ | 61 | Intermediate | No | 9.7 | 3 + 4 | T1c | Prostatectomy | No | No |
| 6 | BRCA1+ | 61 | Intermediate | Yes | 4.5 | 3 + 4 | pT2c | Prostatectomy | Yes | No |
| 7 | BRCA1+ | 69 | Intermediate | Yes | 7.4 | 3 + 3 | T2b | Radiotherapy | Yes | Yes |
| 8 | BRCA1+ | 53 | Intermediate | No | 3.9 | 3 + 3 | T2 | Prostatectomy | No | No |
| 9 | BRCA1+ | 63 | Intermediate | No | 4.2 | 3 + 3 | pT2c | Prostatectomy | Yes | No |
| 10 | BRCA1+ | 49 | Intermediate | Yes | 3.8 | 3 + 3 | pT2c | Prostatectomy | No | No |
| 11 | BRCA1+ | 45 | Intermediate | No | 3.2 | 3 + 4 | T2b | Prostatectomy | Yes | No |
| 12 | BRCA1+ | 61 | Low | Yes | 4.1 | 3 + 3 | T1c | Active surveillance | No | No |
| 13 | BRCA1+ | 56 | Low | No | 5.3 | 3 + 3 | pT2a | Prostatectomy | Yes | No |
| 14 | BRCA1+ | 63 | Low | Yes | 3.4 | 3 + 3 | pT2a | Prostatectomy | No | Yes |
| 15 | BRCA1+ | 57 | Low | No | 3.7 | 3 + 3 | T1c | Prostatectomy | No | No |
| 16 | BRCA1+ | 64 | Low | No | 5 | 3 + 3 | T1c | Active surveillance | No | No |
| 17 | BRCA1+ | 64 | Low | No | 6.2 | 3 + 3 | T1c | Active surveillance | No | No |
| 18 | BRCA1+ | 48 | Low | No | 5.3 | 3 + 3 | * | * | No | No |
| 19 | BRCA1− | 61 | High | Yes | 7.7 | 4 + 3 | pT3a | Prostatectomy | No | No |
| 20 | BRCA1− | 62 | High | Yes | 3.1 | 3 + 4 | pT3a | Prostatectomy | Yes | Yes |
| 21 | BRCA1− | 62 | Intermediate | No | 3.3 | 3 + 3 | pT2c | Prostatectomy | No | No |
| 22 | BRCA1− | 61 | Intermediate | No | 4.8 | 3 + 3 | T2c | Prostatectomy | No | Yes |
| 23 | BRCA1− | 66 | Intermediate | Yes | 5.5 | 4 + 3 | T1c | Radiotherapy | Yes | No |
| 24 | BRCA1− | 57 | Intermediate | No | 4.5 | 3 + 4 | T2c | Prostatectomy | Yes | Yes |
| 25 | BRCA1− | 55 | Intermediate | No | 5.2 | 3 + 4 | pT2 | Prostatectomy | No | No |
| 26 | BRCA1− | 65 | Intermediate | No | 4.7 | 3 + 3 | pT2c | Prostatectomy | No | No |
| 27 | BRCA1− | 59 | Low | No | 4.3 | 3 + 3 | T1c | Active surveillance | Yes | No |
| 28 | BRCA1− | 62 | Low | Unknown | 9.9 | 3 + 3 | T1c | Prostatectomy | No | No |
| 29 | BRCA2+ | 66 | High | Yes | 5 | 3 + 4/4 + 3 | pT3a | Prostatectomy | No | No |
| 30 | BRCA2+ | 51 | High | No | 27 | 4 + 3 | pT3a | Prostatectomy | Yes | No |
| 31 | BRCA2+ | 66 | High | No | 24 | 4 + 4 | T4 | Radiotherapy | No | No |
| 32 | BRCA2+ | 66 | High | Yes | 11 | 4 + 5 | T3a | Prostatectomy | No | No |
| 33 | BRCA2+ | 61 | High | No | 6.3 | 4 + 5 | T1c | Prostatectomy | No | No |
| 34 | BRCA2+ | 67 | High | No | 12.8 | 3 + 3 | T3a | Brachytherapy | No | No |
| 35 | BRCA2+ | 62 | High | Yes | 8.2 | 3 + 4 | pT3a | Prostatectomy | Yes | Yes |
| 36 | BRCA2+ | 49 | Intermediate | Unknown | 4.9 | 3 + 4 | T2c | Prostatectomy | No | No |
| 37 | BRCA2+ | 68 | Intermediate | Yes | 5.3 | 3 + 4 | T2b | Radiotherapy | No | No |
| 38 | BRCA2+ | 54 | Intermediate | No | 3.1 | 3 + 3 | pT2c | Prostatectomy | No | No |
| 39 | BRCA2+ | 56 | Intermediate | No | 5 | 3 + 4 | pT2c | Prostatectomy | Yes | No |
| 40 | BRCA2+ | 59 | Intermediate | Yes | 3 | 3 + 4 | T2c | Prostatectomy | Yes | No |
| 41 | BRCA2+ | 58 | Intermediate | No | 5.1 | 4 + 3 | pT2c | Prostatectomy | No | No |
| 42 | BRCA2+ | 41 | Intermediate | No | 3.5 | 3 + 4 | pT2c | Prostatectomy | Yes | Yes |
| 43 | BRCA2+ | 65 | Intermediate | No | 4.7 | 3 + 4 | T1c | Radiotherapy | No | No |
| 44 | BRCA2+ | 53 | Intermediate | No | 3.6 | 3 + 3 | T2c | Prostatectomy | No | No |
| 45 | BRCA2+ | 63 | Intermediate | No | 3.5 | 3 + 3 | pT2c | Prostatectomy | Yes | Yes |
| 46 | BRCA2+ | 67 | Low | No | 4.8 | 3 + 3 | T2a | Active surveillance | No | No |
| 47 | BRCA2+ | 55 | Low | No | 4.5 | 3 + 3 | T1c | Active surveillance | Yes | Yes |
| 48 | BRCA2+ | 61 | Low | 3.6 | 3 + 3 | T1c | Brachytherapy | No | Yes | |
| 49 | BRCA2+ | 57 | Low | No | 4.9 | 3 + 3 | T1c | Active surveillance | No | Yes |
| 50 | BRCA2+ | 45 | Low | No | 4.7 | 3 + 3 | T1c | Active surveillance | No | No |
| 51 | BRCA2+ | 61 | Low | No | 4.1 | 3 + 3 | T1c | Active surveillance | No | No |
| 52 | BRCA2+ | 54 | Low | Yes | 3.3 | 3 + 3 | T1c | Active surveillance | Yes | No |
| 53 | BRCA2− | 69 | High | No | 14.3 | 4 + 3 | T3 | Radiotherapy | No | No |
| 54 | BRCA2− | 62 | Intermediate | No | 4.8 | 3 + 4 | pT2c | Prostatectomy | No | No |
| 55 | BRCA2− | 65 | Intermediate | Yes | 4.2 | 3 + 3 | T1c | Active surveillance | No | No |
| 56 | BRCA2− | 60 | Low | No | 5.5 | 3 + 3 | T1c | Active surveillance | No | No |
| 57 | BRCA2− | 68 | Low | Yes | 3.3 | 3 + 3 | T1c | Active surveillance | No | No |
| 58 | BRCA2− | 66 | Low | Yes | 6.7 | 3 + 3 | T2a | Prostatectomy | No | No |
| 59 | BRCA2− | 53 | Low | Unknown | 3.4 | 3 + 3 | T2b | Prostatectomy | No | No |
BRCA1 = breast cancer 1, early onset; BRCA2 = breast cancer 2, early onset; PSA = prostate-specific antigen.
Data pending.
3.3. Central analysis of prostate-specific antigen and the kallikrein panel
There was a strong correlation between PSA values measured in the clinical and laboratory settings (Spearman r = 0.85). Serum samples of 57 (24 with PCa) of the 162 men who underwent a biopsy were analysed for MSP and four kallikrein markers (Table 4).
Table 4.
Patient characteristics for kallikrein analysis*
| Characteristics | No cancer (n = 33) | Cancer (n = 24) |
|---|---|---|
| BRCA1 tested, no. (%) | 18 (55) | 11 (46) |
| BRCA1+, no. (%) | 10 (56) | 10 (91) |
| BRCA2 tested, no. (%) | 15 (45) | 13 (54) |
| BRCA2+, no. (%) | 12 (80) | 10 (77) |
| Age at study entry, yr, median (quartiles) | 59 (55, 64) | 61 (57, 66) |
| Specific site total PSA, ng/ml, median (quartiles) | 4.2 (3.4, 5.0) | 4.4 (3.7, 5.2) |
| Central site total PSA, ng/ml, median (quartiles) | 3.9 (3.4, 5.1) | 4.2 (3.3, 5.4) |
| Free PSA, ng/ml, median (quartiles) | 0.93 (0.73, 1.19) | 0.83 (0.53, 0.96) |
| Intact PSA, ng/ml, median (quartiles) | 0.53 (0.42, 0.69) | 0.47 (0.31, 0.67) |
| hK2, ng/ml, median (quartiles) | 0.051 (0.038, 0.076) | 0.062 (0.036, 0.083) |
| MSP, ng/ml, median (quartiles) | 19 (11, 26) | 18 (11, 24) |
| Rotterdam score | 0.235 (0.162, 0.310) | 0.327 (0.243, 0.373) |
| Gleason total score, no. (%) | ||
| 6 | 17 (71) | |
| 7 | 7 (29) | |
| Clinical T stage, no. (%) | ||
| T1C | 8 (33) | |
| T2 | 2 (8.3) | |
| T2A | 1 (4.2) | |
| T2B | 2 (8.3) | |
| T2C | 3 (13) | |
| T3 | 1 (4.2) | |
| Unknown | 7 (29) | |
BRCA1 = breast cancer 1, early onset; BRCA2 = breast cancer 2, early onset; PSA = prostate-specific antigen; hK2 = human kallikrein-related peptidase 2; MSP = microseminoprotein.
Data are frequency (percentage) or median (quartiles).
We found no association between PCa at biopsy and total PSA or MSP (Table 5). We compared the proportion of PCa in mutation carriers with controls and found no association between PCa at biopsy and mutation status. There was an association of PCa at biopsy and Rotterdam score (Wald test p = 0.024). The discrimination of the Rotterdam model was 0.70 (95% confidence interval [CI], 0.56–0.84). For the outcome of high-grade cancer, the Rotterdam score was the only statistically significant predictor (p = 0.009), with a discrimination of 0.86 (95% CI, 0.73–0.99).
Table 5.
Univariate logistic regression for the outcomes of evidence of prostate cancer at biopsy and evidence of high-grade prostate cancer at biopsy*
| Predictor | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Total PSA, ng/ml (n = 57) | |||
| Cancer | 1.02 | 0.75–1.37 | 0.9 |
| High-grade cancer | 1.49 | 1.00–2.23 | 0.051 |
| Rotterdam score (n = 57)** | |||
| Cancer | 2.30 | 1.25–4.22 | 0.007 |
| High-grade cancer | 3.87 | 1.42–10.60 | 0.008 |
| MSP, ng/ml (n = 57) | |||
| Cancer | 1.00 | 0.95–1.04 | 0.8 |
| High-grade cancer | 0.95 | 0.86–1.03 | 0.2 |
| BRCA1 status (n = 29)+ | |||
| Cancer | 8.00 | 0.76–389.69 | 0.10 |
| High-grade cancer† | 0.5 | ||
| BRCA2 status (n = 28)+ | |||
| Cancer | 0.83 | 0.09–7.73 | 1 |
| High-grade cancer | 1.47 | 0.11–83.27 | 1 |
| Mutation status (n = 57)+ | |||
| Cancer | 2.50 | 0.60–12.35 | 0.2 |
| High-grade cancer | 2.33 | 0.24–114.86 | 0.7 |
CI = confidence interval; BRCA1 = breast cancer 1, early onset; BRCA2 = breast cancer 2, early onset; MSP = microseminoprotein; PSA = prostate-specific antigen.
Subset of 57 men biopsied for whom an adequate serum sample was available.
The odds ratio for the Rotterdam score corresponds to a 0.1-unit increase on a 0–1 probability scale.
+ The 95% CI and p values are calculated using the Fisher exact test.
The odds ratio and 95% CI are not estimable because of zero events in the BCRA1-negative group. The p value is calculated from the chi-square test.
For 1202 of 2481 participants with available blood samples, we found a strong correlation of total PSA between measurements taken in the clinical and laboratory settings (Spearman r = 0.95).
3.4. Serious adverse events
Six study-related serious adverse events were reported, all occurring after biopsy. Complications occurred in 6 of 158 participants (3.8%), with five infections (3.2%) reported, two requiring hospitalisation. The sixth participant was hospitalised because of fainting after biopsy.
4. Discussion
In this paper we have presented the results of the first screening round of IMPACT, including the number and features of the PCa detected. With germline mutations in BRCA1 and BRCA2 being rare, the success of IMPACT has been in the formation of an international consortium of 62 centres with both clinical genetics and urologic collaboration. Enrolment was open until the required number of recruits was obtained in all four cohorts, exceeding the numbers required for statistical power in all groups.
Compliance with the protocol was high, with 162 men with PSA >3.0 ng/ml (>81%) proceeding to biopsy. This number compares favourably with the 86% in the ERSPC [35] and the 31.5% in the PLCO study [35], [36], [37]. In the PLCO study, with no strict protocol to guide intervention, 74% of men with an abnormal screening test underwent further diagnostic evaluation, and 64% underwent biopsy within 3 yr [37]. Thus, a similar increase in compliance may be anticipated in IMPACT at subsequent screening rounds. The potential utility of multiparametric magnetic resonance imaging (MRI) as a screening tool before biopsy has been the subject of recent debate [38]; however, the IMPACT protocol was designed prior to the use of MRI in this diagnostic capacity.
In total, 8% of the men had a positive PSA test (>3.0 ng/ml), which is lower than the 16.2% (range: 11.1–22.3% among sites) reported in the ERSPC general population screening study [35]. However, the ERSPC recruited an older cohort of men (55–75 yr), with a mean age of 61 yr compared with 54 yr in IMPACT. It is known that PSA increases with age, so higher PSA levels would be expected. In addition, a number of ERSPC centres used a threshold of 4.0 ng/ml rather than 3.0 ng/ml to determine biopsy, so the two studies are not entirely directly comparable. These results indicate that overbiopsy is not a concern in this younger cohort.
There is controversy about the PSA level used to trigger biopsy, with no clear consensus. The results presented show that while not statistically significant, the PPV of biopsy using a PSA threshold of 3.0 ng/ml is higher for BRCA2 carriers than for controls (48% vs 33%) and higher for BRCA1 carriers than controls (41% vs 23%). For BRCA2 carriers, this percentage is double the 24.1% reported in the ERSPC general population sample. This higher PPV observed in mutation carriers may be explained, at least in part, by the fact that the ERSPC screened older men. Also, given the younger age of the IMPACT cohort, the incidence of benign prostatic hypertrophy (BPH) may have been lower; the incidence of BPH increases with age, and BPH lowers the specificity of PSA screening [27], [39], [40]. These data suggest that lowering the PSA threshold for biopsy in BRCA2 carriers could potentially detect early-stage disease, thus reducing the need for more toxic treatments and ultimately reducing PCa mortality. However, this lowering would need to be balanced against the risk of potentially life-threatening side-effects of biopsy [41], [42].
In IMPACT, men will be offered a prostate biopsy at the end of the study (at the centres with the capacity), which may provide evidence for the optimal PSA threshold for detecting clinically significant PCa in this cohort of higher-risk men.
The observed differences in PPV may also reflect the higher incidence and grade of PCa previously reported, particularly in BRCA2 carriers. The higher PPV in BRCA2 carriers suggests that PSA may have a higher specificity in this high-risk setting. However, as the number of cancers is relatively small, subsequent PSA screening rounds are essential to confirm this hypothesis. Evaluation of the panel of four kallikrein markers in subsequent screening rounds may provide further insights into the panel's potential role in predicting biopsy outcome [30].
The ERSPC reported that 4.2% of men had a cancer diagnosis at the first screening round [43]. In IMPACT, the PCa detection rate was 2.4%, and two-thirds of the men in the cohort were previously unscreened. The younger age of the IMPACT sample is likely to explain this lower detection rate. More than two-thirds of the PCa detected in the BRCA2 carriers were classified as intermediate – or high – risk, supporting retrospective reports of a more aggressive phenotype and poorer prognosis in this group [7], [8], [9], [10], [11], [12]. Sixty-one percent of BRCA1 carriers were classified as having intermediate- or high-risk disease. By comparison, in the ERSPC, only 27.8% of the PCa diagnosed in the screened cohort were Gleason score ≥7 [35]. Longer-term follow-up will determine whether there is a difference in metastatic events and mortality between carriers and controls. From the PLCO study, after 13 yr of follow-up, there is no evidence to support the idea that organised PSA screening reduces mortality compared with opportunistic screening [14]. In contrast, after a median of 11 yr of follow-up, the ERSPC reported a 21% reduction in PCa-specific mortality in the screened cohort [15]. It is important to note that in the PLCO, 56% of men in the control arm had PSA screening, compared with 15% in the ERSPC.
The higher incidence of clinically significant disease in the BRCA2 mutation carriers, together with the significantly younger age of BRCA2 carriers with PSA >3.0 ng/ml, is an important observation in view of the younger age of this cohort compared with the ERSPC study. The only cancers detected in men <50 yr were in BRCA1 and BRCA2 carriers. These data add to the increasing evidence that BRCA1/2 carriers develop more aggressive disease, and at a younger age. Of note, the control groups also had a higher level of intermediate- or high-risk disease compared with the ERSPC. However, the number of cancers is relatively small, and with 19% of men declining biopsy, these data should be interpreted with caution.
The population incidence of PCa in each of the recruiting countries must be considered. The incidence in the majority of the countries is very similar, except in India and Malaysia [44]. Given the relatively low number of recruits from these regions, geographical variation is unlikely to have a major impact on the results. A limitation of IMPACT is that 95% of the men were white. Thus, the results cannot be generalised to all ethnic groups known to have a higher risk of PCa and a more aggressive phenotype (eg, black). A second limitation is that 37% of the cohort had previously had a PSA test. This fact could potentially bias the study to either having men with a lower PSA or having men with higher PSAs due to noncancerous causes. However, no difference in screening levels was observed among those men with and without cancer. A further limitation is that the control group was recruited from families known to have BRCA mutations. It is possible that this group of men has a different PCa risk profile than the general population.
5. Conclusions
The first screening round of IMPACT demonstrates that targeted screening for PCa in men with a genetic predisposition detects clinically significant disease. Using a PSA threshold of 3 ng/ml results in a low biopsy rate (8.0%) and a high PPV, particularly in BRCA2 carriers, for the detection of intermediate- and high-risk disease. Although the observed differences in PCa detection rates between carriers and controls was not statistically significant, the trend is clear. With larger numbers of PCa in the follow-up phase (5 yr), these differences, if sustained, are likely to be significant.
Future screening rounds will determine the optimal frequency of PSA testing, determine the utility of PSA screening in BRCA1 carriers, and provide further data on the value of annual screening in BRCA2 carriers.
A previously published statistical model based on four kallikrein markers was able to predict biopsy outcome in participants with PSA >3 ng/ml with a discrimination of 0.86 for high-grade disease. Longer-term follow-up will be used to validate the role of the kallikrein panel in this population.
IMPACT is the first prospective study to demonstrate the use of germline genetic markers to identify men at higher risk of PCa, which has the potential to enable better risk stratification to inform targeted screening. These early results indicate that the tumours detected are more likely to need treatment based on national guidelines for management of more aggressive PCa. Therefore, our preliminary results support the use of PSA screening for BRCA2 carriers.
Author contributions: Rosalind A. Eeles had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Eeles, Bancroft, Page, Castro, Lilja, Vickers, Mitra, Evans, Eccles, Mitchell, Mæhle, Foster, Johannsson, Lubinski, Aaronson, Ardern-Jones, Dearnaley, Gronberg, Hamdy, Khoo, Kote-Jarai, Falconer, Melia, Moynihan, Rennert, Suri, Wilson, Moss, Blanco, Bangma, Eyfjord.
Acquisition of data: Eeles, Bancroft, Page, Castro, Mitra, Drew, Mæhle, Bulman, Costello, McKinley, Ringelberg, Skytte, Taylor, Salinas, Conner, Selkirk, Hoffman, Powers, Maia, Teixeira, Taherian, Stefansdottir, Copakova, Rothwell, Blanco, Cybulski, McBride, Clowes, Giri, Murthy, Teo, Liljegren, Wokolorczyk, Ramon y Cajal, Gadea, Chen-Shtoyerman, Gallagher.
Analysis and interpretation of data: Bancroft, Page, Castro, Lilja, Vickers, Eeles, Moss, Assel, Sjoberg.
Drafting of the manuscript: Bancroft, Page, Moss, Eeles, Castro, Vickers, Lilja.
Critical revision of the manuscript for important intellectual content: Bancroft, Page, Moss, Eeles, Castro, Vickers, Lilja, Kiemeney, Cybulski.
Statistical analysis: Bancroft, Page, Castro, Lilja, Vickers, Eeles, Moss, Assel, Sjoberg.
Obtaining funding: Eeles, Selkirk, Hulick, Kiemeney, Vasen, van Asperen, Mitchell, Domchek, Strom, Lindeman, Zgajnar, Walker, Liljegren, Buys, Evans, Giri, Foulkes, Tischkowitz.
Administrative, technical, or material support: Drew, Bojesen, Ringelberg, McBride, K. Axcrona, Bulman, Powers, Salinas, Walker, Hodgson, Side, Liljegren, Buys, Conner, Giri, Killick, McKinley, Wokolorczyk, Skytte, Cybulski, Lam, Taylor, Oldenburg, Cremers, Verhaegh, van Zelst-Stams, Oosterwijk, Cook, Rosario, Ausems, Ong, Teixeira, Maia, Kirk, Tucker, Davidson, Izatt, Foulkes, Taherian, Ruijs, Adank, Schmutzler, Helderman-van den Enden, Harris, Douglas, Lindeman, Tischkowitz, Clowes, Susman, Ramon y Cajal, Patcher, Gadea, Spigelman, van Os, Brewer, Brady, Donaldson, Stefansdottir, Friedman, Chen-Shtoyerman, Amor, Barwell, Murthy, Nicolai, Teo, Greenhalgh, Henderson, McGrath, Gallagher, Rothwell, U. Axcrona, Selkirk, Hulick, Hoffman, Domchek, Powers, Zgajnar, Copakova, Costello, The IMPACT Study Collaborators.
Supervision: Eeles.
Other (specify): None.
Financial disclosures: Rosalind A. Eeles certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Hans Lilja holds patents for free PSA, hK2, and intact PSA assays. Rosalind A. Eeles has received educational grants from Janssen Pharmaceuticals, GenProbe (formerly Tepnel), Illumina, and Vista Diagnostics and honoraria from Succinct Communications.
Funding/Support and role of the sponsor: The authors are indebted to the 2481 men who are taking part in this study. This research is coordinated by the Institute of Cancer Research, London, UK, and is supported by grants from the Ronald and Rita McAulay Foundation and Cancer Research UK (grant references C5047/A15007 and C5047/A13232). In Australia, this project was cofunded by Cancer Council Tasmania and Cancer Australia, grant number 1006349 (2011–2013); Prostate Cancer Foundation of Australia, grant number PCFA PR04 (2008); Cancer Councils of Victoria and South Australia, grant number 400048 (2006–2008); the Victorian Cancer Agency Clinical Trial Capacity CTCB08_14; and Translational grants EOI09_50. The Association of International Cancer Research funded data collection in The Netherlands (AICR 10–0596). The authors received funding from the NIHR to the Biomedical Research Center at the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust, and at Central Manchester Foundation Trust; the Basser Research Centre (to Susan Domchek); the National Cancer Institute (R01CA160816, R01 CA175491, and P50-CA92629); the Sidney Kimmel Center for Prostate and Urologic Cancers; David H. Koch through the Prostate Cancer Foundation, the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Program, Swedish Cancer Society project no. 11–0624, a FiDIPro-program award from TEKES in Finland, and Fundacion Federico SA; and the Slovenian Research Agency, research programme P3–0352.
Acknowledgment statement: R. Eeles is the chief investigator of the IMPACT study and has overall responsibility for the study. E. Bancroft, E. Page, E. Castro had overall responsibility for the analyses and writing of the article, together with R. Eeles. S. Moss is the study statistician. H. Lilja, A. Vickers, D. Sjoberg, M. Assel performed the analysis of the panel of four kallikrein markers. C.S. Foster performed the central pathology review. All authors contributed to the study design, provided data and contributed to data interpretation, writing and editing of the report, and approved the final version.
The authors acknowledge Mr. and Mrs. Jack Baker for the study at North Shore University Health System (Evanston, IL, USA) and Myriad Genetics Laboratory (Salt Lake City, UT, USA) for providing research BRCA testing rates for North Shore University Health System participants. The authors are grateful to the members of the Data and Safety Monitoring Committee: S. Duffy (chair), P. White (UK NEQAS representative), and R. Pocock (BAUS representative). The authors acknowledge the contribution of past members of the IMPACT steering committee: D. Easton, S. Peock, F. Schroder, R. Sharifi, and P. Sibley.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eururo.2014.01.003.
Appendix A. Supplementary data
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Leongamornlert D., Mahmud N., Tymrakiewicz M. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106:1697–1701. doi: 10.1038/bjc.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson D., Easton D.F. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 4.Breast Cancer Linkage Consortium Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 5.Kote-Jarai Z., Leongamornlert D., Saunders E. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105:1230–1234. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Asperen C.J., Brohet R.M., Meijers-Heijboer E.J. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42:711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro E., Goh C., Olmos D. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards S.M., Evans D.G., Hope Q. Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br J Cancer. 2010;103:918–924. doi: 10.1038/sj.bjc.6605822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher D.J., Gaudet M.M., Pal P. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16:2115–2121. doi: 10.1158/1078-0432.CCR-09-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitra A., Fisher C., Foster C.S. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer. 2008;98:502–507. doi: 10.1038/sj.bjc.6604132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne H., Willems A.J., Niedermayr E. Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res. 2011;4:1002–1010. doi: 10.1158/1940-6207.CAPR-10-0397. [DOI] [PubMed] [Google Scholar]
- 12.Tryggvadottir L., Vidarsdottir L., Thorgeirsson T. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:929–935. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 13.Giusti R.M., Rutter J.L., Duray P.H. A twofold increase in BRCA mutation related prostate cancer among Ashkenazi Israelis is not associated with distinctive histopathology. J Med Genet. 2003;40:787–792. doi: 10.1136/jmg.40.10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andriole G.L., Crawford E.D., Grubb R.L., III Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder F.H., Hugosson J., Roobol M.J. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalona W.J., Antenor J.A., Roehl K.A., Moul J.W. Screening for prostate cancer in high risk populations. J Urol. 2002;168:1980–1983. doi: 10.1016/S0022-5347(05)64276-0. discussion 1983–4. [DOI] [PubMed] [Google Scholar]
- 17.McWhorter W.P., Hernandez A.D., Meikle A.W. A screening study of prostate cancer in high risk families. J Urol. 1992;148:826–828. doi: 10.1016/s0022-5347(17)36733-2. [DOI] [PubMed] [Google Scholar]
- 18.Neuhausen S.L., Skolnick M.H., Cannon-Albright L. Familial prostate cancer studies in Utah. Br J Urol. 1997;79(Suppl 1):15–20. doi: 10.1111/j.1464-410x.1997.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 19.Bunker C.H., Patrick A.L., Konety B.R. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev. 2002;11:726–729. [PubMed] [Google Scholar]
- 20.Kiemeney L.A., Broeders M.J., Pelger M. Screening for prostate cancer in Dutch hereditary prostate cancer families. Int J Cancer. 2008;122:871–876. doi: 10.1002/ijc.23165. [DOI] [PubMed] [Google Scholar]
- 21.Makinen T., Tammela T.L., Stenman U.H. Family history and prostate cancer screening with prostate-specific antigen. J Clin Oncol. 2002;20:2658–2663. doi: 10.1200/JCO.2002.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Matikainen M.P., Schleutker J., Morsky P., Kallioniemi O.P., Tammela T.L. Detection of subclinical cancers by prostate-specific antigen screening in asymptomatic men from high-risk prostate cancer families. Clin Cancer Res. 1999;5:1275–1279. [PubMed] [Google Scholar]
- 23.Narod S.A., Dupont A., Cusan L. The impact of family history on early detection of prostate cancer. Nat Med. 1995;1:99–101. doi: 10.1038/nm0295-99. [DOI] [PubMed] [Google Scholar]
- 24.Sartor O. Early detection of prostate cancer in African-American men with an increased familial risk of disease. J La State Med Soc. 1996;148:179–185. [PubMed] [Google Scholar]
- 25.Uzzo R.G., Pinover W.H., Horwitz E.M. Free prostate-specific antigen improves prostate cancer detection in a high-risk population of men with a normal total PSA and digitalrectal examination. Urology. 2003;61:754–759. doi: 10.1016/s0090-4295(02)02524-4. [DOI] [PubMed] [Google Scholar]
- 26.Valeri A., Cormier L., Moineau M.P. Targeted screening for prostate cancer in high risk families: early onset is a significant risk factor for disease in first degree relatives. J Urol. 2002;168:483–487. doi: 10.1016/s0022-5347(05)64663-0. [DOI] [PubMed] [Google Scholar]
- 27.Mitra A.V., Bancroft E.K., Barbachano Y. Targeted prostate cancer screening in men with mutations in BRCA1 and BRCA2 detects aggressive prostate cancer: preliminary analysis of the results of the IMPACT study. BJU Int. 2011;107:28–39. doi: 10.1111/j.1464-410X.2010.09648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitra A.V., Bancroft E.K., Eeles R.A. A review of targeted screening for prostate cancer: introducing the IMPACT study. BJU Int. 2007;99:1350–1355. doi: 10.1111/j.1464-410X.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 29.Haiman C.A., Stram D.O., Vickers A.J. Levels of beta-microseminoprotein in blood and risk of prostate cancer in multiple populations. J Natl Cancer Inst. 2013;105:237–243. doi: 10.1093/jnci/djs486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vickers A., Cronin A., Roobol M. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol. 2010;28:2493–2498. doi: 10.1200/JCO.2009.24.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 32.Epstein J.I., Allsbrook W.C., Jr., Amin M.B., Egevad L.L. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 33.Berney D.M., Fisher G., Kattan M.W. Major shifts in the treatment and prognosis of prostate cancer due to changes in pathological diagnosis and grading. BJU Int. 2007;100:1240–1244. doi: 10.1111/j.1464-410X.2007.07199.x. [DOI] [PubMed] [Google Scholar]
- 34.National Institute of Health and Clinical Excellence (NICE) National Collaborating Centre for Cancer; Cardiff, UK: 2008. Prostate cancer: diagnosis and treatment. NICE clinical guidelines, no. 58. [Google Scholar]
- 35.Schroder F.H., Hugosson J., Roobol M.J. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 36.Andriole G.L., Crawford E.D., Grubb R.L., III Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinsky P.F., Andriole G.L., Kramer B.S., Hayes R.B., Prorok P.C., Gohagan J.K. Prostate biopsy following a positive screen in the prostate, lung, colorectal and ovarian cancer screening trial. J Urol. 2005;173:746–750. doi: 10.1097/01.ju.0000152697.25708.71. discussion 750–1. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson L., Ahmed H.U., Allen C. Clinical applications of multiparametric MRI within the prostate cancer diagnostic pathway. Urol Oncol. 2013;31:281–284. doi: 10.1016/j.urolonc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan M.A., Partin A.W., Rittenhouse H.G. Evaluation of proprostate specific antigen for early detection of prostate cancer in men with a total prostate specific antigen range of 4.0 to 10.0 ng/ml. J Urol. 2003;170:723–726. doi: 10.1097/01.ju.0000086940.10392.93. [DOI] [PubMed] [Google Scholar]
- 40.Verhamme K.M., Dieleman J.P., Bleumink G.S. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care—the Triumph project. Eur Urol. 2002;42:323–328. doi: 10.1016/s0302-2838(02)00354-8. [DOI] [PubMed] [Google Scholar]
- 41.Nam R.K., Saskin R., Lee Y. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189:S12–S17. doi: 10.1016/j.juro.2012.11.015. discussion S17–8. [DOI] [PubMed] [Google Scholar]
- 42.Rosario D.J., Lane J.A., Metcalfe C. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ. 2012;344:d7894. doi: 10.1136/bmj.d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoedemaeker R.F., van der Kwast T.H., Boer R. Pathologic features of prostate cancer found at population-based screening with a four-year interval. J Natl Cancer Inst. 2001;93:1153–1158. doi: 10.1093/jnci/93.15.1153. [DOI] [PubMed] [Google Scholar]
- 44.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, editors. Globocan 2008: cancer incidence and mortality worldwide. IARC CancerBase no. 10. International Agency for Research on Cancer Web site. http://globocan.iarc.fr/factsheets/cancers/prostate.asp. Accessed January 3, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.