Abstract
Early origins of adult disease may be defined as adversity or challenges during early life that alter physiological responses and prime the organism to chronic disease in adult life. Adverse childhood experiences or early life stress (ELS) may be considered a silent independent risk factor capable of predicting future cardiovascular disease risk. Maternal separation (Mat-Sep) provides a suitable model to elucidate the underlying molecular mechanisms by which ELS increases the risk to develop cardiovascular disease in adulthood. The aim of this review is to describe the links between behavioural stress early in life and chronic cardiovascular disease risk in adulthood. We will discuss the following: (i) adult cardiovascular outcomes in humans subjected to ELS, (ii) Mat-Sep as an animal model of ELS as well as the limitations and advantages of this model in rodents and (iii) possible ELS-induced mechanisms that predispose individuals to greater cardiovascular risk. Overall, exposure to a behavioural stressor early in life sensitizes the response to a second stressor later in life, thus unmasking an exaggerated cardiovascular dysfunction that may influence quality of life and life expectancy in adulthood.
Keywords: adverse childhood experience, cardiovascular risk, early life stress, maternal separation
Developmental origins of adult disease may be defined as challenges during early life that alter physiological responses and potentially prime the organism to chronic disease in adult life. These atypical signalling pathways result from developmental plasticity, which is the ability of a single genotype to produce more than one alternative form of structure and/or function in response to environmental conditions (Barker et al. 2002, Gluckman & Hanson 2004, McMillen & Robinson 2005, Nuyt 2008, Nuyt & Alexander 2009).
In addition to the traditional adverse environmental factors, such as chemical compounds, toxins and diet, behavioural stress also has a significant impact in the developing organism (Forsdahl 1979, Felitti 1993, Dong et al. 2004). Specifically, early life stress (ELS), defined as adverse childhood experience(s) capable of inducing behavioural and emotional stress, anxiety, fear and discomfort in the individual (Dong et al. 2004, Pace et al. 2006), changes the sensitivity of certain physiological responses that will precipitate an adaptive response to an environmental stressor later in life. Overall, compelling data suggest that repeated exposure to adversity in early life programmes a ‘defensive’ phenotype, which displays a functional resistance to stress-induced responses (Levine 2005, Lyons et al. 2010). However, in the context of late-life chronic disease, dysregulation of certain signalling pathways [i.e. inflammatory system and hypothalamic-pituitary-adrenal (HPA) axis] may favour exaggerated responses that contribute to the pathogenesis of cardiovascular disease, some types of cancer and respiratory disease (Miller et al. 2009).
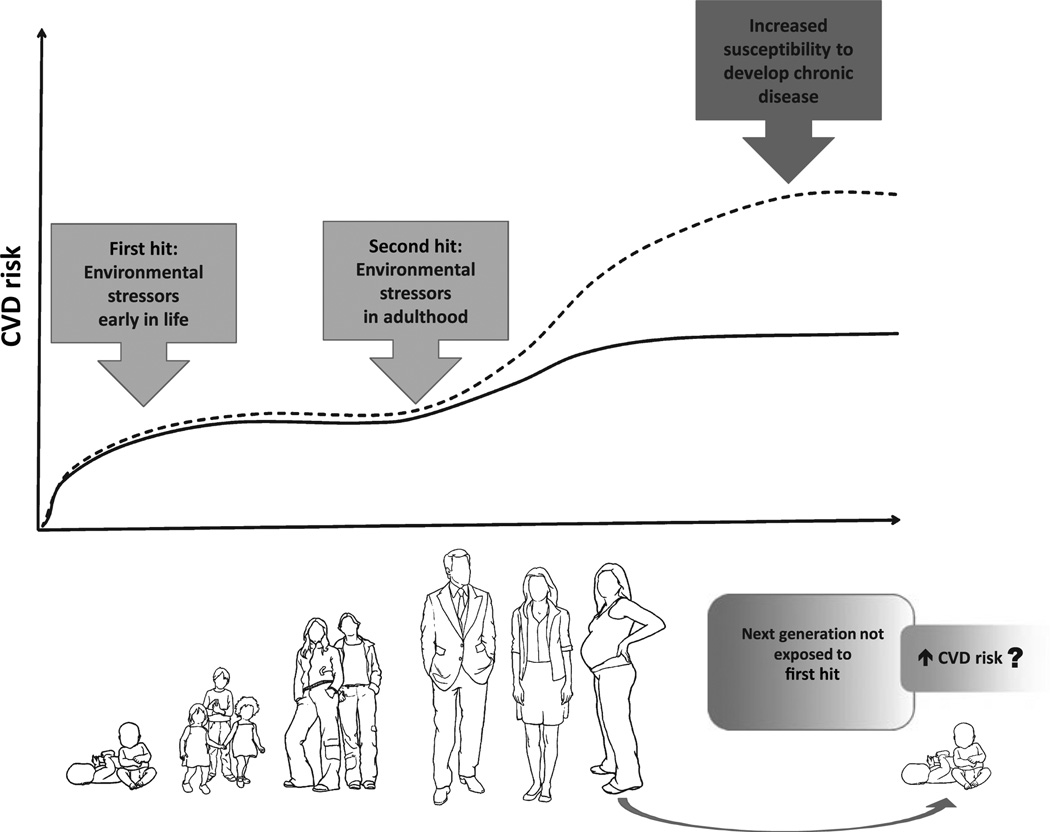
The effects of ELS on the endocrine, inflammatory and neural system have been studied (Lindgarde et al. 1987). We propose the term cardiovascular priming to describe the relationship of early life behavioural stress and the subsequent sensitivity to cardiovascular outcomes. Adverse early life experiences would be considered a first ‘hit’ and may exert cardiovascular priming that may increase the susceptibility to over-or under-react in response to a secondary stressor, or second ‘hit’, in adulthood (Fig. 1). We identify ELS as a key component in the shaping of the adult capacity to maintain cardiovascular homeostasis.
Figure 1.
Schematic of cardiovascular risk during the time course of life. Individuals exposed to a first stressor during early life are more susceptible to secondary stressors later in life. The impact in the phenotype may induce transgenerational effects.
The aim of this review is to describe the current possible mechanistic link(s) of behavioural stress early in life and chronic cardiovascular disease risk in adulthood. We will discuss the following: (i) adult cardiovascular outcomes in humans subjected to ELS, (ii) maternal separation (MatSep) as an animal model of ELS as well as the limitations and advantages of this model in rodents and, (iii) possible ELS-induced mechanisms that predispose individuals to greater cardiovascular risk as well as the role of the epigenome and the possible transgenerational effects of ELS and cardiovascular risk.
ELS and adult cardiovascular outcomes in humans
Early life stress, commonly occurring in the first decade of life, is induced by behavioural stressors or adverse childhood experiences and is highly correlative with ischaemic heart disease in adulthood, more so than the traditional risk factors (Taylor & Seeman 1999, Chida & Steptoe 2009, Alciati et al. 2011). Examples of behavioural stressors include natural disasters such as flooding, fires and earthquakes, physical and sexual abuse, witnessing violence, parents’ divorce, parental loss or intrusive medical interventions. Importantly, low socio-economic status as well is a factor that strongly correlates with the presence of adversity and has been used to approximate ELS in many epidemiological studies (Taylor & Seeman 1999, Holness et al. 2000, Dong et al. 2004, Kelishadi et al. 2009, Chen et al. 2011, Alastalo et al. 2012).
Cohort retrospective studies that describe major associations between childhood adverse experiences and adult cardiovascular risk outcomes are summarized in Table 1A. ELS consistently demonstrated a cardiovascular risk phenotype, including exacerbation of inflammatory markers and lipid profiles that may enhance the underlying risk of cardiovascular disease (Danese et al. 2007, Kelishadi et al. 2009, Chen et al. 2011, Vig et al. 2010, Alastalo et al. 2013).
Table 1.
Clinical studies in cohorts exposed to ELS
| (A) Retrospective studies | ||||
|---|---|---|---|---|
| Cohort study | Stress period | Evaluation | Population | Outcomes |
| Wales and Sweden | 1751–1930 | 1934 | UK Sweden |
Death rates fell as a result of better childhood living conditions (Kermack et al. 1934) |
| The Tromsø Study | 1890 | 1964–1967 1994–2001 |
Norway | Positive correlation between childhood poverty/poor living conditions and adult coronary and atherosclerotic heart disease 3–6 decades later (Forsdahl 1978, Forsdahl et al. 2009) |
| British Birth | 1946 | 1985 | UK | Low socio-economic status correlated with higher blood pressure in 40-year-old patients (Wadsworth et al. 1985, Hardy et al. 2004, Kuh et al. 2008) |
| Helsinki Birth | 1934–1944 | 2011 | Finland | Participants separated during childhood for safety reasons used medications for coronary heart disease more frequently 60 year later (Alastalo et al. 2009, 2012, 2013) |
| Guangzhou Biobank | 2003 | 2008 | China | ELS was negatively associated with some cardiovascular risk factors, particularly among men (Schooling et al. 2011) |
| (B) Prospective studies | ||||
|---|---|---|---|---|
| Study name | Start | End | Population | Outcomes |
| Dunedin health and development |
1972 | 2006 | New Zealand | Childhood maltreatment is an independent risk factor for inflammation, a mediator in the development of CVD (Danese et al. 2007) |
| 1958 British Birth | 1958 | 1991 | UK | Study of combined social and biological risk factors revealed that increments across the distribution of childhood cognition are associated with the improvements in cardiovascular risk profile in midlife (Power et al. 1987, 2002, Power & Elliott 2006) |
| ABCD-Amsterdam | 2003 | 2014 | Holland | Explains socio-economic inequalities in childhood blood pressure and pre-hypertension (van Eijsden et al. 2011) |
| ELS clinic Stanford | Ongoing | USA | Early interventions for children and families who have experienced an acute or chronic stressor or traumatic event |
|
| Generation R | 2002 | Ongoing | Holland | Examines the growth, development and health of 10 000 children in a multi-ethnic population to identify early environmental and genetic causes of normal and abnormal growth and development |
| Adverse childhood experiences (ACE) |
1995 | Ongoing | USA | Over 17 000 members studied revealed a dose–response relationship between ACE and increased ischaemic heart disease risk (Felitti et al. 1998, Dong et al. 2004, Anda et al. 2009) |
Currently, several cohort studies are designed to collect and analyse data provided by children associated with specific risk factors (Table 1B). There is a web site to find information regarding several cohort studies ongoing at http://www.birthcohorts.net. The main objectives of these prospective studies are to investigate children’s health and ethnic disparities at birth as well as in later life, maternal lifestyle, medical, psycho-social and environmental post-natal conditions that may elucidate children’s health in later life. These investigations are critical as prospective studies will allow a better understanding of the cause and effect in the adult cardiovascular outcomes of children exposed to ELS.
Maternal Separation as an animal model of ELS
The mitigating effect of ELS on both behavioural and neuroendocrine parameters in animal models representing extremes in trait anxiety might reflect an evolutionary advantage that is sustained as the genetic variability among individuals of different species. This adaptation would allow adequate responses to potentially dangerous stimuli in adulthood dependent on early life conditions. There are studies to show that ELS confers resiliency to later life stressors (Lyons et al. 2010). However, ELS may also exacerbate stress-induced responses, such as enhanced inflammatory or sympathetic responses, sensitizing the individual to future disease risk.
In humans, ELS has long been linked to anxiety disorders and depression in adulthood (Nugent et al. 2011, Shapero et al. 2013). Animal models of ELS have been extensively used to study the effects of ELS on the development of neurological disorders such as depression and anxiety disorders in adults. Adult and juvenile rats and mice exposed to ELS display heightened reactivity to stressors, increased anxiety as well as depression-like behaviours such as reduced anhedonia (George et al. 2010, Uchida et al. 2010, Schmidt et al. 2011). These ELS-induced pro-anxiety and prodepression traits have been suggested to correlate with the promotion of cardiovascular outcomes such as stroke, atherosclerosis and hypertension (Beutel et al. 2013, Rahman et al. 2013); however, these parameters have not been consistently measured and reported. Thus, validating the use of animal models of ELS to study parameters may contribute to the development of cardiovascular disease in adult life.
There have been many different types of alterations in maternal care with animal models used to approximate childhood adversity in humans. Two major approaches include (i) modifying the time spent with the dam by physically separating the dam and the pup, and (ii) modulating the quality of maternal care. The former approach, called MatSep, is a chronic behavioural stress model that involves temporarily separating or isolating offspring from the dam daily during the early post-natal life. MatSep has been performed in non-human primates, rabbits, pigs, guinea-pig, birds, and most extensively in rats and mice (Table 2). The variety of species, length of separation protocol as well as the time point during which experimental observations were made (i.e. neonatal, juvenile or adult) have resulted in a wide range of aberrant phenotypes described in the literature (Table 2). The most consistently used models are rodents due to their availability, cost-effectiveness and common use in physiological and molecular studies; thus, we will focus the remainder of our discussion on the rodent models.
Table 2.
Comparative models of maternal separation in different species
| Species | Model | Affected parameters | References |
|---|---|---|---|
| Non-human primate |
Maternal separation | Juvenile and adult social responses |
Feng et al. (2011), Spencer-Booth & Hinde (1971), Bernstein & Dobrofsky (1981), Laudenslager et al. (1982), Lyons et al. (2001), Rilling et al. (2001) and Parker et al. (2005, 2007) |
| Serotonin transporter expression | |||
| Pig | Early weaning | Stress-related gene expression in brain |
Kanitz et al. (2004), Tuchscherer et al. (2004, 2006), Moeser et al. (2007), Kanitz et al. (2009) and McLamb et al. (2013) |
| Maternal separation | Neuroendocrine responses | ||
| Immunological responses | |||
| Rabbit | Maternal separation | Neonatal metabolic parameters | Cano et al. (2005a,b) and Rebollar et al. (2006) |
| Circulating stress hormones | |||
| Rat | Maternal separation | Neonatal metabolic parameters |
Tucker & Johnson (1984), Lehmann et al. (2000), Lippmann et al. (2007), Loria et al(2010a,b, 2011, 2013), Desbonnet et al. (2008) and O’Mahony et al. (2008) |
| Adult anxiety | |||
| HPA axis | |||
| Renal hemodynamics | |||
| Vascular inflammation | |||
| Vascular reactivity | |||
| Mouse | Maternal separation | Adult anxiety |
Carlyle et al. (2013), George et al. (2010) and Savignac et al. (2011) |
| Maternal separation with early weaning |
Brain chromatin remodelling senzymes (HDACs) |
||
| Gastrointestinal inflammatory profile Immunological response to infection |
|||
| Guinea-pig | Early weaning | Pup stress hormones |
Hennessy (1988), Hennessy et al(2010, 1989, 2007), Hennessy & Sharp (1990) and Tamborski et al. (1990) |
| Maternal separation | Circulating cortisol levels and vocalization in novel environment |
||
| Core temperature and behaviour | |||
| Bird | Maternal separation | Adult hypothalamic-pituitary-adrenal axis activation | Banerjee et al. (2012) and Spencer et al. (2009) |
| Corticosterone administration | |||
| Mate choice |
Maternal separation in rats involves the removal of pups from the dam for 3–4 h a day starting at postnatal day 2 (P2) and ending at P14 (Lehmann et al. 2000, Lippmann et al. 2007, Loria et al. 2010a). Mat-Sep is overwhelmingly utilized as a model of ELS in behavioural studies. This protocol has yielded fairly consistent results in rats across many studies with regard to the behavioural outcomes in adults. MatSep in mice, however, have yielded highly variable behavioural outcomes in adults, and currently, there is an impetus to determine the optimal MatSep model in mice. It appears that mice are more resistant to Mat-Sep when compared with rats and must be separated for longer periods of time to induce behavioural outcomes as adults (George et al. 2010). Also, strain differences in MatSep mice have been extensively explored (Labarba et al. 1973, Savignac et al. 2011). For example, the innately anxious Balb-c mice appear to be more susceptible to the cognitive ill-effects of MatSep than C57BL6 mice, such as reduced recognition and spatial working memory (Mehta & Schmauss 2011).
Limitations and advantages of maternal separation as a model of ELS
As with many animal models of human disease, there are limitations; thus, we must be intimately aware of these to prevent overinterpretation of results gained from these valuable models. Although MatSep is often considered a model that induces a negative effect in stress-related emotional, metabolic and cardiovascular responses, numerous studies propose that chronic exposure to behavioural stress does not result in vulnerability but instead exaggerates arousal regulation and resilience (Levine 2005, Lyons et al. 2010), which will serve as a adaptive mechanism to fight stressors later in life. Nevertheless, we hypothesize that such exacerbated responses mediate the sensitization of the cardiovascular system.
In rodents, developmental stages during early postnatal life are equivalent to the third trimester in humans. A major criticism of MatSep is that it does not strictly represent childhood adversity in humans in terms of developmental stages. Yet the post-natal period in rodents involves sucking and the exposure to outputs from the external environment. In this regard, the developmental plasticity attained during the rodent’s neonatal period heightens the impact of the ELS, exerting profound changes in the organ function. In essence, there is a mismatch of environment and developmental period. This mismatch may explain why there is a disparity in effects between rodent models and primate models (Lyons et al. 2010). In primates, some researchers have detected resilience rather than a behavioural instability in the face of MatSep (Lyons et al. 2010, Own & Patel 2012). However, this mismatch of development and environment between rodents and humans provides a useful experimental model to perform interventions avoiding in utero manipulations. Moreover, the renal development in rodents is completed in the early post-natal life during the hyporesponsive period. It is known that early development between mice and rats is slightly different. For instance, the stress hyporesponsive period is different between mice (PD1–PD12) and rats (PD4–PD14). These differences may be responsible for the disparity of outcomes, with mice being much more resistant to the effects of MatSep than rats (Savignac et al. 2011).
Concerning behavioural stress, animal models are simplified paradigms of integrated responses compared with humans. Stress in rodents displays a lack of length, memory and spatial perception that influences the short- and long-term adaptation to adversity. Emotional stress in humans is often self-perpetuated from an initial stressor. In other animal species, stressors are often acute and non-self-perpetuated. MatSep certainly does not fully represent the spectrum of early life adversity in humans such as physical abuse, sexual abuse, mental abuse, natural disaster, war-time atrocities, etc.
Alterations in maternal warmth induce changes in molecular mediators regulating the HPA axis sensitivity in offspring (Caldji et al. 2000). The dam’s separation from the pups may change or disturb their normal maternal care, enhancing the effects of the stressor in the pups once they are retuned with the litter (Meaney 2001, Champagne et al. 2004); however, a thorough in-depth study of this phenomenon is lacking. Some insight can be gained from rabbit models of MatSep where maternal lactation and circulating hormones have been shown to change during separation from pups (Cano et al. 2005b, Rebollar et al. 2006).
Although a number of concerns are pointed out, MatSep in rodents is currently one of the best approaches to model ELS in humans. Cardiovascular outcomes of the rodent model of MatSep closely mirrors epidemiological data regardless of the nature of the early life stress, lending support for the legitimacy of the model (Mascitelli et al. 2006). For example, in the human literature, ELS induces heightened inflammatory status that parallels what is observed in rodent models of ELS (Pace et al. 2006, Danese et al. 2007, O’Mahony et al. 2009, Herbert et al. 2012). Also, rodent models mimic the behavioural outcome of ELS in humans such as anxiety and depression (Uchida et al. 2010, Schmidt et al. 2011, Heim & Binder 2012). Our data now provide evidence that similar to humans, rodents exposed to MatSep have increased risk of developing hypertension and cardiovascular pathologies in adulthood. Given these similar outcomes, we conclude that MatSep is an appropriate model to study the molecular mechanisms by which ELS enhances adult cardiovascular disease risk.
Molecular alterations due to Maternal Separation in rodents
Early life stress can influence mechanism(s) of metabolic and mental disorders as well as cardiovascular disease in animal models (Tucker & Johnson 1984, Kaufman et al. 2007, Sanders & Anticevic 2007, Enthoven et al. 2008a, Samuelsson et al. 2008, Loria et al. 2010a). In rodents, MatSep has been shown to alter adult anxiety through changes in the HPA axis, sympathetic-adrenal-medullary (SAM) system, brain, SNS and general neuroendocrine function. These changes are likely to contribute to MatSep-induced cardiovascular outcomes (Loria et al. 2010b, 2011). Table 3 shows specific molecular alterations that Mat-Sep displays in adult rats related to the cardiovascular function and blood pressure control. Our group has reported consistently with others that MatSep rats display greater responsiveness to acute behavioural stress as well as to prohypertensive stimuli as adults. We showed that MatSep rats that lack functioning endothelin type B receptor display a blunted acute air-jet stress-induced rise in blood pressure when compared with MatSep wild-type control rats (Loria et al. 2010a). In addition, others have shown that heart rate is elevated in response to acute stress in MatSep borderline hypertensive rats (Sanders & Anticevic 2007).
Table 3.
Molecular alterations due to MatSep reported in rodents
| Molecular and/or structural alteration | Functional alteration |
|---|---|
| Central nervous system | |
| Hipoccampus: c-Fos, BDNF, serotonin, CREB. | Depression, anxiety, mood disorders |
| Prefrontal cortex: GR receptor, cytochrome oxidase. | Hypercapnic ventilatory response. |
| Dysregulation of the HPA axis sensitivity (Lippmann et al. 2007, Meaney et al. 2007, Genest et al. 2004, O’Mahony et al. 2008). |
|
| HPA axis and SAM system | |
| Increased plasma corticosterone, CRH, ACTH. | Exaggerated behavioral and cardiovascular stress-related response (Enthoven et al. 2008b, Plotsky and Meaney, 1993, Renard et al. 2007). |
| Epigenetic regulation of FKBP5 gene. | |
| Increased risk of adult psychiatric disorders, altered stress regulation, altered immune cell function (Klengel et al. 2013). |
|
| Renal and cardiovascular system | |
| Increased left ventricle weight and capillary density. | Mild effects on cardiomyocyte hypertrophy and myocardial |
| Lower AT2 receptor mRNA expression and function | fibrosis (Trombini et al. 2012). |
| in vasculature. | Reduced NO buffering capacity and increased AngII-induced |
| Greater renal NE-induced rise in blood pressure. | constriction (Loria et al. 2011, Loria et al. 2010a, Loria et al. 2010b). |
| Lower renal filtration capacity. Impaired chronic blood pressure control (Loria et al. 2013). |
|
| Immune system | |
| Increased IL-1, IL-6, TNFa in response to immune challenge. |
Exaggerated response to LPS and E coli infection (Meagher et al. 2010). |
| Metabolic regulation | |
| Reduced insulin and HOMA levels | Insulin resistance and metabolic syndrome |
| Higher adiponutrin, leptin and peroxisome proliferator-activated receptor gamma coactivator 1 alpha. |
Increased body weight and metabolic syndrome (Spivey et al. 2011). |
BDNF, brain derived neurotrophic factor; CREB, cyclic AMP response element binding protein; GR, glucocorticoid receptor; CRH, corticotropin releasing hormone; ACTH, Adrenocorticotropic hormone; FKBP5, FK506 binding protein; AT2: Angioten-sin II receptor type 2; NE, norepinephrine; IL-1, interleukin 1; IL-6, interleukin 6; TNFa, tumor necrosis factor alpha; HOMA, homeostatic model assessment; HPA, hypothalamic pituitary adrenal axis; NO, nitric oxide; LPS, lipopolysaccharide.
Male MatSep WKY rats display an exaggerated response to angiotensin II (AngII)-induced responses ex vivo and in vivo (Loria et al. 2010b, 2011). Aortic vascular tissue from MatSep rats showed a greater vasoconstrictive response than tissue from control rats. In addition, the nitric oxide buffering capacity, necessary for a normal vascular function, is reduced in MatSep rats. In telemetry-instrumented rats, a chronic infusion of AngII induced an exacerbated hypertension, renal vascular damage and renal T cell infiltration. We reported that MatSep rats have reduced renal filtration capacity that is mediated by renal nerve activation (Loria et al. 2013). Thus, we proposed that MatSep in male rats induces a deranged renal response to prohypertensive secondary stressors. Interestingly, female MatSep rats also display enhanced AngII-induced hypertension; however, there is not a clear link suggesting a renal or sex hormone-dependent mechanism. Further investigations are required to elucidate the mechanism by which female MatSep rats are more susceptible to a prohypertensive stimuli.
Is the ELS-induced vascular dysfunction phenotype transmitted to the next generation?
Traditionally, definitions of inheritance have been limited to the passing of genetic information from one generation to the next. The long-term consequences of adverse social experiences during early life on maternal behaviour induce a mechanism by which traits can also be ‘inherited’ by the forthcoming generations. This epigenetic mechanism induces functionally relevant modifications of the genetic code. Examples of such modifications are DNA methylation and histone modification, both of which serve to regulate gene expression without altering the underlying DNA sequence. Thus, epigenetics is an emerging area of study that is relevant to many of the functional outcomes involved in the programming of the adult phenotype (Champagne & Meaney 2001, Cameron et al. 2008, Bogdarina et al. 2010).
A large body of literature shows that maternal behaviour mediates epigenetic changes in the offspring, especially with respect to the HPA axis and SAM system, specifically glucocorticoid receptor (GR) expression in the frontal cortex of the brain and the regulation of GR activity (Weaver et al. 2004, Meaney et al. 2007, Cameron et al. 2008, Szyf et al. 2005, Navailles et al. 2010, Klengel et al. 2013). Hippocampal samples were compared from deceased people who experienced childhood abuse and committed suicide, comparing these with non-abused controls (Suderman et al. 2012). DNA methylation profiles were studied for over 6.5 million base pairs around a locus that houses the GR gene. Childhood abuse alters HPA stress responses and increases the risk of suicide, both of which are associated with the epigenetic regulation of hippocampal GR expression (Fish et al. 2004, McGowan et al. 2009, Murgatroyd et al. 2009). More recently, Klengel et al. (2013) have shown that childhood trauma is linked to increased DNA demethylation of glucocorticoid response elements of the FK506 binding protein 5 (FKBP5) gene, which is correlated with an increased risk of developing adult psychiatric disorders (Klengel et al. 2013). These data lend strong support for the hypothesis that ELS-induced adult cardiovascular disease risk has an epigenetic basis and thus may be transmitted transgenerationally.
Conclusions
Maternal separation in animals provides a model to elucidate the underlying molecular mechanisms by which ELS increases the susceptibility to develop cardiovascular disease. MatSep in rodents is a unique approach to model ELS, although there are limitations (Lippmann et al. 2007) that should be recognized. Some of the ELS-induced changes may not be functionally observed during ‘normal’ conditions of adult life, the exposure to a secondary challenge or stressor may induce an exaggerated physiological response and predispose the individual to greater cardiovascular risk. Therefore, exposure to a behavioural stressor early in life may not significantly influence the overall adult cardiovascular phenotype until a second stressor later in life reveals an exaggerated cardiovascular dysfunction that may influence the quality of life and life expectancy in later in adulthood. Animal studies will contribute to make a positive difference in health improvement by identifying potential mechanisms as targets and/or biomarkers for cardiovascular disease risk.
Footnotes
Conflict of interest
There is no conflict of interest to report.
References
- Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Kajantie E, Heinonen K, Forsen TJ, Eriksson JG. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med. 2009;41:66–72. doi: 10.1080/07853890802301983. [DOI] [PubMed] [Google Scholar]
- Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Heinonen K, Kajantie E, Eriksson JG. Cardiovascular morbidity and mortality in Finnish men and women separated temporarily from their parents in childhood–a life course study. Psychosom Med. 2012;74:583–587. doi: 10.1097/PSY.0b013e31825b3d76. [DOI] [PubMed] [Google Scholar]
- Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Heinonen K, Kajantie E, Eriksson JG. Early life stress and blood pressure levels in late adulthood. J Hum Hypertens. 2013;27:90–94. doi: 10.1038/jhh.2012.6. [DOI] [PubMed] [Google Scholar]
- Alciati A, Gesuele F, Casazza G, Foschi D. The relationship between childhood parental loss and metabolic syndrome in obese subjects. Stress Health. 2011;29:5–13. doi: 10.1002/smi.1435. [DOI] [PubMed] [Google Scholar]
- Anda RF, Dong M, Brown DW, Felitti VJ, Giles WH, Perry GS, Valerie EJ, Dube SR. The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health. 2009;9:106. doi: 10.1186/1471-2458-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SB, Arterbery AS, Fergus DJ, Adkins-Regan E. Deprivation of maternal care has long-lasting consequences for the hypothalamic-pituitary-adrenal axis of zebra finches. Proc Biol Sci. 2012;279:759–766. doi: 10.1098/rspb.2011.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Bernardi JR, Ferreira CF, Senter G, Krolow R, de Aguiar BW, Portella AK, Kauer-Sant’anna M, Kapczinski F, Dalmaz C, Goldani MZ, Silveira PP. Early life stress interacts with the diet deficiency of omega-3 fatty acids during the life course increasing the metabolic vulnerability in adult rats. PLoS One. 2013;8:e62031. doi: 10.1371/journal.pone.0062031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS, Dobrofsky M. Compensatory social responses of older pigtailed monkeys to maternal separation. Dev Psychobiol. 1981;14:163–168. doi: 10.1002/dev.420140210. [DOI] [PubMed] [Google Scholar]
- Beutel ME, Wiltink J, Kirschner Y, Sinning C, Espin-ola-Klein C, Wild PS, Munzel T, Blettner M, Zwiener I, Lackner K, Michal M. History of depression but not current depression is associated with signs of atherosclerosis: data from the Gutenberg Health Study. Psychol Med. 2013:1–7. doi: 10.1017/S0033291713001542. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bogdarina I, Haase A, Langley-Evans S, Clark AJ. Glucocorticoid effects on the programming of AT1b angiotensin receptor gene methylation and expression in the rat. PLoS One. 2010;5:e9237. doi: 10.1371/journal.pone.0009237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J Neuroendocrinol. 2008;20:795–801. doi: 10.1111/j.1365-2826.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- Cano P, Jimenez V, Alvarez MP, Alvarino M, Cardinali DP, Esquifino AI. Effect of litter separation on 24-hour rhythmicity of plasma prolactin, follicle-stimulating hormone and luteinizing hormone levels in lactating rabbit does. J Circadian Rhythms. 2005a;3:9. doi: 10.1186/1740-3391-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano P, Jimenez-Ortega V, Alvarez MP, Alvarino M, Cardinali DP, Esquifino AI. Effect of rabbit doe-litter separation on 24-hour changes of luteinizing hormone, follicle stimulating hormone and prolactin release in female and male suckling pups. Reprod Biol Endocrinol. 2005b;3:50. doi: 10.1186/1477-7827-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, Papademetris X, Hyder F, Taylor JR, Simen AA. Maternal separation with early weaning: a rodent model providing novel insights into neglect associated developmental deficits. Dev Psychopathol. 2013;24:1401–1416. doi: 10.1017/S095457941200079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socio-economic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol. 2009;53:936–946. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci. 2008;26:259–268. doi: 10.1016/j.ijdevneu.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- van Eijsden M, Vrijkotte TG, Gemke RJ, van der Wal MF. Cohort profile: the Amsterdam Born Children and their Development (ABCD) study. Int J Epidemiol. 2011;40:1176–1186. doi: 10.1093/ije/dyq128. [DOI] [PubMed] [Google Scholar]
- Enthoven L, de Kloet ER, Oitzl MS. Differential development of stress system (re)activity at weaning dependent on time of disruption of maternal care. Brain Res. 2008a;1217:62–69. doi: 10.1016/j.brainres.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Enthoven L, Oitzl MS, Koning N, van der Mark M, de Kloet ER. Hypothalamic-pituitary-adrenal axis activity of newborn mice rapidly desensitizes to repeated maternal absence but becomes highly responsive to novelty. Endocrinology. 2008b;149:6366–6377. doi: 10.1210/en.2008-0238. [DOI] [PubMed] [Google Scholar]
- Felitti VJ. Childhood sexual abuse, depression, and family dysfunction in adult obese patients: a case control study. South Med J. 1993;86:732–736. doi: 10.1097/00007611-199307000-00002. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Feng X, Wang L, Yang S, Qin D, Wang J, Li C, Lv L, Ma Y, Hu X. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci USA. 2011;108:14312–14317. doi: 10.1073/pnas.1010943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Forsdahl A. Living conditions in childhood and subsequent development of risk factors for arteriosclerotic heart disease. J Epidemiol Community Health. 1978;32:34–37. doi: 10.1136/jech.32.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdahl A. Are poor living conditions in childhood and adolescence and important risk factor for arteriosclerotic heart disease? Int J Rehabil Res. 1979;2:238–239. doi: 10.1097/00004356-197905000-00008. [DOI] [PubMed] [Google Scholar]
- Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and sex-specific plasticity of the hypoxic ventilatory response in awake rat. J Physiol. 2004;554:543–557. doi: 10.1113/jphysiol.2003.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 2010;11:123. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hardy R, Wadsworth ME, Langenberg C, Kuh D. Birthweight, childhood growth, and blood pressure at 43 years in a British birth cohort. Int J Epidemiol. 2004;33:121–129. doi: 10.1093/ije/dyh027. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Both prevention of physical contact and removal of distal cues mediate cortisol and vocalization responses of guinea pig pups to maternal separation in a novel environment. Physiol Behav. 1988;43:729–733. doi: 10.1016/0031-9384(88)90369-1. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Sharp K. Voluntary and involuntary maternal separation in guinea pig pups with mothers required to forage. Dev Psychobiol. 1990;23:783–796. doi: 10.1002/dev.420230803. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Carlisle CW, O’Brien E. Maternal separation produces, and a second separation enhances, core temperature and passive behavioral responses in guinea pig pups. Physiol Behav. 2010;100:305–310. doi: 10.1016/j.physbeh.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Tamborski A, Schiml P, Lucot J. The influence of maternal separation on plasma concentrations of ACTH, epinephrine, and norepinephrine in guinea pig pups. Physiol Behav. 1989;45:1147–1152. doi: 10.1016/0031-9384(89)90101-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml-Webb PA, Miller EE, Maken DS, Bullinger KL, Deak T. Anti-inflammatory agents attenuate the passive responses of guinea pig pups: evidence for stress-induced sickness behavior during maternal separation. Psychoneuroendocrinology. 2007;32:508–515. doi: 10.1016/j.psyneuen.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert C, Siegle JS, Shadie AM, Nikolaysen S, Garthwaite L, Hansbro NG, Foster PS, Kumar RK. Development of asthmatic inflammation in mice following early-life exposure to ambient environmental particulates and chronic allergen challenge. Dis Model Mech. 2012;6:479–488. doi: 10.1242/dmm.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness MJ, Langdown ML, Sugden MC. Early-life programming of susceptibility to dysregulation of glucose metabolism and the development of Type 2 diabetes mellitus. Biochem J. 2000;349(Pt 3):657–665. doi: 10.1042/bj3490657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanitz E, Tuchscherer M, Puppe B, Tuchscherer A, Stabenow B. Consequences of repeated early isolation in domestic piglets (Sus scrofa) on their behavioural, neuroendocrine, and immunological responses. Brain Behav Immun. 2004;18:35–45. doi: 10.1016/s0889-1591(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Kanitz E, Puppe B, Tuchscherer M, Heberer M, Vier-gutz T, Tuchscherer A. A single exposure to social isolation in domestic piglets activates behavioural arousal, neuroendocrine stress hormones, and stress-related gene expression in the brain. Physiol Behav. 2009;98:176–185. doi: 10.1016/j.physbeh.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Banerji MA, Shorman I, Smith EL, Coplan JD, Rosenblum LA, Kral JG. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes. 2007;56:1382–1386. doi: 10.2337/db06-1409. [DOI] [PubMed] [Google Scholar]
- Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Kermack WO, McKendrick AG, McKinlay PL. Death rates in Great Britain and Sweden. Expression of specific mortality rates as products of two factors and some consequences thereof. J Hyg (Lond) 1934;34:433–457. doi: 10.1017/s0022172400043230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Mishra GD, Black S, Lawlor DA, Davey Smith G, Okell L, Wadsworth M, Hardy R. Offspring birth weight, gestational age and maternal characteristics in relation to glucose status at age 53 years: evidence from a national birth cohort. Diabet Med. 2008;25:530–535. doi: 10.1111/j.1464-5491.2008.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarba RC, Hibbs SE, White JL. Maternal separation and emotional reactivity in BALB-c mice. Psychol Rep. 1973;32:107–110. doi: 10.2466/pr0.1973.32.1.107. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Reite M, Harbeck RJ. Suppressed immune response in infant monkeys associated with maternal separation. Behav Neural Biol. 1982;36:40–48. doi: 10.1016/s0163-1047(82)90223-0. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res. 2000;107:133–144. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Lindgarde F, Furu M, Ljung BO. A longitudinal study on the significance of environmental and individual factors associated with the development of essential hypertension. J Epidemiol Community Health. 1987;41:220–226. doi: 10.1136/jech.41.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Loria AS, D’Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol. 2010a;299:R185–R191. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension. 2010b;55:494–499. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasocon-striction by reduced endothelial nitric oxide buffering capacity. Hypertension. 2011;58:619–626. doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria AS, Brands MW, Pollock DM, Pollock JS. Early life stress sensitizes the renal and systemic sympathetic system in rats. Am J Physiol Renal Physiol. 2013;305:F390–F395. doi: 10.1152/ajprenal.00008.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Sawyer-Glover AM, Moseley ME, Schatzberg AF. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: implications for understanding resilience. Dev Psychobiol. 2010;52:616–624. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascitelli L, Pezzetta F, Sullivan JL. Why women live longer than men: sex differences in longevity. Gend Med. 2006;3:341. doi: 10.1016/s1550-8579(06)80221-4. author reply 342. [DOI] [PubMed] [Google Scholar]
- Mathieu G, Oualian C, Denis I, Lavialle M, Gisquet-Verrier P, Vancassel S. Dietary n-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot Essent Fatty Acids. 2011;85:129–136. doi: 10.1016/j.plefa.2011.07.001. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS One. 2013;8:e59838. doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Sieve AN, Johnson RR, Satterlee D, Belyavskyi M, Mi W, Prentice TW, Welsh TH, Jr, Welsh CJ. Neonatal maternal separation alters immune, endocrine, and behavioral responses to acute The-iler’s virus infection in adult mice. Behav Genet. 2010;40:233–249. doi: 10.1007/s10519-010-9333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mehta M, Schmauss C. Strain-specific cognitive deficits in adult mice exposed to early life stress. Behav Neurosci. 2011;125:29–36. doi: 10.1037/a0021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nich-olls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, Blikslager AT. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G173–G181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bock-muhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Navailles S, Zimnisky R, Schmauss C. Expression of glucocorticoid receptor and early growth response gene 1 during postnatal development of two inbred strains of mice exposed to early life stress. Dev Neurosci. 2010;32:139–148. doi: 10.1159/000293989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene-environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology. 2011;214:175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuyt AM. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin Sci (Lond) 2008;114:1–17. doi: 10.1042/CS20070113. [DOI] [PubMed] [Google Scholar]
- Nuyt AM, Alexander BT. Developmental programming and hypertension. Curr Opin Nephrol Hypertens. 2009;18:144–152. doi: 10.1097/MNH.0b013e328326092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony S, Chua AS, Quigley EM, Clarke G, Shana-han F, Keeling PW, Dinan TG. Evidence of an enhanced central 5HT response in irritable bowel syndrome and in the rat maternal separation model. Neuro-gastroenterol Motil. 2008;20:680–688. doi: 10.1111/j.1365-2982.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Marchesi JR, Scully P, Codling C, Ce-olho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and mic-robiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Own LS, Patel PD. Maternal behavior and offspring resiliency to maternal separation in c57bl/6 mice. Horm Behav. 2012;63:411–417. doi: 10.1016/j.yhbeh.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances pre-frontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Rainwater KL, Buckmaster CL, Schatzberg AF, Lindley SE, Lyons DM. Early life stress and novelty seeking behavior in adolescent monkeys. Psychoneuroendocrinology. 2007;32:785–792. doi: 10.1016/j.psyneuen.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain L, Martisova E, Milagro FI, Ramirez MJ, Martinez JA, Campion J. Postnatal maternal separation modifies the response to an obesogenic diet in adulthood in rats. Dis Model Mech. 2012;5:691–697. doi: 10.1242/dmm.009043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study) Int J Epidemiol. 2006;35:34–41. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- Power C, Barker DJ, Blacklock NJ. Incidence of renal stones in 18 British towns. A collaborative study. Br J Urol. 1987;59:105–110. doi: 10.1111/j.1464-410x.1987.tb04798.x. [DOI] [PubMed] [Google Scholar]
- Power C, Stansfeld SA, Matthews S, Manor O, Hope S. Childhood and adulthood risk factors for socio-economic differentials in psychological distress: evidence from the 1958 British birth cohort. Soc Sci Med. 2002;55:1989–2004. doi: 10.1016/s0277-9536(01)00325-2. [DOI] [PubMed] [Google Scholar]
- Rahman I, Humphreys K, Bennet AM, Ingelsson E, Pe-dersen NL, Magnusson PK. Clinical depression, antidepressant use and risk of future cardiovascular disease. Eur J Epidemiol. 2013;28:589–595. doi: 10.1007/s10654-013-9821-z. [DOI] [PubMed] [Google Scholar]
- Rebollar PG, Milanes A, Pereda N, Millan P, Cano P, Esquifino AI, Villarroel M, Silvan G, Lorenzo PL. Oestrus synchronisation of rabbit does at early post-partum by doe-litter separation or ECG injection: reproductive parameters and endocrine profiles. Anim Reprod Sci. 2006;93:218–230. doi: 10.1016/j.anireprosci.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Renard GM, Rivarola MA, Suarez MM. Sexual dimorphism in rats: effects of early maternal separation and variable chronic stress on pituitary-adrenal axis and behavior. Int J Dev Neurosci. 2007;25:373–379. doi: 10.1016/j.ijdevneu.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Winslow JT, O’Brien D, Gutman DA, Hoffman JM, Kilts CD. Neural correlates of maternal separation in rhesus monkeys. Biol Psychiatry. 2001;49:146–157. doi: 10.1016/s0006-3223(00)00977-x. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Anticevic A. Maternal separation enhances neuronal activation and cardiovascular responses to acute stress in borderline hypertensive rats. Behav Brain Res. 2007;183:25–30. doi: 10.1016/j.bbr.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Dinan TG, Cryan JF. Resistance to early-life stress in mice: effects of genetic background and stress duration. Front Behav Neurosci. 2011;5:13. doi: 10.3389/fnbeh.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Wang XD, Meijer OC. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology. 2011;214:131–140. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- Schooling CM, Jiang C, Lam TH, Zhang W, Cheng KK, Leung GM. Parental death during childhood and adult cardiovascular risk in a developing country: the Guangzhou Biobank Cohort Study. PLoS One. 2011;6:e19675. doi: 10.1371/journal.pone.0019675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapero BG, Black SK, Liu RT, Klugman J, Bender RE, Abramson LY, Alloy LB. Stressful life events and depression symptoms: the effect of childhood emotional abuse on stress reactivity. 2013. J Clin Psychol. doi: 10.1002/jclp.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KA, Evans NP, Monaghan P. Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2009;150:1931–1934. doi: 10.1210/en.2008-1471. [DOI] [PubMed] [Google Scholar]
- Spencer-Booth Y, Hinde RA. The effects of 13 days maternal separation on infant rhesus monkeys compared with those of shorter and repeated separations. Anim Behav. 1971;19:595–605. doi: 10.1016/s0003-3472(71)80117-3. [DOI] [PubMed] [Google Scholar]
- Spivey JM, Padilla E, Shumake JD, Gonzalez-Lima F. Effects of maternal separation, early handling, and gonadal sex on regional metabolic capacity of the pre-weanling rat brain. Brain Res. 2011;1367:198–206. doi: 10.1016/j.brainres.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suderman M, McGowan PO, Sasaki A, Huang TC, Hallett MT, Meaney MJ, Turecki G, Szyf M. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17266–17272. doi: 10.1073/pnas.1121260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26:139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Tamborski A, Lucot JB, Hennessy MB. Central dopamine turnover in guinea pig pups during separation from their mothers in a novel environment. Behav Neuro-sci. 1990;104:607–611. doi: 10.1037//0735-7044.104.4.607. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE. Psychosocial resources and the SES-health relationship. Ann N Y Acad Sci. 1999;896:210–225. doi: 10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- Trombini M, Hulshof HJ, Graiani G, Carnevali L, Meerlo P, Quaini F, Sgoifo A. Early maternal separation has mild effects on cardiac autonomic balance and heart structure in adult male rats. Stress. 2012;15:457–470. doi: 10.3109/10253890.2011.639414. [DOI] [PubMed] [Google Scholar]
- Tuchscherer M, Kanitz E, Puppe B, Tuchscherer A, Stabenow B. Effects of postnatal social isolation on hormonal and immune responses of pigs to an acute endo-toxin challenge. Physiol Behav. 2004;82:503–511. doi: 10.1016/j.physbeh.2004.04.056. [DOI] [PubMed] [Google Scholar]
- Tuchscherer M, Kanitz E, Puppe B, Tuchscherer A. Early social isolation alters behavioral and physiological responses to an endotoxin challenge in piglets. Horm Behav. 2006;50:753–761. doi: 10.1016/j.yhbeh.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Tucker DC, Johnson AK. Influence of neonatal handling on blood pressure, locomotor activity, and pre-weanling heart rate in spontaneously hypertensive and Wi-star Kyoto rats. Dev Psychobiol. 1984;17:587–600. doi: 10.1002/dev.420170603. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig R, Gordon JR, Thebaud B, Befus AD, Vliagoftis H. The effect of early-life stress on airway inflammation in adult mice. NeuroImmunoModulation. 2010;17:229–239. doi: 10.1159/000290039. [DOI] [PubMed] [Google Scholar]
- Wadsworth ME, Cripps HA, Midwinter RE, Colley JR. Blood pressure in a national birth cohort at the age of 36 related to social and familial factors, smoking, and body mass. Br Med J (Clin Res Ed) 1985;291:1534–1538. doi: 10.1136/bmj.291.6508.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Yoo SB, Ryu V, Park EY, Kim BT, Kang DW, Lee JH, Jahng JW. The arcuate NPY, POMC, and CART expressions responding to food deprivation are exaggerated in young female rats that experienced neonatal maternal separation. Neuropeptides. 2011;45:343–349. doi: 10.1016/j.npep.2011.07.005. [DOI] [PubMed] [Google Scholar]