Abstract
The human genome contains over 4 million variant sites, as compared to the reference genome, including rare sequence variants, which have the potential to exert large phenotypic effects, such as susceptibility to drug toxicity. We report identification and functional characterization of a rare non-synonymous (p.A1427S) variant in the SCN5A gene that was associated with incessant and lethal ventricular tachycardia and fibrillation after administration of lidocaine to a patient with acute myocardial infarction. The variant, located in a highly conserved domain distinct from the predicted lidocaine binding site, decreased peak current density of the sodium channel. With the increasing availability of the whole exome and whole genome sequencing data, it would be possible to identify and characterize rare variants in SCN5A that might predispose to lethal ventricular arrhythmias.
Keywords: Genetics, Arrhythmias, Lidocaine, Rare Variants, SCN5A
INTRODUCTION
The human genome contains over 4 million variant sites, as compared to the reference genome, including unique and rare DNA sequence variants that might exert large phenotypic effects 1. The recent technological advances enable identification of the functional variants in each genome that might be clinically consequential.
Lidocaine, a class 1B agent, is often used as the first-line anti-arrhythmic therapy in the treatment of ventricular arrhythmias in patients with acute myocardial infarction (AMI). Lidocaine and its analogous agents are also an effective anesthetic that are commonly used for pain management. At the commonly administered doses lidocaine is a relatively safe anti-arrhythmic and anesthetic agent. However, administration of lidocaine also has been associated with induction or propagation of ventricular arrhythmias, typically in the context of the Brugada syndrome 2–4. The molecular basis of the diabolically opposing effects of lidocaine from being an effective anti-arrhythmic to a pro-arrhythmic drug remains largely unknown but has been attributed to genetic variations in genes encoding the ion channels 2, 3.
We report identification and characterization of a rare loss-of-Function (LoF) variant in the SCN5A gene that impaired sodium current density and was associated with incessant and lethal ventricular tachycardia/fibrillation (VT/VF) after administration of lidocaine to a patient with acute myocardial infarction (AMI).
METHODS
This study was approved by the regional institutional review board and carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Appropriate informed consent was obtained to conduct the study.
Diagnosis of AMI
Diagnosis of AMI was established per the conventional methods by the presence of chest pain, electrocardiographic changes and blood biomarkers.
Genetic screening
Genomic DNA was extracted from the peripheral blood lymphocytes. All exons and exon-intron boundaries of the candidate genes were amplified by polymerase chain reaction (PCR), the amplicons were purified with SAP-EXON I purification method, and sequenced in both strands by capillary sequencing. In addition, 200 healthy control individuals from the same ethnic background were sequenced by the same method.
Site-directed mutagenesis and transfection
Wild type human SCN5A cDNA was amplified by PCR and cloned into a pcDNA3.1+ vector (Invitrogen, Carlsbad, CA, USA). Site directed mutagenesis was performed to introduce the variant nucleotide using the GeneTailor Site-Directed Mutagenesis System (Invitrogen Corp, Carlsbad, CA, USA). The clones were sequenced completely on both strands to ensure the presence of the mutation and the absence of other substitutions introduced by PCR.
Cellular Electrophysiology
HEK 293 cells were transfected with the WT or mutant SCN5A constructs using Lipofectamine TM 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Membrane current was measured by whole-cell patch clamp with EPC 10 amplifier (HEKA instruments). The pipette solution contained (mmol/L): CsF 110, CsCl 20, HEPES 10, NaF 10, EGTA10 (pH 7.35 adjusted with CsOH). The bath solution consisted of (mmol/L): NaCl 145, KCl 4, HEPES 10, MgCl2 1.0, CaCl2 1.8, glucose 10 (pH 7.35 adjusted with NaOH). Recordings were made at room temperature. Voltage dependent sodium currents recorded at various membrane potentials from −80mv to 60mv in 10mv increment for 50ms, holding potential was −120mv. Steady-state activation was estimated by measuring the peak sodium currents. Conductance G (v) was calculated by the equation: (I: peak currents, Vm: membrane potentials), Eres: the measured reversal potentials). Steady-state inactivation was estimated by a prepulse protocol, which varies membrane potentials from −140mv to −50mv for 500ms followed by depolarizing to −20mv for 20ms. Data for steady-state activation and steady-state inactivation were fitted with the Boltzmann equation.
Data recording and statistics
All signals were acquired at 20kHz with Patchmaster software (HEKA Instruments, Bellmore, NY, USA). The data were analyzed with Fitmaster, Sigmaplot 10.0 and EXCEL data handling. Results are presented as mean ± standard errors of the mean (SEM). The mean values and SEM were compared by the T-test.
RESULTS
Lidocaine-associated VT/VF
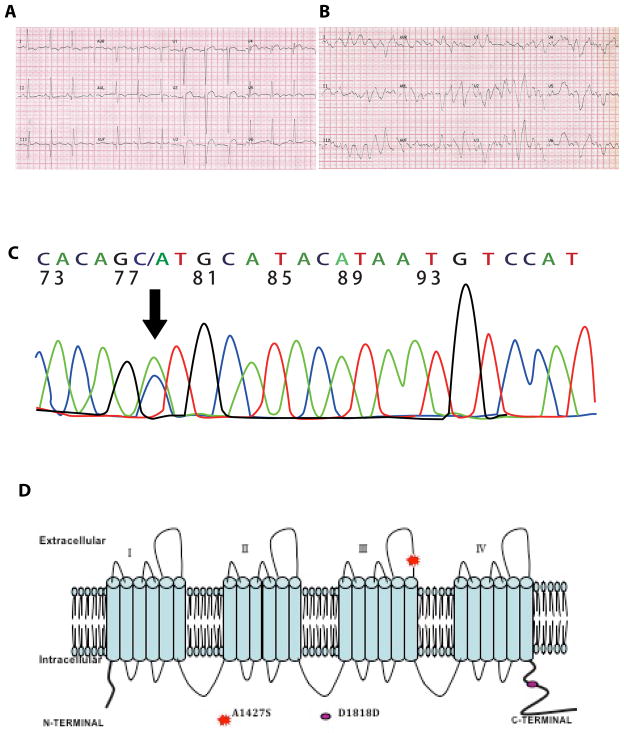
A 56-year female with a known history of 3-vessel coronary artery disease and a normal left ventricular ejection fraction of 60% presented with a ST segment elevation AMI (Figure 1A). She declined invasive interventions and was treated with heparin, aspirin, clopidogrel, metoprolol, benazepril hydrochloride, isosorbide mononitrate and fluvastatin. She had polymorphic ventricular ectopic beats and episodes of non-sustained VT (Figure 1B). Routine blood tests including serum calcium, magnesium and potassium levels were normal. She was given a 50 mg bolus dose of lidocaine intravenously. Within three minutes of administration of lidocaine, the patient developed incessant polymorphic VT/VF and died. She did not complain of chest pain or angina-like symptoms and had no new ECG abnormalities or a rise in serum levels of cardiac troponin I preceding the terminal event.
Figure 1. Lidocaine-associated ventricular tachycardia/fibrillation.
A. 12-lead electrocardiogram on admission showing pathological Q waves, ST-segment elevation, intraventricular conduction delay and a normal QTc; B. Eletcrocardiogram showing an episode of polymorphic VT/VF; C. Electropherogram showing a c.1427G>T transversion (arrow, anti-sense strand) in the SCN5A gene; D. Schematic representation of p.A1427S variant location in the SCN5A protein.
Identification of the SCN5A mutation
Sequencing of the KCNQ1, KCNH2, KCNE1, KCNE2 and SCN5A genes led to identification of a novel c.4279G>T transversion in the SCN5A gene, which encodes SCN5A protein, the α-subunit of the sodium channel (Figure 1C). The mutation corresponds to p.A1427S (codon GCT/TCT) change in the protein (Figure 1C). The variant affects a highly conserved domain of the SCN5A protein (Figure 1D). The variant was absent in 200 healthy individuals from the same ethnic background and in the NHLBI GO Exome Sequencing Project database. The frequency of this variant (rs200034939 or rs199473244) in the “1000 Genomes – A Deep Catalog of Human Genetic Variation “ was <0.01. (http://browser.1000genomes.org/)”
No other potentially pathogenic variant was identified.
Cell electrophysiology
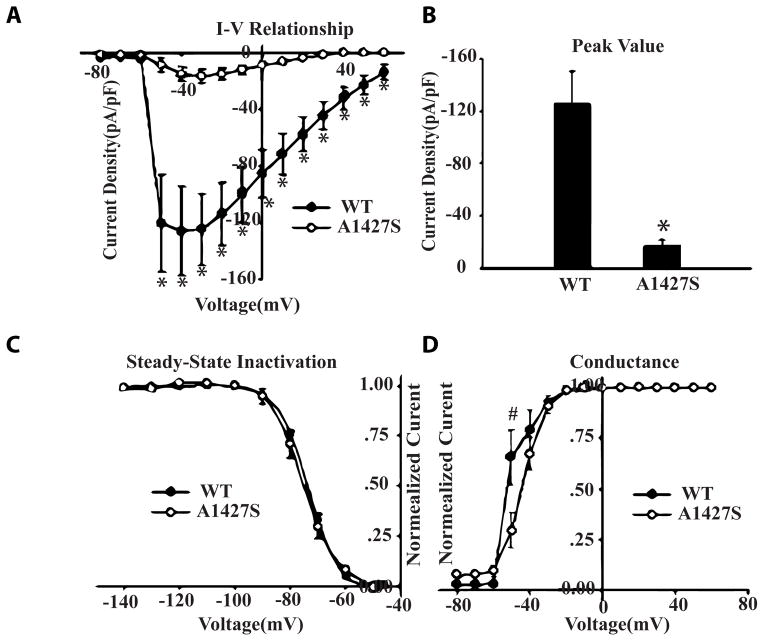
Whole-cell patch clamp studies, upon expression of the wild type and mutant SCN5A protein in the HEK293 cells, showed significantly decreased peak current density (Figure 2A and B). The steady-state inactivation of sodium current was unchanged (Figure 2C). The mutant variant also showed a right shift positive potential in the voltage dependence of peak conductance (Figure 2D).
Figure 2. Loss-of-Function effects of the mutation on sodium current density.
A. Current-voltage relationships for WT and p.A1427S variant. B. Peak value of sodium current for WT and p.A1427S mutation. C. Steady-state inactivation for WT and p.A1427S mutation, the normalized currents were plotted as function of membrane potentials. D. Voltage dependence of peak conductance for WT and p.A1427S mutation, the normalized peak conductance was plotted against membrane potentials. * p<0.01 compared with WT. # p<0.05 compared with WT.
DISCUSSION
The case presents the diabolically opposing effects of lidocaine from being an anti-arrhythmic therapy to a malignant pro-arrhythmic agent in the presence of myocardial ischemia and a rare missense LoF variant in the SCN5A gene. The p.A1427S variant is located at the S5–S6 extracellular linker of domain III in the SCN5A protein. The affected domain is highly conserved among members of the voltage-gated sodium channel alpha subunit family and among species from chicken to man (Figure 1D). Lidocaine blocks the sodium current by modification of the sodium channel’s voltage sensors predominately associated with channel activation 5, 6. The effect leads to channel opening and results in allosteric coupling of the binding sites of lidocaine to the voltage sensors formed by the S4 segments in domains III and IV 5, 6. The p.A1427S variant is located outside the predicted lidocaine-binding site 7 and imparts distinct effects on gating and intermediate inactivation. The effects are expected to accentuate the blocking effect of lidocaine on the sodium channels.
Mutations in the SCN5A gene cause Brugada and the Long QT syndromes 8, 9. Lidocaine was found to unmask the electrocardiographic pattern of the Brudada syndrome in a patient with a compound polymorphism in the SCN5A gene 2. Our patient did not exhibit electrocardiographic patterns of the Brugada or the long QT syndromes. She also had no history of ventricular tachyarrhythmias. Thus, the findings portend a novel phenotype associated with the SCN5A variants.
The patient had polymorphic ventricular ectopic beats and episodes of non-sustained VT prior to lidocaine administration. Thus, despite the functional effect of the p.A1427S variant being in accord with the electrophysiological effects of lidocaine, temporal association of the VT/VF episode with lidocaine administration, and biological plausibility of the findings, we can not exclude fortuitous occurrence of VT/VF in the setting of myocardial ischemia, independent of lidocaine administration. Although the patient did not have evidence of active ischemia preceding the terminal even, the role of silent myocardial ischemia in perpetuating lidocaine-induced VT/VF in the presence of the p.A1427S could not be excluded. Thus, whether the SCN5A variant alone in the absence of other susceptibility factors or upon administration of lidocaine as an anesthetic agent, predisposes to malignant ventricular arrhythmias remains unknown. Despite these shortcomings, the findings raise awareness for the potential pro-arrhythmic effects of drugs in the presence of rare variants in genes involved in cardiac electrophysiological properties.
We conclude that rare LoF missense SCN5A variants might predispose to VT/VF after lidocaine administration in the setting of myocardial ischemia. The findings offer a glimpse into the potential clinical implications of rare and functional variants in the personal genomes.
Supplementary Material
Acknowledgments
KH is supported in part by grants from the Ministry of Chinese Education Innovation Team Development Plan [IRT1141]; National Basic Research Program of China [973 Program: 2007CB512002; 2008CB517305], the National Natural Science Foundation of China [81070148, 81160023, 30760076].
AJM is supported in part by grants from NHLBI (R01-HL088498 and R34HL-105563); NIA (R21 AG038597-01), TexGen Fund from Greater Houston Community Foundation and George and Mary Josephine Hamman Foundation.
Footnotes
Conflict of interest:
The authors declare there is no financial interest in relation to the work described in the present manuscript.
References
- 1.Marian AJ, Belmont J. Strategic approaches to unraveling genetic causes of cardiovascular diseases. Circulation Research. 2011;108(10):1252–69. doi: 10.1161/CIRCRESAHA.110.236067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barajas-Martinez HM, Hu D, Cordeiro JM, Wu Y, Kovacs RJ, Meltser H, et al. Lidocaine-induced Brugada syndrome phenotype linked to a novel double mutation in the cardiac sodium channel. Circulation Research. 2008;103(4):396–404. doi: 10.1161/CIRCRESAHA.108.172619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin MT, Wu MH, Chang CC, Chiu SN, Theriault O, Huang H, et al. In utero onset of long QT syndrome with atrioventricular block and spontaneous or lidocaine-induced ventricular tachycardia: compound effects of hERG pore region mutation and SCN5A N-terminus variant. Heart rhythm: the official journal of the Heart Rhythm Society. 2008;5(11):1567–74. doi: 10.1016/j.hrthm.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Ludot H, Tharin JY, Belouadah M, Mazoit JX, Malinovsky JM. Successful resuscitation after ropivacaine and lidocaine-induced ventricular arrhythmia following posterior lumbar plexus block in a child. Anesthesia and analgesia. 2008;106(5):1572–4. doi: 10.1213/01.ane.0000286176.55971.f0. table of contents. [DOI] [PubMed] [Google Scholar]
- 5.Sheets MF, Hanck DA. Outward stabilization of the S4 segments in domains III and IV enhances lidocaine block of sodium channels. The Journal of Physiology. 2007;582(Pt 1):317–34. doi: 10.1113/jphysiol.2007.134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheets MF, Hanck DA. Molecular action of lidocaine on the voltage sensors of sodium channels. The Journal of general physiology. 2003;121(2):163–75. doi: 10.1085/jgp.20028651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunami A, Glaaser IW, Fozzard HA. A critical residue for isoform difference in tetrodotoxin affinity is a molecular determinant of the external access path for local anesthetics in the cardiac sodium channel. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2000;97(5):2326–31. doi: 10.1073/pnas.030438797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80(5):805–11. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392(6673):293–6. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.