Abstract
G protein-coupled receptors (GPCRs) are membrane proteins that traverse the plasma membrane seven times (hence, are also called 7TM receptors). The polytopic structure of GPCRs makes the folding of GPCRs difficult and complex. Indeed, many wild-type GPCRs are not folded optimally, and defects in folding are the most common cause of genetic diseases due to GPCR mutations. Both general and receptor-specific molecular chaperones aid the folding of GPCRs. Chemical chaperones have been shown to be able to correct the misfolding in mutant GPCRs, proving to be important tools for studying the structure-function relationship of GPCRs. However, their potential therapeutic value is very limited. Pharmacological chaperones (pharmacoperones) are potentially important novel therapeutics for treating genetic diseases caused by mutations in GPCR genes that resulted in misfolded mutant proteins. Pharmacoperones also increase cell surface expression of wild-type GPCRs; therefore, they could be used to treat diseases that do not harbor mutations in GPCRs. Recent studies have shown that indeed pharmacoperones work in both experimental animals and patients. High-throughput assays have been developed to identify new pharmacoperones that could be used as therapeutics for a number of endocrine and other genetic diseases.
Introduction
Cellular Quality Control
-
Folding and Maturation of G Protein-Coupled Receptors
Some wild-type G protein-coupled receptors are not efficiently folded
Defects in folding and maturation of mutant receptors are the major cause of genetic diseases caused by mutations in G protein-coupled receptors
Motifs involved in retaining G protein-coupled receptors intracellularly
-
Molecular Chaperones in the Folding and Maturation of G Protein-Coupled Receptors
General molecular chaperones
Receptor-specific molecular chaperones
Small G proteins in the folding and maturation of G protein-coupled receptors
-
Chemical Chaperones in the Folding and Maturation of G Protein-Coupled Receptors
Low temperature
Chemical chaperones
-
Pharmacoperones in the Folding and Maturation of G Protein-Coupled Receptors
Pharmacoperones for the gonadotropin-releasing hormone receptor
Pharmacoperones for the arginine V2 vasopressin receptor
Pharmacoperones for rhodopsin
Pharmacoperones for the melanocortin-4 receptor
Pharmacoperones for other G protein-coupled receptors
Pharmacoperones as tools to study structure-function relationship of G protein-coupled receptors
Pharmacoperones as potential therapeutics
Conclusions and Future Directions
I. Introduction
The superfamily of G protein-coupled receptors (GPCRs) consists of the most numerous membrane proteins in the mammalian genomes. With the completion of the human genome, essentially all the GPCR genes can be identified. The International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification published a complete list of nonsensory GPCRs in humans, with newly deorphanized receptors updated in the most recent review (1, 2). In humans, there are about 800 GPCRs, with at least 342 functional nonolfactory receptors (3) (another study identified 367 receptors with endogenous ligands [4]; this has subsequently been increased to 400 [5]). Most of the olfactory receptors (ORs) are still orphan receptors, receptors whose endogenous ligands are unknown.
Of the nonolfactory receptors, there are three major families (6). Family A (class 1), rhodopsin-like GPCRs, is the most numerous of the nonolfactory GPCRs, including the prototypical and most extensively studied rhodopsin and β2-adrenergic receptor (AR), as well as the receptors for numerous hormones. They have some highly conserved residues, including two signature motifs: the D(E)RY(W) motif toward the end of transmembrane domain (TM) 3 and the N(D)PXXY motif in TM7, as well as highly conserved proline residues in TM5, TM6, and TM7. A total of 276 members are listed (not including the seven opsin-like receptors) in this family (1). Family B (class 2), secretin-like GPCRs, include the glucagon receptor (GCGR), the glucagon-like peptide-1 receptor (GLP1-R), the calcitonin (CT) receptor (CTR), the calcitonin receptor-like receptor (CLR), the PTH receptors (PTHRs), the secretin receptor, and vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating peptide receptors (VPACRs), numbering about 53. Family C (class 3) GPCRs consist of the type B metabotropic γ-aminobutyric acid (GABA) type B receptors (GABABRs), the Ca2+-sensing receptor (CaSR), several metabotropic glutamate receptors (mGluRs), a large group of taste receptors (with at least 39 members), and several orphan receptors.
There are about 400 potentially functional ORs in the human genome based on the sequence analysis (1). However, the great majority of these receptors have not been deorphanized, with a major obstacle being the difficulty in expressing them at the cell surface for functional studies (elaborated more in Sections III.A. and IV.B.3.). A number of Frizzled receptors and Smoothened are also GPCRs, listed as a separate family, although they do not couple to G proteins. Therefore, some prefer the term “seven-transmembrane domain receptors” instead of GPCRs (7). Adiponectin receptors also contain seven TMs, but they have the opposite topology with the N terminus intracellular and C terminus extracellular, and their signaling does not involve G proteins (8). They are not typically considered as GPCRs or seven-transmembrane domain receptors. Another popular classification scheme for GPCRs, based on phylogenetic analyses of functional nonolfactory human GPCR sequences, is the GRAFS system, standing for glutamate, rhodopsin, adhesion, Frizzled/tase2, and secretin (3).
Evolutionarily, GPCRs are ancient and very successful (9) and are found in all eukaryotic organisms, including yeast (10), plants (11), and invertebrates (12). In archaebacteria, there is a 7TM protein that acts as a photon sensor, bacteriorhodopsin, which had been used extensively as a template for homology modeling of GPCRs before the crystal structures of GPCRs were available. However, it has very low homology with GPCRs and does not signal through G proteins (it acts as a proton pump); therefore, it is not considered a GPCR.
The ligands activating GPCRs are extremely diverse, including photons, ions, amines, organic odorants, nucleotides, nucleosides, lipids, small peptides, as well as large glycoproteins (9). The numerous GPCRs regulate almost every physiological function in the body, such as cardiovascular function (13, 14), energy and glucose homeostasis (15–17), reproduction (18), and cell proliferation and cancer pathogenesis (19, 20), to name just a few. They mediate functions of most of the peptide and protein hormones, with several prominent examples described in detail herein. Even for some steroid hormones that are well known to exert their functions through nuclear receptors, GPCRs have been identified to mediate their functions, with the most established example being that for estrogen. In addition to the classical estrogen receptor-α and -β, the GPCR, G protein-coupled estrogen receptor also mediates some of estrogen's action (21). Membrane receptors for progesterone and T have also been identified (22).
Dysfunction in the signaling of GPCRs, due to either genetic or somatic causes, can cause numerous diseases including cancer (elaborated in detail in Section III.B.). The GPCRs are also the most druggable targets, with estimates of one-third to one-half of currently used pharmaceutical drugs targeting the GPCRs, accounting for about a quarter of the top 100 best-selling drugs worldwide (23). It should be pointed out that these drugs target only a handful of GPCRs (about 10%), with the vast majority so far untapped; therefore there is enormous potential in targeting these untapped receptors.
The GPCRs have the classical seven α-helical TMs connected with alternating extracellular and intracellular loops (ECLs and ICLs), with the N terminus extracellular and the C-terminal tail intracellular. The crystal structures of a number of GPCRs published during the past 14 years, starting with that of rhodopsin in 2000 and including the Nobel Prize-winning work of Brian Kobilka, demonstrated that there is an additional helical extension after TM7, which has been called Helix 8 or ICL4, that is parallel to the surface of the inner membrane and perpendicular to the other seven TMs (24–26). This is frequently due to the palmitoylation of cysteine residues at the C-terminal tail that anchor it to the plasma membrane. Some GPCRs are activated by lipids, including free fatty acids and phospholipids. The binding pockets for these receptors are located in the TMs situated in the lipid bilayers (27). Even these receptors need to be transported to the cell surface for functioning. Defects in translocation to the cell surface in these receptors also cause human diseases (see Ref. 28 for an example). It should be noted that similar to the tyrosine kinase receptors, some GPCRs have also been found in the nuclear and mitochondrial membranes (29, 30). We will focus on cell surface trafficking in this article.
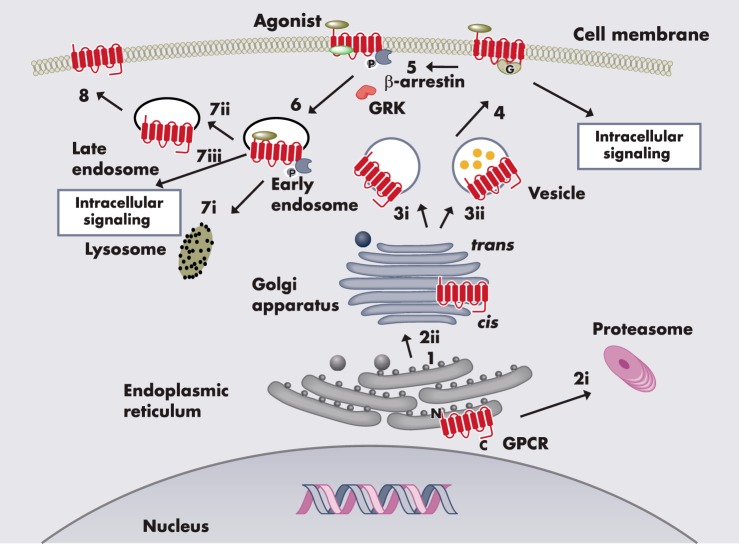
The life cycle of a GPCR starts at the synthesis on the ribosome (Figure 1). For those GPCRs with a signal peptide, the signal sequence binds to the signal recognition particle directing the ribosome and the growing polypeptide to the endoplasmic reticulum (ER) membrane. The nascent receptor is inserted in the ER cotranslationally. For the more than 90% of the GPCRs without signal peptide, a TM, usually TM1, takes over the role of signal peptide by binding to signal recognition particle (31). Once the receptor has achieved its native conformation, the one with the lowest Gibbs free energy, the receptor exits the ER and is further transported through the ER/Golgi intermediate compartment, cis-Golgi, Golgi apparatus, trans-Golgi network, and reaches the plasma membrane. GPCRs function as ligand-activated molecular switches coupling ligand binding in the extracellular space to GTP/GDP exchange by the cognate G protein interacting with the ICLs. The endogenous ligands for GPCRs are mostly hydrophilic and cannot easily cross the lipid bilayer of the plasma membrane. Therefore, to convey the information of the ligands to the inside of the cell, GPCRs must be expressed at the cell surface.
Figure 1.
The life cycle of GPCRs. The nascent receptor is synthesized on the ribosome and inserted into the ER where it is folded (1). Misfolded protein is polyubiquitinated and targeted for degradation by the proteasome (2i). Correctly folded protein is packaged into transport vesicles and moved to the Golgi apparatus where it undergoes further posttranslational modifications (2ii). The mature protein in small vesicles undergoes either constitutive (3i) or regulated (3ii) transport and is fused into the cell membrane (4). Upon agonist binding, the receptor undergoes conformational change and initiates intracellular signaling. The agonist-activated receptor is phosphorylated by GPCR kinase (GRK) that attracts β-arrestin binding (5) and induces receptor internalization (6). The internalized receptor is either degraded by the lysosome (7i) or dephosphorylated and recycled to the cell membrane, resulting in resensitization (7ii, 8). Internalized receptor in the endosome can also initiate signaling (7iii).
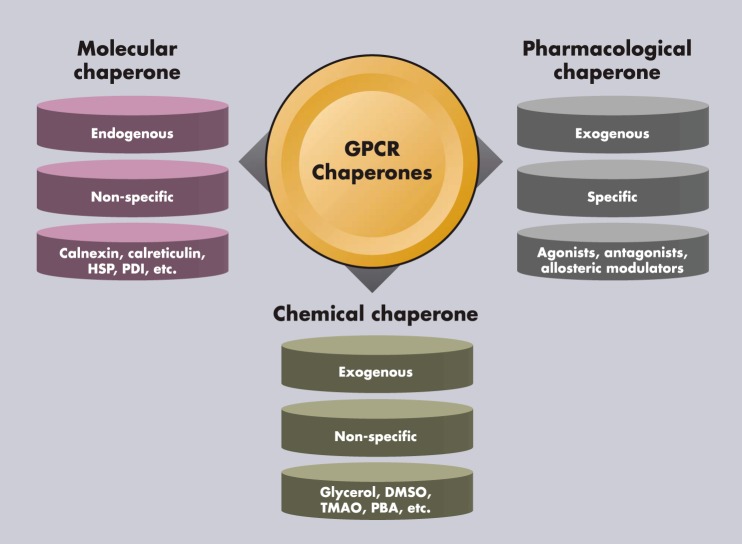
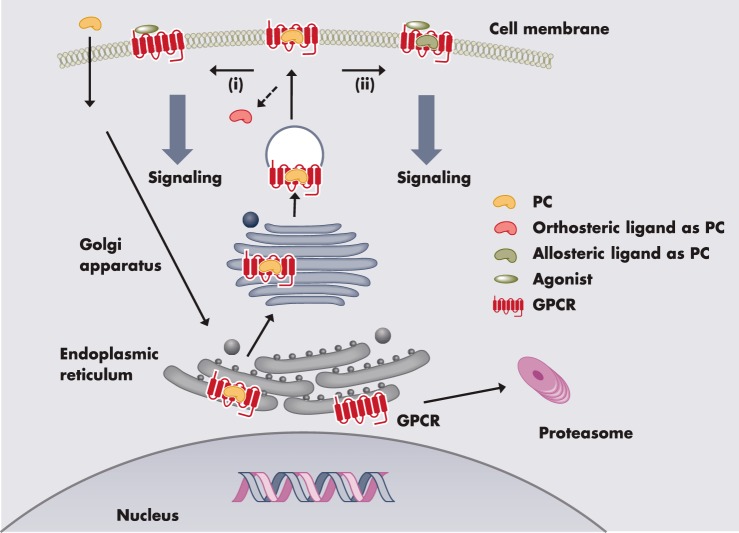
Once activated by agonist binding, GPCRs undergo desensitization and internalization (32, 33). Internalized receptor can either recycle to the cell surface resulting in resensitization or degradation, primarily in lysosome, resulting in down-regulation (Figure 1). The intensity of signaling is frequently related to the receptor density at the cell surface: the quantity of GPCRs expressed on the cell surface that is accessible for ligand stimulation is an important factor regulating receptor signaling. Maturation, internalization, recycling, and degradation together determine the net amount of cell surface receptor level. Extensive studies were performed on receptor trafficking after ligand-induced activation; however, until recently, much less attention has been paid to the anterograde trafficking of GPCRs (34), the delivery of ligand-naive nascent receptor to the cell surface from its site of synthesis in the ER. This is the focus of our review. Starting with a brief general introduction of cellular quality control, we will discuss why it is relevant to study anterograde trafficking. Molecular, chemical, and pharmacological chaperones that help GPCR folding and maturation will be discussed (Figure 2). The therapeutic potential of pharmacological chaperones (pharmacoperones) will be highlighted.
Figure 2.
Molecular, chemical, and pharmacological chaperones that aid in the trafficking of GPCRs. Molecular chaperones are endogenous proteins that help protein folding nonspecifically. Chemical chaperones are exogenous compounds that help protein folding nonspecifically. Pharmacological chaperones are GPCR ligands that help GPCR folding specifically.
II. Cellular Quality Control
The ER is a major site of lipid biosynthesis and calcium storage and release (35, 36). It is also a major site for the folding of nascent proteins. In eukaryotic cells, nascent secretory proteins are cotranslationally translocated into the ER lumen, whereas nascent integral membrane proteins are cotranslationally inserted into the ER membrane, with both mediated by the Sec61 translocon (37). These unfolded polypeptide chains undergo post-translational modifications, including glycosylation, disulfide bond formation, and dimerization/oligomerization. Before these proteins can exit the ER, they must meet the stringent ER quality control standards (38–40). Proteins that cannot achieve the native conformation and pass the ER quality control inspection are prevented from aggregation with the help of chaperones and provided with additional opportunities to fold. If they fail multiple rounds of folding attempts, they are retrotranslocated through the translocon Sec61 to the cytosol, ubiquitinated, and degraded by the proteasome, the so-called ER-associated degradation (ERAD) (41). ERAD is important to maintain global protein homeostasis in the cell; there is a delicate balance between protein folding and ERAD (41). Overexpression of misfolded protein in the ER can lead to the production of reactive oxygen species and ER stress, finally leading to unfolded protein response (UPR), the activation of intracellular signal transduction pathways that increase the ER folding capacity (increased gene transcription of molecular chaperones) and decrease the load (decreased protein translation and degradation of ER-bound mRNAs) (42, 43). If UPR cannot resolve the ER stress, the cells undergo apoptosis (43).
The major machinery for quality control includes heat shock protein (HSP) of 70 kDa (HSP70), chaperone Ig binding protein (BiP) and its cochaperones that detect exposed hydrophobic surfaces indicative of misfolding (44, 45), and calnexin/calreticulin that monitor both N-linked carbohydrates and unfolded regions (46). BiP is the major system used for monitoring the folding of nonglycosylated proteins because they are not monitored by calnexin/calreticulin.
For glycosylated membrane proteins, the glycans play a critical role in folding or sorting for degradation (47). When the glycoproteins are irreversibly misfolded, the glycan structure will be changed, allowing the glycoprotein to move from association with calnexin/calreticulin for folding to ER degradation enhancing α-mannosidase-like protein (EDEM) for degradation by the 26S proteasome (48). EDEM binds to glycoproteins after the glycans have been trimmed to a form that cannot be recognized by calnexin/calreticulin.
III. Folding and Maturation of G Protein-Coupled Receptors
The assembly of GPCRs, as exemplified by rhodopsin, like other transmembrane proteins, begins with the entry of the nascent polypeptide chain into the ER lumen where they undergo post-translational modification such as glycosylation and initial folding. The transmembrane α-helices are formed and inserted into the ER membrane. The formation of a highly conserved disulfide bond connecting the top of TM3 and the second ECL has been hypothesized to be important for the alignment of the seven TMs in the ER membrane and establishing interhelical interactions (49).
A. Some wild-type G protein-coupled receptors are not efficiently folded
Some wild-type (WT) GPCRs are not efficiently folded and processed therefore localized intracellularly and prone to degradation. Earlier studies showed that muscarinic and opioid receptors have significant intracellular presence (50, 51). Less than 50% of the human δ-opioid receptor (DOR) and D1 dopamine receptor (DR) are transported out of the ER and obtain complex glycosylation (Golgi and post-Golgi), with the exit from the ER being the limiting step in the whole process of maturation (52, 53). Those that fail to exit ER are retrotranslocated to the cytosol and degraded by the ubiquitin-proteasome pathway (54). Serotonin 5-HT2A receptor is primarily expressed intracellularly in cortical neurons and associated with the cytoskeleton (55) and partially localized in the ER in dopaminergic neurons in the ventral tegmental area (56).
The rat LH receptor (LHR) is another recently described example of poorly expressed WT receptor. In HEK293 cells stably transfected with rat LHR, only about 20% of the receptor precursors mature to reach the plasma membrane; the rest are retained in the ER and eventually degraded by the proteasome pathway (57) (the intracellularly retained portion is expected to be higher than 80% in transiently transfected cells). Blocking proteasome degradation with proteasome inhibitors leads to increased ER export and maturation of the receptors. For TSH receptor (TSHR) transfected in L cells, only one-third of the newly synthesized receptor can attain the mature form (58). In lactotrophs and in AtT20 corticotrophs, TRH receptor (TRHR) is primarily expressed at the plasma membrane. However, TRHRs expressed in HEK293 and COS7 cells are primarily located intracellularly, in the ER and Golgi apparatus; this intracellular retention is not due to transient overexpression (59). Similar observations were made for human CTR expressed in COS cells (60).
The ORs are notoriously difficult to express functionally in heterologous cell lines. For example, when expressed in HEK293 cells, the ORs are poorly trafficked to the plasma membrane; rather, these receptors are located in the ER but not in Golgi apparatus or endosomes (61, 62).
Closely related receptors can have very different folding efficiency. For example, of the two A2 adenosine receptors, A2A receptor and A2B receptor, A2A receptor is efficiently expressed on the cell surface, whereas the cell surface expression of A2B receptor is significantly lower, with a substantial portion of nascent receptor retained intracellularly in the ER and eventually degraded by the proteasome (63). Different subtypes of ARs are located intracellularly to different degrees (64), with α2C-AR significantly retained intracellularly. Compared with the melanocortin-4 receptor (MC4R), the melanocortin-3 receptor (MC3R) has a more significant intracellular expression in stably expressed HEK293 cells (65). As discussed in detail in Section IV.B.5., the melanocortin-2 receptor (MC2R) is completely retained intracellularly when expressed in HEK293 cells.
Differentially spliced forms of the same receptor can also have different degrees of intracellular retention. For example, of the two alternatively spliced isoforms of D2 DR, D2S and D2L (with 29 extra amino acids in ICL3 compared with D2S), D2S is more readily processed to the mature form, whereas D2L has a significant portion persistently trapped intracellularly, even at the decreased temperature of 20°C, and it does not reach the plasma membrane (66). Of the three mouse prostaglandin E3 receptor isoforms that differ in the C termini, two are localized intracellularly but not in plasma membrane, whereas the other isoform is localized in both plasma membrane and intracellular compartment (67).
For the same receptor, orthologs from different species can also have very different folding efficiency. Although human GPCR family C, group 6, subtype A is poorly expressed on the cell surface when expressed in mammalian cells, the mouse ortholog is expressed at the cell surface (68, 69). Of human and rat LHRs, the human LHR is more efficiently processed and is expressed at the cell surface at a higher level than the rat LHR, and the unusually long extracellular domain of the LHRs contributes partly to this difference in maturation and cell surface expression (70, 71). Human arginine vasopressin type 2 receptor (AVPR2) is efficiently expressed at the plasma membrane, whereas the mouse AVPR2 is localized primarily in the ER (72).
The inefficient trafficking of WT GPCRs may have important physiological implications, representing a mechanism for the cell to regulate receptor sensitization and modulate strength of signal transduction (73). GnRH receptor (GnRHR) from lower vertebrates is expressed better at the cell surface than mammalian GnRHR (74). We have argued that the inefficient expression of primate GnRHRs at the plasma membrane represents an effective mechanism by nature to cope with the increased complexity and cost per unit of production of offspring (75). It has been suggested that rat LHR cell surface expression may be controlled at the ER level by regulating the number of nascent proteins that exit the ER rather than proteasome degradation (57). Indeed, the maturation of rat LHR is developmentally regulated, with the developing gonads and some extragonadal tissues expressing only the immature form, whereas the mature gonads express both the immature and the mature forms (76). The maturation can also be regulated physiologically because increased maturation is observed in adrenal gland and kidney of pregnant rats when the differentiation of fetal urogenital tissues is taking place (but not at term pregnancy) (76). As mentioned above, α2C-AR is usually retained intracellularly in fibroblasts and vascular smooth muscle cells at a body temperature of 37°C. Reducing temperature will facilitate the transport of the intracellular pool to the cell surface, which can cause enhanced peripheral vasoconstriction, the so-called Raynaud syndrome (77).
DOR is also primarily located intracellularly in basal condition, with only a small portion located at the plasma membrane (78, 79) (reviewed in Ref. 80). In small dorsal root ganglion neurons, DORs are mainly associated with Golgi apparatus and the membrane of large dense-core vesicles containing neuropeptides such as CT gene-related peptide (CGRP) and substance P (79). The intracellular pool can be transported to the cell surface and inserted into the plasma membrane upon stimulation (81). For example, activation of surface DORs with DOR agonists causes a slow and long-lasting exocytosis of large dense-core vesicles and DOR insertion into plasma membrane, whereas membrane depolarization or activation of vanilloid and purinergic receptor P2Y1 receptors induces rapid DOR insertion into plasma membrane (82). Concomitant release of excitatory neuropeptides such as CGRP potentiates pain perception, suggesting that DORs need to be blocked in the treatment of inflammatory pain (82).
In proximal tubular-like cell line, LLCPK1 cells, increased dopamine availability in the cell (such as treating with agonist, dopamine precursor, or inhibitor of dopamine metabolism) leads to rapid translocation of D1DRs from the cytosol to the plasma membrane (83). Peptide hormones can induce heterologous sensitization by recruiting intracellular catecholamine receptors to the plasma membrane, contributing to the potentiating effects of atrial natriuretic peptide on dopamine (D1DR) and neuropeptide Y on norepinephrine (α1A-AR), respectively (73). Both homologous and heterologous recruitment modulates receptor density at the plasma membrane.
In cultured neurons, the chemokine receptor for CXCL12, CXCR7, is mainly located in the cytoplasm, partially overlapping with ER marker, with little to no expression at the plasma membrane, whereas another chemokine receptor for the same chemokine, CXCR4, is expressed at the plasma membrane (84). As an atypical chemokine receptor, the CXCR7 does not stimulate typical G protein-dependent pathways but may activate β-arrestin-mediated signaling (85). It can also act as a chemokine scavenger (86); by sequestering CXCL12, it generates a CXCL12 gradient in the extracellular space, critical during development or in the tumor microenvironment (84). By heterodimerization with CXCR4 in the ER, the CXCR7 can also regulate CXCR4 density at the cell surface (84). Another decoy receptor, D6, is also expressed at low level at the cell surface, with >80% found in intracellular vesicular structures (87, 88). Cognate chemokines induce a dose-dependent redistribution of the intracellular receptor to the plasma membrane, increasing chemokine-scavenging activity (88). This represents a rapid and unique mechanism for D6 to control inflammation.
The CaSR is a family C GPCR with a significant intracellular pool at the ER that can be mobilized to the cell surface by Ca2+-induced signaling, leading to what is called “agonist-driven insertional signaling” (89). The receptor already at the plasma membrane undergoes constitutive endocytosis without substantial recycling; therefore the net amount of the CaSR and its signaling can be dynamically regulated by the trafficking of the intracellular receptor to the plasma membrane (90). Indeed, different from results in other GPCRs that demonstrate the dominant negative effect of intracellularly retained mutants on the WT receptor expression (see Section III.B.), in the CaSR, expression of the WT receptor can substantially rescue the cell surface expression of mutants retained intracellularly (91).
Although GPCR signaling is traditionally believed to be mediated at the cell surface, activation of intracellular pool of GPCRs has also been reported. For example, activation of intracellular mGluR5 generates distinct Ca2+ responses and downstream signaling cascades different from the cell surface mGluR5, resulting in sustained synaptic transmission (92, 93). ER-located MC4R is also capable of initiating signaling (94). Some internalized GPCRs, such as β2-AR, PTHR, and TSHR, also continue to signal in endosomes or in vesicles associated with the Golgi complex or trans-Golgi network, generating a new wave of signaling (95–100), which is turned off not by β-arrestin, but by retromer complex (97) (Figure 1).
B. Defects in folding and maturation of mutant receptors are the major cause of genetic diseases caused by mutations in G protein-coupled receptors
After the cloning of GPCR genes, first with the rhodopsin (101) and then with the β2-AR (102), followed by numerous other GPCRs, it was soon found that both gain-of-function (primarily constitutively active) and loss-of-function mutations were identified to cause human diseases, including many endocrine diseases. Both inherited and somatic mutations were identified. There are several extensive reviews covering the general aspects of GPCR mutations (see Refs. 103–110 for examples) as well as numerous review articles covering individual receptors. Interested readers are referred to these resources for further details. Because the topic of this article is GPCR trafficking, we will discuss only the inactivating mutations herein.
Table 1 lists some examples of inactivating mutations in GPCRs causing human diseases. It is not meant to be exhaustive, and with the next-generation high-throughput sequencing, new reports of mutations in GPCR genes are appearing in the literature all the time. The list does highlight a number of prominent endocrine diseases caused by GPCR gene mutations. It should be noted that mutations in GPCR genes in other animals have also been reported to be associated with diseases. Some of the examples, in addition to mutations in rodents not discussed here, include horse Hirschsprung disease due to endothelin B receptor mutation (111, 112), dog retinal degeneration (similar to human retinitis pigmentosa) due to rhodopsin mutation (113), dog narcolepsy due to orexin 2 receptor mutation (114), pig growth traits due to MC4R mutations (115, 116), dog obesity due to MC4R mutations (117, 118), and CCR5 (C-C chemokine receptor type 5) mutations in African green monkey changing susceptibility to simian immunodeficiency virus infection (119). Fascinating studies on the melanocortin-1 receptor (MC1R) mutations and their association with color pattern in many different species of animals, including rodents, pigs, cows, and foxes, among others, clearly showed that constitutively active mutations cause a black coat color, whereas loss-of-function mutations are associated with yellow or red coat colors (120).
Table 1.
Diseases Caused by Inactivating Mutations in GPCR Genes
| GPCR | Disease | Refs. |
|---|---|---|
| Rhodopsin | Retinitis pigmentosa | 486, 487 |
| Retinal GPCR (RGR) | Retinitis pigmentosa | 488 |
| Blue opsin | Color blindness (tritanopia) | 489, 490 |
| GPR56 | Bilateral frontoparietal polymicrogyria | 491–493 |
| PROKR2 | Kallmann syndrome | 494, 495 |
| GnRHR | Hypogonadotropic hypogonadism | 18, 496, 497 |
| GHRH receptor | Dwarfism | 498, 499 |
| TRHR | Isolated central hypothyroidism | 500 |
| GPR54 | Isolated hypogonadotropic hypogonadism | 501, 502 |
| TSHR | Hypothyroidism | 503 |
| LHR | LHR hypergonadotropic hypogonadism | 504, 505 |
| FSHR | Hypergonadotropic hypogonadism | 506 |
| TACR3 | Familial hypogonadotropic hypogonadism | 507 |
| RXFP2 (relaxin family peptide 2) receptor | Cryptorchidism | 508 |
| AVPR2 | Nephrogenic diabetes insipidus | 435 |
| CaSR | Hyperparathyroidism | 509 |
| PTHR1 | Blomstrand chondrodysplasia/ Ollier disease | 510–512 |
| MC1R | Hypopigmentation | 513 |
| MC2R | Familial glucocorticoid deficiency syndrome | 514, 515 |
| MC3R | Obesity | 150, 516 |
| MC4R | Obesity | 517–520 |
| CCR5 | Susceptibility to HIV infection | 521, 522 |
| CXCR4 | WHIM syndrome | 523 |
| CX3CR1 | AIDS progression | 524 |
| Duffy antigen/receptors for chemokines | Susceptibility to malarial infection | 525 |
| GLP-1R | Diabetes | 526 |
| GCGR | Type 2 diabetes/Mahvash disease | 459, 527 |
| GHSR | Obesity and short statue | 528, 529 |
| GPR120 | Obesity and diabetes | 28, 530 |
| GPR40 | Abnormal insulin secretion | 531 |
| Endothelin type B receptor | Hirschsprung's disease | 532 |
| Orexin 2 receptor | Narcolepsy (canine and human) | 114, 533 |
| Thromboxane A2 receptor | Bleeding disorder | 534 |
| P2Y12 receptor | Bleeding disorder | 535 |
| Multiple receptors | Cancer | 20, 536 |
| N-formyl peptide receptor | Juvenile periodontitis | 537 |
| MASS1 | Febrile and afebrile seizures | 538, 539 |
| ATGR2 | X-lined mental retardation | 540 |
| Histamine H1 receptor | Autoimmune disease | 541 |
| Prostacyclin receptor | Accelerated cardiovascular disease progression | 542 |
| Cannabinoid receptor CB2 | Osteoporosis | 543 |
| GPR143 | Ocular albinism type 1/Congenital nystagmus | 544 |
Abbreviation: WHIM, warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis. This list is comprehensive but not exhaustive. For a complete list of the natural variants in GPCRs, consult the GPCR natural variants database at http://nava.liacs.nl/ (545).
Single nucleotide polymorphisms (SNPs) have also been identified in numerous GPCRs that are associated with different phenotypes or disease progression. There are a number of excellent reviews summarizing these studies (106, 121, 122). Functional studies on these variants have also been done with usually more subtle changes. Our focus herein is on the overt mutations; therefore we will not discuss SNPs further.
Pseudogenization can also be considered as a natural mutation leading to a total loss of function, a natural knockout model (106). It is estimated that approximately 17% of nonolfactory GPCRs in humans are functionally inactive (123). In some animals, similar pseudogenization occurs. For example, the taste receptors have evolved with the major diets of the animals. In cats, which are indifferent to sweet food, the sweet receptor T2R1 is a pseudogene (124). In carnivorous mammals, the taste receptors are inactivated (125). And in giant panda, which primarily feeds on bamboo, the umami receptor T1R1 is not functional (126).
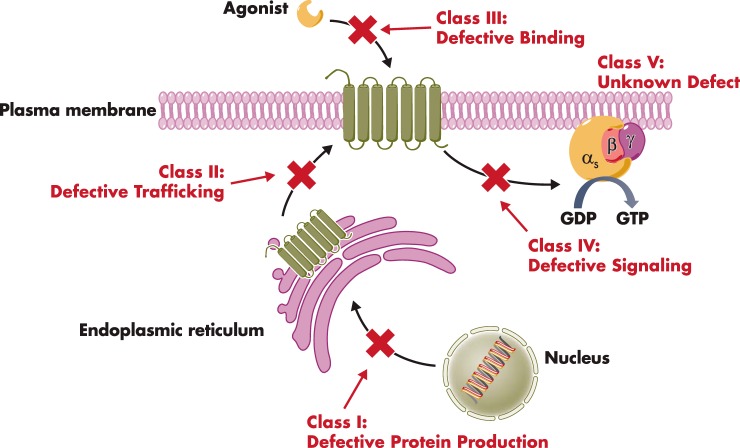
Extensive functional studies of the naturally occurring mutations in those GPCRs associated with diseases/phenotypes have provided unique insights into the structure-function of these receptors. We proposed that these inactivating mutations can be classified into five different classes (107, 127, 128) (Figure 3). Class I mutants have decreased protein levels due to decreased protein synthesis and/or increased protein degradation, mostly associated with nonsense or frameshift mutations. Class II mutants are retained intracellularly, mostly due to misfolding and retained in the ER, with some retained in the Golgi apparatus. Class III mutants are transported onto the cell surface but cannot bind to the ligand. Class IV mutants are transported onto the cell surface, can bind to the ligand, but cannot generate signaling due to defects in either G protein coupling or activation. Class V mutants have no significant effect on receptor expression and signaling. However, defects in other aspects of the receptor's functions including desensitization, internalization, resensitization, or activity at an alternative signaling pathway are usually not investigated and could potentially be the cause of the phenotypes observed. Most of the mutations are Class II mutations that are retained in the ER.
Figure 3.
Classification of naturally occurring inactivating mutations of GPCRs. This scheme was first proposed by Tao and Segaloff (127) for the MC4R and subsequently elaborated and extended to other GPCRs (see Refs. 107 and 128).
Of special interest are the findings on a unique atypical GPCR, GPR143 (ocular albinism type 1). Unlike the other GPCRs that need to be present at the cell membrane for signaling (see rare exceptions mentioned in Section III.A.), GPR143 is located at the membrane of an intracellular organelle, melanosome (129, 130); it can bind to Gi family of heterotrimeric G proteins and regulate melanosome transport in pigment cells (130, 131). Characterization of 19 missense mutations in GPR143 identified from patients with ocular albinism type 1 in COS-7 cells showed that 11 (∼60%) mutants are misfolded and retained in the ER, defective in intracellular transport and glycosylation (without the mature glycosylation achieved in Golgi apparatus), whereas the other eight mutants have normal processing and trafficking as the WT receptor, suggesting that protein misfolding and defective trafficking is also a major pathogenic mechanism in the naturally occurring mutations in this intracellular GPCR (132).
ER-retained mutants frequently exert dominant negative effects on the coexpressed WT receptors due to heterodimerization between the WT and mutant receptors, which likely takes place during or shortly after their biosynthesis in the ER (133–135). Dominant negative effect has been observed with numerous GPCRs. Examples include rhodopsin (134, 136), β2-AR (133), AVPR2 (137), CCR5 (138), LHR (139), TSHR (140), MC1R (141), GnRHR (142), prostacyclin receptor (143), and the Frizzled family of Wnt receptors (144), among many others.
However, dominant negative activity is not always observed with ER-retained mutants. A prominent example is the MC4R. Most of the obese patients harboring MC4R mutations are heterozygous. Therefore, it is of significant interest in understanding whether heterozygous MC4R mutations cause obesity by dominant negative activity or due to haploinsufficiency. Because functional studies showed that many mutant receptors are retained intracellularly (127, 145–147), it was surprising that most of these misfolded mutants do not exert dominant negative activity (reviewed in Refs. 128 and 148). Some mutants were shown to have dominant negative activity, but these mutants are expressed on the cell surface (149). In the related MC3R, where mutations have also been implicated in the pathogenesis of human obesity or adiposity (150), there is also no dominant negative activity, whether the mutant receptors are expressed on the cell surface or not (65, 151, 152), although dimerization of the MC3R and MC4R has been demonstrated (149, 153). However, more detailed studies need to be done to explain the apparent lack of dominant negative activity in the naturally occurring MC3R and MC4R mutations that are retained intracellularly. Whether these two melanocortin receptors (MCRs) dimerize in the ER, like other family A GPCRs (154), is of special interest.
Kallmann syndrome includes hypogonadism due to decreased GnRH secretion and anosmia or hyposmia due to defect in olfactory bulb morphogenesis. PROKR2 (prokineticin receptor 2) mutations cause Kallmann syndrome. Functional studies showed that some PROKR2 mutants have decreased cell surface expression, but these mutants do not have a dominant negative effect on the WT receptor cell surface expression (155).
Although most of the misfolded mutant receptors are retained in the ER, there are some mutants that can escape the ER quality control mechanism but are retained in the Golgi apparatus. For example, rhodopsin mutant E150K associated with autosomal recessive retinitis pigmentosa is partially colocalized with the cis/medial Golgi compartment markers such as GM130 and Vti1b, but not with the trans-Golgi network such as P230 (156). This mutant receptor also does not exert dominant negative activity on the coexpressed WT rhodopsin (156). The ORs expressed in undifferentiated odora cells are exported from the ER but are retained in the Golgi apparatus and not transported to the plasma membrane (157). Mutants that disrupt the N-terminal YS motif at the α2A- and α2B-AR are also retained in the Golgi apparatus, not at the ER (158). Of eight naturally occurring AVPR2 mutations studied in one report, five can exit the ER and reach the ER/Golgi intermediate compartment and are retrogradely transported back to the ER (159). These results are consistent with the idea that in addition to the quality control at the ER, there is additional post-ER quality control (160).
C. Motifs involved in retaining G protein-coupled receptors intracellularly
Several studies have identified a series of motifs, most of them highly conserved in GPCRs, that are involved in retaining GPCRs intracellularly. These motifs include tyrosine-based motifs, dileucine motifs, hydrophobic FxxxFxxxF motifs, basic domains, and polyproline motifs, among others. These studies are summarized in several excellent reviews (34, 135, 161, 162) and will not be repeated here.
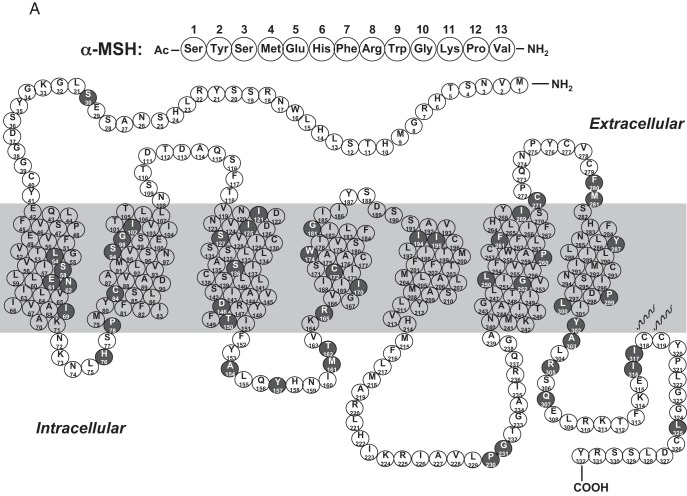
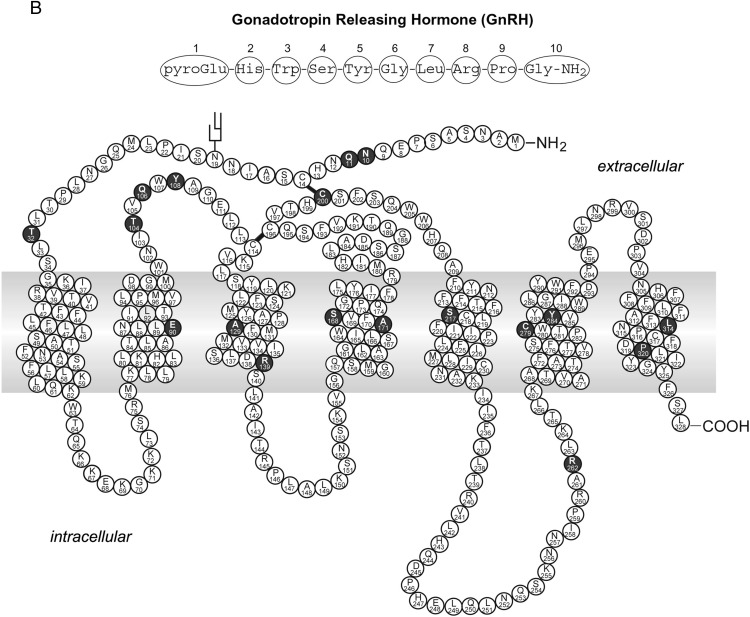
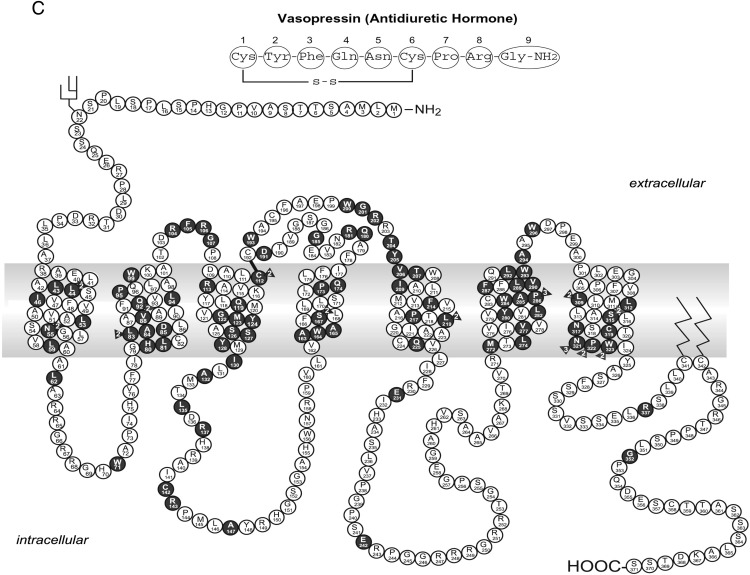
It should be emphasized that naturally occurring mutations in these motifs causing human diseases are very rare. Most of the studies that identified these motifs are structure-function studies using laboratory-generated mutants. Functional studies on disease-causing loss of function mutations showed that defects in trafficking could occur anywhere in the receptor. In Figure 4, we list the Class II mutations identified in MC4R (Figure 4A), GNRHR (Figure 4B), and AVPR2 (Figure 4C) that clearly demonstrate that they are widely distributed in the receptors, including all seven TMs, the ECLs, and ICLs, as well as the N and C termini. These mutations, instead of disrupting particular motifs, have defects in folding.
Figure 4.
Schematic representation of Class II mutations in the MC4R (A), GNRHR (B), and AVPR2 (C). Naturally occurring mutations that are defective in cell surface expression are highlighted in dark background. Panel A is based on the tabulation in Ref. 520. Panels B and C are reprinted from P. M. Conn et al: G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–250 (108), with permission. © American Society for Pharmacology and Experimental Therapeutics.
Because the anterograde trafficking of GPCRs is tightly regulated, we will discuss next the endogenous (molecular) and exogenous (chemical and pharmacological) chaperones that help the GPCRs moving from their site of synthesis at the ER to the plasma membrane (Figure 2).
IV. Molecular Chaperones in the Folding and Maturation of G Protein-Coupled Receptors
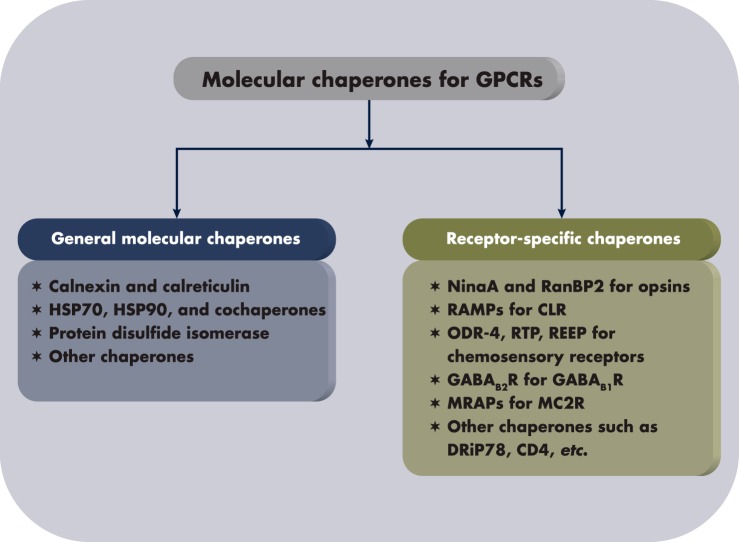
Anfinsen's Nobel Prize-winning work showed that the tertiary structure of a protein is determined by its primary structure, the sequence of the amino acids on the peptide chain. In his Nobel Lecture, he wrote: “the native conformation is determined by the totality of interatomic interactions and hence by the amino acid sequence in a given environment,” the “normal physiological milieu” (163). The forces that determine the three-dimensional structure are hydrogen bonds, van der Waals interactions, backbone angle preferences, electrostatic interactions, and hydrophobic interactions (164). However, the classical self-assembly principle is not sufficient to explain the efficient folding of nascent proteins in the cell, because of the enormous theoretical “folding space” (165) and the much higher concentrations of proteins in living cells than that used in Anfinsen's classical experiments that frequently leads to protein aggregation due to the exposed hydrophobic surface in folding intermediates (166). A seminal study demonstrated that in live cells, mitochondrial proteins can be synthesized and imported into the mitochondria but cannot be folded when the mitochondrial chaperone is mutated (167). The subsequent discovery of a network of molecular chaperones that assist the folding of nascent proteins provides a logical explanation. Molecular chaperones promote folding and prevent aggregation (the name “chaperone” derives from its role in preventing or reversing incorrect interactions, analogous to the human chaperone at a high school prom), ensuring protein homeostasis (proteostasis), the balance of protein folding, misfolding, and degradation (40). Figure 5 lists the general and receptor-specific molecular chaperones that will be discussed in this article.
Figure 5.
General and receptor-specific molecular chaperones that are involved in folding and maturation of GPCRs summarized in this article.
A. General molecular chaperones
1. Calnexin and calreticulin
Calnexin is an ER membrane chaperone, and calreticulin is an ER luminal chaperone. Working with glycosyltransferase, they help the folding of glycosylated proteins (168). As true molecular chaperones, they also interact with non-native unglycosylated proteins, decreasing their aggregation and maintaining unfolded proteins in a conformation that can be refolded (169–171).
Most of the GPCRs are glycoproteins containing the consensus sequence for N-linked glycosylation (sequon), Asn-X-Ser/Thr (where X is any amino acid except proline), at the extracellular N terminus and ECLs. Earlier biochemical studies using pure receptors showed that β-ARs are glycoproteins containing both high mannose immature carbohydrates (sensitive to endoglycosidase H treatment and binds to concanavalin A) and complex type mature carbohydrates (sensitive to neuraminidase treatment and binds to wheat germ agglutinin) (172, 173). Since then, extensive literature exists on the functional roles of glycosylation on receptor function, with different effects observed in different receptors. For example, treatment of cells expressing β-ARs with tunicamycin, an inhibitor of N-glycosylation, does not affect β-AR expression and function, whereas it leads to loss of prostaglandin E1 receptor function (173). This was confirmed by another study using the lipid vesicle reconstitution technique (174).
Even in related receptors, N-linked glycosylation can have very different importance in receptor expression and function. For example, in the rat LHR, N-linked glycosylation is not essential (although it is beneficial) for proper folding (175); however, in the closely related rat FSH receptor (FSHR), N-linked glycosylation is absolutely required for folding into mature conformation capable of ligand binding (176). These studies showed that N-linked glycosylation exerts different functions in different GPCRs, with an effect on folding and cell surface expression most consistently observed.
Calnexin is a well-characterized molecular chaperone that interacts with nascent chains of N-glycosylated proteins in the ER lumen. Interaction of calnexin with its client proteins allows correctly folded molecules to reach their normal destination and retain misfolded molecules in the ER. Several GPCRs have been shown to interact with calnexin and/or calreticulin, including immature LHR (177, 178), immature FSHR (177), TSHR (179), GnRHR (180), AVPR2 (181), and D1 and D2 DRs (182). The immature form of WT and mutant AVPR2s associate with calnexin, and ER-retained mutants have prolonged association with calnexin, suggesting that calnexin is serving as a monitor for quality control, retaining the misfolded AVPR2 in the ER (181). Mutant MCHR1 (melanin-concentrating hormone receptor type 1) retained in the ER was also found to associate with calnexin more extensively compared with WT MCHR1 (183). With D1 and D2 DRs, both calnexin inhibitors and calnexin overexpression increase intracellular retention and decrease cell surface expression of the receptors, suggesting that optimal receptor-calnexin interaction is critical for optimal cell surface expression of the receptors (182). Overexpression of calnexin decreases plasma membrane expression of human and rat GnRHRs and subsequent signaling (184). Recently, a 4-amino acid, noncontiguous “motif” (L112, Q208, L300, D302) in GnRHR that might be a determinant of calnexin recognition was identified (184). Overexpression of several ER resident chaperones including calnexin does not increase cell surface expression of mammalian GPCRs expressed in yeast (185). These results suggest that calnexin binds to glycosylated GPCRs, serving as a monitor of receptor folding.
Different unglycosylated GPCRs have varying abilities to bind to calnexin. Unglycosylated human DOR does not interact with calnexin and is transported out of the ER and processed to the mature form at an accelerated rate (186). Some of these receptors at the cell surface cannot bind the ligand, and they have increased turnover and lysosomal degradation. The end result is that the unglycosylated receptor is expressed at the cell surface at a reduced level (186). However, unglycosylated angiotensin type 1 receptor (AT1R) can associate with calnexin (187), consistent with the pure chaperone function of calnexin.
Drosophila and mammalian rhodopsins seem to have different requirements for calnexin in their maturation. Calnexin is required for Drosophila rhodopsin maturation (188). However, mammalian rhodopsin does not have an absolute requirement for calnexin in its biogenesis and maturation (189). These differences may be explained by the different requirements for glycosylation in the maturation of Drosophila (requiring glycosylation) and mammalian (not requiring glycosylation) rhodopsin. Nevertheless, calnexin preferentially associates with misfolded rhodopsin, and overexpression of calnexin in the presence of the pharmacoperone 11-cis-retinal increases the folding of mutant rhodopsin (190).
In HEK293T cells, mouse odorant receptors mI7 and mOREG are retained in the ER, colocalizing with ER markers and containing endoglycosidase H-sensitive carbohydrates; they cannot be detected in Golgi apparatus or plasma membrane (191). They interact with calnexin but not calreticulin and are eventually degraded by proteasome or autophagosome (191).
The vomeronasal organ, one of the three chemosensory systems, is dedicated to the detection of pheromones (192). Receptors for peptide pheromones, vomeronasal receptor type 2 (V2Rs), are not expressed on the cell surface in heterologous cells frequently used for studying GPCRs. (Note: V2R is also frequently used as an abbreviation for type 2 arginine vasopressin receptor, for which we used AVPR2 as the abbreviation to avoid confusion with the pheromone receptor also discussed in this article.) Calreticulin, a housekeeping chaperone usually expressed in most eukaryotic cells, is not expressed at a significant level in the vomeronasal sensory neurons. Calreticulin knockdown in heterologous cell lines results in transfected V2Rs to reach the plasma membrane efficiently (193). Therefore, in vomeronasal sensory neurons, calreticulin is deleterious for cell surface expression of V2Rs. A calreticulin homolog, calreticulin 4, functions as a replacement for calreticulin but does not exert a negative effect on membrane export of V2Rs (193).
In summary, calnexin and calreticulin can interact with many different GPCRs, especially the glycosylated form (although coimmunoprecipitation experiments do not show interactions between prostaglandin receptor 1 (DP1) overexpressed in HEK293 cells and calnexin or calreticulin [194]). The effects of overexpressing these chaperones on the cell surface expression of GPCRs vary with the GPCRs. Frequently, too high and too low expression of these chaperones are both deleterious for the forward trafficking of GPCRs.
2. Heat shock proteins
When exposed to increased temperature, a universal response in cultured cells and every organism investigated is the production of a small number of highly conserved proteins, the HSPs (195). The HSPs are also induced by a wide variety of other stresses, including the presence of misfolded proteins. These HSPs help misfolded proteins to achieve their native conformation to relieve the stress (this explains why some of the molecular chaperones are stress proteins). The genes encoding the HSPs are highly conserved during evolution, not only in the coding region but also in the regulatory region (195).
HSP70 is the most highly conserved HSP and has been the most extensively studied (195). HSP70 aids in protein folding, stabilization, translocation, and degradation. The nascent peptide is recognized cotranslationally by HSP70, prevented from premature misfolding (196, 197). HSP70 and HSP90 chaperone systems collaborate in the cellular processing of clients. HSP70 interacts with some GPCRs such as α2A-AR, which results in uncoupling of the receptor from G protein (198). DP1 overexpressed in HEK293 cells do not interact with several molecular chaperones such as BiP, cytosolic 70-kDa heat-shock cognate protein (Hsc70), and HSP90 (194). A recent study using tandem-affinity purification and mass spectrometry showed that the adenosine A2A receptor interacts with HSP90α and HSP70–1A, and these interactions result in the retention of partially folded receptor from exiting the ER prematurely, acting as a mechanism of quality control (199). Disruption of receptor-HSP90 interaction is required before the recruitment of coat protein complex II (COPII) components. HSP90 inhibitors (such as radicicol and 17-dimethylaminoethylamino-17-demethoxygeldanamycin) or decreasing cellular HSP90 levels using small interfering RNA (siRNA) knockdown of HSP90 increase the cell surface expression levels of adenosine A2A receptor endogenously expressed in PC12 cells (199) or α2C-AR expressed in HEK293 cells (200).
Coimmunoprecipitation experiments showed that Hsc70 associates with MC4R (201). Hsc70 overexpression corrects the misfolding of naturally occurring mutant receptors and increases their plasma membrane expression and signaling capacity. For MC4R, inhibition of endogenous HSP90 by geldanamycin reduces receptor levels. By contrast, expression of the HSP90 cochaperone Aha1 (activator of HSP90 ATPase) increases cellular levels of MC4R. These data indicate that cytosolic chaperone systems can facilitate folding and forward trafficking of intracellularly retained MC4R mutants (201).
BiP, also known as GRP78 (or glucose-regulated protein of 78 kDa), is a member of the HSP70 family of proteins in the ER lumen. It has been shown to be important for the folding and assembly of nascent proteins, identifying misfolded proteins for ERAD, preserving the permeability barrier of Sec61 translocon during early stages of protein translocation, as well as maintaining ER calcium stores (45). WT rat LHR and FSHR do not associate with BiP (177). Although WT human LHR does not associate with BiP, two naturally occurring mutant human LHRs, A593P and S616Y, associate with BiP (178). It was suggested that the association of BiP with WT human LHR that is in the process of normal folding is likely to be transient, more difficult to observe with coimmunoprecipitation technique; however, the association of misfolded mutant receptors with BiP is likely more persistent, and therefore easier to observe (178). Another recent study showed that both human and rat LHRs but not human FSHR interact with BiP; increased BiP expression increases total (both cell surface and intracellular) LHR but not FSHR levels (202). The exact reason for this discrepancy between these two studies is not clear. TSHR interacts with BiP, and overexpression of BiP increases TSHR degradation and decreases mature TSHR level at the cell surface (179).
In vitro, overexpression of BiP has no effect on the folding and trafficking of the most common rhodopsin gene mutation in North America, P23H (203). Extensive studies on this mutation have shown that P23H rhodopsin is misfolded (204). Expression of P23H in cells causes ER stress, activates the UPR, and induces apoptosis (203, 205) (similar findings were reported for another misfolding rhodopsin mutation, T17M [206]). In transgenic animals expressing P23H and patients harboring this mutation, retinal degeneration and autosomal dominant retinitis pigmentosa ensue (207, 208), accompanied with decreased BiP expression (205). Overexpression of BiP in transgenic rats can alleviate ER stress and decrease UPR and photoreceptor apoptosis (203). A recent study demonstrated that BiP is important for preventing aggregation and maintaining solubility of rhodopsin in the ER (209).
HSP90 interacts with the C-terminal tail of protease-activated receptor-1 (210). This interaction does not affect cell surface expression of the receptor; rather, it modulates receptor signaling (210). This was suggested to be consistent with the fact that in nonstress conditions, HSP90 interacts with several signaling molecules to affect these signal transduction cascades (211).
To summarize, a number of HSPs, especially BiP, have been shown to affect the cell surface expression of WT and mutant GPCRs, with different effects observed in distinct GPCRs. No general conclusions can be reached at this time. More studies on different GPCRs are needed.
3. Protein disulfide isomerase
Disulfide bonds are known to increase protein thermodynamic stability (212). The ER is the major compartment in which disulfide bonds are formed during protein folding (disulfide bonds are also formed in the mitochondrial intermembrane space) (213, 214). Two enzymes in the eukaryotic ER, ER oxidoreductin-1 and protein disulfide isomerase (PDI), are responsible for introducing disulfide bonds into proteins being folded in the ER (214). Molecular oxygen oxidizes ER oxidoreductin-1 generating disulfide bond de novo, which then oxidizes PDI, acquiring the disulfide bond by dithiol/disulfide exchange. PDI then passes the disulfide bonds onto substrate proteins (215). First discovered by Anfinsen and colleagues (216), PDI is a resident protein in the ER of eukaryotes, acting both as a molecular chaperone and an isomerase (217–219). It can affect protein folding and protein aggregation through its peptide-binding domain, including proteins without any disulfide bonds. As an ER oxidoreductase, it also facilitates formation of the correct disulfide bonds in the client proteins by promoting rapid reshuffling of disulfide pairings, including reduction of incorrect cysteine pairings and reoxidation of cysteines for correct disulfide bonding (220).
A signature motif in most family A GPCRs is the existence of a disulfide bond connecting the top of TM3 with the second ECL. This disulfide bond has been experimentally shown to be critical for normal folding of a number of GPCRs. For example, in rhodopsin, a disulfide bond between C110 and C187 is required for normal rhodopsin formation, whereas a disulfide bond between other cysteines, for example between C185 and C187, leads to misfolding, characterized by the loss of ability of opsin to bind 11-cis-retinal (221–224). Similarly, extracellular cysteines were found to be essential for normal cell surface expression of the human platelet-activating factor receptor (225). Studies with the naturally occurring MC4R mutation, C271R, suggested that formation of a functionally disastrous disulfide bridge also results in misfolding and intracellular retention (226).
Very few studies have been done on the role of PDI in GPCR folding. In coimmunoprecipitation experiments, PDI was found to associate with immature forms of human glycoprotein hormone receptors, FSHR, LHR, and TSHR (178). Interestingly, of two naturally occurring mutations in human LHR that are retained intracellularly, A593P and S616Y (139, 178), S616Y is associated with PDI but A593P is not (178). To study the potential function of this association, PDI was overexpressed in a yeast system. For the GPCRs that contain a disulfide bond, overexpression of PDI does not increase cell surface expression (185). However, overexpression of ERP-57 led to increased intracellular retention of human GnRHR (227). Indeed, it is known that under certain conditions, PDI also facilitates protein misfolding and aggregation, the so-called antichaperone activity or unfoldase activity (228, 229). It was suggested that this antichaperone activity might be a mechanism to retain misfolded proteins in the ER as large aggregates bound to PDI (or other ER resident chaperones) when the folding capacity is exceeded (230).
Using a membrane-based split ubiquitin yeast two-hybrid assay with full-length GLP1-R as the bait, ERp29, a PDI-like ER luminal protein but without thioredoxin-like catalytic moiety, was identified to interact with GLP1-R (231), although the function of this interaction is not clear. Interestingly, none of the general molecular chaperones described above were identified to interact with GLP1-R.
4. Other chaperones
In addition to the molecular chaperones described above that were extensively studied, there are some reports of other less-known chaperones that can also interact with GPCRs and potentially affect forward trafficking of GPCRs. For example, Ribophorin I is a component of the oligosaccharide transferase complex that has been shown to directly interact with μ-opioid receptor (MOR), promoting its export from the ER and hence increasing cell surface expression of the receptor (232). This chaperone activity is dependent on N-linked glycosylation because Ribophorin I does not affect the trafficking of mutant GPCRs that are deficient in N-linked glycosylation (232).
Ankyrin repeat domain-containing protein 13C (ANKRD13) is a protein associated with the cytosolic side of ER membranes. Its interaction with DP can promote the biogenesis of DP by inhibiting the degradation of newly synthesized receptors. However, a prolonged interaction between ANKRD13C and DP1 results in ER retention of misfolded or unassembled forms of DP1 and their proteasome-mediated degradation (194). ANKRD13C also regulates the expression of several other GPCRs, including CRTH2 (chemoattractant receptor homologous molecule expressed on T-helper type 2 cells), thromboxane A2 receptor, and β2-AR, and this interaction is specific because ANKRD13C does not affect the expression of several other non-GPCRs (194). These results suggest that ANKRD13C, acting as a molecular chaperone, can regulate the maturation of some GPCRs. A pharmacoperone for DP1, MK-0524, enhances DP1-ANKRD13C interaction, leading to increased proteasomal degradation of DP1 (194).
A Golgi membrane-associated protein, AT2R binding protein of 50 kDa (ATBP50; also called AT2 interacting protein 1, or ATIP1), binds to an ER export signal in the C terminus of angiotensin type 2 receptor (AT2R) as demonstrated with yeast two-hybrid assay and coimmunoprecipitation (233, 234) (see Ref. 235 for a review on AT2R interacting proteins). In vivo, AT2R and ATBP50 are coexpressed, with high expression in the uterus and adrenal gland. siRNA knockdown of ATBP50 leads to ER retention of AT2R and decreased cell surface expression, suggesting that this protein functions as a chaperone to traffic the AT2R to the plasma membrane (234). Golgin-160, localized primarily in the cis and medial regions of the Golgi apparatus, promotes the cell surface expression of β1-AR (236).
These selected examples are just a glimpse of the proteins that can modulate forward trafficking of GPCRs. Future studies, especially using proteomic approaches, will certainly identify additional interaction partners of GPCRs that affect the anterograde trafficking in the different compartments, especially the exiting of the ER quality control system.
B. Receptor-specific molecular chaperones
The general molecular chaperones described above help multiple GPCRs (as well as other cellular proteins) to fold and travel to their destiny. Below, we list several molecular chaperones that have evolved to be specialized proteins escorting a very limited number of GPCRs.
1. Specific chaperones for opsins
Neither inactivation nor afterpotential A (NinaA), a cyclophilin homolog with peptidyl-prolyl cis-trans isomerase activity, is essential for biogenesis of the major Drosophila rhodopsin, Rh1 (237–239). In transgenic Drosophila, NinaA forms a stable complex with Rh1 and contributes to Rh1 biogenesis in a quantitative manner (239). Therefore, NinaA acts as a chaperone to promote Rh1 export from the ER. A cyclophilin-related protein, Ran-binding protein 2 (RanBP2), acts as a chaperone for mammalian red/green opsin, causing cis-trans isomerization of one or more proline residues in the opsin protein (240, 241). Both NinaA and RanBP2 have a single hydrophobic TM attaching them to a subcellular membrane. These two proteins represent the first examples of receptor-specific chaperones.
2. Specific chaperones for calcitonin receptor-like receptor
When an orphan receptor related to CTR named “calcitonin receptor-like receptor” (CLR) was cloned from rat and human (242–244), it was found not to be expressed on the cell surface and not to be activated by any known ligand when transfected into cell lines frequently used in expression studies. In 1998, Foord and colleagues (245) identified single transmembrane proteins called receptor activity-modifying proteins (RAMPs) that promote translocation of CLR to the cell surface, from 3% to approximately 25%. RAMPs are type I transmembrane proteins with an extracellular N terminus and a cytoplasmic C terminus, encoded by three distinct genes. When expressed alone, neither RAMPs nor CLR can reach the cell surface. When expressed together, RAMPs form a complex with CLR in the ER and are translocated to the cell surface together. Therefore, translocation of RAMPs to the cell surface can be used as a measure of RAMP–GPCR interaction.
Interestingly, RAMPs not only affect receptor trafficking; they also affect receptor pharmacology, including ligand binding and G protein coupling. CLR associated with RAMP1 is a CGRP receptor, whereas CLR associated with RAMP2 or RAMP3 is one of the two different subtypes of adrenomedullin receptor (245). RAMP1 or RAMP3 (but not RAMP2) coexpressed with CTR generates an amylin receptor. The binding affinity of the RAMP1-CTR complex is the highest for salmon CT, high to moderate for rat amylin and human CGRPα, and low for human CT, whereas for the RAMP3-CTR complex, similar affinities for amylin and the CTs are observed, but that of human CGRPα is markedly decreased (246). Compared with CTR expressed alone, CTR coexpressed with RAMPs has dramatically increased amylin potency of Gs signaling (20- to 30-fold as measured by increased intracellular cAMP) compared with Gq signaling (2- to 5-fold as measured with increased intracellular Ca2+) or ERK1/2 phosphorylation (2- to 5-fold using non-Western blot-based Surefire phospho-ERK kit), consistent with increased affinity (247). These data suggest that RAMPs directly affect the G protein-coupling efficiency of CTR (247).
RAMPs also affect receptor internalization and recycling (248, 249). RAMP3 (but not RAMP1 or RAMP2) has PSD-95/Discs-large/ZO-1 homology (PZD) domain at the C terminus that can interact with Na+/H+ exchanger regulatory factor-1 (NHERF-1) to affect receptor internalization (in the absence of RAMP3, the receptor is desensitized but not internalized) or with N-ethylmaleimide-sensitive factor (NSF) to affect receptor recycling after internalization (in the absence of RAMP3, the receptor is degraded).
Subsequent studies showed that RAMPs also associate with other members of family B GPCRs and even a family C GPCR. All three RAMPs interact with VPAC1R and VPAC2R, affecting G protein coupling (250). Of the three RAMPs, only RAMP2 interacts with GCGR, PTHR1, and corticotropin-releasing factor (CRF) 1 receptor (CRF1R); only RAMP3 interacts with PTHR2 and secretin receptor; and GLP1-R does not interact with any RAMPs (250–252). The transmembrane region of RAMP3 associates with TM6 and TM7 of secretin receptor. Although RAMP3 association does not change the forward trafficking of the WT secretin receptor (which traffics normally in the absence of RAMP3), it does rescue an intracellularly retained secretin receptor mutant (G241C) to the cell surface. There is no change in signaling (including intracellular cAMP, calcium, and ERK1/2 phosphorylation) or internalization when the WT secretin receptor is associated with RAMP3 (252). RAMP2 association with the CRF1R increases the cell surface expression of CRF1R but does not affect agonist-stimulated cAMP generation; however, it enhances intracellular calcium response to CRF and urocortin 1, but not to sauvagine.
The CaSR is a family C GPCR with a large extracellular N terminus for binding to Ca2+ in a Venus flytrap mode, and it plays a crucial role in calcium homeostasis (253). The RAMPs also promote the trafficking of the CaSR (254). In COS7 cells that do not contain endogenous RAMPs, the CaSR is retained in the ER; coexpression of RAMP1 or RAMP3, but not RAMP2, escorts the CaSR to the cell surface. In HEK293 cells that express RAMP1, the CaSR is expressed at the plasma membrane; siRNA knockdown of RAMP1 alters CaSR trafficking. Coimmunoprecipitation experiments showed that RAMP1 and RAMP3 associate with the CaSR. These results suggest that CaSR association with RAMPs is necessary and sufficient for cell surface expression of the CaSR (254).
The physiological significance of these interactions has not been elucidated in all cases. However, there is evidence indicating that they are physiologically relevant. For example, in mice heterozygous for Ramp2, there is reduced CRF responsiveness to release ACTH, suggesting that RAMP2 interaction with CRF1R is of physiological significance (250).
It is interesting to note that a coreceptor structurally and functionally related to RAMPs, termed RAMP-like triterpene glycoside receptor (RL-TGR), has been cloned in zebrafish that is suggested to be involved in generating signaling to triterpene glycosides, deterrent compounds isolated from marine sponges to defend them from predation (255). RL-TGR interacts with β2-AR as demonstrated by coimmunoprecipitation, and β2-AR induces the trafficking of RL-TGR from the cytoplasm to the plasma membrane (255), the same way as RAMPs trafficking from the cytosol to the plasma membrane when coexpressed with any interacting GPCRs. The GPCR that interacts with RL-TGR has not been identified in the fish.
3. Specific chaperones for chemosensory receptors
There are three chemosensory receptors in mammals—the odorant receptors, the taste receptors, and the pheromone receptors. All three types of receptors are difficult to be expressed on the cell surface in heterologous cell lines (61, 256, 257), with only occasional cells expressing the receptor at the cell surface (258). We describe below the different strategies of the three types of chemosensory receptors used for achieving cell surface expression.
The Nobel Prize-winning work of Axel and Buck on the cloning of the large family of ORs (259–261) represents a major breakthrough in the studies of olfaction. In humans, there are about 400 ORs, and in rodents there are about 1000. Most of these receptors remain orphan receptors. The studies on these receptors have been very difficult because they are barely expressed on the cell surface when expressed heterologously in most of the cell types frequently used for functional studies of GPCRs. Different strategies were used to artificially increase the plasma membrane expression, including N-terminal extension by the addition of an N-terminal fragment of rhodopsin (262, 263) or the addition of an N-terminal cleavable signal sequence (264, 265) (reviewed in Ref. 266). In mature cells of the OR neuron lineage, the ORs are expressed at the plasma membrane (157, 267, 268). It seems that the accessory proteins that allow the ORs to be trafficked to the plasma membrane are expressed only in mature olfactory sensory neurons. These accessory proteins regulate the exit of the ORs from the ER as well as the further trafficking from post-Golgi compartments to the plasma membrane of the olfactory cilia where the receptors are exposed to odorants. Several specific chaperones for these receptors have been cloned.
In the nematode Caenorhabditis elegans, odr-4 encodes a type II membrane protein expressed exclusively on intracellular membranes of chemosensory neurons in the cell body and dendrites, but not at the plasma membrane (269). ODR-4 is located in the ER, Golgi apparatus, and transport vesicles, with its C-terminal hydrophobic tail predicted to be a TM anchoring the protein into a subcellular membrane. ODR-4 promotes the trafficking of the OR ODR10 to the cell surface in chemosensory neurons, likely affecting folding, sorting, or targeting, with the acidic residues in ODR-4 interacting with the basic residues in the ICLs of the ORs. Mutation in odr-4 results in intracellular retention of ODR10. Further analysis showed that odr-4 is required for correct localization of a subset of ORs (269). ODR-4 also promotes the trafficking of rat OR U131 in the olfactory cell line odora and CHO cells to the plasma membrane (157).
Single pass transmembrane proteins specifically expressed in olfactory neurons named receptor-transporting protein (RTP), including RTP1 and RTP2, act as chaperones to promote functional cell surface expression of the ORs expressed in heterologous cells such as HEK293T cells, hence signaling responses to odorants (270). Coimmunoprecipitation experiments showed that RTPs associate with the ORs. When expressed together, the accessory proteins, including RTP1S (a short form of RTP1 that is more effective at promoting cell surface expression of the ORs than RTP1), Ric8B (resistance to inhibitors of cholinesterase 8B, a putative guanine nucleotide exchange factor specifically expressed in mature olfactory neurons that has been shown to promote functional expression of the ORs [271, 272]), and Gαolf (the heterotrimeric olfactory-specific G protein), exert synergistic effects on the cell surface expression of the ORs with enhanced OR responses (273). Ric8 proteins (including Ric8A and Ric8B) have been shown to be molecular chaperones required for the initial association of nascent Gα subunits with cellular membranes (274) but do not increase the cell surface OR numbers (271). Both Ric8A and Ric8B enhance OR-mediated signaling (191, 271). Another protein, REEP1 (receptor expression enhancing protein 1), has similar but weaker effects than RTPs (270). Recently, it was shown that some REEPs selectively enhance the cell surface expression of other difficult-to-express GPCRs (275).
Molecular chaperones can also regulate the biogenesis of GPCR heterodimers. RTP4, a Golgi chaperone, protects the MOR-DOR heterodimers from ubiquitination and degradation, leading to increased plasma membrane heterodimer levels and increased signaling activity (276). Because the pharmacological properties of the heterodimers are different from either the MOR or DOR homodimers, these important findings suggest that RTP4 can modulate the pharmacology of their endogenous ligands (276).
Another strategy to increase the cell surface expression of ORs is through coexpression of another GPCR. In Drosophila, Or83b is a ubiquitously expressed OR and is highly conserved between insect species (64–88% amino acid identity). Two studies showed that Or83b might be an atypical OR essential for the function of most of the other conventional ORs through heterodimerization with the other ORs (277, 278). In the mammalian system, heterodimerization with the β2-AR (but not any of the other eight subtypes of ARs) leads to increased cell surface expression of OR M71 in heterologous cells (279).
Mammals sense five tastes: sweet, bitter, sour, salty, and umami. The sensing of sweet, bitter, and umami is mediated by GPCRs, whereas that of sour and salty is not (280). Three subtypes of taste receptors have been cloned in the taste buds: first the T1R1 and T1R2 (281), and then the T1R3 independently by six groups (282–287). They are all members of family C GPCRs. When these candidate taste receptors are individually expressed in heterologous cells, they do not respond to sweet stimuli. However, when the T1R3 is coexpressed with the T1R2, a robust response to sweet stimulants including saccharin, sucrose, and other sweet tastants is obtained, suggesting that heterodimerization between the two subtypes of taste receptors is required for the formation of functional sweet taste receptors (286, 288). T1R1 and T1R3 heterodimerization results in a functional receptor responsive to amino acids and monosodium glutamate, the so-called umami taste (umami means “delicious flavor” in Japanese), with the human heteromer selectively tuned to detect glutamate, and that of the mouse sensing all amino acids with similar affinity (288, 289). Studies in knockout mice demonstrate that indeed formation of the T1R1 and T1R3 heterodimer is needed for umami sensation (290).
Depending on the species, there are about 30 members of T2Rs that mediate the bitter taste; it has been shown that they are necessary and sufficient for bitter sensing (280, 291). Unlike the T1Rs, the T2Rs do not have long N termini and are not members of family C GPCRs; they are distantly related to opsins. These receptors are also difficult to express on the cell surface (291). However, the mechanism and accessory factor(s) mediating their expression at the plasma membrane in vivo have not been elucidated. Most of the T2Rs are expressed in each bitter sensing cell (292–294). Whether heterodimerization between the different T2Rs promotes forward trafficking remains to be investigated.
The V2Rs expressed in vomeronasal organ are not related in sequence to the ORs expressed in the main olfactory epithelium. In mouse vomeronasal organ, one or a few members of the M10 family of major histocompatibility complex (MHC) class Ib that share about 50% sequence identity to classical MHC molecules are specifically coexpressed with a given V2R. In vomeronasal organ sensory dendrites, M10s, pheromone receptors, and β2-microglobulin (a binding partner for MHC class I proteins) associate to form a large complex. In vitro, M10s promote V2R trafficking to the cell surface; the V2R is not expressed on the cell surface in cells lacking M10s (295). Crystal structure revealed how M10s, rather than presenting MHC-binding peptides, by binding to the V2Rs, might act as molecular chaperones to the V2Rs promoting their forward trafficking (296) (reviewed in Ref. 297), although the exact role in folding, cargo sorting, vesicle transport, and vesicle fusion is unknown. In humans, there is no homolog of M10s, and most of the human pheromone receptor genes have been pseudogenized (298).
4. Specific chaperones for GABAB1 receptor
The principal inhibitory neurotransmitter in the mammalian central nervous system, GABA, exerts its effects through both inotropic (GABAA/C) receptors that produce fast synaptic inhibition and metabotropic (GABAB) receptors that produce slow but prolonged inhibitory signals. GABAA receptors are ion channels, whereas GABAB receptors (GABABRs) are GPCRs.
When two GABAB1R splice variants generated from alternative splicing of a single gene, resulting in different N termini, were first cloned and expressed in vitro, 150-fold lower affinity for agonists compared with native GABABRs was observed, and coupling to certain effector systems was difficult to measure (299). Another study showed that in heterologous cells or cortical neurons, GABAB1Rs are retained in the ER and fail to reach the cell surface (300). Very quickly, several groups reported the cloning of a second GABABR, GABAB2R (301–305). These studies showed that when the two GABABRs, GABAB1R and GABAB2R, are coexpressed, they form a heterodimer, and the GABAB1R is now expressed as a mature receptor on the cell surface that can bind to GABA and generate second messenger as expected.
Further studies showed that there is an ER retention motif at the C terminus of the GABAB1R (RSRR) that can be masked by the GABAB2R upon heterodimerization via the C-terminal coiled-coil α-helices (306). Chimera of GABAB2R containing the entire C-terminal tail of GABAB1R is retained in the ER when expressed alone (307). The GABAB2R can be expressed on the cell surface when expressed alone but cannot bind to GABA. In this context, the GABAB2R serves as a specific chaperone for the GABAB1R, escorting the trafficking of the GABAB1R from the ER to the cell surface. It should be mentioned that the intracellular domains of the GABAB2R are also necessary for G protein coupling and receptor signaling, whereas the intracellular domains of the GABAB1R are not (308).
In vivo, these receptors are coexpressed. In native brain membranes, homodimers of GABAB1Ra, GABAB1Rb, or GABAB2R cannot be detected; all GABAB2R protein forms heterodimeric complexes with either the GABAB1Ra or the GABAB1Rb, demonstrating that almost all GABABRs are heterodimers in situ (309). These studies demonstrating that the GABABRs are obligatory heterodimers in vivo are extremely important in solidly establishing the concept that GPCR dimerization/oligomerization is not an artifact (310), confirming our study three decades ago suggesting that GnRHR dimerizes before the cloning of the receptor or knowing that it is a GPCR (311). Numerous other examples for regulation of GPCR cell surface expression through heterodimerization with another GPCR have been reported since then. Here we provide several examples.
Coimmunoprecipitation experiments showed that the GABABRs can heterodimerize with the CaSR and the heterodimerization modulates CaSR surface expression (312, 313). Coexpression of the GABAB2R increases CaSR cell surface (and total) expression whereas coexpression of the GABAB1R decreases CaSR cell surface (and total) expression. GABAB1R-null mice have increased CaSR plasma membrane expression. Colocalization of the CaSR with the GABAB1R and GABAB2R in neurons and growth-plate chondrocytes are observed (312, 313), suggesting that the interactions may have physiological relevance. The mGluRs are also family C GPCRs. They have also been shown to interact with the CaSR by coimmunoprecipitation (314). The CaSR is colocalized with the mGluRs in specific populations of neurons in the brain. Heterodimerization with the mGluR1α does not affect the CaSR surface expression, but heterodimerization with mGluR5 increases surface expression of the CaSR (314). For a comprehensive review on the dimerization of family C GPCRs, please refer to the article by Pin et al (315).
The adenosine A2B receptor is poorly expressed on the cell surface. Both in vitro and in vivo experiments showed that the A2A receptor increases surface expression of the A2B receptor: cotransfection of the A2A receptor with the A2B receptor enhances surface expression of the A2B receptor through the F(X)(6)LL motif in the C terminus of the A2A receptor; significantly lower levels of splenocyte A2B receptor signaling in A2A receptor-null mice, compared to those in WT mice, are observed (63). In a similar vein, the α1D-AR is poorly expressed at the cell surface. Both β2-AR and α1B-AR can heterodimerize with the α1D-AR and act as chaperones escorting α1D-AR to the cell surface (316). Heterodimerization of mouse OR M71 with β2-AR (but not any other AR subtypes) leads to dramatically increased M71 expression at the cell surface transfected in HEK293 cells and signaling response (279). Coimmunoprecipitation experiments showed that the two receptors interact and they internalize together when stimulated by the respective ligands. In vivo, the two receptors are colocalized in mouse olfactory epithelium (279). In a follow-up study, potential interaction of 42 distinct GPCRs with M71 was examined. The vast majority of these receptors do not affect M71 expression at the plasma membrane. Only three subtypes of purinergic receptor dramatically increase M71 expression at the plasma membrane (317). One OR in Drosophila, Or83b, also likely acts a chaperone for the other odorant receptors through heterodimerization to escort them to the dendrites (277).
These are just selected examples of GPCR heterodimerization affecting anterograde trafficking. Single transmembrane splice variant can even heterodimerize with the full-length MOR, decreasing the ERAD of the full-length receptor (318). It should be mentioned that other functions of GPCR heterodimerization have been described in the literature, including receptor binding, signaling, internalization, and desensitization. Interested readers are referred to several recent articles (154, 319–323).
5. Specific chaperones for melanocortin-2 receptor
Of the five subtypes of MCRs, MC2R, the classical ACTH receptor, is unique in that it is not possible to express it at the cell surface in cell lines frequently used for expressing GPCRs, such as HEK293 or CHO cells. The MC2R expressed in these cells are retained in the ER (324). Only adrenal cell lines such as Y6/OS3 or cells expressing endogenous MCRs such as melanoma cells that express MC1R endogenously can be used to express the MC2R (324). These results suggest that corticoadrenal cells express protein(s) that help the nascent MC2R to fold and traffic to the plasma membrane. This is consistent with the fact that mutations in the MC2R gene only account for about 25% of familial glucocorticoid deficiency syndrome cases. Mutations in other genes, such as the hypothetical chaperone, might be responsible for some of the other cases. Indeed, using SNP array genotyping, Clark and colleagues (325) showed that mutations in a gene encoding a 19-kDa single-TM protein that they named melanocortin 2 receptor accessory protein (MRAP) cause type 2 familial glucocorticoid deficiency syndrome. MRAP interacts with the MC2R to enhance the trafficking of the MC2R from the ER to the plasma membrane (325).
Originally cloned from adipocytes and named fat tissue-specific low molecular weight protein, MRAP in humans exists as two alternatively spliced isoforms, with the same N terminus but different C termini (326). It has a putative TM and strictly localizes at a compact perinuclear membrane compartment (326), likely ER. Subsequent experiments showed that MRAPs form antiparallel homodimers and the homodimers associate with the MC2R promoting its cell surface targeting (327). The enhancement of cell surface expression is specific because MRAP does not increase surface expression of the β2-AR and the TRHR (327). Similar to RAMPs, in addition to its role in forward trafficking, MRAP is also involved in MC2R binding to ACTH and subsequent signaling (328).
Interestingly, MRAP also interacts with the other four subtypes of MCRs, changing their cell surface expression, signaling, and dimerization (327, 329–333), consistent with expression of MRAPs outside of the adrenal gland and additional physiological roles of these proteins (332). For example, ligand-induced signaling at human MC1R and human MC3R is increased by interaction with MRAPa, whereas ligand-induced signaling is decreased at human MC5R by interaction with MRAPa. In zebrafish, there are three MRAPs, MRAP1 and two isoforms of MRAP2—MRAP2a expressed during the larval stage, and MRAP2b expressed later in development (334, 335). These MRAPs promote the cell surface expression of the zebrafish MC2R. Interaction of MRAP2a with the MC4R leads to decreased ability of the MC4R to bind to the endogenous agonist α-MSH and increased growth during larval development, whereas the interaction of MRAP2b with the MC4R leads to increased sensitivity to α-MSH and enhanced signaling (335). Clinical relevance of MRAP2 interaction with the MC4R was demonstrated by mutations in MRAP2 as a potential cause for severe early-onset obesity (336).
6. Specific chaperones for other receptors
In addition to the extensively studied receptor-specific chaperones described above, there are several other examples of chaperones for one or a few specific GPCRs. Below we provide a brief summary of these studies.
Glutathione S-transferase pull-down and yeast two-hybrid assays showed that an ER-membrane protein, DR interacting protein of 78 kDa (DRiP78), binds to a hydrophobic motif (FxxxFxxxF) at the proximal C terminus in Helix 8 (which serves as an ER export signal) in D1DR (53). DRiP78 has two centrally located TMs with both the N and C termini located in the cytosol. Either overexpression or sequestration of DRiP78 leads to D1DR retention in the ER and delay in receptor glycosylation and maturation, suggesting that DRiP78 is a chaperone for D1DR with a dual function, promoting folding by regulating its ER export by binding to the hydrophobic ER export signal, as well as playing a role in quality control (53). The M2 muscarinic receptor and AT1R seem to be similarly regulated by DRiP78 (53, 337). Coexpression of DRiP78 with the WT AT1R increases receptor plasma membrane expression but has no effect on the plasma membrane expression of the mutant receptors when the aromatic residues are mutated to alanine and interaction with DRiP78 is disrupted (337).
A member of the 4.1 family of cytoskeletal proteins enriched in neurons, protein 4.1N, specifically interacts with the D2 and D3 DRs, as shown by yeast two-hybrid assay, glutathione S-transferase pull down, and coimmunoprecipitation (338). They are colocalized at the plasma membrane. Overexpression of a protein 4.1N truncation fragment decreases the cell surface D2 and D3 DR expression, suggesting that protein 4.1N is involved in the localization or stability of DRs at the plasma membrane (338). Whether the interaction between the DR and protein 4.1N starts in the ER is not known.
CD4 and CCR5 associate to form the main receptor for HIV infection. In primary T lymphocytes and in a monocytic cell line, the CCR5 is expressed at low density on the cell surface, with the majority retained intracellularly (339). In the ER, CD4 specifically associates with the CCR5, promoting CCR5 export out of the ER and trafficking to the plasma membrane. This effect was specific for the CCR5 because CD4 does not affect cellular distribution of the CXCR4, the other HIV coreceptor (339).
C. Small G proteins in the folding and maturation of G protein-coupled receptors
The Ras-like small GTPase proteins, Rabs, are essential for vesicle transport, with each member (numbering more than 60) associated specifically with a particular organelle or pathway. For example, Rab1 is involved in vesicle transport between the ER and Golgi apparatus. In Drosophila, expression of dominant negative Rab1 blocks rhodopsin transport from ER, resulting in accumulation of immature rhodopsin (340). Similar findings were reported for the AT1R, the AT2R, the β2-AR, and the CaSR, but not the α2B-AR (341–344). Anterograde transport of Drosophila rhodopsin also requires GTPase function of Rab6 (a GTP binding protein that regulates vesicular trafficking within the Golgi and post-Golgi compartments) because expression of GTPase-defective mutant leads to dramatically reduced rhodopsin (345). Rab8 is important for docking and fusion of rhodopsin-containing post-Golgi membranes. Expression of mutant Rab8 in transgenic Xenopus rods causes cell death (346). Rab8 also regulates α2B-AR and β2-AR cell surface expression (347). Rab11 is important for post-Golgi trafficking of rhodopsin (348) and recycling of thromboxane A2 receptor (349) and β2-AR (350). Rab26 also regulates Golgi to plasma membrane transport of the α2-AR (351). An excellent recent review article can be consulted for detailed information on the regulation of forward trafficking of GPCRs by Rabs (352).
Coated vesicles concentrate and package cargo molecules to efficiently transport the cargoes between different intracellular compartments. COPI (coat protein I) mediates the retrograde flow of proteins from Golgi to the ER, whereas COPII mediates the bulk of ER export for proteins of the secretory pathway (353–355), including GPCRs. COPII comprises Sar1, Sec23/24, and Sec13/31. Continuous cycling of Sar1 through GTPase cycles facilitates cargo concentration before ER exit (356). So far, very limited studies on the Sar1 regulation of GPCR anterograde trafficking have been reported. H79G Sar1 is a constitutively active Sar1 because GTP cannot be hydrolyzed and there is no GDP/GTP exchange. Thus, in cells overexpressing H79G Sar1, COPII vesicles cannot be released from ER membrane (357). Expression of H79G Sar1 significantly decreases the cell surface expression of α2B-AR, β2-AR, and AT1R (358), and CaSR (343). Knocking down Sar1 expression also decreases the CaSR cell surface expression (343). These results suggest that Sar1 is involved in ER export of GPCRs. Another member of small GTPases, ADP-ribosylation factor 6, is also involved in the exit of GPCRs from the ER (359, 360).
Binding of cAMP to Epac (the exchange protein directly activated by cyclic AMP) increases the rate of exchange of GTP for GDP at Rap1 (Ras-related protein-1), therefore activating Rap1. Similar to incubation at low temperature, increasing intracellular cAMP levels with forskolin or activating Epac with an Epac activator, by activation of RhoA, reorganization of the actin cytoskeleton, and phosphorylation of filamin-2, promotes the translocation of α2C-AR from the Golgi to intracellular filaments and the plasma membrane (77, 361, 362). These effects are mediated by Rap1 because they are not present in cells lacking Rap1, whereas constitutively active Rap1 mutant increases translocation of the α2C-AR to the cell surface (77).
V. Chemical Chaperones in the Folding and Maturation of G Protein-Coupled Receptors
A. Low temperature
Temperature is known to affect protein folding. Decreasing the temperature used to culture cells has been shown to improve protein folding. Decreasing temperature to 30°C from 37°C usually used in culturing cells increases the maturation of temperature-sensitive, misfolded vesicular stomatitis virus G proteins (363). For the most common CFTR (cystic fibrosis transmembrane conductance regulator) mutation, ΔF508, channel activity can be measured when the mutant CFTR is expressed in Xenopus oocytes and Sf9 insect cells, which are usually maintained at lower temperatures than mammalian cells, although no channel activity is observed when it is expressed in mammalian cells cultured at 37°C. When the cell culture temperature was decreased, ΔF508 processing was similar to the WT CFTR (364).
In GPCRs, similar observations were made with a number of receptors. For example, incubating cells at 26°C for 48 hours markedly increases the cell surface expression of intracellularly retained human LHR mutants (365). Similar data have also been obtained in AVPR2 in one study (366), although in another study, growing cells at 27°C does not change cell surface expression of any of the nine mutants studied (367). The reason for this discrepancy is not clear at present. The GCGR mutation that causes Mahvash disease, P86S, is retained in the ER; incubation of the mutant receptor at 27°C promotes the normal processing and plasma membrane expression (368). The α2C-AR can be efficiently expressed in some neuroendocrine cells such as PC12 and AtT20 cells. However, it is retained in the ER and cis/medial Golgi in fibroblasts (369, 370). Exposure of fibroblasts expressing the α2C-AR to low-temperature (28°C) facilitates receptor transport to the plasma membrane (370), likely by releasing the inhibitory activity of HSP90 on the receptor traffic (200). In summary, low temperature culture has variable effect on cell surface expression of mutant receptors, with the majority reporting positive effects on promoting forward trafficking. Obviously, this is not a realistic therapeutic option for patients.
B. Chemical chaperones
Chemical chaperones are small, low molecular weight chemicals that can facilitate protein folding. Examples of chemical chaperones are osmolytes such as glycerol, solvents such as dimethyl sulfoxide (DMSO), methylamines such as trimethylamine-N-oxide (TMAO), and fatty acids such as 4-phenylbutyric acid (4-PBA) (371). They promote protein folding through several different mechanisms, including solvating hydrophobic regions and preventing aggregation of folding intermediates by glycerol and DMSO, modulating ER calcium levels by thapsigargin, and changing expression levels of endogenous molecular chaperones by PBA (372).
A number of chemical chaperones have been used to study the potential rescuing effect on misfolded GPCRs. In one report, correction of cell surface expression of nine naturally occurring AVPR2 mutations by chemical chaperones was studied. Only one mutant, V206D, has improved maturation and plasma membrane expression by glycerol, DMSO, thapsigargin/curcumin, and ionomycin (367). This revealed that rescue is mutant specific and that this mutant is prone to rescue by multiple compounds (367). Another study showed that all three AVPR2 mutations are corrected by DMSO and TMAO (366). None of the four chemical chaperones tested, including DMSO, TMAO, PBA, and trehalose, promotes the translocation of P23H rhodopsin to the plasma membrane (371). The osmotic chemical chaperones are not effective at rescuing P86S GCGR mutation either (368).
In the MC4R, PBA was shown to partially increase the cell surface expression of three naturally occurring mutations, P78L, I316S, and I317T, with increased signaling capacity of the rescued mutants (373). PBA decreases the misfolding of the mutant receptors, with decreased proportion of ubiquitinated receptors and decreased ER stress. Indeed, PBA and proteasome inhibitor cotreatment leads to further increase in cell surface expression of mutant receptor. PBA also increases the cell surface expression of the WT MC4R (373), consistent with our data on pharmacoperone, suggesting that the WT MC4R is not folded optimally (374) (see Section VI.D.).
In summary, chemical chaperones are nonspecific modulators of protein folding. Their effects on correcting mutant receptors are variable and generally less effective than pharmacoperones (see Section VI.). Because of the lack of specificity, the extremely high concentration needed, and the associated significant side effects, the clinical utility of chemical chaperones is very limited.
VI. Pharmacoperones in the Folding and Maturation of G Protein-Coupled Receptors
A pharmacoperone (pharmacological chaperone or pharmacochaperone) is a small molecule that enters cells and serves as a “molecular scaffold” to promote correct folding of otherwise misfolded mutant proteins within the cell (375, 376). It “is a small molecule that stabilizes a protein by binding, as either a substrate, agonist, antagonist, or allosteric modulator, at a physiologically relevant site on the target protein, but the binding primarily occurs within an organelle and usually during biosynthesis and trafficking of the target protein” (377). Misfolded proteins are frequently retained by the cellular ER quality control system, do not reach their normal site of function (378, 379), and may result in disease (380). Pharmacoperones can rescue misfolded proteins and restore them to function, which is a potentially useful therapeutic approach when the target is a misfolded/misrouted protein. One could envision drugs given in a prophylactic manner (in vitamins, for example) that prevent the misfolding that leads to neurodegenerative disorders such as Alzheimer's (misfolded amyloid) (381), Parkinson's (misfolded α-synuclein) (382), and cataracts (misfolded lens crystalline) (383). In this regard, diseases may be prevented before clinical signs are present.
Pharmacoperone rescue potentially applies to a diverse array of human diseases that result from misfolding. These include cystic fibrosis (384, 385), drug resistance (386), hypercholesterolemia (387), long QT syndrome (388), cataracts (389), neurodegenerative diseases such as Alzheimer's, Huntington's, and Parkinson's (390–393), cancer (394), α1-antitrypsin deficiency (395, 396), lysosomal storage diseases such as Fabry, Gaucher, Pompe, and Schindler/Kanzaki disease (397–400), psychotic disorder and depression (377, 401, 402), mucopolysaccharidosis type IIIC (403), methylmalonic aciduria cblB type (404), phenylketonuria (405, 406), infantile Batten disease (407), and many others (for recent reviews, see Refs. 372, 377, 408, and 409). With CFTR, VX-809 developed by Vertex Pharmaceuticals has been shown to partially correct the processing defect of the most common mutation, ΔF508, in vitro (410). The mutant CFTR rescued by VX-809 has similar biochemical and functional characteristics as the WT CFTR (410). Despite its high efficacy and selectivity in vitro, data from the clinical trial have not been very promising, with improvement in CFTR function in sweat gland but not in nasal epithelium (411).
We will detail below studies directed against diseases caused by GPCR mutations including hypogonadotropic hypogonadism, nephrogenic diabetes insipidus (NDI), retinitis pigmentosa, and obesity. Science writers commenting on these studies have observed that rescue with pharmacoperones is a viable “alternative to gene therapy” because it serves as a means of “skirting gene therapy to correct genetic defects” (412, 413). This view is supported by the consideration that correction of defective protein folding appears significantly less challenging than replacement of a defective gene (or gene product) by a perfect one. The quality control system is not protein-specific; it recognizes general aspects of misfolding (eg, exposure of hydrophobic plates in aqueous environments), frequently with relatively low affinity. Accordingly, GPCRs that retain ligand binding and effector coupling but are recognized as misfolded by such general criteria are often retained in the ER. Their rescue with pharmacoperones leads to proper folding, passage through the quality control system, restoration to the proper site, and return of function. In the case of certain proteins (eg, the GnRHR, AVPR2, rhodopsin, and MC4R), this approach has succeeded with a striking number of different mutants, supporting the view that pharmacoperones will become powerful weapons in our therapeutic arsenal (108). Figure 6 illustrates schematically how pharmacoperone works.
Figure 6.
Schematic presentation of pharmacoperone action. Pharmacoperones are permeant ligands (agonists, antagonists, or allosteric modulators) that can enter the cells and the ER, bind to misfolded receptors in the ER, and escort the receptors to the cell surface. Once there, pharmacoperones that are antagonists need to be displaced by endogenous agonists before the receptors initiate signaling. Pharmacoperones that are agonists can also initiate signaling. Pharmacoperones that are allosteric modulators do not need to be displaced before endogenous agonists bind and initiate signaling.
A. Pharmacoperones for the gonadotropin-releasing hormone receptor
The GnRHR resides in the pituitary gonadotropes and is responsible for producing responses to hypothalamic GnRH, such as the release of the gonadotropins, LH and FSH. Mutation in GNRHR causes hypogonadotropic hypogonadism (Table 1). The human GnRHR has been a central focus of drug development and understanding the mechanism of GnRH action. These studies have already led to useful drugs (agonists and antagonists). Because this target is causally and mechanistically associated with pathophysiological responses, intervention with pharmacoperones is a valuable therapeutic approach.
Pharmacoperones may also have uses in other diseases associated with the GnRHR. For example, GnRHR is expressed in virtually all melanomas, about 80% of human endometrial and ovarian cancers, and about 50% of breast cancers including triple-negative breast cancer, as well as bladder, colorectal, and pancreatic cancers; sarcomas; lymphomas; prostatic cancers; and renal cell carcinomas (414). For these cells, GnRH agonists are negative regulators of cancer growth. For example, activation of the GnRHR by exogenous agonists inhibits the proliferation of melanoma growth both in vitro and in vivo, indicating a direct antitumor activity of this class of compounds. Additionally, toxins conjugated to GnRH agonists are effectively targeted to melanoma cells where they show antiangiogenic, antimetastatic, and antioncogenic behavior (414–419). When GnRHR agonists or GnRH-toxin conjugates are used to treat melanoma, it is desirable to use the lowest dose consonant with therapeutic response so as to limit side effects (ie, androgen deprivation due to pituitary desensitization or nonspecific actions of the toxins). Pharmacoperones increase trafficking of the WT human GnRHR to the plasma membrane, a process that is otherwise about 50% efficient (ie, about 50% is retained in the ER). Because selectively increasing the number of melanoma GnRHRs also increases the sensitivity of these cells to GnRH agonist, we expect that pharmacoperones will increase the sensitivity of these cells to GnRH agonist treatment, as well as to the toxin-GnRH conjugates. An additional use involves a subset of infertile women with suboptimal responses to GnRH, suggesting a low plasma membrane expression of GnRHR (420, 421). This is a candidate target for increased expression of WT GnRHR by pharmacoperones.
Mutant GnRHR E90K has been a model mutant for the study of pharmacoperones in this system. This mutant causes human hypogonadotropic hypogonadism and has been recreated in mice, where it has a similar effect (422, 423). Modeling studies for the human GnRHR and experimental data support the view that the E90-K121 salt bridge is a fundamental and evolutionarily conserved determinant required for correct protein trafficking to the plasma membrane in all mammals examined (424–426). This bridge links TM2 to TM3. Because this salt bridge is a requirement for correct routing, mutation E90K results in a routing defect in both mouse and human GnRHRs (427, 428). This leads to full but pharmacoperone-rescuable ER retention (376) and the predicted phenotype in humans (429) and mice (422). We described the molecular and biochemical mechanism of action of pharmacoperone action in human GnRHR mutants (424, 430, 431) and showed that pharmacoperones that rescue one mutant also rescue the other mutants, although the mutations are not proximal to each other (432).
Two additional observations are important because these extend the therapeutic potential of these drugs. First, pharmacoperone drugs need not be present at the time of protein synthesis, but can rescue ER-retained proteins that have already accumulated (433). This observation increases the therapeutic reach, because misfolded mutants need not be (first) degraded and then replaced by newly synthesized protein (ie, the portion synthesized in the presence of pharmacoperone). In addition, whereas pharmacoperones are specific for individual proteins, those that rescue one mutant of an individual protein typically rescue most mutants of the same protein, likely by stabilizing a core region that makes the protein acceptable to the quality control system of the cell. This observation improves the therapeutic reach of these drugs (108, 432, 434) because each mutant of an individual protein will not require a separate drug.
We recently extended these in vitro findings to in vivo in knock-in mice with E90K mutant GnRHR. We showed that pulsatile administration of the pharmacoperone IN3 to these mice rescues the E90K mutant receptor from ER to the plasma membrane, and the rescued receptor can respond to the endogenous ligand, GnRH, resulting in restored steroidogenesis and spermatogenesis (423) (Figure 7). These exciting findings suggest that pharmacoperone therapy may indeed be used to treat disorders that can benefit from increased cell surface expression of GnRHR as described above in this section.
Figure 7.
In vivo action of a pharmacoperone (IN3) at GnRHR. A, Testes from the indicated genotypes were collected at 90 days of age and imaged with a stereomicroscope, and histology was examined by hematoxylin and eosin (H&E) staining. WT and E90Kneo/+ testes are indistinguishable in size, appearance, abundance of Leydig cells, and the presence of spermatozoa in the seminiferous tubules. The testes of E90Kneo/E90Kneo males are smaller and exhibit varying degrees of hypogonadism. There are few to no eosin-stained Leydig cells or elongated spermatids. Treatment of E90Kneo/E90Kneo males with pharmacoperone IN3 for 30 days results in increased testis size and restored spermatogenesis. The stereomicroscopic image is shown in the left column (scale bar, 2 mm), and the H&E-stained sections are shown on the middle and right (scale bar, 0.1 mm). B, Spermatogenic activity was assayed by measuring seminiferous tubule diameter. Mean seminiferous tubule diameter is not different between WT and E90Kneo heterozygotes. Seminiferous tubule diameter is reduced in E90Kneo/E90Kneo males. IN3 treatment increases the mean seminiferous tubule diameter of E90Kneo/E90Kneo males, but does not restore it to WT levels. Significant differences (P < .05) are denoted by the lowercase letters above each bar. Equivalent means have the same letter; different letters indicate statistically significant differences. Error bars show SEM. For WT, E90Kneo/+, E90Kneo/E90Kneo, and IN3 groups, n = 6, 12, 10, and 12, respectively. C, IN3 restores T levels in E90Kneo/E90Kneo males. The serum concentration of T was measured by RIA. E90Kneo/E90Kneo males exhibit less serum T than heterozygous and WT males. Testosterone levels are restored in E90Kneo/E90Kneo males after pharmacoperone treatment. Significant differences (P < .05) are denoted by the lowercase letters above each bar. Equivalent means have the same letter; different letters indicate statistically significant differences. Error bars show SEM for the mean of n ≥ 3. [Modified from J. A. Janovick et al: Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc Natl Acad Sci USA. 2013;110:21030–21035 (423), with permission. © The National Academy of Sciences.]
B. Pharmacoperones for the arginine V2 vasopressin receptor
Mutations in AVPR2 gene cause X-linked NDI because the AVPR2 gene is located at Xq28 (435). A recent tabulation included 222 mutations, of which 138 are missense or nonsense mutations (436). Most of these mutations are Class II mutations that are retained intracellularly, primarily in the ER but also in some other organelles such as Golgi apparatus.
One of the first demonstrations of pharmacoperones in GPCRs was provided by Bouvier's group (434) in AVPR2. In this study, eight Class II mutant AVPR2s were treated with selective, nonpeptidic AVPR2 antagonists and then measured for cell surface expression and signaling. The antagonists dramatically increase cell surface expression and signaling of the AVPR2 mutants. Peptide AVPR2 antagonists cannot mimic these effects or block the effect of the small molecule ligands, suggesting that the site of action is intracellular (however, cell-penetrating peptides can be used to correct mutants that can reach post-ER compartments) (437). This study demonstrates that small cell-permeable ligands can act as pharmacoperones, promoting the folding, maturation, and cell surface expression of mutant receptors that are otherwise retained intracellularly (434). Pharmacoperones act post-translationally (438). Pharmacoperone does not prevent β-arrestin-mediated constitutive endocytosis and stabilization of the receptor at the cell surface in another AVPR2 mutant, R137H; rather, it acts by promoting maturation and cell surface trafficking (439). Comparison of several antagonists suggests that ligands with higher affinity are better therapeutics (440). However, there is a trade-off between the affinity and the ease of release from the receptor once it has reached the cell surface (so the endogenous agonist can activate the receptor) (436).
Subsequently, agonists were also shown to act as pharmacoperones promoting maturation and subsequent signaling of mutant AVPR2s (441). Other cell-permeable agonists can initiate signaling of intracellular receptor without promoting cell surface expression of the mutant receptors (442, 443) and, therefore, should not be considered as pharmacoperones.
Importantly, the first clinical trial of GPCR pharmacoperones was done in NDI patients with Class II AVPR2 mutations. The five patients treated with the pharmacoperone SR49059 had significantly decreased water intake and urine volume and increased urine osmolality (444). Due to side effects, this strategy was not pursued further. However, other pharmacoperones with fewer side effects have the potential of treating the NDI patients with Class II AVPR2 mutations. The recently approved cell-permeable AVPR2 antagonist OPC41061 (Tolvaptan) for treating hyponatremia in the syndrome of inappropriate antidiuretic hormone secretion and congestive heart failure has a good safety profile, is an effective pharmacoperone at clinically feasible concentrations, and can be used in future clinical trials for treating NDI patients with Class II AVPR2 mutations (443). Different from its action as an antagonist in treating patients with the syndrome of inappropriate ADH secretion, for treating NDI patients, Tolvaptan could rescue the mutant AVPR2 to the cell surface, which can then be activated by endogenous or exogenous vasopressin. Therefore, paradoxically, “antagonists come to the rescue” (445).
C. Pharmacoperones for rhodopsin
Rhodopsin is a unique GPCR with the inverse agonist 11-cis-retinal, a vitamin A derivative, covalently bound to the apoprotein, opsin. Vitamin A supplementation in two lines of transgenic mice—one expressing the folding defective mutant T17M, one expressing the mutant with no significant in vitro defect P347S—showed that mice expressing T17M slow the decline of electroretinogram amplitude and improved photoreceptor morphology, whereas no effect on photoreceptor degeneration is observed in P347S mice (446). In vitro, treatment of 11-cis-retinal partially alleviates the instability defect of the T17M mutant, whereas P347S is not corrected (446). Similar correction was observed in transgenic Xenopus laevis expressing P23H rhodopsin that is defective in forward trafficking (447). Cell surface expression of P23H, but not K296E, is increased by 9-cis-retinal (448).
Kaushal and colleagues (449) showed that 11-cis-7-ring retinal, an 11-cis-retinal analog with a seven-membered ring, acts as a pharmacoperone that quantitatively induces the in vivo folding of the most common folding defective rhodopsin mutation, P23H. After 11-cis-7-ring retinal treatment, P23H rhodopsin can exit the ER, can acquire mature glycosylation, and is transported to the cell surface, suggesting that 11-cis-7-ring retinal may be used to prevent retinal degeneration for patients with P23H mutation (449). Further studies showed that 9- and 11-cis-retinal also act as pharmacoperones in correcting the P23H mutation (450) (again, 11-cis-retinal is the inverse agonist that is covalently linked to the apoprotein opsin forming rhodopsin). P23H rhodopsin levels are increased by 5- or 6-fold when treated with 9- or 11-cis-retinal, respectively. Retinal promotes ER export and cell surface transport of mutant rhodopsin. Data from additional experiments suggest that retinal binds to P23H opsin in the ER and stabilizes the protein to facilitate its folding and forward trafficking (450). When multiple mutants were tested, it was shown that the degree of correction is higher in mutants with less severe misfolding (451).
D. Pharmacoperones for the melanocortin-4 receptor
The MC4R is a critical regulator of energy homeostasis, regulating both food intake and energy expenditure (452). Several functional studies of naturally occurring MC4R mutations showed that defect in cell surface expression is the most common cause for loss of function. In a recent study of 20 mutations, we showed that 11 are severely defective in cell surface expression (453). We first showed that a small molecule MC4R antagonist, ML00253764, has pharmacoperone activity in two naturally occurring mutations identified from Italian patients (374). In HEK293 cells stably expressing C84R or W174C human MC4R, treatment with 10−5 m ML00253764 for 24 hours lead to increased cell surface expression (at approximately 35% of the WT receptor), and the rescued mutants are functional in responding to agonist stimulation (up to 80% of the WT receptor response) (374). We subsequently showed that ML00253764 also increases cell surface expression of several additional naturally occurring MC4R mutations (452).
Bouvier's group (454) reported that five other MC4R ligands also act as pharmacoperones. Ten MC4R mutations were studied, and the five molecules used have distinct efficacy profiles for the different mutations (454). One pharmacoperone restores function to most of the mutant receptors tested (454). Recently, four related compounds were identified that can increase the expression of three intracellularly retained mutant MC4Rs at the plasma membrane, with V50M and S58C showing some signaling response (455). The three mutants have 40–80% surface expression of the WT receptor. Whether these compounds can affect trafficking of more severely misfolded mutants is not clear, and the chemical structures of these compounds were not disclosed.
Very recently, Baldini and colleagues (94) devised a clever strategy to deliver the endogenous agonist α-MSH to the ER. They showed that the cell surface expression of a naturally occurring mutation, I316S, which is normally retained in the ER, is increased by the ER-delivered α-MSH (94). Therefore, a peptide agonist can act as a pharmacoperone if it can be delivered into the ER where it can interact with the misfolded receptor.
We initially showed that the cell surface expression of WT MC4R is also increased with pharmacoperone treatment (374). This observation has two important implications. One is that the maturation of WT MC4R is not optimal, consistent with the fact that intracellular staining of the WT MC4R in stably transfected cells is frequently observed (127, 456). Secondly, because the amount of cell surface expression is tightly coupled to regulation of energy homeostasis, with haploinsufficiency being the cause of genetic obesity in patients harboring MC4R mutations, the pharmacoperones that can increase the WT MC4R surface expression can also conceivably be used to treat obese patients without MC4R mutations (374). Subsequent studies confirmed that the other compounds identified as pharmacoperones also increase the WT MC4R maturation (454, 455).
Mutations in the related MC3R that are potentially related to human obesity or adiposity also decrease cell surface expression (65, 152, 457, 458) with only rare exception (151) (reviewed in Ref. 150). Whether pharmacoperones can be identified that can correct the defective forward trafficking in the MC3R remains to be investigated.
E. Pharmacoperones for other G protein-coupled receptors
Homozygous inactivating mutation in GCGR causes Mahvash disease (459). Three lipophilic antagonists were shown to be able to partially rescue the mutant GCGR P86S to the cell surface (368). Whether the rescued mutant is functional in responding to glucagon stimulation with increased cAMP production was not studied, although the mutants rescued using other strategies were shown to be functional (368).
Treatment of cells expressing DP1 with a DP1 inverse agonist, MK-0524 (also known as laropiprant), lead to redistribution of DP1 from intracellular compartment to the plasma membrane, and this promotion of cell surface expression is blocked by brefeldin A, an inhibitor of transport from the ER-Golgi to the plasma membrane (460), suggesting that MK-0524 promotes folding rather than later processes such as trafficking through the Golgi apparatus.
Several studies reported the rescue of CaSR mutants by pharmacoperones. Overnight treatment of cells expressing the WT or mutant CaSR with allosteric activator NPS R-568 increases plasma membrane expression and signaling (461, 462). Of the 30 loss-of-function mutations studied, half are rescued with most of the rescued mutants attaining signaling capability (461, 462). Some mutants are defective in ligand binding and/or signaling; therefore, despite trafficking to the cell surface after pharmacoperone treatment, there is still no ligand-stimulated signaling. In another study, NPS R-568 rescues signaling of four out of seven mutants, although the cell surface expression of the mutant receptors was not measured (463). Recently, NPS R-568 was shown to rescue the signaling of two novel inactivating mutants without increasing cell surface expression levels (464). The positive and negative allosteric modulators, cinacalcet and NPS-2143, respectively, also effectively rescue intracellularly retained mutants and restore signaling (465).
In LHR, inactivating mutations result in hypergonadotropic hypogonadism. A small molecule allosteric agonist, Org 42599, was found to be able to rescue the cell surface expression of two naturally occurring mutations, A593P and S616Y, that are retained intracellularly, and activate the rescued receptors (466). Org 42599 does not displace the binding of radiolabeled LH to the WT LHR but can activate the receptor; therefore, it is an allosteric agonist. Short-term treatment with Org 42599 also increases the activation of mutant receptors by the endogenous agonist, LH (466). This class of chaperones might be especially useful for Class III mutations that disrupt the binding of (endogenous) ligand.
A number of AR agonists can stabilize the intracellular pool of β1-AR, exhibiting pharmacoperone activity (467). Pharmacoperones have also been identified in MOR (468), κ-opioid receptor (469, 470), DOR (206, 471), MCHR1 (183), V1a (472) and V1b (473) vasopressin receptors, kinin B1 receptor (474), adenosine A1 receptor (475), leukotriene B4 type-2 receptor (476), and D4 DR (477). In some of these receptors, plasma membrane expression of both the WT and mutant receptors is increased by pharmacoperones, and both agonists and antagonists can act as pharmacoperones (Table 2).
Table 2.
Pharmacoperones for GPCRs
| GPCR | Ligands | Refs. |
|---|---|---|
| GnRHR | Antagonists | 376, 424, 432, 546 |
| AVPR2 | Both agonists and antagonists | 434, 437, 438, 440, 441 |
| Rhodopsin | Vitamin A derivatives | 446, 449–451 |
| MC4R | Antagonists and ER-targeted endogenous agonist | 94, 374, 452, 454 |
| GCGR | Antagonists | 368 |
| DP1 | Inverse agonist | 460 |
| CaSR | Allosteric modulators | 461–465 |
| LHR | Allosteric agonist | 466 |
| β1-AR | Agonists | 467 |
| MOR | Both agonists and antagonists | 468 |
| κ-opioid receptor | Both agonists and antagonists | 469, 470 |
| DOR | Both agonists and antagonists | 471 |
| V1a vasopressin receptor | Antagonist | 472 |
| V1b/V3 vasopressin receptor | Antagonist | 473 |
| Kinin B1 receptor | Antagonist | 474 |
| Adenosine A1 receptor | Both agonists and antagonists | 475 |
| Leukotriene B4 type-2 receptor | Both agonist and antagonist | 476 |
| D4 DR | Both agonist and antagonists | 477 |
F. Pharmacoperones as tools to study structure-function relationship of G protein-coupled receptors
Pharmacoperones can also be used as important tools for studying the structure-function relationship of GPCRs. Decreased or absent cell surface expression with mutant receptors is frequently observed. Pharmacological studies on these mutants could not be done. However, pharmacoperones can be used to treat these mutants to rescue them to the cell surface, and then the pharmacological properties of the rescued mutants could be studied. Using this strategy, we identified the first constitutively active mutation in the human GnRHR (425). Before our study was published, although numerous GnRHR mutations were generated and studied, none was found to be constitutively active (109). The human GnRHR mutant E90K that we have studied extensively is retained in the ER. When we rescued this mutant to the plasma membrane, we showed that the mutant is constitutively active (425). The mutation breaks E90-K121 salt bridge destabilizing the TM2-TM3 association, suggesting that this salt bridge is involved in constraining the GnRHR in inactive conformation (425).
Although most of the mutants rescued to the cell surface are functional, some mutants can be rescued to the cell surface efficiently but could not bind to the ligand and/or generate signaling, suggesting that additional defects in ligand binding or signaling exist in the mutant receptors. For example, we have found that G98R human MC4R can be rescued to the cell surface (452), but no ligand-stimulated signaling can be measured (H. Huang and Y.-X. Tao, unpublished observations), suggesting that the mutant is also defective in ligand binding/signaling.
G. Pharmacoperones as potential therapeutics
For more than 20 years, there has been a great deal of interest in the use of gene therapy to correct genetic diseases. Issues related to the integration of therapeutic DNA into the genome, immune responses, technical problems with vectors (toxicity, immune and inflammatory responses, gene control, and targeting issues), chances of inducing tumors (insertional mutagenesis), and other problems have made it challenging to reduce this approach to routine practice. Correcting the folding of misfolded mutant proteins and restoring them to function (with pharmacoperone drugs) is a potential alternative to replacing them by gene therapy. It is likely that valuable drugs reside in chemical libraries, yet have been missed because screening approaches that rely on identification of agonists and antagonists would have failed to identify pharmacoperones. There are several advantages of using pharmacoperones; among these is the ability to restore misfolded proteins to function and not leave residual proteins behind that can result in activation of the UPR (478), an event that causes other metabolic problems. If left unchecked, UPR leads to cell death, an event believed to have evolved to remove unregulated cells from organisms (43). Other examples include the observations that in patients with retinitis pigmentosa, retinal cells undergo apoptosis due to retention of the causative rhodopsin mutant (479). In type 2 diabetes, β-cells become damaged by elevated demand for insulin and UPR activation (480). Pharmacoperone drugs may provide a new way to accomplish this goal.
Most pharmacoperones identified to date and all pharmacoperones of the GnRHR have been identified from screens that were developed to select antagonists (Table 2). Accordingly, these drugs have both pharmacoperone and antagonist activity, which is therapeutically undesirable, requiring a subtle balance between their chaperoning capacity and ability to be displaced by the endogenous ligand for plasma membrane-located receptor to be activated (Figure 6). Therefore, pulsatile administration is likely required (423). High throughput screen has been designed to identify pharmacoperones that lack antagonism.
We have described high throughput screening assays for pharmacoperones of the GnRHR and the AVPR2 (481–484). In these assays, the level of functional (mutant) receptor present in each test well is quantitated using a luminescent-based assay for second messengers. This allows the screen to identify compounds that increase the trafficking of the mutant. To triage assay artifact and compounds with intrinsic off-target activity, compounds are counter screened with the same cell line as the primary assay in the presence of doxycycline, which shuts off the mutant expression because it is expressed until tetracycline-off control. To eliminate artifacts resulting from the luminescence assay, an orthogonal independent assay is used to confirm hits. Compounds are then profiled for nonspecific cytotoxicity and specificity for the GPCR under study over other GPCR systems. Increase of total mutant at the cell surface is confirmed with radioligand binding and fluorescently tagged receptor localization studies. All validated hits are screened for agonist or antagonist activity and profiled for specificity using a panel of other cellular receptors. These screens will likely yield pharmacoperones that are allosteric modulators but not agonists or antagonists of the GPCRs with better therapeutic potential. A screening assay was also described for rhodopsin that identified both potent and weak pharmacoperones (485).
VII. Conclusions and Future Directions
The transport of GPCR from its site of synthesis at the ER to the cell surface is an extremely complicated process. Numerous chaperones (both generic and receptor-specific) and transport proteins are likely involved in the different stages of the transport process. To prevent potential damaging effects of misfolded receptors, the cell exerts stringent quality control at several stages in the transport process, from protein folding to post-translational modifications, to cargo sorting and vesicle budding (53). Many diseases caused by mutations in GPCR genes are primarily due to the misfolding of mutant receptors that are being detected by these quality control mechanisms, predominantly in the ER but also in the Golgi apparatus. Gaining a better understanding of these quality control mechanisms and compounds that can correct the misfolding may lead to treatment of these protein conformational diseases caused by mutations in GPCR genes. If in vivo, the cell surface expression level is critical for the particular receptor system and pharmacoperones can promote surface expression of the WT receptor, these drugs may also be used to treat common disease without any genetic mutation (as in the case for GnRHR and MC4R). Receptor antagonists may not work well with this strategy. Drugs identified by high-throughput screening that are not antagonists, such as allosteric modulators, may have better therapeutic potential. Research along this vein has significant translational implications.
Acknowledgments
We thank Hui Huang and Xiu-Lei Mo for their help in preparing the figures.
This work was supported by grants from the National Institutes of Health (OD012220, DK085040, and DK099090, to P.M.C.; and DK077213), the American Diabetes Association (Grant 1-12-BS212), the Diabetes Action Research and Education Foundation, the Auburn University Intramural Grant Program and the Interdisciplinary Grant Program of the College of Veterinary Medicine at Auburn University (to Y.-X.T.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ANKRD13
- ankyrin repeat domain-containing protein 13C
- AR
- adrenergic receptor
- ATBP50
- AT2R binding protein of 50 kDa
- AT1R
- angiotensin type 1 receptor
- AT2R
- angiotensin type 2 receptor
- AVPR2
- arginine vasopressin receptor type 2
- BiP
- Ig binding protein
- CaSR
- Ca2+-sensing receptor
- CCR5
- C-C chemokine receptor type 5
- CFTR
- cystic fibrosis transmembrane conductance regulator
- CGRP
- CT gene-related peptide
- CLR
- CT receptor-like receptor
- COPII
- coat protein complex II
- CRF
- corticotropin-releasing factor
- CRF1R
- CRF 1 receptor
- CT
- calcitonin
- CTR
- CT receptor
- DMSO
- dimethyl sulfoxide
- DOR
- δ-opioid receptor
- DP
- prostaglandin D receptor
- DR
- dopamine receptor
- DRiP78
- DR interacting protein of 78 kDa
- ECL
- extracellular loop
- EDEM
- ER degradation enhancing α-mannosidase-like protein
- ER
- endoplasmic reticulum
- ERAD
- ER-associated degradation
- FSHR
- FSH receptor
- GABA
- γ-aminobutyric acid
- GABABR
- GABA type B receptor
- GCGR
- glucagon receptor
- GLP1-R
- glucagon-like peptide-1 receptor
- GnRHR
- GnRH receptor
- GPCR
- G protein-coupled receptor
- Hsc
- heat-shock cognate protein
- HSP
- heat shock protein
- ICL
- intracellular loop
- LHR
- LH receptor
- MCHR1
- melanin-concentrating hormone receptor type 1
- MCR
- melanocortin receptor
- MC1R
- melanocortin-1 receptor
- MC2R
- melanocortin-2 receptor
- MC3R
- melanocortin-3 receptor
- MC4R
- melanocortin-4 receptor
- mGluR
- metabotropic glutamate receptor
- MHC
- major histocompatibility complex
- MOR
- μ-opioid receptor
- MRAP
- melanocortin 2 receptor accessory protein
- NDI
- nephrogenic diabetes insipidus
- NinaA
- neither inactivation nor afterpotential A
- OR
- olfactory receptor
- PBA
- phenylbutyric acid
- PDI
- protein disulfide isomerase
- PROKR2
- prokineticin receptor 2
- PTHR
- PTH receptor
- RAMP
- receptor activity-modifying protein
- RanBP2
- Ran-binding protein 2
- Rap1
- Ras-related protein-1
- REEP1
- receptor expression enhancing protein 1
- Ric8
- resistance to inhibitors of cholinesterase 8
- RL-TGR
- RAMP-like triterpene glycoside receptor
- RTP
- receptor-transporting protein
- siRNA
- small interfering RNA
- SNP
- single nucleotide polymorphism
- TM
- transmembrane domain
- TMAO
- trimethylamine-N-oxide
- TRHR
- TRH receptor
- TSHR
- TSH receptor
- UPR
- unfolded protein response
- VPACR
- vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating peptide receptor
- V2R
- vomeronasal receptor type 2
- WT
- wild-type.
References
- 1. Foord SM, Bonner TI, Neubig RR, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. 2005;57:279–288 [DOI] [PubMed] [Google Scholar]
- 2. Davenport AP, Alexander SP, Sharman JL, et al. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev. 2013;65:967–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272 [DOI] [PubMed] [Google Scholar]
- 4. Vassilatis DK, Hohmann JG, Zeng H, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci USA. 2003;100:4903–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjarnadóttir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schiöth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–273 [DOI] [PubMed] [Google Scholar]
- 6. Horn F, Weare J, Beukers MW, et al. GPCRDB: an information system for G protein-coupled receptors. Nucleic Acids Res. 1998;26:275–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall RA, Premont RT, Lefkowitz RJ. Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol. 1999;145:927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769 [DOI] [PubMed] [Google Scholar]
- 9. Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yun CW, Tamaki H, Nakayama R, Yamamoto K, Kumagai H. G-Protein coupled receptor from yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1997;240:287–292 [DOI] [PubMed] [Google Scholar]
- 11. Josefsson LG, Rask L. Cloning of a putative G-protein-coupled receptor from Arabidopsis thaliana. Eur J Biochem. 1997;249:415–420 [DOI] [PubMed] [Google Scholar]
- 12. Hill CA, Fox AN, Pitts RJ, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178 [DOI] [PubMed] [Google Scholar]
- 13. Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212 [DOI] [PubMed] [Google Scholar]
- 14. Maguire JJ, Davenport AP. Regulation of vascular reactivity by established and emerging GPCRs. Trends Pharmacol Sci. 2005;26:448–454 [DOI] [PubMed] [Google Scholar]
- 15. Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385 [DOI] [PubMed] [Google Scholar]
- 16. Tao YX, Yuan ZH, Xie J. G Protein-coupled receptors as regulators of energy homeostasis. Prog Mol Biol Transl Sci. 2013;114:1–43 [DOI] [PubMed] [Google Scholar]
- 17. Tao YX, Liang XF. G Protein-coupled receptors as regulators of glucose homeostasis and therapeutic targets for diabetes mellitus. Prog Mol Biol Transl Sci. 2014;121:1–21 [DOI] [PubMed] [Google Scholar]
- 18. Ulloa-Aguirre A, Zariñán T, Dias JA, Conn PM. Mutations in G protein-coupled receptors that impact receptor trafficking and reproductive function. Mol Cell Endocrinol. 2014;382:411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dorsam RT, Gutkind JS. G-Protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94 [DOI] [PubMed] [Google Scholar]
- 20. O'Hayre M, Vázquez-Prado J, Kufareva I, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630 [DOI] [PubMed] [Google Scholar]
- 22. Thomas P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen Comp Endocrinol. 2012;175:367–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flower DR. Modelling G-protein-coupled receptors for drug design. Biochim Biophys Acta. 1999;1422:207–234 [DOI] [PubMed] [Google Scholar]
- 24. Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745 [DOI] [PubMed] [Google Scholar]
- 25. Rasmussen SG, Choi HJ, Rosenbaum DM, et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387 [DOI] [PubMed] [Google Scholar]
- 26. Rosenbaum DM, Cherezov V, Hanson MA, et al. GPCR engineering yields high resolution structural insights into β2-adrenergic receptor function. Science. 2007;318:1266–1273 [DOI] [PubMed] [Google Scholar]
- 27. Hanson MA, Roth CB, Jo E, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ichimura A, Hirasawa A, Poulain-Godefroy O, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–354 [DOI] [PubMed] [Google Scholar]
- 29. Tadevosyan A, Vaniotis G, Allen BG, et al. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J Physiol. 2012;590:1313–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bénard G, Massa F, Puente N, et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat Neurosci. 2012;15:558–564 [DOI] [PubMed] [Google Scholar]
- 31. Nanoff C, Freissmuth M. ER-bound steps in the biosynthesis of G protein-coupled receptors. Subcell Biochem. 2012;63:1–21 [DOI] [PubMed] [Google Scholar]
- 32. Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24 [PubMed] [Google Scholar]
- 33. Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel lecture). Angew Chem Int Ed Engl. 2013;52:6366–6378 [DOI] [PubMed] [Google Scholar]
- 34. Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2004;44:559–609 [DOI] [PubMed] [Google Scholar]
- 35. Chesterton CJ. Distribution of cholesterol precursors and other lipids among rat liver intracellular structures. J Biol Chem. 1968;243:1147–1151 [PubMed] [Google Scholar]
- 36. Lam AK, Galione A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim Biophys Acta. 2013;1833:2542–2559 [DOI] [PubMed] [Google Scholar]
- 37. Blobel G. Protein targeting (Nobel lecture). Chembiochem. 2000;1:86–102 [DOI] [PubMed] [Google Scholar]
- 38. Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894 [DOI] [PubMed] [Google Scholar]
- 39. Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99 [DOI] [PubMed] [Google Scholar]
- 40. Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332 [DOI] [PubMed] [Google Scholar]
- 41. Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92:537–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Malhotra JD, Miao H, Zhang K, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA. 2008;105:18525–18530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086 [DOI] [PubMed] [Google Scholar]
- 44. Flynn GC, Pohl J, Flocco MT, Rothman JE. Peptide-binding specificity of the molecular chaperone BiP. Nature. 1991;353:726–730 [DOI] [PubMed] [Google Scholar]
- 45. Otero JH, Lizák B, Hendershot LM. Life and death of a BiP substrate. Semin Cell Dev Biol. 2010;21:472–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hebert DN, Molinari M. Flagging and docking: dual roles for N-glycans in protein quality control and cellular proteostasis. Trends Biochem Sci. 2012;37:404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369 [DOI] [PubMed] [Google Scholar]
- 48. Kanehara K, Kawaguchi S, Ng DT. The EDEM and Yos9p families of lectin-like ERAD factors. Semin Cell Dev Biol. 2007;18:743–750 [DOI] [PubMed] [Google Scholar]
- 49. Khorana HG. Rhodopsin, photoreceptor of the rod cell. An emerging pattern for structure and function. J Biol Chem. 1992;267:1–4 [PubMed] [Google Scholar]
- 50. Laduron P. Axoplasmic transport of muscarinic receptors. Nature. 1980;286:287–288 [DOI] [PubMed] [Google Scholar]
- 51. Young WS, 3rd, Wamsley JK, Zarbin MA, Kuhar MJ. Opioid receptors undergo axonal flow. Science. 1980;210:76–78 [DOI] [PubMed] [Google Scholar]
- 52. Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human δ opioid receptor. J Biol Chem. 2000;275:13727–13736 [DOI] [PubMed] [Google Scholar]
- 53. Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol. 2001;3:492–498 [DOI] [PubMed] [Google Scholar]
- 54. Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–4423 [DOI] [PubMed] [Google Scholar]
- 55. Cornea-Hébert V, Watkins KC, Roth BL, et al. Similar ultrastructural distribution of the 5-HT(2A) serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience. 2002;113:23–35 [DOI] [PubMed] [Google Scholar]
- 56. Doherty MD, Pickel VM. Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res. 2000;864:176–185 [DOI] [PubMed] [Google Scholar]
- 57. Pietilä EM, Tuusa JT, Apaja PM, et al. Inefficient maturation of the rat luteinizing hormone receptor. A putative way to regulate receptor numbers at the cell surface. J Biol Chem. 2005;280:26622–26629 [DOI] [PubMed] [Google Scholar]
- 58. Misrahi M, Ghinea N, Sar S, et al. Processing of the precursors of the human thyroid-stimulating hormone receptor in various eukaryotic cells (human thyrocytes, transfected L cells and baculovirus-infected insect cells). Eur J Biochem. 1994;222:711–719 [DOI] [PubMed] [Google Scholar]
- 59. Yu R, Hinkle PM. Effect of cell type on the subcellular localization of the thyrotropin-releasing hormone receptor. Mol Pharmacol. 1997;51:785–793 [DOI] [PubMed] [Google Scholar]
- 60. Dermer SJ, Cohen DP, Thaw CN, Nussenzveig DR, Gershengorn MC. Intracellular retention and rapid degradation of human calcitonin receptors overexpressed in COS cells. Endocrinology. 1996;137:5502–5508 [DOI] [PubMed] [Google Scholar]
- 61. McClintock TS, Landers TM, Gimelbrant AA, et al. Functional expression of olfactory-adrenergic receptor chimeras and intracellular retention of heterologously expressed olfactory receptors. Brain Res Mol Brain Res. 1997;48:270–278 [DOI] [PubMed] [Google Scholar]
- 62. Gimelbrant AA, Stoss TD, Landers TM, McClintock TS. Truncation releases olfactory receptors from the endoplasmic reticulum of heterologous cells. J Neurochem. 1999;72:2301–2311 [DOI] [PubMed] [Google Scholar]
- 63. Moriyama K, Sitkovsky MV. Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem. 2010;285:39271–39288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. von Zastrow M, Link R, Daunt D, Barsh G, Kobilka B. Subtype-specific differences in the intracellular sorting of G protein-coupled receptors. J Biol Chem. 1993;268:763–766 [PubMed] [Google Scholar]
- 65. Tao YX. Functional characterization of novel melanocortin-3 receptor mutations identified from obese subjects. Biochim Biophys Acta. 2007;1772:1167–1174 [DOI] [PubMed] [Google Scholar]
- 66. Fishburn CS, Elazar Z, Fuchs S. Differential glycosylation and intracellular trafficking for the long and short isoforms of the D2 dopamine receptor. J Biol Chem. 1995;270:29819–29824 [DOI] [PubMed] [Google Scholar]
- 67. Hasegawa H, Katoh H, Yamaguchi Y, Nakamura K, Futakawa S, Negishi M. Different membrane targeting of prostaglandin EP3 receptor isoforms dependent on their carboxy-terminal tail structures. FEBS Lett. 2000;473:76–80 [DOI] [PubMed] [Google Scholar]
- 68. Wellendorph P, Bräuner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46 [DOI] [PubMed] [Google Scholar]
- 69. Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Bräuner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597 [DOI] [PubMed] [Google Scholar]
- 70. Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174 [DOI] [PubMed] [Google Scholar]
- 71. Lin CC, Clouser C, Peegel H, Menon B, Menon KM. The extracellular domain of luteinizing hormone receptor dictates its efficiency of maturation. Biochem Biophys Res Commun. 2008;377:307–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Oksche A, Leder G, Valet S, et al. Variant amino acids in the extracellular loops of murine and human vasopressin V2 receptors account for differences in cell surface expression and ligand affinity. Mol Endocrinol. 2002;16:799–813 [DOI] [PubMed] [Google Scholar]
- 73. Holtbäck U, Brismar H, DiBona GF, Fu M, Greengard P, Aperia A. Receptor recruitment: a mechanism for interactions between G protein-coupled receptors. Proc Natl Acad Sci USA. 1999;96:7271–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin X, Janovick JA, Brothers S, Blömenrohr M, Bogerd J, Conn PM. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol. 1998;12:161–171 [DOI] [PubMed] [Google Scholar]
- 75. Conn PM, Janovick JA, Brothers SP, Knollman PE. ‘Effective inefficiency’: cellular control of protein trafficking as a mechanism of post-translational regulation. J Endocrinol. 2006;190:13–16 [DOI] [PubMed] [Google Scholar]
- 76. Apaja PM, Aatsinki JT, Rajaniemi HJ, Petäjä-Repo UE. Expression of the mature luteinizing hormone receptor in rodent urogenital and adrenal tissues is developmentally regulated at a posttranslational level. Endocrinology. 2005;146:3224–3232 [DOI] [PubMed] [Google Scholar]
- 77. Jeyaraj SC, Unger NT, Eid AH, et al. Cyclic AMP-Rap1A signaling activates RhoA to induce α2C-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am J Physiol Cell Physiol. 2012;303:C499–C511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cheng PY, Svingos AL, Wang H, et al. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of δ-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci. 1995;15:5976–5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang X, Bao L, Arvidsson U, Elde R, Hökfelt T. Localization and regulation of the δ-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82:1225–1242 [DOI] [PubMed] [Google Scholar]
- 80. Cahill CM, Holdridge SV, Morinville A. Trafficking of δ-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31 [DOI] [PubMed] [Google Scholar]
- 81. Zhang X, Bao L, Guan JS. Role of delivery and trafficking of δ-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329 [DOI] [PubMed] [Google Scholar]
- 82. Bao L, Jin SX, Zhang C, et al. Activation of δ opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133 [DOI] [PubMed] [Google Scholar]
- 83. Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci USA. 1998;95:5573–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shimizu S, Brown M, Sengupta R, Penfold ME, Meucci O. CXCR7 protein expression in human adult brain and differentiated neurons. PLoS One. 2011;6:e20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rajagopal S, Kim J, Ahn S, et al. β-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci USA. 2010;107:628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boldajipour B, Mahabaleshwar H, Kardash E, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473 [DOI] [PubMed] [Google Scholar]
- 87. Blackburn PE, Simpson CV, Nibbs RJ, et al. Purification and biochemical characterization of the D6 chemokine receptor. Biochem J. 2004;379:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bonecchi R, Borroni EM, Anselmo A, et al. Regulation of D6 chemokine scavenging activity by ligand- and Rab11-dependent surface up-regulation. Blood. 2008;112:493–503 [DOI] [PubMed] [Google Scholar]
- 89. Breitwieser GE. Minireview: the intimate link between calcium sensing receptor trafficking and signaling: implications for disorders of calcium homeostasis. Mol Endocrinol. 2012;26:1482–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Grant MP, Stepanchick A, Cavanaugh A, Breitwieser GE. Agonist-driven maturation and plasma membrane insertion of calcium-sensing receptors dynamically control signal amplitude. Sci Signal. 2011;4:ra78. [DOI] [PubMed] [Google Scholar]
- 91. Grant MP, Stepanchick A, Breitwieser GE. Calcium signaling regulates trafficking of familial hypocalciuric hypercalcemia (FHH) mutants of the calcium sensing receptor. Mol Endocrinol. 2012;26:2081–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jong YJ, Kumar V, O'Malley KL. Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J Biol Chem. 2009;284:35827–35838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kumar V, Fahey PG, Jong YJ, Ramanan N, O'Malley KL. Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up-regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein. J Biol Chem. 2012;287:5412–5425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Granell S, Molden BM, Baldini G. Exposure of MC4R to agonist in the endoplasmic reticulum stabilizes an active conformation of the receptor that does not desensitize. Proc Natl Acad Sci USA. 2013;110:E4733–E4742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Calebiro D, Nikolaev VO, Gagliani MC, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ferrandon S, Feinstein TN, Castro M, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Feinstein TN, Wehbi VL, Ardura JA, et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Werthmann RC, Volpe S, Lohse MJ, Calebiro D. Persistent cAMP signaling by internalized TSH receptors occurs in thyroid but not in HEK293 cells. FASEB J. 2012;26:2043–2048 [DOI] [PubMed] [Google Scholar]
- 99. Irannejad R, Tomshine JC, Tomshine JR, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lohse MJ, Calebiro D. Cell biology: receptor signals come in waves. Nature. 2013;495:457–458 [DOI] [PubMed] [Google Scholar]
- 101. Nathans J, Hogness DS. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983;34:807–814 [DOI] [PubMed] [Google Scholar]
- 102. Dixon RA, Kobilka BK, Strader DJ, et al. Cloning of the gene and cDNA for mammalian β-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79 [DOI] [PubMed] [Google Scholar]
- 103. Spiegel AM. Defects in G protein-coupled signal transduction in human disease. Annu Rev Physiol. 1996;58:143–170 [DOI] [PubMed] [Google Scholar]
- 104. Schöneberg T, Schulz A, Gudermann T. The structural basis of G-protein-coupled receptor function and dysfunction in human diseases. Rev Physiol Biochem Pharmacol. 2002;144:143–227 [PubMed] [Google Scholar]
- 105. Spiegel AM, Weinstein LS. Inherited diseases involving G proteins and G protein-coupled receptors. Annu Rev Med. 2004;55:27–39 [DOI] [PubMed] [Google Scholar]
- 106. Schöneberg T, Schulz A, Biebermann H, Hermsdorf T, Römpler H, Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther. 2004;104:173–206 [DOI] [PubMed] [Google Scholar]
- 107. Tao YX. Inactivating mutations of G protein-coupled receptors and diseases: structure-function insights and therapeutic implications. Pharmacol Ther. 2006;111:949–973 [DOI] [PubMed] [Google Scholar]
- 108. Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–250 [DOI] [PubMed] [Google Scholar]
- 109. Tao YX. Constitutive activation of G protein-coupled receptors and diseases: insights into mechanisms of activation and therapeutics. Pharmacol Ther. 2008;120:129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol. 2011;7:362–372 [DOI] [PubMed] [Google Scholar]
- 111. Metallinos DL, Bowling AT, Rine J. A missense mutation in the endothelin-B receptor gene is associated with Lethal White Foal Syndrome: an equine version of Hirschsprung disease. Mamm Genome. 1998;9:426–431 [DOI] [PubMed] [Google Scholar]
- 112. Yang GC, Croaker D, Zhang AL, Manglick P, Cartmill T, Cass D. A dinucleotide mutation in the endothelin-B receptor gene is associated with lethal white foal syndrome (LWFS); a horse variant of Hirschsprung disease. Hum Mol Genet. 1998;7:1047–1052 [DOI] [PubMed] [Google Scholar]
- 113. Kijas JW, Cideciyan AV, Aleman TS, et al. Naturally occurring rhodopsin mutation in the dog causes retinal dysfunction and degeneration mimicking human dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 2002;99:6328–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376 [DOI] [PubMed] [Google Scholar]
- 115. Kim KS, Larsen N, Short T, Plastow G, Rothschild MF. A missense variant of the porcine melanocortin-4 receptor (MC4R) gene is associated with fatness, growth, and feed intake traits. Mamm Genome. 2000;11:131–135 [DOI] [PubMed] [Google Scholar]
- 116. Fan ZC, Sartin JL, Tao YX. Pharmacological analyses of two naturally occurring porcine melanocortin-4 receptor mutations in domestic pigs. Domest Anim Endocrinol. 2008;34:383–390 [DOI] [PubMed] [Google Scholar]
- 117. Skorczyk A, Stachowiak M, Szczerbal I, et al. Polymorphism and chromosomal location of the MC4R (melanocortin-4 receptor) gene in the dog and red fox. Gene. 2007;392:247–252 [DOI] [PubMed] [Google Scholar]
- 118. Yan J, Tao YX. Pharmacological characterization of canine melancortin-4 receptor and its natural variant V213F. Domest Anim Endocrinol. 2011;41:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kuhmann SE, Platt EJ, Kozak SL, Kabat D. Polymorphisms in the CCR5 genes of African green monkeys and mice implicate specific amino acids in infections by simian and human immunodeficiency viruses. J Virol. 1997;71:8642–8656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cone RD, Lu D, Koppula S, et al. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317 [PubMed] [Google Scholar]
- 121. Rana BK, Shiina T, Insel PA. Genetic variations and polymorphisms of G protein-coupled receptors: functional and therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:593–624 [DOI] [PubMed] [Google Scholar]
- 122. Insel PA, Tang CM, Hahntow I, Michel MC. Impact of GPCRs in clinical medicine: monogenic diseases, genetic variants and drug targets. Biochim Biophys Acta. 2007;1768:994–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Foord SM. Receptor classification: post genome. Curr Opin Pharmacol. 2002;2:561–566 [DOI] [PubMed] [Google Scholar]
- 124. Li X, Li W, Wang H, et al. Pseudogenization of a sweet-receptor gene accounts for cats' indifference toward sugar. PLoS Genet. 2005;1:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jiang P, Josue J, Li X, et al. Major taste loss in carnivorous mammals. Proc Natl Acad Sci USA. 2012;109:4956–4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Li R, Fan W, Tian G, et al. The sequence and de novo assembly of the giant panda genome. Nature. 2010;463:311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tao YX, Segaloff DL. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology. 2003;144:4544–4551 [DOI] [PubMed] [Google Scholar]
- 128. Tao YX. Molecular mechanisms of the neural melanocortin receptor dysfunction in severe early onset obesity. Mol Cell Endocrinol. 2005;239:1–14 [DOI] [PubMed] [Google Scholar]
- 129. Schiaffino MV, Baschirotto C, Pellegrini G, et al. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc Natl Acad Sci USA. 1996;93:9055–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schiaffino MV, d'Addio M, Alloni A, et al. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nat Genet. 1999;23:108–112 [DOI] [PubMed] [Google Scholar]
- 131. Palmisano I, Bagnato P, Palmigiano A, et al. The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells. Hum Mol Genet. 2008;17:3487–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. d'Addio M, Pizzigoni A, Bassi MT, et al. Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum Mol Genet. 2000;9:3011–3018 [DOI] [PubMed] [Google Scholar]
- 133. Salahpour A, Angers S, Mercier JF, Lagacé M, Marullo S, Bouvier M. Homodimerization of the β2-adrenergic receptor as a prerequisite for cell surface targeting. J Biol Chem. 2004;279:33390–33397 [DOI] [PubMed] [Google Scholar]
- 134. Rajan RS, Kopito RR. Suppression of wild-type rhodopsin maturation by mutants linked to autosomal dominant retinitis pigmentosa. J Biol Chem. 2005;280:1284–1291 [DOI] [PubMed] [Google Scholar]
- 135. Duvernay MT, Filipeanu CM, Wu G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell Signal. 2005;17:1457–1465 [DOI] [PubMed] [Google Scholar]
- 136. Kurada P, O'Tousa JE. Retinal degeneration caused by dominant rhodopsin mutations in Drosophila. Neuron. 1995;14:571–579 [DOI] [PubMed] [Google Scholar]
- 137. Zhu X, Wess J. Truncated V2 vasopressin receptors as negative regulators of wild-type V2 receptor function. Biochemistry. 1998;37:15773–15784 [DOI] [PubMed] [Google Scholar]
- 138. Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT. Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5Δ32. J Biol Chem. 1997;272:30603–30606 [DOI] [PubMed] [Google Scholar]
- 139. Tao YX, Johnson NB, Segaloff DL. Constitutive and agonist-dependent self-association of the cell surface human lutropin receptor. J Biol Chem. 2004;279:5904–5914 [DOI] [PubMed] [Google Scholar]
- 140. Calebiro D, de Filippis T, Lucchi S, et al. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum Mol Genet. 2005;14:2991–3002 [DOI] [PubMed] [Google Scholar]
- 141. Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 2005;14:2145–2154 [DOI] [PubMed] [Google Scholar]
- 142. Brothers SP, Cornea A, Janovick JA, Conn PM. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol. 2004;18:1787–1797 [DOI] [PubMed] [Google Scholar]
- 143. Ibrahim S, Tetruashvily M, Frey AJ, et al. Dominant negative actions of human prostacyclin receptor variant through dimerization: implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2010;30:1802–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kaykas A, Yang-Snyder J, Héroux M, Shah KV, Bouvier M, Moon RT. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat Cell Biol. 2004;6:52–58 [DOI] [PubMed] [Google Scholar]
- 145. Lubrano-Berthelier C, Durand E, Dubern B, et al. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet. 2003;12:145–153 [DOI] [PubMed] [Google Scholar]
- 146. Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet. 2003;12:561–574 [DOI] [PubMed] [Google Scholar]
- 147. Nijenhuis WA, Garner KM, van Rozen RJ, Adan RA. Poor cell surface expression of human melanocortin-4 receptor mutations associated with obesity. J Biol Chem. 2003;278:22939–22945 [DOI] [PubMed] [Google Scholar]
- 148. Tao YX. Mutations in melanocortin-4 receptor and human obesity. Prog Mol Biol Transl Sci. 2009;88:173–204 [DOI] [PubMed] [Google Scholar]
- 149. Biebermann H, Krude H, Elsner A, Chubanov V, Gudermann T, Grüters A. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes. 2003;52:2984–2988 [DOI] [PubMed] [Google Scholar]
- 150. Tao YX. Mutations in the melanocortin-3 receptor (MC3R) gene: impact on human obesity or adiposity. Curr Opin Investig Drugs. 2010;11:1092–1096 [PubMed] [Google Scholar]
- 151. Tao YX, Segaloff DL. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J Clin Endocrinol Metab. 2004;89:3936–3942 [DOI] [PubMed] [Google Scholar]
- 152. Yang F, Tao YX. Functional characterization of nine novel naturally occurring human melanocortin-3 receptor mutations. Biochim Biophys Acta. 2012;1822:1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mandrika I, Petrovska R, Wikberg J. Melanocortin receptors form constitutive homo- and heterodimers. Biochem Biophys Res Commun. 2005;326:349–354 [DOI] [PubMed] [Google Scholar]
- 154. Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137 [DOI] [PubMed] [Google Scholar]
- 155. Monnier C, Dodé C, Fabre L, et al. PROKR2 missense mutations associated with Kallmann syndrome impair receptor signalling activity. Hum Mol Genet. 2009;18:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhu L, Imanishi Y, Filipek S, et al. Autosomal recessive retinitis pigmentosa and E150K mutation in the opsin gene. J Biol Chem. 2006;281:22289–22298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gimelbrant AA, Haley SL, McClintock TS. Olfactory receptor trafficking involves conserved regulatory steps. J Biol Chem. 2001;276:7285–7290 [DOI] [PubMed] [Google Scholar]
- 158. Dong C, Wu G. Regulation of anterograde transport of α2-adrenergic receptors by the N termini at multiple intracellular compartments. J Biol Chem. 2006;281:38543–38554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hermosilla R, Oueslati M, Donalies U, et al. Disease-causing V2 vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic. 2004;5:993–1005 [DOI] [PubMed] [Google Scholar]
- 160. Krebs MP, Noorwez SM, Malhotra R, Kaushal S. Quality control of integral membrane proteins. Trends Biochem Sci. 2004;29:648–655 [DOI] [PubMed] [Google Scholar]
- 161. Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wu G. Anterograde trafficking of nascent α2B-adrenergic receptor: structural basis, roles of small GTPases. Curr Top Membr. 2011;67:79–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230 [DOI] [PubMed] [Google Scholar]
- 164. Dill KA, MacCallum JL. The protein-folding problem, 50 years on. Science. 2012;338:1042–1046 [DOI] [PubMed] [Google Scholar]
- 165. Gidalevitz T, Stevens F, Argon Y. Orchestration of secretory protein folding by ER chaperones. Biochim Biophys Acta. 2013;1833:2410–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Jaenicke R. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry. 1991;30:3147–3161 [DOI] [PubMed] [Google Scholar]
- 167. Cheng MY, Hartl FU, Martin J, et al. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989;337:620–625 [DOI] [PubMed] [Google Scholar]
- 168. Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. J Biol Chem. 2008;283:10221–10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Saito Y, Ihara Y, Leach MR, Cohen-Doyle MF, Williams DB. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999;18:6718–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Swanton E, High S, Woodman P. Role of calnexin in the glycan-independent quality control of proteolipid protein. EMBO J. 2003;22:2948–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119:615–623 [DOI] [PubMed] [Google Scholar]
- 172. Stiles GL, Benovic JL, Caron MG, Lefkowitz RJ. Mammalian β-adrenergic receptors. Distinct glycoprotein populations containing high mannose or complex type carbohydrate chains. J Biol Chem. 1984;259:8655–8663 [PubMed] [Google Scholar]
- 173. George ST, Ruoho AE, Malbon CC. N-Glycosylation in expression and function of β-adrenergic receptors. J Biol Chem. 1986;261:16559–16564 [PubMed] [Google Scholar]
- 174. Benovic JL, Staniszewski C, Cerione RA, Codina J, Lefkowitz RJ, Caron MG. The mammalian β-adrenergic receptor: structural and functional characterization of the carbohydrate moiety. J Recept Res. 1987;7:257–281 [DOI] [PubMed] [Google Scholar]
- 175. Davis DP, Rozell TG, Liu X, Segaloff DL. The six N-linked carbohydrates of the lutropin/choriogonadotropin receptor are not absolutely required for correct folding, cell surface expression, hormone binding, or signal transduction. Mol Endocrinol. 1997;11:550–562 [DOI] [PubMed] [Google Scholar]
- 176. Davis D, Liu X, Segaloff DL. Identification of the sites of N-linked glycosylation on the follicle-stimulating hormone (FSH) receptor and assessment of their role in FSH receptor function. Mol Endocrinol. 1995;9:159–170 [DOI] [PubMed] [Google Scholar]
- 177. Rozell TG, Davis DP, Chai Y, Segaloff DL. Association of gonadotropin receptor precursors with the protein folding chaperone calnexin. Endocrinology. 1998;139:1588–1593 [DOI] [PubMed] [Google Scholar]
- 178. Mizrachi D, Segaloff DL. Intracellularly located misfolded glycoprotein hormone receptors associate with different chaperone proteins than their cognate wild-type receptors. Mol Endocrinol. 2004;18:1768–1777 [DOI] [PubMed] [Google Scholar]
- 179. Siffroi-Fernandez S, Giraud A, Lanet J, Franc JL. Association of the thyrotropin receptor with calnexin, calreticulin and BiP. Effects on the maturation of the receptor. Eur J Biochem. 2002;269:4930–4937 [DOI] [PubMed] [Google Scholar]
- 180. Brothers SP, Janovick JA, Conn PM. Calnexin regulated gonadotropin-releasing hormone receptor plasma membrane expression. J Mol Endocrinol. 2006;37:479–488 [DOI] [PubMed] [Google Scholar]
- 181. Morello JP, Salahpour A, Petäjä-Repo UE, et al. Association of calnexin with wild type and mutant AVPR2 that causes nephrogenic diabetes insipidus. Biochemistry. 2001;40:6766–6775 [DOI] [PubMed] [Google Scholar]
- 182. Free RB, Hazelwood LA, Cabrera DM, et al. D1 and D2 dopamine receptor expression is regulated by direct interaction with the chaperone protein calnexin. J Biol Chem. 2007;282:21285–21300 [DOI] [PubMed] [Google Scholar]
- 183. Fan J, Perry SJ, Gao Y, Schwarz DA, Maki RA. A point mutation in the human melanin concentrating hormone receptor 1 reveals an important domain for cellular trafficking. Mol Endocrinol. 2005;19:2579–2590 [DOI] [PubMed] [Google Scholar]
- 184. Cabrera-Wrooman A, Janovick JA, Conn PM. Species sequence differences determine the interaction of GnRH receptor with the cellular quality control system. Mol Cell Endocrinol. 2013;381:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Butz JA, Niebauer RT, Robinson AS. Co-expression of molecular chaperones does not improve the heterologous expression of mammalian G-protein coupled receptor expression in yeast. Biotechnol Bioeng. 2003;84:292–304 [DOI] [PubMed] [Google Scholar]
- 186. Markkanen PM, Petäjä-Repo UE. N-Glycan-mediated quality control in the endoplasmic reticulum is required for the expression of correctly folded δ-opioid receptors at the cell surface. J Biol Chem. 2008;283:29086–29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lanctôt PM, Leclerc PC, Escher E, Guillemette G, Leduc R. Role of N-glycan-dependent quality control in the cell-surface expression of the AT1 receptor. Biochem Biophys Res Commun. 2006;340:395–402 [DOI] [PubMed] [Google Scholar]
- 188. Rosenbaum EE, Hardie RC, Colley NJ. Calnexin is essential for rhodopsin maturation, Ca2+ regulation, and photoreceptor cell survival. Neuron. 2006;49:229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kosmaoglou M, Cheetham ME. Calnexin is not essential for mammalian rod opsin biogenesis. Mol Vis. 2008;14:2466–2474 [PMC free article] [PubMed] [Google Scholar]
- 190. Noorwez SM, Sama RR, Kaushal S. Calnexin improves the folding efficiency of mutant rhodopsin in the presence of pharmacological chaperone 11-cis-retinal. J Biol Chem. 2009;284:33333–33342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Yoshikawa K, Touhara K. Myr-Ric-8A enhances Gα15-mediated Ca2+ response of vertebrate olfactory receptors. Chem Senses. 2009;34:15–23 [DOI] [PubMed] [Google Scholar]
- 192. Matsunami H, Amrein H. Taste and pheromone perception in mammals and flies. Genome Biol. 2003;4:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Dey S, Matsunami H. Calreticulin chaperones regulate functional expression of vomeronasal type 2 pheromone receptors. Proc Natl Acad Sci USA. 2011;108:16651–16656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Parent A, Roy SJ, Iorio-Morin C, et al. ANKRD13C acts as a molecular chaperone for G protein-coupled receptors. J Biol Chem. 2010;285:40838–40851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191 [DOI] [PubMed] [Google Scholar]
- 196. Langer T, Lu C, Echols H, Flanagan J, Hayer MK, Hartl FU. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356:683–689 [DOI] [PubMed] [Google Scholar]
- 197. Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370:111–117 [DOI] [PubMed] [Google Scholar]
- 198. Lim WK, Kanelakis KC, Neubig RR. Regulation of G protein signaling by the 70kDa heat shock protein. Cell Signal. 2013;25:389–396 [DOI] [PubMed] [Google Scholar]
- 199. Bergmayr C, Thurner P, Keuerleber S, et al. Recruitment of a cytoplasmic chaperone relay by the A2A adenosine receptor. J Biol Chem. 2013;288:28831–28844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Filipeanu CM, de Vries R, Danser AH, Kapusta DR. Modulation of α2C adrenergic receptor temperature-sensitive trafficking by HSP90. Biochim Biophys Acta. 2011;1813:346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Meimaridou E, Gooljar SB, Ramnarace N, Anthonypillai L, Clark AJ, Chapple JP. The cytosolic chaperone Hsc70 promotes traffic to the cell surface of intracellular retained melanocortin-4 receptor mutants. Mol Endocrinol. 2011;25:1650–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kogure K, Nakamura K, Ikeda S, et al. Glucose-regulated protein, 78-kilodalton is a modulator of luteinizing hormone receptor expression in luteinizing granulosa cells in rats. Biol Reprod. 2013;88:8. [DOI] [PubMed] [Google Scholar]
- 203. Gorbatyuk MS, Knox T, LaVail MM, et al. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci USA. 2010;107:5961–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Liu X, Garriga P, Khorana HG. Structure and function in rhodopsin: correct folding and misfolding in two point mutants in the intradiscal domain of rhodopsin identified in retinitis pigmentosa. Proc Natl Acad Sci USA. 1996;93:4554–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lin JH, Li H, Yasumura D, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Leskelä TT, Lackman JJ, Vierimaa MM, et al. Cys-27 variant of human δ-opioid receptor modulates maturation and cell surface delivery of Phe-27 variant via heteromerization. J Biol Chem. 2012;287:5008–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Dryja TP, Hahn LB, Cowley GS, McGee TL, Berson EL. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA. 1991;88:9370–9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Olsson JE, Gordon JW, Pawlyk BS, et al. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992;9:815–830 [DOI] [PubMed] [Google Scholar]
- 209. Athanasiou D, Kosmaoglou M, Kanuga N, et al. BiP prevents rod opsin aggregation. Mol Biol Cell. 2012;23:3522–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Pai KS, Mahajan VB, Lau A, Cunningham DD. Thrombin receptor signaling to cytoskeleton requires Hsp90. J Biol Chem. 2001;276:32642–32647 [DOI] [PubMed] [Google Scholar]
- 211. Pratt WB. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol. 1997;37:297–326 [DOI] [PubMed] [Google Scholar]
- 212. Fass D. Disulfide bonding in protein biophysics. Annu Rev Biophys. 2012;41:63–79 [DOI] [PubMed] [Google Scholar]
- 213. Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Riemer J, Bulleid N, Herrmann JM. Disulfide formation in the ER and mitochondria: two solutions to a common process. Science. 2009;324:1284–1287 [DOI] [PubMed] [Google Scholar]
- 215. Alon A, Grossman I, Gat Y, et al. The dynamic disulphide relay of quiescin sulphydryl oxidase. Nature. 2012;488:414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Goldberger RF, Epstein CJ, Anfinsen CB. Purification and properties of a microsomal enzyme system catalyzing the reactivation of reduced ribonuclease and lysozyme. J Biol Chem. 1964;239:1406–1410 [PubMed] [Google Scholar]
- 217. Cai H, Wang CC, Tsou CL. Chaperone-like activity of protein disulfide isomerase in the refolding of a protein with no disulfide bonds. J Biol Chem. 1994;269:24550–24552 [PubMed] [Google Scholar]
- 218. Wang CC, Tsou CL. Enzymes as chaperones and chaperones as enzymes. FEBS Lett. 1998;425:382–384 [DOI] [PubMed] [Google Scholar]
- 219. Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta. 2004;1699:35–44 [DOI] [PubMed] [Google Scholar]
- 220. Rajpal G, Schuiki I, Liu M, Volchuk A, Arvan P. Action of protein disulfide isomerase on proinsulin exit from endoplasmic reticulum of pancreatic β-cells. J Biol Chem. 2012;287:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Karnik SS, Sakmar TP, Chen HB, Khorana HG. Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin. Proc Natl Acad Sci USA. 1988;85:8459–8463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Karnik SS, Khorana HG. Assembly of functional rhodopsin requires a disulfide bond between cysteine residues 110 and 187. J Biol Chem. 1990;265:17520–17524 [PubMed] [Google Scholar]
- 223. Hwa J, Reeves PJ, Klein-Seetharaman J, Davidson F, Khorana HG. Structure and function in rhodopsin: further elucidation of the role of the intradiscal cysteines, Cys-110, -185, and -187, in rhodopsin folding and function. Proc Natl Acad Sci USA. 1999;96:1932–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. McKibbin C, Toye AM, Reeves PJ, et al. Opsin stability and folding: the role of Cys185 and abnormal disulfide bond formation in the intradiscal domain. J Mol Biol. 2007;374:1309–1318 [DOI] [PubMed] [Google Scholar]
- 225. Le Gouill C, Parent JL, Rola-Pleszczynski M, Stanková J. Role of the Cys90, Cys95 and Cys173 residues in the structure and function of the human platelet-activating factor receptor. FEBS Lett. 1997;402:203–208 [DOI] [PubMed] [Google Scholar]
- 226. Tarnow P, Schoneberg T, Krude H, Gruters A, Biebermann H. Mutationally induced disulfide bond formation within the third extracellular loop causes melanocortin 4 receptor inactivation in patients with obesity. J Biol Chem. 2003;278:48666–48673 [DOI] [PubMed] [Google Scholar]
- 227. Ayala Yáñez R, Conn PM. Protein disulfide isomerase chaperone ERP-57 decreases plasma membrane expression of the human GnRH receptor. Cell Biochem Funct. 2010;28:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Puig A, Gilbert HF. Protein disulfide isomerase exhibits chaperone and anti-chaperone activity in the oxidative refolding of lysozyme. J Biol Chem. 1994;269:7764–7771 [PubMed] [Google Scholar]
- 229. Tsai B, Rodighiero C, Lencer WI, Rapoport TA. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell. 2001;104:937–948 [DOI] [PubMed] [Google Scholar]
- 230. Puig A, Lyles MM, Noiva R, Gilbert HF. The role of the thiol/disulfide centers and peptide binding site in the chaperone and anti-chaperone activities of protein disulfide isomerase. J Biol Chem. 1994;269:19128–19135 [PubMed] [Google Scholar]
- 231. Huang X, Dai FF, Gaisano G, et al. The identification of novel proteins that interact with the GLP-1 receptor and restrain its activity. Mol Endocrinol. 2013;27:1550–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Ge X, Loh HH, Law PY. μ-Opioid receptor cell surface expression is regulated by its direct interaction with Ribophorin I. Mol Pharmacol. 2009;75:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Nouet S, Amzallag N, Li JM, et al. Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor-interacting protein, ATIP. J Biol Chem. 2004;279:28989–28997 [DOI] [PubMed] [Google Scholar]
- 234. Wruck CJ, Funke-Kaiser H, Pufe T, et al. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25:57–64 [DOI] [PubMed] [Google Scholar]
- 235. Rodrigues-Ferreira S, Nahmias C. An ATIPical family of angiotensin II AT2 receptor-interacting proteins. Trends Endocrinol Metab. 2010;21:684–690 [DOI] [PubMed] [Google Scholar]
- 236. Hicks SW, Horn TA, McCaffery JM, Zuckerman DM, Machamer CE. Golgin-160 promotes cell surface expression of the β-1 adrenergic receptor. Traffic. 2006;7:1666–1677 [DOI] [PubMed] [Google Scholar]
- 237. Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog NinaA is required in the secretory pathway. Cell. 1991;67:255–263 [DOI] [PubMed] [Google Scholar]
- 238. Stamnes MA, Shieh BH, Chuman L, Harris GL, Zuker CS. The cyclophilin homolog NinaA is a tissue-specific integral membrane protein required for the proper synthesis of a subset of Drosophila rhodopsins. Cell. 1991;65:219–227 [DOI] [PubMed] [Google Scholar]
- 239. Baker EK, Colley NJ, Zuker CS. The cyclophilin homolog NinaA functions as a chaperone, forming a stable complex in vivo with its protein target rhodopsin. EMBO J. 1994;13:4886–4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ferreira PA, Nakayama TA, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383:637–640 [DOI] [PubMed] [Google Scholar]
- 241. Ferreira PA, Nakayama TA, Travis GH. Interconversion of red opsin isoforms by the cyclophilin-related chaperone protein Ran-binding protein 2. Proc Natl Acad Sci USA. 1997;94:1556–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Chang CP, Pearse RV, 2nd, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195 [DOI] [PubMed] [Google Scholar]
- 243. Njuki F, Nicholl CG, Howard A, et al. A new calcitonin-receptor-like sequence in rat pulmonary blood vessels. Clin Sci (Lond). 1993;85:385–388 [DOI] [PubMed] [Google Scholar]
- 244. Flühmann B, Muff R, Hunziker W, Fischer JA, Born W. A human orphan calcitonin receptor-like structure. Biochem Biophys Res Commun. 1995;206:341–347 [DOI] [PubMed] [Google Scholar]
- 245. McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339 [DOI] [PubMed] [Google Scholar]
- 246. Christopoulos G, Perry KJ, Morfis M, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–242 [DOI] [PubMed] [Google Scholar]
- 247. Morfis M, Tilakaratne N, Furness SG, et al. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology. 2008;149:5423–5431 [DOI] [PubMed] [Google Scholar]
- 248. Bomberger JM, Parameswaran N, Hall CS, Aiyar N, Spielman WS. Novel function for receptor activity-modifying proteins (RAMPs) in post-endocytic receptor trafficking. J Biol Chem. 2005;280:9297–9307 [DOI] [PubMed] [Google Scholar]
- 249. Bomberger JM, Spielman WS, Hall CS, Weinman EJ, Parameswaran N. Receptor activity-modifying protein (RAMP) isoform-specific regulation of adrenomedullin receptor trafficking by NHERF-1. J Biol Chem. 2005;280:23926–23935 [DOI] [PubMed] [Google Scholar]
- 250. Wootten D, Lindmark H, Kadmiel M, et al. Receptor activity modifying proteins (RAMPs) interact with the VPAC2 receptor and CRF1 receptors and modulate their function. Br J Pharmacol. 2013;168:822–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Christopoulos A, Christopoulos G, Morfis M, et al. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297 [DOI] [PubMed] [Google Scholar]
- 252. Harikumar KG, Simms J, Christopoulos G, Sexton PM, Miller LJ. Molecular basis of association of receptor activity-modifying protein 3 with the family B G protein-coupled secretin receptor. Biochemistry. 2009;48:11773–11785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: a molecular perspective. Endocr Rev. 2011;32:3–30 [DOI] [PubMed] [Google Scholar]
- 254. Bouschet T, Martin S, Henley JM. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci. 2005;118:4709–4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Cohen SP, Haack KK, Halstead-Nussloch GE, et al. Identification of RL-TGR, a coreceptor involved in aversive chemical signaling. Proc Natl Acad Sci USA. 2010;107:12339–12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lu M, Echeverri F, Moyer BD. Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic. 2003;4:416–433 [DOI] [PubMed] [Google Scholar]
- 257. Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5:263–278 [DOI] [PubMed] [Google Scholar]
- 258. Zhang Y, Chou JH, Bradley J, Bargmann CI, Zinn K. The Caenorhabditis elegans seven-transmembrane protein ODR-10 functions as an odorant receptor in mammalian cells. Proc Natl Acad Sci USA. 1997;94:12162–12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187 [DOI] [PubMed] [Google Scholar]
- 260. Axel R. Scents and sensibility: a molecular logic of olfactory perception (Nobel lecture). Angew Chem Int Ed Engl. 2005;44:6110–6127 [DOI] [PubMed] [Google Scholar]
- 261. Buck LB. Unraveling the sense of smell (Nobel lecture). Angew Chem Int Ed Engl. 2005;44:6128–6140 [DOI] [PubMed] [Google Scholar]
- 262. Krautwurst D, Yau KW, Reed RR. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell. 1998;95:917–926 [DOI] [PubMed] [Google Scholar]
- 263. Kajiya K, Inaki K, Tanaka M, Haga T, Kataoka H, Touhara K. Molecular bases of odor discrimination: reconstitution of olfactory receptors that recognize overlapping sets of odorants. J Neurosci. 2001;21:6018–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Wellerdieck C, Oles M, Pott L, Korsching S, Gisselmann G, Hatt H. Functional expression of odorant receptors of the zebrafish Danio rerio and of the nematode C. elegans in HEK293 cells. Chem Senses. 1997;22:467–476 [DOI] [PubMed] [Google Scholar]
- 265. Wetzel CH, Oles M, Wellerdieck C, Kuczkowiak M, Gisselmann G, Hatt H. Specificity and sensitivity of a human olfactory receptor functionally expressed in human embryonic kidney 293 cells and Xenopus Laevis oocytes. J Neurosci. 1999;19:7426–7433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. McClintock TS, Sammeta N. Trafficking prerogatives of olfactory receptors. Neuroreport. 2003;14:1547–1552 [DOI] [PubMed] [Google Scholar]
- 267. Zhao H, Ivic L, Otaki JM, Hashimoto M, Mikoshiba K, Firestein S. Functional expression of a mammalian odorant receptor. Science. 1998;279:237–242 [DOI] [PubMed] [Google Scholar]
- 268. Touhara K, Sengoku S, Inaki K, et al. Functional identification and reconstitution of an odorant receptor in single olfactory neurons. Proc Natl Acad Sci USA. 1999;96:4040–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93:455–466 [DOI] [PubMed] [Google Scholar]
- 270. Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–691 [DOI] [PubMed] [Google Scholar]
- 271. Von Dannecker LE, Mercadante AF, Malnic B. Ric-8B promotes functional expression of odorant receptors. Proc Natl Acad Sci USA. 2006;103:9310–9314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Von Dannecker LE, Mercadante AF, Malnic B. Ric-8B, an olfactory putative GTP exchange factor, amplifies signal transduction through the olfactory-specific G-protein Gαolf. J Neurosci. 2005;25:3793–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Zhuang H, Matsunami H. Synergism of accessory factors in functional expression of mammalian odorant receptors. J Biol Chem. 2007;282:15284–15293 [DOI] [PubMed] [Google Scholar]
- 274. Gabay M, Pinter ME, Wright FA, et al. Ric-8 proteins are molecular chaperones that direct nascent G protein α subunit membrane association. Sci Signal. 2011;4:ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Björk S, Hurt CM, Ho VK, Angelotti T. REEPs are membrane shaping adapter proteins that modulate specific G protein-coupled receptor trafficking by affecting ER cargo capacity. PLoS One. 2013;8:e76366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Décaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of μ-δ opioid receptor heterodimers by RTP4. Proc Natl Acad Sci USA. 2008;105:16045–16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714 [DOI] [PubMed] [Google Scholar]
- 278. Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Störtkuhl K, Hatt H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat Neurosci. 2005;8:15–17 [DOI] [PubMed] [Google Scholar]
- 279. Hague C, Uberti MA, Chen Z, et al. Olfactory receptor surface expression is driven by association with the β2-adrenergic receptor. Proc Natl Acad Sci USA. 2004;101:13672–13676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551 [DOI] [PubMed] [Google Scholar]
- 282. Bachmanov AA, Li X, Reed DR, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283:236–242 [DOI] [PubMed] [Google Scholar]
- 284. Max M, Shanker YG, Huang L, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63 [DOI] [PubMed] [Google Scholar]
- 285. Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498 [DOI] [PubMed] [Google Scholar]
- 286. Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390 [DOI] [PubMed] [Google Scholar]
- 287. Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem. 2001;77:896–903 [DOI] [PubMed] [Google Scholar]
- 288. Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416:199–202 [DOI] [PubMed] [Google Scholar]
- 290. Zhao GQ, Zhang Y, Hoon MA, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266 [DOI] [PubMed] [Google Scholar]
- 291. Chandrashekar J, Mueller KL, Hoon MA, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711 [DOI] [PubMed] [Google Scholar]
- 292. Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702 [DOI] [PubMed] [Google Scholar]
- 293. Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604 [DOI] [PubMed] [Google Scholar]
- 294. Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229 [DOI] [PubMed] [Google Scholar]
- 295. Loconto J, Papes F, Chang E, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618 [DOI] [PubMed] [Google Scholar]
- 296. Olson R, Huey-Tubman KE, Dulac C, Bjorkman PJ. Structure of a pheromone receptor-associated MHC molecule with an open and empty groove. PLoS Biol. 2005;3:e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Olson R, Dulac C, Bjorkman PJ. MHC homologs in the nervous system–they haven't lost their groove. Curr Opin Neurobiol. 2006;16:351–357 [DOI] [PubMed] [Google Scholar]
- 298. Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562 [DOI] [PubMed] [Google Scholar]
- 299. Kaupmann K, Huggel K, Heid J, et al. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246 [DOI] [PubMed] [Google Scholar]
- 300. Couve A, Filippov AK, Connolly CN, Bettler B, Brown DA, Moss SJ. Intracellular retention of recombinant GABAB receptors. J Biol Chem. 1998;273:26361–26367 [DOI] [PubMed] [Google Scholar]
- 301. Jones KA, Borowsky B, Tamm JA, et al. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature. 1998;396:674–679 [DOI] [PubMed] [Google Scholar]
- 302. White JH, Wise A, Main MJ, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682 [DOI] [PubMed] [Google Scholar]
- 303. Kuner R, Köhr G, Grünewald S, Eisenhardt G, Bach A, Kornau HC. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–77 [DOI] [PubMed] [Google Scholar]
- 304. Ng GY, Clark J, Coulombe N, et al. Identification of a GABAB receptor subunit, gb2, required for functional GABAB receptor activity. J Biol Chem. 1999;274:7607–7610 [DOI] [PubMed] [Google Scholar]
- 305. Martin SC, Russek SJ, Farb DH. Molecular identification of the human GABABR2: cell surface expression and coupling to adenylyl cyclase in the absence of GABABR1. Mol Cell Neurosci. 1999;13:180–191 [DOI] [PubMed] [Google Scholar]
- 306. Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106 [DOI] [PubMed] [Google Scholar]
- 307. Calver AR, Robbins MJ, Cosio C, et al. The C-terminal domains of the GABAB receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J Neurosci. 2001;21:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Margeta-Mitrovic M, Jan YN, Jan LY. Function of GB1 and GB2 subunits in G protein coupling of GABAB receptors. Proc Natl Acad Sci USA. 2001;98:14649–14654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Benke D, Honer M, Michel C, Bettler B, Mohler H. γ-Aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution. J Biol Chem. 1999;274:27323–27330 [DOI] [PubMed] [Google Scholar]
- 310. Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol. 2004;66:1–7 [DOI] [PubMed] [Google Scholar]
- 311. Conn PM, Rogers DC, Stewart JM, Niedel J, Sheffield T. Conversion of a gonadotropin-releasing hormone antagonist to an agonist. Nature. 1982;296:653–655 [DOI] [PubMed] [Google Scholar]
- 312. Chang W, Tu C, Cheng Z, et al. Complex formation with the type B γ-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem. 2007;282:25030–25040 [DOI] [PubMed] [Google Scholar]
- 313. Cheng Z, Tu C, Rodriguez L, et al. Type B γ-aminobutyric acid receptors modulate the function of the extracellular Ca2+-sensing receptor and cell differentiation in murine growth plate chondrocytes. Endocrinology. 2007;148:4984–4992 [DOI] [PubMed] [Google Scholar]
- 314. Gama L, Wilt SG, Breitwieser GE. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem. 2001;276:39053–39059 [DOI] [PubMed] [Google Scholar]
- 315. Pin JP, Galvez T, Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98:325–354 [DOI] [PubMed] [Google Scholar]
- 316. Hague C, Uberti MA, Chen Z, Hall RA, Minneman KP. Cell surface expression of α1D-adrenergic receptors is controlled by heterodimerization with α1B-adrenergic receptors. J Biol Chem. 2004;279:15541–15549 [DOI] [PubMed] [Google Scholar]
- 317. Bush CF, Jones SV, Lyle AN, Minneman KP, Ressler KJ, Hall RA. Specificity of olfactory receptor interactions with other G protein-coupled receptors. J Biol Chem. 2007;282:19042–19051 [DOI] [PubMed] [Google Scholar]
- 318. Xu J, Xu M, Brown T, et al. Stabilization of the μ-opioid receptor by truncated single transmembrane splice variants through a chaperone-like action. J Biol Chem. 2013;288:21211–21227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157 [DOI] [PubMed] [Google Scholar]
- 320. Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–537 [DOI] [PubMed] [Google Scholar]
- 321. Prinster SC, Hague C, Hall RA. Heterodimerization of G protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57:289–298 [DOI] [PubMed] [Google Scholar]
- 322. Pin JP, Neubig R, Bouvier M, et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev. 2007;59:5–13 [DOI] [PubMed] [Google Scholar]
- 323. Milligan G. G Protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Noon LA, Franklin JM, King PJ, Goulding NJ, Hunyady L, Clark AJ. Failed export of the adrenocorticotrophin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J Endocrinol. 2002;174:17–25 [DOI] [PubMed] [Google Scholar]
- 325. Metherell LA, Chapple JP, Cooray S, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–170 [DOI] [PubMed] [Google Scholar]
- 326. Xu A, Choi KL, Wang Y, et al. Identification of novel putative membrane proteins selectively expressed during adipose conversion of 3T3–L1 cells. Biochem Biophys Res Commun. 2002;293:1161–1167 [DOI] [PubMed] [Google Scholar]
- 327. Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci USA. 2007;104:20244–20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Sebag JA, Hinkle PM. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J Biol Chem. 2009;284:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Chan LF, Webb TR, Chung TT, et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA. 2009;106:6146–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Kay EI, Botha R, Montgomery JM, Mountjoy KG. hMRAPa specifically alters hMC4R molecular mass and N-linked complex glycosylation in HEK293 cells. J Mol Endocrinol. 2013;50:217–227 [DOI] [PubMed] [Google Scholar]
- 331. Kay EI, Botha R, Montgomery JM, Mountjoy KG. hMRAPa increases αMSH-induced hMC1R and hMC3R functional coupling and hMC4R constitutive activity. J Mol Endocrinol. 2013;50:203–215 [DOI] [PubMed] [Google Scholar]
- 332. Novoselova TV, Jackson D, Campbell DC, Clark AJ, Chan LF. Melanocortin receptor accessory proteins in adrenal gland physiology and beyond. J Endocrinol. 2013;217:R1–R11 [DOI] [PubMed] [Google Scholar]
- 333. Sebag JA, Hinkle PM. Opposite effects of the melanocortin-2 (MC2) receptor accessory protein MRAP on MC2 and MC5 receptor dimerization and trafficking. J Biol Chem. 2009;284:22641–22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Agulleiro MJ, Roy S, Sánchez E, Puchol S, Gallo-Payet N, Cerdá-Reverter JM. Role of melanocortin receptor accessory proteins in the function of zebrafish melanocortin receptor type 2. Mol Cell Endocrinol. 2010;320:145–152 [DOI] [PubMed] [Google Scholar]
- 335. Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341:278–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Asai M, Ramachandrappa S, Joachim M, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Leclerc PC, Auger-Messier M, Lanctot PM, Escher E, Leduc R, Guillemette G. A polyaromatic caveolin-binding-like motif in the cytoplasmic tail of the type 1 receptor for angiotensin II plays an important role in receptor trafficking and signaling. Endocrinology. 2002;143:4702–4710 [DOI] [PubMed] [Google Scholar]
- 338. Binda AV, Kabbani N, Lin R, Levenson R. D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol Pharmacol. 2002;62:507–513 [DOI] [PubMed] [Google Scholar]
- 339. Achour L, Scott MG, Shirvani H, et al. CD4-CCR5 interaction in intracellular compartments contributes to receptor expression at the cell surface. Blood. 2009;113:1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Satoh A, Tokunaga F, Kawamura S, Ozaki K. In situ inhibition of vesicle transport and protein processing in the dominant negative Rab1 mutant of Drosophila. J Cell Sci. 1997;110:2943–2953 [DOI] [PubMed] [Google Scholar]
- 341. Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G protein-coupled receptor. J Biol Chem. 2003;278:47062–47069 [DOI] [PubMed] [Google Scholar]
- 342. Zhang X, Wang G, Dupré DJ, et al. Rab1 GTPase and dimerization in the cell surface expression of angiotensin II type 2 receptor. J Pharmacol Exp Ther. 2009;330:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Zhuang X, Adipietro KA, Datta S, Northup JK, Ray K. Rab1 small GTP-binding protein regulates cell surface trafficking of the human calcium-sensing receptor. Endocrinology. 2010;151:5114–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Hammad MM, Kuang YQ, Morse A, Dupré DJ. Rab1 interacts directly with the β2-adrenergic receptor to regulate receptor anterograde trafficking. Biol Chem. 2012;393:541–546 [DOI] [PubMed] [Google Scholar]
- 345. Shetty KM, Kurada P, O'Tousa JE. Rab6 regulation of rhodopsin transport in Drosophila. J Biol Chem. 1998;273:20425–20430 [DOI] [PubMed] [Google Scholar]
- 346. Moritz OL, Tam BM, Hurd LL, Peränen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Dong C, Yang L, Zhang X, et al. Rab8 interacts with distinct motifs in α2B- and β2-adrenergic receptors and differentially modulates their transport. J Biol Chem. 2010;285:20369–20380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Satoh AK, O'Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497 [DOI] [PubMed] [Google Scholar]
- 349. Hamelin E, Thériault C, Laroche G, Parent JL. The intracellular trafficking of the G protein-coupled receptor TPβ depends on a direct interaction with Rab11. J Biol Chem. 2005;280:36195–36205 [DOI] [PubMed] [Google Scholar]
- 350. Parent A, Hamelin E, Germain P, Parent JL. Rab11 regulates the recycling of the β2-adrenergic receptor through a direct interaction. Biochem J. 2009;418:163–172 [DOI] [PubMed] [Google Scholar]
- 351. Li C, Fan Y, Lan TH, Lambert NA, Wu G. Rab26 modulates the cell surface transport of α2-adrenergic receptors from the Golgi. J Biol Chem. 2012;287:42784–42794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Wang G, Wu G. Small GTPase regulation of GPCR anterograde trafficking. Trends Pharmacol Sci. 2012;33:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Barlowe C, Orci L, Yeung T, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907 [DOI] [PubMed] [Google Scholar]
- 354. Rothman JE. The future of Golgi research. Mol Biol Cell. 2010;21:3776–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Gillon AD, Latham CF, Miller EA. Vesicle-mediated ER export of proteins and lipids. Biochim Biophys Acta. 2012;1821:1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Miller EA, Barlowe C. Regulation of coat assembly–sorting things out at the ER. Curr Opin Cell Biol. 2010;22:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Pucadyil TJ, Schmid SL. Conserved functions of membrane active GTPases in coated vesicle formation. Science. 2009;325:1217–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Dong C, Zhou F, Fugetta EK, Filipeanu CM, Wu G. Endoplasmic reticulum export of adrenergic and angiotensin II receptors is differentially regulated by Sar1 GTPase. Cell Signal. 2008;20:1035–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Madziva MT, Birnbaumer M. A role for ADP-ribosylation factor 6 in the processing of G-protein-coupled receptors. J Biol Chem. 2006;281:12178–12186 [DOI] [PubMed] [Google Scholar]
- 360. Dong C, Zhang X, Zhou F, et al. ADP-ribosylation factors modulate the cell surface transport of G protein-coupled receptors. J Pharmacol Exp Ther. 2010;333:174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of α2C-adrenoceptor translocation. Circ Res. 2004;94:1367–1374 [DOI] [PubMed] [Google Scholar]
- 362. Motawea HK, Jeyaraj SC, Eid AH, et al. Cyclic AMP-Rap1A signaling mediates cell surface translocation of microvascular smooth muscle α2C-adrenoceptors through the actin-binding protein filamin-2. Am J Physiol Cell Physiol. 2013;305:C829–C845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Machamer CE, Rose JK. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988;263:5955–5960 [PubMed] [Google Scholar]
- 364. Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764 [DOI] [PubMed] [Google Scholar]
- 365. Jaquette J, Segaloff DL. Temperature sensitivity of some mutants of the lutropin/choriogonadotropin receptor. Endocrinology. 1997;138:85–91 [DOI] [PubMed] [Google Scholar]
- 366. Cheong HI, Cho HY, Park HW, Ha IS, Choi Y. Molecular genetic study of congenital nephrogenic diabetes insipidus and rescue of mutant vasopressin V2 receptor by chemical chaperones. Nephrology (Carlton). 2007;12:113–117 [DOI] [PubMed] [Google Scholar]
- 367. Robben JH, Sze M, Knoers NV, Deen PM. Rescue of vasopressin V2 receptor mutants by chemical chaperones: specificity and mechanism. Mol Biol Cell. 2006;17:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Yu R, Chen CR, Liu X, Kodra JT. Rescue of a pathogenic mutant human glucagon receptor by pharmacological chaperones. J Mol Endocrinol. 2012;49:69–78 [DOI] [PubMed] [Google Scholar]
- 369. Daunt DA, Hurt C, Hein L, Kallio J, Feng F, Kobilka BK. Subtype-specific intracellular trafficking of α2-adrenergic receptors. Mol Pharmacol. 1997;51:711–720 [DOI] [PubMed] [Google Scholar]
- 370. Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of α2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:1195–1200 [DOI] [PubMed] [Google Scholar]
- 371. Mendes HF, Cheetham ME. Pharmacological manipulation of gain-of-function and dominant-negative mechanisms in rhodopsin retinitis pigmentosa. Hum Mol Genet. 2008;17:3043–3054 [DOI] [PubMed] [Google Scholar]
- 372. Babcock JJ, Li M. Inside job: ligand-receptor pharmacology beneath the plasma membrane. Acta Pharmacol Sin. 2013;34:859–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Granell S, Mohammad S, Ramanagoudr-Bhojappa R, Baldini G. Obesity-linked variants of melanocortin-4 receptor are misfolded in the endoplasmic reticulum and can be rescued to the cell surface by a chemical chaperone. Mol Endocrinol. 2010;24:1805–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Fan ZC, Tao YX. Functional characterization and pharmacological rescue of melanocortin-4 receptor mutations identified from obese patients. J Cell Mol Med. 2009;13:3268–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Conn PM, Leaños-Miranda A, Janovick JA. Protein origami: therapeutic rescue of misfolded gene products. Mol Interv. 2002;2:308–316 [DOI] [PubMed] [Google Scholar]
- 376. Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–3262 [DOI] [PubMed] [Google Scholar]
- 377. Lester HA, Miwa JM, Srinivasan R. Psychiatric drugs bind to classical targets within early exocytotic pathways: therapeutic effects. Biol Psychiatry. 2012;72:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004;5:821–837 [DOI] [PubMed] [Google Scholar]
- 379. Bernier V, Lagacé M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–228 [DOI] [PubMed] [Google Scholar]
- 380. Castro-Fernández C, Maya-Núñez G, Conn PM. Beyond the signal sequence: protein routing in health and disease. Endocr Rev. 2005;26:479–503 [DOI] [PubMed] [Google Scholar]
- 381. Sun Y, Breydo L, Makarava N, Yang Q, Bocharova OV, Baskakov IV. Site-specific conformational studies of prion protein (PrP) amyloid fibrils revealed two cooperative folding domains within amyloid structure. J Biol Chem. 2007;282:9090–9097 [DOI] [PubMed] [Google Scholar]
- 382. Stefani M. Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim Biophys Acta. 2004;1739:5–25 [DOI] [PubMed] [Google Scholar]
- 383. Pande A, Pande J, Asherie N, et al. Crystal cataracts: human genetic cataract caused by protein crystallization. Proc Natl Acad Sci USA. 2001;98:6116–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Zhang XM, Wang XT, Yue H, et al. Organic solutes rescue the functional defect in δ F508 cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2003;278:51232–51242 [DOI] [PubMed] [Google Scholar]
- 385. Amaral MD. Therapy through chaperones: sense or antisense? Cystic fibrosis as a model disease. J Inherit Metab Dis. 2006;29:477–487 [DOI] [PubMed] [Google Scholar]
- 386. Loo TW, Clarke DM. Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J Biol Chem. 1997;272:709–712 [DOI] [PubMed] [Google Scholar]
- 387. Wang QY, Manicassamy B, Yu X, Dolmer K, Gettins PG, Rong L. Characterization of the LDL-A module mutants of Tva, the subgroup A Rous sarcoma virus receptor, and the implications in protein folding. Protein Sci. 2002;11:2596–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Zhou Z, Gong Q, January CT. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem. 1999;274:31123–31126 [DOI] [PubMed] [Google Scholar]
- 389. Benedek GB, Pande J, Thurston GM, Clark JI. Theoretical and experimental basis for the inhibition of cataract. Prog Retin Eye Res. 1999;18:391–402 [DOI] [PubMed] [Google Scholar]
- 390. Heiser V, Scherzinger E, Boeddrich A, et al. Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implications for Huntington's disease therapy. Proc Natl Acad Sci USA. 2000;97:6739–6744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Permanne B, Adessi C, Saborio GP, et al. Reduction of amyloid load and cerebral damage in a transgenic mouse model of Alzheimer's disease by treatment with a β-sheet breaker peptide. FASEB J. 2002;16:860–862 [DOI] [PubMed] [Google Scholar]
- 392. Soto C, Kascsak RJ, Saborío GP, et al. Reversion of prion protein conformational changes by synthetic β-sheet breaker peptides. Lancet. 2000;355:192–197 [DOI] [PubMed] [Google Scholar]
- 393. Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22 [DOI] [PubMed] [Google Scholar]
- 394. Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286:2507–2510 [DOI] [PubMed] [Google Scholar]
- 395. Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant α 1-antitrypsin (α 1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in α 1-AT deficiency. Proc Natl Acad Sci USA. 2000;97:1796–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Bottomley SP. The structural diversity in α1-antitrypsin misfolding. EMBO Rep. 2011;12:983–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5:112–115 [DOI] [PubMed] [Google Scholar]
- 398. Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S β-glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci USA. 2002;99:15428–15433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Parenti G, Zuppaldi A, Gabriela Pittis M, et al. Pharmacological enhancement of mutated α-glucosidase activity in fibroblasts from patients with Pompe disease. Mol Ther. 2007;15:508–514 [DOI] [PubMed] [Google Scholar]
- 400. Clark NE, Metcalf MC, Best D, Fleet GW, Garman SC. Pharmacological chaperones for human α-N-acetylgalactosaminidase. Proc Natl Acad Sci USA. 2012;109:17400–17405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human α4β2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851 [DOI] [PubMed] [Google Scholar]
- 402. Srinivasan R, Richards CI, Xiao C, et al. Pharmacological chaperoning of nicotinic acetylcholine receptors reduces the endoplasmic reticulum stress response. Mol Pharmacol. 2012;81:759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Feldhammer M, Durand S, Pshezhetsky AV. Protein misfolding as an underlying molecular defect in mucopolysaccharidosis III type C. PLoS One. 2009;4:e7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Jorge-Finnigan A, Brasil S, Underhaug J, et al. Pharmacological chaperones as a potential therapeutic option in methylmalonic aciduria cblB type. Hum Mol Genet. 2013;22:3680–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Martinez A, Calvo AC, Teigen K, Pey AL. Rescuing proteins of low kinetic stability by chaperones and natural ligands phenylketonuria, a case study. Prog Mol Biol Transl Sci. 2008;83:89–134 [DOI] [PubMed] [Google Scholar]
- 406. Santos-Sierra S, Kirchmair J, Perna AM, et al. Novel pharmacological chaperones that correct phenylketonuria in mice. Hum Mol Genet. 2012;21:1877–1887 [DOI] [PubMed] [Google Scholar]
- 407. Dawson G, Schroeder C, Dawson PE. Palmitoyl:protein thioesterase (PPT1) inhibitors can act as pharmacological chaperones in infantile Batten disease. Biochem Biophys Res Commun. 2010;395:66–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Loo TW, Clarke DM. Chemical and pharmacological chaperones as new therapeutic agents. Expert Rev Mol Med. 2007;9:1–18 [DOI] [PubMed] [Google Scholar]
- 409. Gavrin LK, Denny RA, Saiah E. Small molecules that target protein misfolding. J Med Chem. 2012;55:10823–10843 [DOI] [PubMed] [Google Scholar]
- 410. Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA. 2011;108:18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Clancy JP, Rowe SM, Accurso FJ, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax. 2012;67:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Kresge N. Pharmacological chaperones show. Am Soc Biochem Mol Biol Today. 2004;3:10–11 [Google Scholar]
- 413. Hunter PJ. Receptor redemption: skirting gene therapy to correct genetic defects. Scientist. 2004;18:30 [Google Scholar]
- 414. Engel J, Emons G, Pinski J, Schally AV. AEZS-108: a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs. 2012;21:891–899 [DOI] [PubMed] [Google Scholar]
- 415. Kovacs M, Schally AV, Nagy A, Koppan M, Groot K. Recovery of pituitary function after treatment with a targeted cytotoxic analog of luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA. 1997;94:1420–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Comaru-Schally AM, Brannan W, Schally AV, Colcolough M, Monga M. Efficacy and safety of luteinizing hormone-releasing hormone antagonist cetrorelix in the treatment of symptomatic benign prostatic hyperplasia. J Clin Endocrinol Metab. 1998;83:3826–3831 [DOI] [PubMed] [Google Scholar]
- 417. Limonta P, Moretti RM, Marelli MM, Dondi D, Parenti M, Motta M. The luteinizing hormone-releasing hormone receptor in human prostate cancer cells: messenger ribonucleic acid expression, molecular size, and signal transduction pathway. Endocrinology. 1999;140:5250–5256 [DOI] [PubMed] [Google Scholar]
- 418. Keller G, Schally AV, Gaiser T, et al. Human malignant melanomas express receptors for luteinizing hormone releasing hormone allowing targeted therapy with cytotoxic luteinizing hormone releasing hormone analogue. Cancer Res. 2005;65:5857–5863 [DOI] [PubMed] [Google Scholar]
- 419. Limonta P, Montagnani Marelli M, Mai S, Motta M, Martini L, Moretti RM. GnRH receptors in cancer: from cell biology to novel targeted therapeutic strategies. Endocr Rev. 2012;33:784–811 [DOI] [PubMed] [Google Scholar]
- 420. Waldstreicher J, Seminara SB, Jameson JL, et al. The genetic and clinical heterogeneity of gonadotropin-releasing hormone deficiency in the human. J Clin Endocrinol Metab. 1996;81:4388–4395 [DOI] [PubMed] [Google Scholar]
- 421. Seminara SB, Beranova M, Oliveira LM, Martin KA, Crowley WF, Jr, Hall JE. Successful use of pulsatile gonadotropin-releasing hormone (GnRH) for ovulation induction and pregnancy in a patient with GnRH receptor mutations. J Clin Endocrinol Metab. 2000;85:556–562 [DOI] [PubMed] [Google Scholar]
- 422. Stewart MD, Deng JM, Stewart CA, et al. Mice harboring Gnrhr E90K, a mutation that causes protein misfolding and hypogonadotropic hypogonadism in humans, exhibit testis size reduction and ovulation failure. Mol Endocrinol. 2012;26:1847–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Janovick JA, Stewart MD, Jacob D, et al. Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc Natl Acad Sci USA. 2013;110:21030–21035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Janovick JA, Patny A, Mosley R, et al. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Mol Endocrinol. 2009;23:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Janovick JA, Conn PM. Salt bridge integrates GPCR activation with protein trafficking. Proc Natl Acad Sci USA. 2010;107:4454–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Janovick JA, Pogozheva ID, Mosberg HI, Conn PM. Salt bridges overlapping the gonadotropin-releasing hormone receptor agonist binding site reveal a coincidence detector for G protein-coupled receptor activation. J Pharmacol Exp Ther. 2011;338:430–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Knollman PE, Janovick JA, Brothers SP, Conn PM. Parallel regulation of membrane trafficking and dominant-negative effects by misrouted gonadotropin-releasing hormone receptor mutants. J Biol Chem. 2005;280:24506–24514 [DOI] [PubMed] [Google Scholar]
- 428. Janovick JA, Knollman PE, Brothers SP, Ayala-Yáñez R, Aziz AS, Conn PM. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem. 2006;281:8417–8425 [DOI] [PubMed] [Google Scholar]
- 429. Soderlund D, Canto P, de la Chesnaye E, Ulloa-Aguirre A, Méndez JP. A novel homozygous mutation in the second transmembrane domain of the gonadotrophin releasing hormone receptor gene. Clin Endocrinol (Oxf). 2001;54:493–498 [DOI] [PubMed] [Google Scholar]
- 430. Conn PM, Janovick JA. Drug development and the cellular quality control system. Trends Pharmacol Sci. 2009;30:228–233 [DOI] [PubMed] [Google Scholar]
- 431. Conn PM, Ulloa-Aguirre A. Trafficking of G-protein-coupled receptors to the plasma membrane: insights for pharmacoperone drugs. Trends Endocrinol Metab. 2010;21:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003;305:608–614 [DOI] [PubMed] [Google Scholar]
- 433. Janovick JA, Brothers SP, Cornea A, et al. Refolding of misfolded mutant GPCR: post-translational pharmacoperone action in vitro. Mol Cell Endocrinol. 2007;272:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Morello JP, Salahpour A, Laperrière A, et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105:887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Rosenthal W, Seibold A, Antaramian A, et al. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature. 1992;359:233–235 [DOI] [PubMed] [Google Scholar]
- 436. Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev. 2013;34:278–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Oueslati M, Hermosilla R, Schönenberger E, et al. Rescue of a nephrogenic diabetes insipidus-causing vasopressin V2 receptor mutant by cell-penetrating peptides. J Biol Chem. 2007;282:20676–20685 [DOI] [PubMed] [Google Scholar]
- 438. Wüller S, Wiesner B, Löffler A, et al. Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem. 2004;279:47254–47263 [DOI] [PubMed] [Google Scholar]
- 439. Bernier V, Lagacé M, Lonergan M, Arthus MF, Bichet DG, Bouvier M. Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol Endocrinol. 2004;18:2074–2084 [DOI] [PubMed] [Google Scholar]
- 440. Robben JH, Sze M, Knoers NV, Deen PM. Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2007;292:F253–F260 [DOI] [PubMed] [Google Scholar]
- 441. Jean-Alphonse F, Perkovska S, Frantz MC, et al. Biased agonist pharmacochaperones of the AVP V2 receptor may treat congenital nephrogenic diabetes insipidus. J Am Soc Nephrol. 2009;20:2190–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Robben JH, Kortenoeven ML, Sze M, et al. Intracellular activation of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus by nonpeptide agonists. Proc Natl Acad Sci USA. 2009;106:12195–12200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Los EL, Deen PM, Robben JH. Potential of nonpeptide (ant)agonists to rescue vasopressin V2 receptor mutants for the treatment of X-linked nephrogenic diabetes insipidus. J Neuroendocrinol. 2010;22:393–399 [DOI] [PubMed] [Google Scholar]
- 444. Bernier V, Morello JP, Zarruk A, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17:232–243 [DOI] [PubMed] [Google Scholar]
- 445. Welch WJ, Howard M. Antagonists to the rescue. J Clin Invest. 2000;105:853–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Li T, Sandberg MA, Pawlyk BS, et al. Effect of vitamin A supplementation on rhodopsin mutants threonine-17 –> methionine and proline-347 –> serine in transgenic mice and in cell cultures. Proc Natl Acad Sci USA. 1998;95:11933–11938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Tam BM, Moritz OL. Dark rearing rescues P23H rhodopsin-induced retinal degeneration in a transgenic Xenopus laevis model of retinitis pigmentosa: a chromophore-dependent mechanism characterized by production of N-terminally truncated mutant rhodopsin. J Neurosci. 2007;27:9043–9053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Saliba RS, Munro PM, Luthert PJ, Cheetham ME. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci. 2002;115:2907–2918 [DOI] [PubMed] [Google Scholar]
- 449. Noorwez SM, Kuksa V, Imanishi Y, et al. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J Biol Chem. 2003;278:14442–14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Noorwez SM, Malhotra R, McDowell JH, Smith KA, Krebs MP, Kaushal S. Retinoids assist the cellular folding of the autosomal dominant retinitis pigmentosa opsin mutant P23H. J Biol Chem. 2004;279:16278–16284 [DOI] [PubMed] [Google Scholar]
- 451. Krebs MP, Holden DC, Joshi P, Clark CL, 3rd, Lee AH, Kaushal S. Molecular mechanisms of rhodopsin retinitis pigmentosa and the efficacy of pharmacological rescue. J Mol Biol. 2010;395:1063–1078 [DOI] [PubMed] [Google Scholar]
- 452. Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Wang ZQ, Tao YX. Functional studies on twenty novel naturally occurring melanocortin-4 receptor mutations. Biochim Biophys Acta. 2011;1812:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. René P, Le Gouill C, Pogozheva ID, et al. Pharmacological chaperones restore function to MC4R mutants responsible for severe early-onset obesity. J Pharmacol Exp Ther. 2010;335:520–532 [DOI] [PubMed] [Google Scholar]
- 455. Ward NA, Hirst S, Williams J, Findlay JB. Pharmacological chaperones increase the cell-surface expression of intracellularly retained mutants of the melanocortin 4 receptor with unique rescuing efficacy profiles. Biochem Soc Trans. 2012;40:717–720 [DOI] [PubMed] [Google Scholar]
- 456. Tao YX, Segaloff DL. Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J Clin Endocrinol Metab. 2005;90:5632–5638 [DOI] [PubMed] [Google Scholar]
- 457. Lee YS, Poh LK, Kek BL, Loke KY. The role of melanocortin 3 receptor gene in childhood obesity. Diabetes. 2007;56:2622–2630 [DOI] [PubMed] [Google Scholar]
- 458. Mencarelli M, Walker GE, Maestrini S, et al. Sporadic mutations in melanocortin receptor 3 in morbid obese individuals. Eur J Hum Genet. 2008;16:581–586 [DOI] [PubMed] [Google Scholar]
- 459. Zhou C, Dhall D, Nissen NN, Chen CR, Yu R. Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, α cell hyperplasia, and islet cell tumor. Pancreas. 2009;38:941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Labrecque P, Roy SJ, Frechette L, Iorio-Morin C, Gallant MA, Parent JL. Inverse agonist and pharmacochaperone properties of MK-0524 on the prostanoid DP1 receptor. PLoS One. 2013;8:e65767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem. 2007;282:9517–9525 [DOI] [PubMed] [Google Scholar]
- 462. White E, McKenna J, Cavanaugh A, Breitwieser GE. Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants. Mol Endocrinol. 2009;23:1115–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Rus R, Haag C, Bumke-Vogt C, et al. Novel inactivating mutations of the calcium-sensing receptor: the calcimimetic NPS R-568 improves signal transduction of mutant receptors. J Clin Endocrinol Metab. 2008;93:4797–4803 [DOI] [PubMed] [Google Scholar]
- 464. Nakamura A, Hotsubo T, Kobayashi K, Mochizuki H, Ishizu K, Tajima T. Loss-of-function and gain-of-function mutations of calcium-sensing receptor: functional analysis and the effect of allosteric modulators NPS R-568 and NPS 2143. J Clin Endocrinol Metab. 2013;98:E1692–E1701 [DOI] [PubMed] [Google Scholar]
- 465. Leach K, Wen A, Cook AE, Sexton PM, Conigrave AD, Christopoulos A. Impact of clinically relevant mutations on the pharmacoregulation and signaling bias of the calcium-sensing receptor by positive and negative allosteric modulators. Endocrinology. 2013;154:1105–1116 [DOI] [PubMed] [Google Scholar]
- 466. Newton CL, Whay AM, McArdle CA, et al. Rescue of expression and signaling of human luteinizing hormone G protein-coupled receptor mutants with an allosterically binding small-molecule agonist. Proc Natl Acad Sci USA. 2011;108:7172–7176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Hakalahti AE, Khan H, Vierimaa MM, et al. β-Adrenergic agonists mediate enhancement of β1-adrenergic receptor N-terminal cleavage and stabilization in vivo and in vitro. Mol Pharmacol. 2013;83:129–141 [DOI] [PubMed] [Google Scholar]
- 468. Chaipatikul V, Erickson-Herbrandson LJ, Loh HH, Law PY. Rescuing the traffic-deficient mutants of rat μ-opioid receptors with hydrophobic ligands. Mol Pharmacol. 2003;64:32–41 [DOI] [PubMed] [Google Scholar]
- 469. Chen Y, Chen C, Wang Y, Liu-Chen LY. Ligands regulate cell surface level of the human κ opioid receptor by activation-induced down-regulation and pharmacological chaperone-mediated enhancement: differential effects of nonpeptide and peptide agonists. J Pharmacol Exp Ther. 2006;319:765–775 [DOI] [PubMed] [Google Scholar]
- 470. Wannemacher KM, Yadav PN, Howells RD. A select set of opioid ligands induce up-regulation by promoting the maturation and stability of the rat κ-opioid receptor in human embryonic kidney 293 cells. J Pharmacol Exp Ther. 2007;323:614–625 [DOI] [PubMed] [Google Scholar]
- 471. Petäjä-Repo UE, Hogue M, Bhalla S, Laperrière A, Morello JP, Bouvier M. Ligands act as pharmacological chaperones and increase the efficiency of δ opioid receptor maturation. EMBO J. 2002;21:1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Hawtin SR. Pharmacological chaperone activity of SR49059 to functionally recover misfolded mutations of the vasopressin V1a receptor. J Biol Chem. 2006;281:14604–14614 [DOI] [PubMed] [Google Scholar]
- 473. Robert J, Auzan C, Ventura MA, Clauser E. Mechanisms of cell-surface rerouting of an endoplasmic reticulum-retained mutant of the vasopressin V1b/V3 receptor by a pharmacological chaperone. J Biol Chem. 2005;280:42198–42206 [DOI] [PubMed] [Google Scholar]
- 474. Fortin JP, Dziadulewicz EK, Gera L, Marceau F. A nonpeptide antagonist reveals a highly glycosylated state of the rabbit kinin B1 receptor. Mol Pharmacol. 2006;69:1146–1157 [DOI] [PubMed] [Google Scholar]
- 475. Málaga-Diéguez L, Yang Q, Bauer J, Pankevych H, Freissmuth M, Nanoff C. Pharmacochaperoning of the A1 adenosine receptor is contingent on the endoplasmic reticulum. Mol Pharmacol. 2010;77:940–952 [DOI] [PubMed] [Google Scholar]
- 476. Yasuda D, Okuno T, Yokomizo T, et al. Helix 8 of leukotriene B4 type-2 receptor is required for the folding to pass the quality control in the endoplasmic reticulum. FASEB J. 2009;23:1470–1481 [DOI] [PubMed] [Google Scholar]
- 477. Van Craenenbroeck K, Clark SD, Cox MJ, Oak JN, Liu F, Van Tol HH. Folding efficiency is rate-limiting in dopamine D4 receptor biogenesis. J Biol Chem. 2005;280:19350–19357 [DOI] [PubMed] [Google Scholar]
- 478. Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol Rev. 2011;91:1219–1243 [DOI] [PubMed] [Google Scholar]
- 479. Lin JH, Lavail MM. Misfolded proteins and retinal dystrophies. Adv Exp Med Biol. 2010;664:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011;22:266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481. Conn PM, Janovick JA. Pharmacoperone identification for therapeutic rescue of misfolded mutant proteins. Front Endocrinol (Lausanne). 2011;2:pii: 00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Janovick JA, Park BS, Conn PM. Therapeutic rescue of misfolded mutants: validation of primary high throughput screens for identification of pharmacoperone drugs. PLoS One. 2011;6:e22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483. Smithson DC, Janovick JA, Conn PM. Therapeutic rescue of misfolded/mistrafficked mutants: automation-friendly high-throughput assays for identification of pharmacoperone drugs of GPCRs. Methods Enzymol. 2013;521:3–16 [DOI] [PubMed] [Google Scholar]
- 484. Conn PM, Smith E, Hodder P, Janovick JA, Smithson DC. High-throughput screen for pharmacoperones of the vasopressin type 2 receptor. J Biomol Screen. 2013;18:930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Noorwez SM, Ostrov DA, McDowell JH, Krebs MP, Kaushal S. A high-throughput screening method for small-molecule pharmacologic chaperones of misfolded rhodopsin. Invest Ophthalmol Vis Sci. 2008;49:3224–3230 [DOI] [PubMed] [Google Scholar]
- 486. Dryja TP, McGee TL, Hahn LB, et al. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990;323:1302–1307 [DOI] [PubMed] [Google Scholar]
- 487. Dryja TP, McGee TL, Reichel E, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366 [DOI] [PubMed] [Google Scholar]
- 488. Morimura H, Saindelle-Ribeaudeau F, Berson EL, Dryja TP. Mutations in RGR, encoding a light-sensitive opsin homologue, in patients with retinitis pigmentosa. Nat Genet. 1999;23:393–394 [DOI] [PubMed] [Google Scholar]
- 489. Weitz CJ, Miyake Y, Shinzato K, et al. Human tritanopia associated with two amino acid substitutions in the blue-sensitive opsin. Am J Hum Genet. 1992;50:498–507 [PMC free article] [PubMed] [Google Scholar]
- 490. Nathans J. In the eye of the beholder: visual pigments and inherited variation in human vision. Cell. 1994;78:357–360 [DOI] [PubMed] [Google Scholar]
- 491. Piao X, Hill RS, Bodell A, et al. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–2036 [DOI] [PubMed] [Google Scholar]
- 492. Jin Z, Tietjen I, Bu L, et al. Disease-associated mutations affect GPR56 protein trafficking and cell surface expression. Hum Mol Genet. 2007;16:1972–198517576745 [Google Scholar]
- 493. Jin Z, Luo R, Piao X. GPR56 and its related diseases. Prog Mol Biol Transl Sci. 2009;89:1–13 [DOI] [PubMed] [Google Scholar]
- 494. Cole LW, Sidis Y, Zhang C, et al. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab. 2008;93:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. Martin C, Balasubramanian R, Dwyer AA, et al. The role of the prokineticin 2 pathway in human reproduction: evidence from the study of human and murine gene mutations. Endocr Rev. 2011;32:225–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496. de Roux N, Young J, Misrahi M, et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–1602 [DOI] [PubMed] [Google Scholar]
- 497. Layman LC, Cohen DP, Jin M, et al. Mutations in gonadotropin-releasing hormone receptor gene cause hypogonadotropic hypogonadism. Nat Genet. 1998;18:14–15 [DOI] [PubMed] [Google Scholar]
- 498. Wajnrajch MP, Gertner JM, Harbison MD, Chua SC, Jr, Leibel RL. Nonsense mutation in the human growth hormone-releasing hormone receptor causes growth failure analogous to the little (lit) mouse. Nat Genet. 1996;12:88–90 [DOI] [PubMed] [Google Scholar]
- 499. Martari M, Salvatori R. Diseases associated with growth hormone-releasing hormone receptor (GHRHR) mutations. Prog Mol Biol Transl Sci. 2009;88:57–84 [DOI] [PubMed] [Google Scholar]
- 500. Collu R, Tang J, Castagné J, et al. A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropin-releasing hormone receptor gene. J Clin Endocrinol Metab. 1997;82:1561–1565 [DOI] [PubMed] [Google Scholar]
- 501. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 502. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Biebermann H, Grüters A, Schöneberg T, Gudermann T. Congenital hypothyroidism caused by mutations in the thyrotropin-receptor gene. N Engl J Med. 1997;336:1390–1391 [DOI] [PubMed] [Google Scholar]
- 504. Kremer H, Kraaij R, Toledo SP, et al. Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat Genet. 1995;9:160–164 [DOI] [PubMed] [Google Scholar]
- 505. Laue L, Wu SM, Kudo M, et al. A nonsense mutation of the human luteinizing hormone receptor gene in Leydig cell hypoplasia. Hum Mol Genet. 1995;4:1429–1433 [DOI] [PubMed] [Google Scholar]
- 506. Aittomäki K, Lucena JL, Pakarinen P, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968 [DOI] [PubMed] [Google Scholar]
- 507. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Gorlov IP, Kamat A, Bogatcheva NV, et al. Mutations of the GREAT gene cause cryptorchidism. Hum Mol Genet. 2002;11:2309–2318 [DOI] [PubMed] [Google Scholar]
- 509. Pollak MR, Brown EM, Chou YH, et al. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297–1303 [DOI] [PubMed] [Google Scholar]
- 510. Zhang P, Jobert AS, Couvineau A, Silve C. A homozygous inactivating mutation in the parathyroid hormone/parathyroid hormone-related peptide receptor causing Blomstrand chondrodysplasia. J Clin Endocrinol Metab. 1998;83:3365–3368 [DOI] [PubMed] [Google Scholar]
- 511. Jobert AS, Zhang P, Couvineau A, et al. Absence of functional receptors for parathyroid hormone and parathyroid hormone-related peptide in Blomstrand chondrodysplasia. J Clin Invest. 1998;102:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Couvineau A, Wouters V, Bertrand G, et al. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum Mol Genet. 2008;17:2766–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513. Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–330 [DOI] [PubMed] [Google Scholar]
- 514. Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993;341:461–462 [DOI] [PubMed] [Google Scholar]
- 515. Tsigos C, Arai K, Hung W, Chrousos GP. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J Clin Invest. 1993;92:2458–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. Lee YS, Poh LK, Loke KY. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J Clin Endocrinol Metab. 2002;87:1423–1426 [DOI] [PubMed] [Google Scholar]
- 517. Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114 [DOI] [PubMed] [Google Scholar]
- 518. Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112 [DOI] [PubMed] [Google Scholar]
- 519. Hinney A, Schmidt A, Nottebom K, et al. Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J Clin Endocrinol Metab. 1999;84:1483–1486 [DOI] [PubMed] [Google Scholar]
- 520. Hinney A, Volckmar AL, Knoll N. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci. 2013;114:147–191 [DOI] [PubMed] [Google Scholar]
- 521. Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377 [DOI] [PubMed] [Google Scholar]
- 522. Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725 [DOI] [PubMed] [Google Scholar]
- 523. Hernandez PA, Gorlin RJ, Lukens JN, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74 [DOI] [PubMed] [Google Scholar]
- 524. Faure S, Meyer L, Costagliola D, et al. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–2277 [DOI] [PubMed] [Google Scholar]
- 525. Tournamille C, Le Van Kim C, Gane P, et al. Arg89Cys substitution results in very low membrane expression of the Duffy antigen/receptor for chemokines in Fy(x) individuals. Blood. 1998;92:2147–2156 [PubMed] [Google Scholar]
- 526. Tokuyama Y, Matsui K, Egashira T, Nozaki O, Ishizuka T, Kanatsuka A. Five missense mutations in glucagon-like peptide 1 receptor gene in Japanese population. Diabetes Res Clin Pract. 2004;66:63–69 [DOI] [PubMed] [Google Scholar]
- 527. Hager J, Hansen L, Vaisse C, et al. A missense mutation in the glucagon receptor gene is associated with non-insulin-dependent diabetes mellitus. Nat Genet. 1995;9:299–304 [DOI] [PubMed] [Google Scholar]
- 528. Wang HJ, Geller F, Dempfle A, et al. Ghrelin receptor gene: identification of several sequence variants in extremely obese children and adolescents, healthy normal-weight and underweight students, and children with short normal stature. J Clin Endocrinol Metab. 2004;89:157–162 [DOI] [PubMed] [Google Scholar]
- 529. Pantel J, Legendre M, Cabrol S, et al. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest. 2006;116:760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Mo XL, Wei HK, Peng J, Tao YX. Free fatty acid receptor GPR120 and pathogenesis of obesity and type 2 diabetes mellitus. Prog Mol Biol Transl Sci. 2013;114:251–276 [DOI] [PubMed] [Google Scholar]
- 531. Vettor R, Granzotto M, De Stefani D, et al. Loss-of-function mutation of the GPR40 gene associates with abnormal stimulated insulin secretion by acting on intracellular calcium mobilization. J Clin Endocrinol Metab. 2008;93:3541–3550 [DOI] [PubMed] [Google Scholar]
- 532. Puffenberger EG, Hosoda K, Washington SS, et al. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell. 1994;79:1257–1266 [DOI] [PubMed] [Google Scholar]
- 533. Thompson MD, Comings DE, Abu-Ghazalah R, et al. Variants of the orexin2/hcrt2 receptor gene identified in patients with excessive daytime sleepiness and patients with Tourette's syndrome comorbidity. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:69–75 [DOI] [PubMed] [Google Scholar]
- 534. Hirata T, Kakizuka A, Ushikubi F, Fuse I, Okuma M, Narumiya S. Arg60 to Leu mutation of the human thromboxane A2 receptor in a dominantly inherited bleeding disorder. J Clin Invest. 1994;94:1662–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207 [DOI] [PubMed] [Google Scholar]
- 536. Kan Z, Jaiswal BS, Stinson J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873 [DOI] [PubMed] [Google Scholar]
- 537. Gwinn MR, Sharma A, De Nardin E. Single nucleotide polymorphisms of the N-formyl peptide receptor in localized juvenile periodontitis. J Periodontol. 1999;70:1194–1201 [DOI] [PubMed] [Google Scholar]
- 538. Nakayama J, Fu YH, Clark AM, et al. A nonsense mutation of the MASS1 gene in a family with febrile and afebrile seizures. Ann Neurol. 2002;52:654–657 [DOI] [PubMed] [Google Scholar]
- 539. Weston MD, Luijendijk MW, Humphrey KD, Möller C, Kimberling WJ. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet. 2004;74:357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Vervoort VS, Beachem MA, Edwards PS, et al. AGTR2 mutations in X-linked mental retardation. Science. 2002;296:2401–2403 [DOI] [PubMed] [Google Scholar]
- 541. Ma RZ, Gao J, Meeker ND, et al. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science. 2002;297:620–623 [DOI] [PubMed] [Google Scholar]
- 542. Arehart E, Stitham J, Asselbergs FW, et al. Acceleration of cardiovascular disease by a dysfunctional prostacyclin receptor mutation: potential implications for cyclooxygenase-2 inhibition. Circ Res. 2008;102:986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Karsak M, Cohen-Solal M, Freudenberg J, et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet. 2005;14:3389–3396 [DOI] [PubMed] [Google Scholar]
- 544. Bassi MT, Schiaffino MV, Renieri A, et al. Cloning of the gene for ocular albinism type 1 from the distal short arm of the X chromosome. Nat Genet. 1995;10:13–19 [DOI] [PubMed] [Google Scholar]
- 545. Kazius J, Wurdinger K, van Iterson M, Kok J, Bäck T, Ijzerman AP. GPCR NaVa database: natural variants in human G protein-coupled receptors. Hum Mutat. 2008;29:39–44 [DOI] [PubMed] [Google Scholar]
- 546. Leaños-Miranda A, Ulloa-Aguirre A, Janovick JA, Conn PM. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormone receptors causing hypogonadotropic hypogonadism in humans expressing compound heterozygous alleles. J Clin Endocrinol Metab. 2005;90:3001–3008 [DOI] [PubMed] [Google Scholar]