Summary
Mammalian sex determination initiates in the fetal gonad with specification of bipotential precursor cells into male Sertoli cells or female granulosa cells. This choice was long presumed to be irreversible, but genetic analysis in the mouse recently revealed that sexual fates must be maintained throughout life. Somatic cells in the testis or ovary, even in adults, can be induced to transdifferentiate to their opposite-sex equivalents by loss of a single transcription factor, DMRT1 in the testis or FOXL2 in the ovary. Here we ask what mechanism DMRT1 prevents from triggering transdifferentiation. We find that DMRT1 blocks testicular retinoic acid (RA) signaling from activating genes normally involved in female sex determination and ovarian development and show that inappropriate activation of these genes can drive sexual transdifferentiation. By preventing activation of potential feminizing genes, DMRT1 allows Sertoli cells to participate in RA signaling, which is essential for reproduction, without being sexually reprogrammed.
Keywords: DMRT1, transdifferentiation, sexual differentiation, gonad, testis, Sertoli
Introduction
In mammals, gonadal sex is determined during fetal development in the bipotential precursors of male Sertoli cells and female granulosa cells (McClelland et al., 2012). If the Y chromosome gene Sry is expressed in the bipotential gonad during a critical window of fetal development it activates the related Sox9 gene and triggers testis differentiation. Otherwise a female-promoting regulatory network prevails and triggers ovary differentiation. Despite this early cell fate commitment, genetic studies in the mouse have shown that sexual fates in the gonad must be actively maintained in both sexes throughout life. The transcriptional regulators Dmrt1 and Foxl2 are essential for sex maintenance in the postnatal testis and ovary, respectively. Loss of either gene, even in the adult gonad, can trigger a dramatic transdifferentiation of cell fate involving extensive reprogramming of sex-specific gene regulation (Matson et al., 2011; Uhlenhaut et al., 2009). Previous studies suggested mutual antagonism between the two genes: loss of Dmrt1 in the adult mouse testis activates Foxl2 expression, whereas loss of Foxl2 in the adult ovary activates Dmrt1 (Matson et al., 2011; Matson and Zarkower, 2012; Uhlenhaut et al., 2009). Thus Dmrt1 and Foxl2 appear to anchor mutually antagonistic regulatory networks that lock in sexual differentiation and then continuously maintain appropriate cell fates.
While previous genetic analysis clearly revealed the existence of male and female sexual fate maintenance networks, the functional composition of these networks is poorly understood. In particular, it is unknown whether the regulatory mechanisms that can cause Sertoli cells to transdifferentiate into granulosa cells in the mutant testis are related to those that normally direct granulosa cell differentiation in the fetal ovary. Moreover, the physiological reason why sexual fates must be continuously maintained postnatally, long after they are specified, is unknown.
Here we address both questions. First, we use genetic analyses to ask which genes are functionally required in fate maintenance and reprogramming of the testis. We show that DMRT1 maintains male sex postnatally in concert with the male fetal sex determination gene Sox9 and that the feminizing genes it must silence include components of the fetal sex determination network. Our results therefore indicate that postnatal sex maintenance and transdifferentiation are mechanistically related to fetal male and female sex determination. Second, although RA (RA) signaling between Sertoli cells and germ cells is essential for mammalian spermatogenesis, we show that when DMRT1 is absent RA signaling also can activate genes that drive male-to-female transdifferentiation. Thus DMRT1 allows Sertoli cells to participate in RA signaling while avoiding consequent cell fate reprogramming. Our results reveal that cell signaling can entail risk to the cell identities of the participants, and we suggest that other cell types likewise may require mechanisms to protect against reprogramming.
Results
Ectopic FOXL2 drives male-to-female transdifferentiation in Dmrt1 mutant Sertoli cells
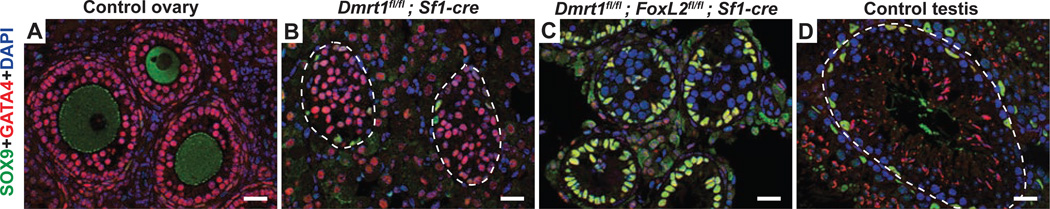
Dmrt1 mutant Sertoli cells express FOXL2 early in transdifferentiation and chromatin immunoprecipitation (ChIP) suggested that DMRT1 directly represses Foxl2 transcription in the postnatal testis (Matson et al., 2011). However, it is unknown whether the ectopic expression of FOXL2 is important for driving transdifferentiation or is merely a consequence of activating transdifferentiation. To distinguish between these possibilities we deleted Dmrt1 and Foxl2 in somatic cells of the fetal testis using Sf1-cre, which is active in fetal pre-Sertoli cells around the time of sex determination (Bingham et al., 2006; Lavery et al., 2011). To track cell fates we examined expression of SOX9, which is expressed only in Sertoli cells, and GATA4, which is expressed in both Sertoli and granulosa cells. Loss of Foxl2 dramatically suppressed feminization of adult Dmrt1 mutant testes: double mutant gonads retained GATA4/SOX9 double-positive Sertoli cells, lacked GATA4 single-positive granulosa cells, and had seminiferous tubules (Fig. 1). Since DMRT1 is dispensable for maintenance of male cell fate if Foxl2 is inactivated, we conclude that ectopic Foxl2 does indeed drive female transdifferentiation. Repression of Foxl2 cannot be the only function of DMRT1 in postnatal Sertoli cells, however, since Dmrt1;Foxl2 conditional mutant adult gonads had small seminiferous tubules and severely disrupted spermatogenesis. We therefore examined additional markers. In addition to SOX9, the Sertoli cells in double mutants expressed GATA1 (Fig. S1) and double mutant testes also had highly elevated expression of the Sertoli cell marker Sox8 relative to Dmrt1 single mutants (16-fold qRT-PCR difference; P=0.038, Student’s two-tailed t-test; 2 individuals of each genotype). However, double mutant Sertoli cells appeared not to have completed polarization and did not express androgen receptor (AR) (Fig. S1) which we showed previously is directly activated by DMRT1 (Murphy et al., 2010). AR is required in Sertoli cells for support of spermatogenesis and its deletion in Sertoli cells disrupts germ cell meiotic prophase (Chang et al., 2004; De Gendt et al., 2004; Holdcraft and Braun, 2004). Dmrt1;Foxl2 mutant testes had disrupted meiotic prophase but also severely reduced numbers of spermatogonia, so lack of AR expression in the mutant Sertoli cells can only partially explain the spermatogenesis defect. We conclude that Dmrt1 is essential in the postnatal testis to prevent male-to-female (Sertoli-to-granulosa) transdifferentiation and also for full Sertoli cell differentiation and function.
Figure 1. Ectopic Foxl2 drives male-to-female transdifferentiation in Dmrt1 mutant testes.
A-D, Immunofluorescence detection of SOX9 and GATA4 proteins in control and mutant adult gonads. Granulosa cells of control ovaries express GATA4 but not SOX9 (A), as do granulosa cells arising from postnatal transdifferentiation after Dmrt1 is deleted in fetal XY gonads with Sf1-cre (B). (Green staining of oocytes in control ovaries is a non-specific antibody artifact; GATA4 also is expressed in interstitial Leydig cells outside the tubules). Deletion of Foxl2 together with Dmrt1 in XY gonads (C) suppresses transdifferentiation; the resulting adult gonads have tubules with many Sertoli cells expressing abundant SOX9 adjacent to the tubule periphery, similar to those of control testis (D), as well as germ cells negative for both SOX9 and GATA4. (Red staining of elongated spermatids is a non-specific antibody artifact). Control gonads in this figure were from floxed animals lacking cre. Scale bars, 20 µm. DAPI, 4’6-diamidino-2-phenylindole is a DNA stain. Dashed lines indicate tubule periphery. (See also Fig. S1)
Ectopic estrogen signaling and Wnt signaling drive male-to-female transdifferentiation in Dmrt1 mutant testes
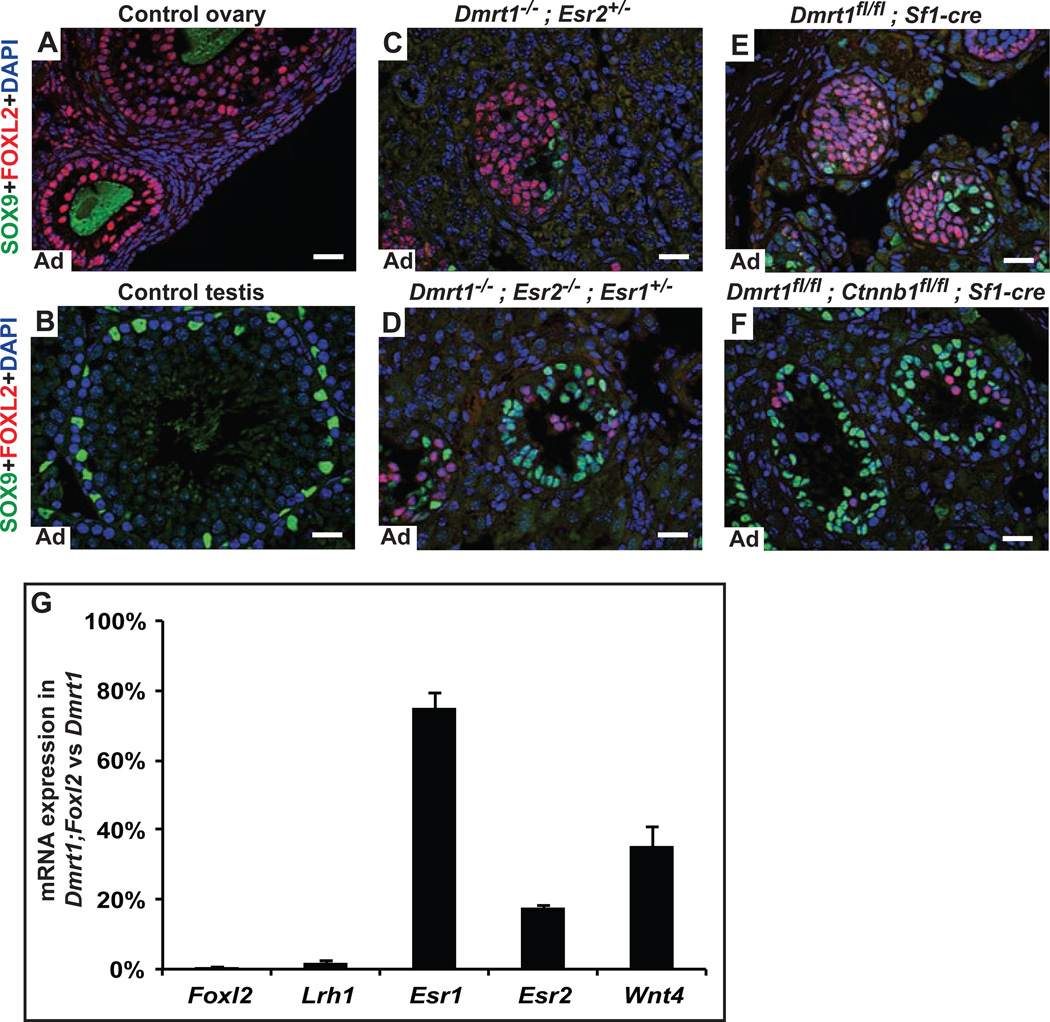
Like Foxl2, the estrogen receptors Esr1 and Esr2 help maintain female sex (Couse et al., 1999), and they cooperate with Foxl2 to repress Sox9 transcription in granulosa cells (Uhlenhaut et al., 2009). Loss of Dmrt1 in Sertoli cells activates Esr1, Esr2, and the estrogenic enzyme CYP19A1/Aromatase, and Dmrt1 mutant males have elevated serum estradiol (Matson et al., 2011). To test whether this ectopically activated estrogen signaling promotes male-to-female transdifferentiation we examined XY mice carrying null mutations in Dmrt1 and each estrogen receptor. Loss of Esr1 had no effect on feminization of Dmrt1 mutant gonads (Fig. S2A,B,E) but loss of Esr2 greatly reduced feminization in animals heterozygous or null for Esr1 (Fig. 2A-D; Fig. S2C,E). We conclude that estrogen signaling is an important driver of transdifferentiation when Dmrt1 is lost. Based on our results ERβ (encoded by Esr2) is clearly important for transdifferentiation. However, as we were unable to obtain Dmrt1;Esr2 mutant males fully wild type for Esr1, it remains possible that there is some functional redundancy between ERα and ERβ. Dmrt1 mutant testes lacking both estrogen receptors were still partially feminized (Fig. S2D), suggesting that another feminizing pathway remained active. Together with the Dmrt1;Foxl2 phenotype described above, the requirement for Esr2 demonstrates that the female sex maintenance network drives transdifferentiation when ectopically activated in males.
Figure 2. Activated estrogen signaling and Wnt/β-catenin signaling drive male-to-female transdifferentiation in Dmrt1 mutant testes.
A-F, Immunofluorescence detection of SOX9 and FOXL2 proteins in control and mutant adult gonads. Granulosa cells in wild type (WT) ovaries express abundant FOXL2 (A), as do elongated theca cells surrounding follicles. (As in Fig. 1, green staining of oocytes is a nonspecific antibody artifact). In wild type testes (B), only SOX9-positive Sertoli cells are present. In males, homozygous deletion of Dmrt1 (in this case with loss of one copy of Esr2) (C) causes postnatal transdifferentiation of most Sertoli cells into morphologically distinct FOXL2 positive granulosa-like cells. Deletion of Esr2 and one copy of Esr1 in males of the same genotype suppresses this feminization, and many more SOX9-positive Sertoli cells are present (D). Adult males conditionally mutant for Dmrt1 in Sertoli cells are almost fully feminized (E) but conditional deletion of the β-catenin gene Ctnnb1 together with Dmrt1 using the same Sf1-cre transgene strongly suppresses the feminization (F). G, qRT-PCR showing reduced expression of feminizing gene mRNAs in Dmrt1;Foxl2 conditional double mutant testes relative to expression in Dmrt1 single mutant testes. Data are averaged from two animals of each genotype; degree of feminization can vary between Dmrt1 mutants, but loss of Foxl2 reduced expression of feminizing genes below that seen in Dmrt1 mutants. Scale bars, 20 µm. (See also Fig. S2)
In the fetal gonad, female primary sex determination is controlled by a WNT signaling pathway in which the WNT4 and RSPO1 ligands signal through the β-catenin transcription factor. Loss of Wnt4 or Rspo1 in XX fetal gonads causes female-to-male sex reversal, while constitutively active β-catenin can trigger male-to-female sex reversal in the XY fetal gonad (Chassot et al., 2008; Kim et al., 2006; Maatouk et al., 2008). DMRT1 represses transcription of Wnt4 and Rspo1 in the postnatal testis (Matson et al., 2011). To ask whether ectopically activated WNT signaling in Dmrt1 mutant testes promotes transdifferentiation we conditionally deleted Dmrt1 and β-catenin (encoded by Ctnnb1) in fetal pre-Sertoli cells using Sf1-cre. Loss of Ctnnb1 strongly suppressed feminization of Dmrt1 mutant XY gonads (Fig. 2F; Fig. S2F). We also deleted Dmrt1 and Ctnnb1 with Dhh-cre, which is active in differentiating fetal Sertoli cells (Bitgood et al., 1996) and observed the same phenotype (not shown). Thus at least part of the female sex determination network is activated by loss of Dmrt1, suggesting that transdifferentiation of postnatal Sertoli cells is mechanistically related to normal fetal specification of ovarian cells. The residual feminization in Dmrt1;Ctnnb1 double mutant gonads suggests that other genes repressed by Dmrt1 can promote feminization independently of β-catenin.
Reciprocal regulation of feminizing genes
As shown earlier, loss of Foxl2 more completely suppresses feminization of Dmrt1 mutants than loss of Esr1/2 or β-catenin. To ask whether Foxl2 is an upstream activator of other feminizing genes we examined their expression in Dmrt1;Foxl2 double mutant testes. We found that Dmrt1;Foxl2 double mutant testes had greatly reduced expression of the granulosa marker Lrh1 as well as Esr2 and Wnt4 and slightly reduced expression of Esr1 relative to Dmrt1 single mutants (Fig. 2G). This result suggests that ectopic Foxl2 expression in the testis activates estrogen signaling and Wnt signaling. In addition, the loss of FOXL2 expression in Dmrt1;Esr1/2, and Dmrt1;β-catenin mutant testes (Fig. 2D,F,H) indicates that estrogen signaling and Wnt signaling also regulate Foxl2. Thus the female regulatory network activated by loss of Dmrt1 involves significant reciprocal regulation.
DMRT1 acts with SOX9 to maintain male gonadal cell fates
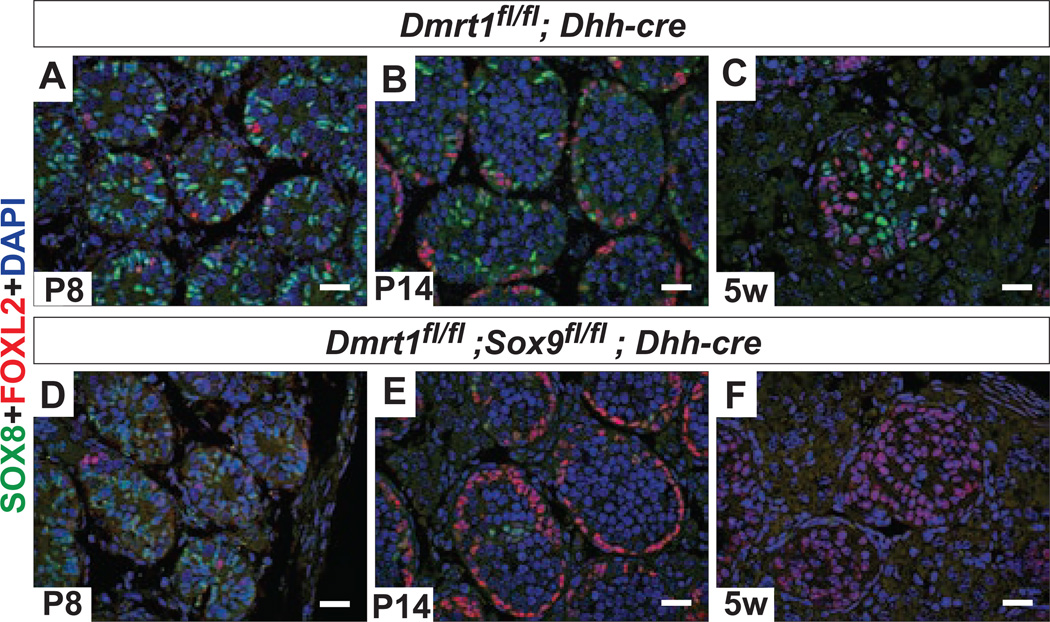
Given the apparent role of female sex-determining genes in transdifferentiation, we investigated whether these genes are opposed in the postnatal testis by the male fetal sex determination network. Sox9 plays a central role in male fetal sex determination: its expression in the early fetal gonad is both necessary and sufficient for male development (Chaboissier et al., 2004; Lavery et al., 2011; Qin et al., 2004; Vidal et al., 2001). Sox9 is dispensable for male fates after sex determination (Barrionuevo et al., 2009; Chang et al., 2008). However, we found that Sox9 is partially redundant with Dmrt1 in postnatal sex maintenance: deleting Sox9 together with Dmrt1 after sex determination using Dhh-cre enhanced transdifferentiation (Fig. 3). The start of transdifferentiation was unaffected in double mutants, beginning around P8 in both Dmrt1 and Dmrt1;Sox9 testes (Fig. 3A,D). However, by P14 the Dmrt1;Sox9 gonads had considerably more FOXL2-positive cells and by 5 weeks virtually all Sertoli cells had transdifferentiated (Fig. 3B,C,E,F). The involvement of Sox9 suggests that male sex maintenance, like transdifferentiation, is mechanistically related to normal fetal sex determination.
Figure 3. DMRT1 and SOX9 act together to maintain male fates.
A-F, Immunofluorescence detection of the Sertoli cell marker SOX8 and granulosa cell marker FOXL2 in control and conditional mutant adult gonads. Deletion of Dmrt1 with Dhh-cre in the XY fetal gonad after sex determination causes transdifferentiation and FOXL2 expression beginning around postnatal day 8 (P8) (A). Transdifferentiation is still quite limited at P14 (B), and apparent in many but not all intratubular somatic cells by 5 weeks (C). XY gonads with both Dmrt1 and Sox9 deleted with Dhh-cre have similar numbers of FOXL2 positive cells present at P8 (D), but many more at P14 (E) and 5 weeks (F) compared to Dmrt1 single mutant gonads. Scale bars, 20 µm.
Enhanced RA signaling accelerates transdifferentiation in Dmrt1 mutant testes
Maintenance of gonadal cell fates requires sustained activity of sex maintenance networks throughout postnatal life (Matson et al., 2011; Uhlenhaut et al., 2009). It is not obvious why fully differentiated postmitotic Sertoli cells should require this continuous protection. Sertoli cells surround and support germ cells and the two cell types communicate via varied paracrine signaling interactions that facilitate reproduction; we hypothesized that one or more of these signaling interactions might have potential to reprogram Sertoli cell fate if DMRT1 is not present.
In particular, we considered a possible role for retinoic acid (RA) signaling, based in part on the timing of the transdifferentiation phenotype. RA is essential for aspects of spermatogenesis including spermatogonial differentiation and spermiation (Hasegawa and Saga, 2012; Raverdeau et al., 2012; van Pelt and de Rooij, 1990) and is a potent cell fate inducer during development (Rhinn and Dolle, 2012). Strikingly, RA from neonatal Sertoli cells triggers spermatogonial differentiation during the first postnatal week, close to when transdifferentiation starts in Dmrt1 mutant testes (Raverdeau et al., 2012). RA also influences cyclical gene expression in adult Sertoli cells during steady state spermatogenesis and promotes differentiation of cultured juvenile Sertoli cells (Hasegawa and Saga, 2012; Nicholls et al., 2013; Sugimoto et al., 2012; Vernet et al., 2006). We found previously that DMRT1 limits RA signaling in spermatogonia in order to promote spermatogonial differentiation and control the mitosis-tomeiosis switch (Matson et al., 2010). We therefore considered that DMRT1 also might limit RA response in Sertoli cells.
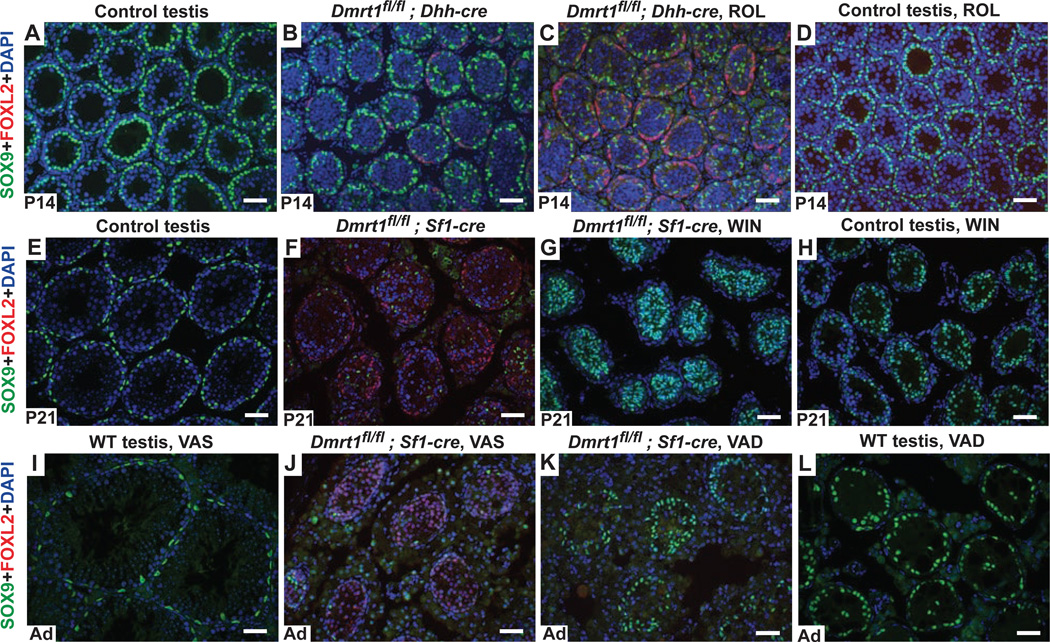
We first tested the prediction that elevated RA enhances transdifferentiation. We injected the RA precursor retinol acetate (ROL) at P4 into Dmrt1 conditional mutant pups deleted with Dhh-Cre and examined their gonads at P14, a time when transdifferentiation is just starting in untreated Dmrt1 mutant testes. ROL injection in wild type did not affect Sertoli cell fates (Fig. 4A,D), but ROL injection of Dmrt1 mutants strongly enhanced feminization relative to vehicle-injected controls (Fig. 4B,C).
Figure 4. Retinoid signaling promotes transdifferentiation of Dmrt1 mutant Sertoli cells.
A-D, Retinol treatment enhances transdifferentiation. At P14 control testes from Dmrt1fl/+ animals injected with vehicle (PBS) had no FOXL2 positive transdifferentiated cells at P14 (A) and vehicle-injected conditional mutants deleted with Dhh-Cre (B) had small numbers of transdifferentiated cells at this stage. Retinol acetate (ROL) injection at P4 into conditional mutant testes resulted in many FOXL2 positive cells at P14 (C), whereas injection of ROL into control testes did not induce transdifferentiation (D). E-H, Inhibition of RA synthesis suppresses transdifferentiation. At P21 testes of Dmrtfl/fl control animals (E) treated from P5 with vehicle (1% gum tragacanth) had no FOXL2-positive transdifferentiated cells while vehicle-treated conditional mutants deleted with Sf1-Cre were extensively transdifferentiated (F). Treatment of conditional mutants with WIN 18,446 almost completely suppressed transdifferentiation (G). Treatment of controls with WIN 18,446 disrupted spermatogenesis (H), confirming that the RA synthesis was blocked. I-L, Dietary vitamin A depletion suppresses transdifferentiation. Relative to testes from wild type adult mice on vitamin A sufficient (VAS) diets (I), VAS adult testes with Dmrt1 deleted using Sf1-cre (J) had many transdifferentiated cells expressing FOXL2. (Note that conditional loss of Dmrt1 in Sertoli cells also causes postnatal germ cell loss.) Dietary vitamin A depletion of conditional Dmrt1 mutant mice, starting in utero, reduced the number of FOXL2 positive cells and increased the number of SOX9 positive cells (K). VAD treatment of wild type mice had no effect on Sertoli cell differentiation or maintenance but blocked spermatogonial differentiation, confirming the effectiveness of VAD and resulting in largely empty seminiferous tubules (L). Scale bars, 40 µm.
Reduced RA signaling suppresses transdifferentiation in Dmrt1 mutant testes
If RA signaling stimulates transdifferentiation, as suggested by the ROL injections, then reducing RA levels in Dmrt1 mutants should suppress the process. We confirmed this prediction using two strategies. First, to block intratubular RA synthesis we treated neonates with the retinaldehyde dehydrogenase inhibitor WIN 18,446 (Amory et al., 2011; Hogarth et al., 2013; Hogarth et al., 2011). Pups conditionally deleted for Dmrt1 in Sertoli cells with Sf1-cre were treated with WIN 18,446 or vehicle daily from P2 to P21 and then the extent of transdifferentiation was assessed. In contrast to vehicle-treated mutant testes which were extensively feminized at P21, those from WIN 18,446-treated mutants had virtually no FOXL2-positive granulosa cells and many SOX9-positive Sertoli cells (Fig. 4E-H). As a second approach to reduce RA levels, we used dietary vitamin A depletion (VAD), placing pregnant females on vitamin A deficient chow and maintaining their pups on this chow until adulthood (Fig. 4I-L). RA is essential for spermatogonial differentiation; consequently VAD wild type adult males had normal Sertoli cells but very few germ cells, confirming the effectiveness of the depletion (Fig. 4I,L; arrowheads). In Dmrt1 mutant VAD adult males feminization was strongly suppressed relative to vitamin A sufficient (VAS) mutants (Fig. 4J,K). In summary, increasing RA enhanced transdifferentiation, whereas reducing RA levels by two different methods suppressed transdifferentiation of Dmrt1 mutant testes. We conclude that RA can stimulate transdifferentiation and that DMRT1 normally blocks its ability to do so.
Loss of RARα in Sertoli cells suppresses transdifferentiation
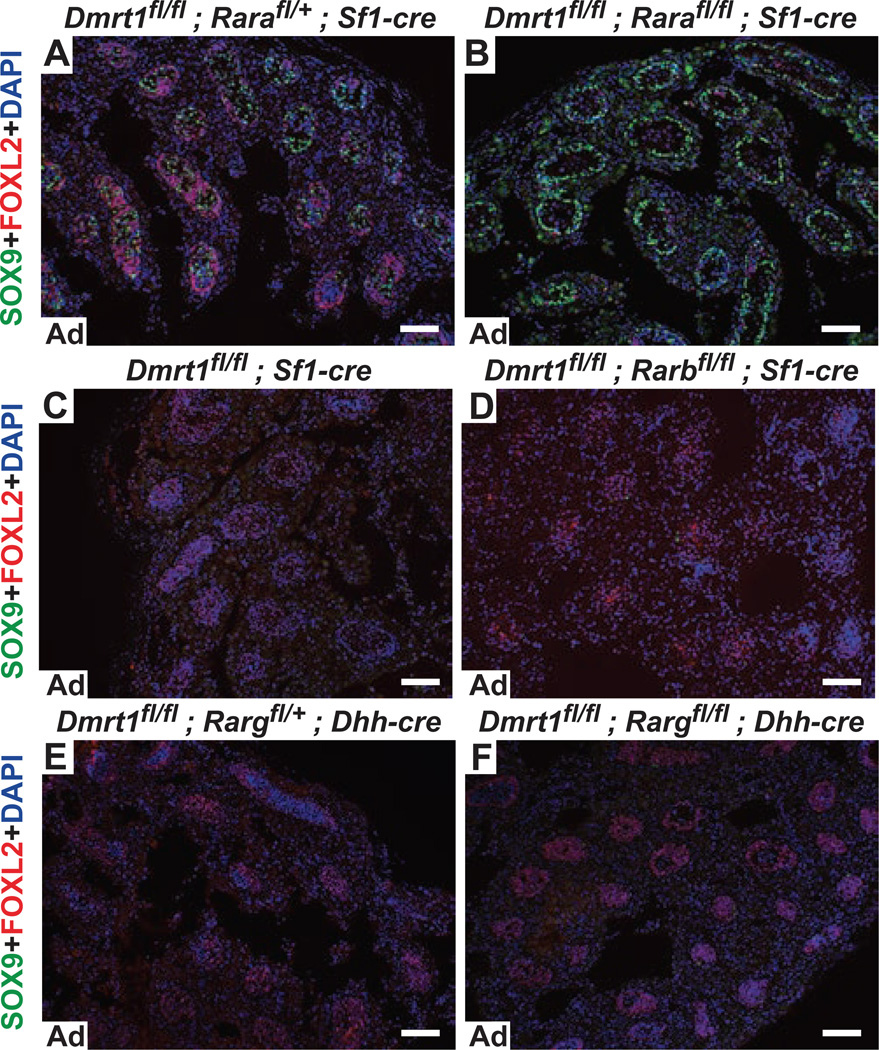
Next we examined the role of retinoic acid receptors (RAR) in transdifferentiation. To test which receptor mediates transdifferentiation we used conditional alleles of the two predominant RAR-encoding genes of Sertoli cells and spermatogonia, Rara and Rarg, as well as the third RAR-encoding gene Rarb (Chapellier et al., 2002a; Chapellier et al., 2002b; Chapellier et al., 2002c). We deleted Dmrt1 together with each receptor gene in somatic cells of the fetal gonad using Sf1-Cre or Dhh-Cre and assessed transdifferentiation in double mutant testes. Deletion of Rara with Dmrt1 strongly suppressed feminization (Fig. 5A,B), whereas deletion of Rarb or Rarg plus Dmrt1 had no discernible effect on transdifferentiation (Fig. 5C-E). Deleting Rara with either Sf1-Cre (Fig. 5B) or the Sertoli-specific Dhh-Cre (not shown) suppressed feminization, confirming that RA acts directly on Dmrt1 mutant Sertoli cells. Sertoli cells lacking Dmrt1 and one copy of Rara were more feminized than Dmrt1 single mutants (Fig. 5A versus 5C) or Dmrt1 mutants lacking one copy of Rarg (Fig. 5E), suggesting that Rara may be partially haploinsufficient for feminization. Collectively our data indicate that retinoid signaling, acting via RARα, plays an important role in promoting Sertoli cell transdifferentiation in Dmrt1 mutants and that Dmrt1 is largely dispensable for sex maintenance in the absence of retinoid signaling.
Figure 5. RARα, but not RARβ or RARγ, is required for transdifferentiation.
A-F, Immunofluorescence detection of SOX9 and FOXL2 proteins in adult testes. Conditional deletion of Dmrt1 with one copy of Rara using Sf1-Cre caused strong transdifferentiation (A), whereas deletion of Dmrt1 and both alleles of Rara with Sf1-Cre strongly suppressed transdifferentiation (B), indicating that Rara is required. By contrast, deletion of Dmrt1 alone or with Rarb using Sf1-Cre (C,D) or deletion of Dmrt1 with one (C) or both (D) alleles of Rarg using Dhh-Cre caused transdifferentiation, indicating that Rarb and Rarg are not required for transdifferentiation of Dmrt1 mutant Sertoli cells. Scale bars, 80 µm.
Germ cells are not required for transdifferentiation in juvenile testes
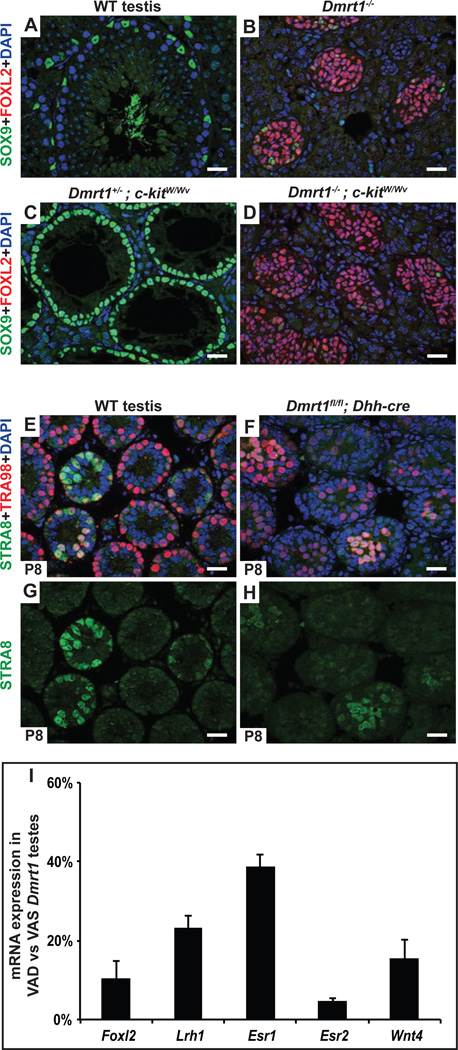
Both cell types inside the seminiferous tubules, Sertoli cells and germ cells, are believed to produce RA during at least some stages of development (Raverdeau et al., 2012). We tested whether germ cells might contribute to transdifferentiation of Dmrt1 mutants as sources of RA or other signaling molecules by genetic ablation, using mutations in the germ cell survival factor c-kit. Dmrt1;kitW/Wv mutant testes were strongly feminized despite lacking germ cells since prior to gonadal differentiation (Fig. 6A-D), indicating that germ cells do not play a significant role as a source of feminizing RA in the juvenile testis, although we cannot exclude that germ cells play a role in transdifferentiation caused by loss of Dmrt1 in adults (Matson et al., 2011).
Figure 6. Feminizing RA is not produced by germ cells and appears to be at normal levels.
A-D, Germ cells are not required for transdifferentiation of Dmrt1 mutant Sertoli cells. Deletion of Dmrt1 causes most SOX9-positive Sertoli cells (A) to transdifferentiate into FOXL2 positive granulosa cells (B). Loss of c-kit function in eliminates virtually all germ cells during fetal development, leaving seminiferous tubules containing only differentiated SOX9-positive Sertoli cells in Dmrt1 heterozygous control testes (C). Testes of Dmrt1 homozygous kitW/Wv mutants were extensively feminized despite lacking germ cells (D). (Gonads in A-D were from adults). E-H, Loss of Dmrt1 in Sertoli cells does not induce STRA8 expression in spermatogonia. Expression of RA-inducible germ cell marker STRA8 and spermatogonial marker TRA98 in juvenile (P8) testes. In wild type testes (E,G) a subset of tubules contain germ cells with abundant STRA8 expression, reflecting the asynchronous initiation of spermatogenesis that is triggered by RA from Sertoli cells at this stage. When Dmrt1 is deleted in Sertoli cells (F,H) there is no obvious increase in the proportion of germ cells expressing STRA8 suggesting that intratubular RA levels are not elevated (RA is freely diffusible between cells). I, qRT-PCR showing reduced expression of feminizing gene mRNAs in VAD Dmrt1 mutants relative to VAS Dmrt1 mutants. Results are from two animals of each condition. (See also Fig. S3.)
Intratubular RA levels appear normal in Dmrt1 mutant juvenile testes
Our results indicate that DMRT1 prevents transdifferentiation by somehow limiting RARα activity in Sertoli cells. DMRT1 might inhibit RARα by limiting RA levels or by repressing transcription of selected RARα targets, including feminizing genes activated by RARα in Sertoli cells. It is not feasible to directly assay RA levels in Sertoli cells, but RA is freely diffusible between cells (Shimozono et al., 2013). Spermatogonia are extremely sensitive to RA and thus can be used as sensors to ask whether Dmrt1 mutant Sertoli cells are producing excessive intratubular RA. Two experiments examining spermatogonial gene expression suggested that intratubular RA levels are not elevated in Dmrt1 mutant testes. First, we examined the RA-inducible gene Stra8. RA from juvenile Sertoli cells normally triggers STRA8 expression in a subset of spermatogonia to initiate asynchronous spermatogenesis (Raverdeau et al., 2012), and RA supplementation in neonates can activate strong STRA8 expression in virtually all juvenile spermatogonia, demonstrating that they are very sensitive to RA during this period (Davis et al., 2013; Snyder et al., 2011). Dmrt1 mutant juvenile testes have reduced numbers of spermatogonia, but deletion of Dmrt1 in Sertoli cells did not increase the proportion of spermatogonia that were strongly STRA8-positive or the proportion of tubules containing STRA8-positive germ cells (Fig. 6 E-H). Second, we examined expression of the RA-responsive transcriptional reporter gene RAREhsplacZ (Rossant et al., 1991). This reporter does not express in Sertoli cells but is exquisitely sensitive to RA signaling in spermatogonia (Matson et al., 2011; Snyder et al., 2011). RAREhsplacZ expression in wild type and Dmrt1 mutant testes, like STRA8, was high only in a subset of spermatogonia regardless of their proximity to mutant Sertoli cells (Fig. S3A-F). We also examined mRNA expression profiling data from P9 testes conditionally deleted for Dmrt1 in Sertoli cells (Murphy et al., 2010) and these showed no significant misregulation of RA pathway genes and no increase in expression of known RA-inducible genes. From these results we conclude that DMRT1 in Sertoli cells does not function to limit the level of intratubular RA. Instead we favor alternative models in which DMRT1 restricts RARα activity in Sertoli cells without regulating RA levels.
Retinoid signaling activates expression of feminizing genes if DMRT1 is absent
In principle RARα could act upstream to activate some or all of the feminizing genes, or it could instead act in parallel to those genes to promote transdifferentiation. If RARα acts upstream of feminizing genes then their expression should be reduced in VAD Dmrt1 mutants; by contrast, if RARα acts in parallel to the feminizing genes, VAD mutants should retain elevated expression of feminizing genes but maintain male fates because RARα is not able to cooperate with the feminizing genes. VAD treatment reduced expression of Foxl2, Lrh1, Esr2, and Wnt4 mRNAs (Fig. 6I) in Dmrt1 mutant testes, indicating that RAR does act upstream to promote expression of feminizing mRNAs when Dmrt1 is missing. We suggest that DMRT1 silences inappropriate RARα target genes that can transform sexual cell fate, while permitting RARα-dependent expression of appropriate target genes that are essential for Sertoli cell differentiation and for support of spermatogenesis. We note, however, that it remains possible that RARα also synergizes with the feminizing genes to promote transdifferentiation once they are activated (see Discussion).
Discussion
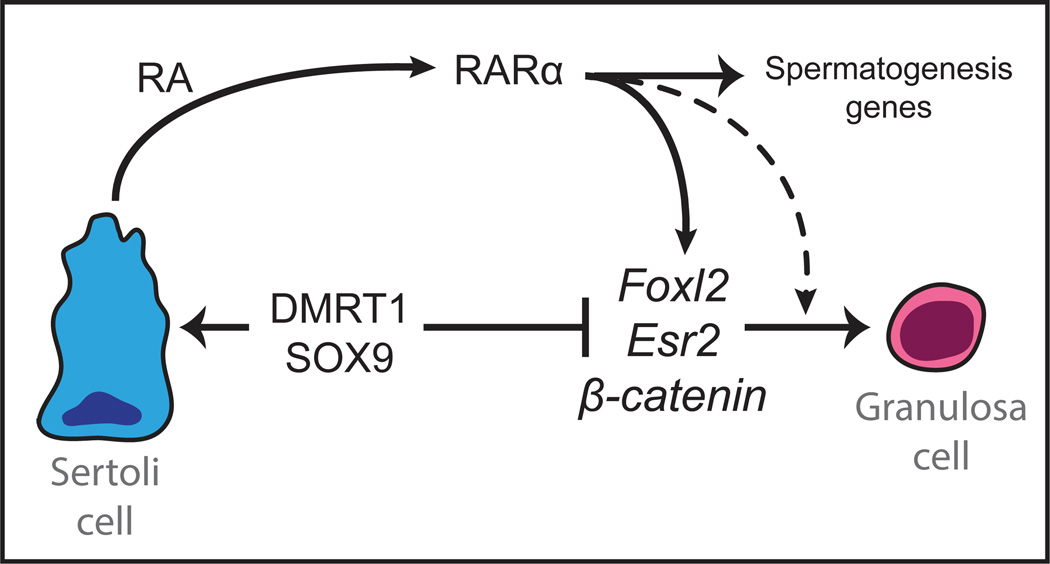
Here we have found that DMRT1 is required to prevent RA signaling from promoting reprogramming of Sertoli cells to granulosa cells in the postnatal testis. RA signaling is essential for male gametogenesis and has been implicated in Sertoli cell differentiation; however we found that RA also can activate genes of both the female sex maintenance network and the female sex determination network if DMRT1 is absent from Sertoli cells. We found previously that DMRT1 binds to chromatin near many of the key feminizing genes identified here, including Foxl2 and Esr2 (Matson et al., 2011). Thus the simplest model for sexual fate maintenance is that DMRT1 and RARα are antagonistic regulators of feminizing genes whose inappropriate expression in the testis can cause transdifferentiation. By silencing this subset of RARα targets DMRT1 allows Sertoli cells to participate in the RA signaling that is essential for spermatogenesis while avoiding transdifferentiation (diagrammed in Fig. 7).
Figure 7. Model for role of DMRT1 in sex maintenance.
DMRT1 directly represses transcription in Sertoli cells of potential feminizing genes including Foxl2, Esr2, and the Wnt/β-catenin pathway genes Wnt4 and Rspo1 (Matson et al., 2011). This paper shows that DMRT1 thereby allows Sertoli cells to produce RA that is necessary for spermatogenesis without causing RARα to activate these feminizing genes, which also activate one another. The model also indicates that it is possible, based on data from other systems, that RARα synergizes with products of some of the feminizing genes to drive transdifferentiation. In addition to the genes shown, DMRT1 also represses Cyp19a1/aromatase, which makes estradiol that stimulates ER activity (Matson et al., 2011).
While the ability of RA to activate feminizing genes when DMRT1 is missing indicates a role for RARα upstream of the feminization network and in parallel to DMRT1, studies of RARα in other contexts suggest that it may also act together with the feminizing genes to cooperatively regulate gene expression. For example, ER and RARα are nuclear hormone receptors (NHRs) that cooperate to bind DNA and regulate gene expression in breast cancer cells, with ER recruiting RARα at many target genes (Ross-Innes et al., 2010). We suggest that ER and RARα may cooperate by a similar molecular mechanism in Dmrt1 mutant Sertoli cells to promote female transdifferentiation. ER also can be recruited to many cell type-specific sites by “tethering” to a variety of transcription factors (Gertz et al., 2013). Similarly, β-catenin can physically interact with RARα and other NHRs that are ectopically expressed in Dmrt1 mutant Sertoli cells including ER and LRH1, and it can serve as an NHR coactivator (Mulholland et al., 2005). We found that the feminizing genes can mutually activate each others’ expression. This reciprocal regulation and the potential for synergistic function once they are expressed suggest that the critical feminizing genes we have identified may functionally cooperate in a complex regulatory web. It will be important in future work to examine in greater detail the physical interactions, DNA binding, and gene regulatory functions of these proteins in the context of Sertoli cell transdifferentiation.
Genetic analysis demonstrated that several genes normally involved in female sex determination and ovarian differentiation are critical for transdifferentiation in the testis when they are inappropriately activated by RARα. This suggests a mechanistic link between the normal specification and differentiation of granulosa cells in the fetal and postnatal ovary and Sertoli-to-granulosa transdifferentiation in the postnatal testis. Similarly, the involvement of Sox9 in sex maintenance suggests a link between male sex maintenance and male fetal sex determination. Doubtless there are important differences in how Sertoli cell transdifferentiation and normal granulosa cell differentiation are regulated, but our data make it clear that transdifferentiation occurs by a process related to normal ovarian differentiation. This link between transdifferentiation and normal female development may answer an important question: why do feminizing genes remain potential RARα targets in the testis when their inappropriate activation in the testis is highly deleterious to reproduction? An attractive possibility is that this represents a sexual conflict: if RAR promotes granulosa cell differentiation in the fetal ovary by activating these feminizing genes, then their capacity for regulation by RAR (with the attendant risk to male reproduction) must be maintained to allow female reproduction.
In addition to DMRT1, other sexual regulators implicated in this study as controlling postnatal sexual fates are conserved in gonadal regulation in other vertebrates (eg SOX9, ER, and FOXL2), raising the possibility that the process is conserved outside mammals. This appears to be the case in fish: loss of Dmrt1 causes male to female sexual transdifferentiation in medaka (Masuyama et al., 2012) while disruption of estrogen signaling with the Aromatase inhibitor fadrazole can cause female to male transdifferentiation in Nile tilapia (Sun et al., 2014). It is unknown whether sexual transdifferentiation in fish involves RA signaling but the possibility should be investigated, since RA signaling recently has been suggested to play a role in normal gonadal development and/or gametogenesis in zebrafish (Rodriguez-Mari et al., 2013). Homologs of DMRT1 also regulate sex in many other metazoans (Matson and Zarkower, 2012) and it will be important to determine whether sex maintenance is conserved outside vertebrates.
In the past decade much progress has been made in artificially reprogramming cell fates. It is now possible, by forced expression of defined sets of transcription factors and/or miRNAs, to achieve direct cell fate conversion in culture between many cell types, even across germ layers (Ladewig et al., 2013). However, reports of in vivo cell fate reprogramming resulting from altered expression of one gene are still quite rare and the reasons are unclear (Cobaleda et al., 2007; Holmberg and Perlmann, 2012; Johnson et al., 2008). Are Sertoli and granulosa cells unusual in being readily interconvertible or is such plasticity more widespread but poorly recognized? Although they are morphologically very different, Sertoli and granulosa cells derive from a common bipotential progenitor and they may retain epigenetic and other similarities. Thus one possibility is that their barrier to transdifferentiation is unusually low. However, it is also possible that other cell types are potentially interconvertable and require active maintenance to prevent transdifferentiation, whether in response to RA or other signaling molecules.
Detecting a role for a regulatory gene in cell fate maintenance can require allowing differentiation to be completed and then conditionally inactivating the gene, which is not a test that is routinely performed. Moreover, RA functions during and after development in many tissues (Rhinn and Dolle, 2012). We therefore speculate that additional mammalian cell types might require active protection from transdifferentiation triggered by RA or possibly other potent signaling molecules, and that the critical genes maintaining their fates await discovery. There is some evidence for this possibility. In the pancreas, for example, ablation of beta cells causes an apparent alpha-to-beta transdifferentiation (Thorel et al., 2010) although the signals responsible and the genetic mechanism by which the alpha cell fate is maintained in the presence of beta cells have yet to be defined. Candidate regulators of cell fate maintenance include not only other DMRT transcription factors (Matson and Zarkower, 2012), but also homeobox transcription factors (Johnson et al., 2008). Because the gonad is dispensable for viability and has highly plastic differentiated cells, it may provide a useful model for understanding mechanisms of cell fate maintenance and transdifferentiation and for developing strategies to artificially reprogram other cell types in vivo.
Experimental Procedures
Mouse breeding
Mice were of mixed C57BL/6J and 129Sv genetic background. Genotyping was performed as described ((Chaboissier et al., 2004; Chapellier et al., 2002a; Chapellier et al., 2002b; Chapellier et al., 2002c; Uhlenhaut et al., 2009) and http://jaxmice.jax.org). Experimental protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Vitamin A depletion and retinol injection
Dietary vitamin A depletion was performed as described (Matson et al., 2010; van Pelt and de Rooij, 1990). The retinaldehyde dehydrogenase inhibitor WIN 18,446 (gift of C. Hogarth and M. Griswold) was administered by pipette feeding of 100 ug/gram body weight WIN 18,446 in 1% gum tragacanth, as described (Hogarth et al., 2013). For retinol supplementation, 100 ul retinolacetate (2.5 mg/ml in phosphate-buffered saline) was injected intraperitoneally at P4.
mRNA and protein expression analysis
mRNA extraction, cDNA synthesis, qRT-PCR, immunofluorescence and immunohistochemistry were performed as described (Matson et al., 2010). Antibodies and dilutions are listed in Table S1.
Supplementary Material
Highlights.
RA is essential for spermatogenesis but can cause Sertoli cell transdifferentiation.
DMRT1 blocks RA signaling from activating female gonadal genes.
DMRT1 permits cell signaling while protecting from cell fate reprogramming.
Related gene networks control ovary development and transdifferentiation.
Acknowledgements
We thank R. Behringer, D. Largaespada, K. Parker, S. Kliewer, A. Schedl, and M. Treier for providing mice, M. Griswold and M. Wegner for antibodies, and D. Greenstein and E. Matunis for critical reading of the manuscript. We also thank K. Hogarth and M. Griswold for WIN 18,446 and much helpful advice. This work was supported by the NIH (5 RO1 GM59152 and 1 F32 GM106484), an NSF predoctoral fellowship (C.K.M), the Minnesota Medical Foundation, and the French Agence Nationale de la Recherche under the frame program Investissements d’Avenir labelled ANR-10-LABX-0030-INRT (N.B.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amory JK, Muller CH, Shimshoni JA, Isoherranen N, Paik J, Moreb JS, Amory DW, Sr, Evanoff R, Goldstein AS, Griswold MD. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J Androl. 2011;32:111–119. doi: 10.2164/jandrol.110.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, Scherer G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. 2009;327:301–312. doi: 10.1016/j.ydbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR. Wt1 negatively regulates beta-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapellier B, Mark M, Bastien J, Dierich A, LeMeur M, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor beta (RARbeta) gene. Genesis. 2002a;32:91–94. doi: 10.1002/gene.10073. [DOI] [PubMed] [Google Scholar]
- Chapellier B, Mark M, Garnier JM, Dierich A, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor gamma (RARgamma) gene. Genesis. 2002b;32:95–98. doi: 10.1002/gene.10072. [DOI] [PubMed] [Google Scholar]
- Chapellier B, Mark M, Garnier JM, LeMeur M, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis. 2002c;32:87–90. doi: 10.1002/gene.10071. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Davis JC, Snyder EM, Hogarth CA, Small C, Griswold MD. Induction of spermatogenic synchrony by retinoic acid in neonatal mice. Spermatogenesis. 2013;3:e23180. doi: 10.4161/spmg.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz J, Savic D, Varley KE, Partridge EC, Safi A, Jain P, Cooper GM, Reddy TE, Crawford GE, Myers RM. Distinct properties of cell-type-specific and shared transcription factor binding sites. Mol Cell. 2013;52:25–36. doi: 10.1016/j.molcel.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Saga Y. Retinoic acid signaling in Sertoli cells regulates organization of the blood-testis barrier through cyclical changes in gene expression. Development. 2012;139:4347–4355. doi: 10.1242/dev.080119. [DOI] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Mitchell D, Kent T, Small C, Amory JK, Griswold MD. Turning a spermatogenic wave into a tsunami: synchronizing murine spermatogenesis using WIN 18,446. Biol Reprod. 2013;88:40. doi: 10.1095/biolreprod.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D, Small C, Amory JK, Griswold MD. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol Reprod. 2011;84:957–965. doi: 10.1095/biolreprod.110.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Perlmann T. Maintaining differentiated cellular identity. Nat Rev Genet. 2012;13:429–439. doi: 10.1038/nrg3209. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22:3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladewig J, Koch P, Brustle O. Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat Rev Mol Cell Biol. 2013;14:225–236. doi: 10.1038/nrm3543. [DOI] [PubMed] [Google Scholar]
- Lavery R, Lardenois A, Ranc-Jianmotamedi F, Pauper E, Gregoire EP, Vigier C, Moreilhon C, Primig M, Chaboissier MC. XY Sox9 embryonic loss-of-function mouse mutants show complete sex reversal and produce partially fertile XY oocytes. Dev Biol. 2011;354:111–122. doi: 10.1016/j.ydbio.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H, Yamada M, Kamei Y, Fujiwara-Ishikawa T, Todo T, Nagahama Y, Matsuda M. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res. 2012;20:163–176. doi: 10.1007/s10577-011-9264-x. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, Zarkower D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev Cell. 2010;19:612–624. doi: 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet. 2012;13:163–174. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland K, Bowles J, Koopman P. Male sex determination: insights into molecular mechanisms. Asian J Androl. 2012;14:164–171. doi: 10.1038/aja.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Rev. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- Murphy MW, Sarver AL, Rice D, Hatzi K, Ye K, Melnick A, Heckert LL, Zarkower D, Bardwell VJ. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc Natl Acad Sci U S A. 2010;107:13360–13365. doi: 10.1073/pnas.1006243107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls PK, Harrison CA, Rainczuk KE, Wayne Vogl A, Stanton PG. Retinoic acid promotes Sertoli cell differentiation and antagonises activin-induced proliferation. Mol Cell Endocrinol. 2013;377:33–43. doi: 10.1016/j.mce.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Qin Y, Kong LK, Poirier C, Truong C, Overbeek PA, Bishop CE. Long-range activation of Sox9 in Odd Sex (Ods) mice. Hum Mol Genet. 2004;13:1213–1218. doi: 10.1093/hmg/ddh141. [DOI] [PubMed] [Google Scholar]
- Raverdeau M, Gely-Pernot A, Feret B, Dennefeld C, Benoit G, Davidson I, Chambon P, Mark M, Ghyselinck NB. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci U S A. 2012;109:16582–16587. doi: 10.1073/pnas.1214936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, Dolle P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mari A, Canestro C, Bremiller RA, Catchen JM, Yan YL, Postlethwait JH. Retinoic Acid metabolic genes, meiosis, and gonadal sex differentiation in zebrafish. PLoS One. 2013;8:e73951. doi: 10.1371/journal.pone.0073951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Holmes KA, Schmidt D, Spyrou C, Russell R, Massie CE, Vowler SL, Eldridge M, Carroll JS. Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev. 2010;24:171–182. doi: 10.1101/gad.552910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Shimozono S, Iimura T, Kitaguchi T, Higashijima S, Miyawaki A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature. 2013;496:363–366. doi: 10.1038/nature12037. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol Reprod. 2011;84:886–893. doi: 10.1095/biolreprod.110.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto R, Nabeshima Y, Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev. 2012;128:610–624. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Sun LN, Jiang XL, Xie QP, Yuan J, Huang BF, Tao WJ, Zhou LY, Nagahama Y, Wang DS. Transdifferentiation of differentiated ovary into functional testis by long term treatment of aromatase inhibitor in Nile tilapia. Endocrinology. 2014:en20131959. doi: 10.1210/en.2013-1959. [DOI] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod. 1990;43:363–367. doi: 10.1095/biolreprod43.3.363. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Guillou F, Chambon P, Ghyselinck NB, Mark M. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006;25:5816–5825. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.