Abstract
Purpose
The standard surgical procedure recommended to treat gastric cancer in advanced cases is dissection of D2 lymph nodes (LNs). However, the optimum number of LNs that should be retrieved in advanced gastric cancer (AGC) remains debatable. Therefore, this study aimed to investigate the optimum number of retrieved LNs and determine the clinical implications of retrieved LN numbers on the treatment of AGC.
Materials and Methods
Of 575 AGC patients reviewed, 369 who underwent open curative gastrectomy with D2 or more extensive LN dissection at our institution were analyzed according to their clinicopathologic characteristics and number of LNs retrieved.
Results
Multivariate regression analysis revealed that tumor size (P=0.006), depth of invasion (P=0.000), LN metastasis (P=0.000), and stage (P=0.000) were independent variables with predictive value. The 5-year survival rates were differed significantly according to the numbers of LNs retrieved ([1] 15~25 vs. >25 and [2] 15~39 vs. ≥40) in patients with differentiated carcinoma.
Conclusions
Tumor size, depth of invasion, LN metastasis, and stage were independent predictive factors for survival. The number of retrieved LNs was significantly associated with a long-term survival benefit in patients with differentiated carcinoma. Therefore, our data suggest that the retrieval of a minimum of 15 LNs may not be sufficient to warrant a recommendation for further curative surgery and that extensive LN dissection should be considered in advanced carcinoma of the differentiated type.
Keywords: Lymph nodes, Retrieval, Advanced gastric cancer
Introduction
In general, curative surgery for gastric carcinoma requires en bloc resection with a sufficient negative margin and the dissection of corresponding lymph nodes (LNs). The standard surgical procedure to treat gastric cancer is recommended to be D2 LN dissection in advanced cases. However, D2 dissection refers to the anatomic extent to which LNs are removed instead of the number of LNs being removed. Recently, such an anatomic classification of LN removal has been abandoned in accordance with the tumor-node-metastasis (TNM) classification manual (fifth edition) guidelines released by the International Union Against Cancer/American Joint Cancer Committee (UICC/AJCC).1 The 14th edition of the Japanese Gastric Cancer Association (JGCA) staging system, released in 2010, also uses a numeric classification scheme identical to the one used in the UICC/AJCC TNM system.2 Therefore, use of the anatomic extent of metastatic LN removal is no longer practical, and use of the numeric system has become universal. However, the optimum number of LNs that should be retrieved for gastric cancer staging is a topic of intense debate; moreover, the lack of a defined cut-off value for LN retrieval in the standard treatment for gastric carcinoma further complicates this approach. In advanced gastric cancer (AGC), the retrieval of more than 25 LNs has been shown to be associated with an overall survival advantage.3,4 Smith et al.5 reported that patient survival was significantly correlated with the total number of LNs examined, associating the best survival with 40 or more LNs removed. Although no fixed number of retrieved LNs has been demonstrated to be necessary for the accurate staging of gastric cancer, the retrieval of a minimum of 15 is recommended to avoid stage migration according to version 2 of the National Comprehensive Cancer Network guidelines (2013).5,6,7,8 Therefore, the present study aimed to investigate the optimum number of retrieved LNs and determine the clinical implications of retrieved LN numbers on the treatment of AGC.
Materials and Methods
This retrospective study was performed using a database of patients who underwent radical gastrectomy to treat gastric carcinoma at Ewha Womans University Hospital (Dongdaemun and Mokdong), Seoul, Korea, from January 1995 to December 2009. Of 575 AGC patients reviewed, 369 who underwent curative gastrectomy with D2 or more extensive LN dissection were enrolled. All patients who underwent laparoscopic surgery were excluded to eliminate the bias of surgical techniques. Although a universally accepted minimum number of retrieved LNs necessary for the accurate staging of gastric cancer has not been determined, 15 is often considered sufficient for stage assignment using the sixth UICC guidelines.9 Therefore, patients from whom fewer than 15 LNs were retrieved were also excluded and we attempted to minimize the bias of LN examination related with insufficient dissection. Sex, age, tumor location, tumor size, macroscopic type, histological classification, depth of invasion, LN metastasis, TNM stage, and type of surgery were analyzed. Tumors were classified into 2 histologic subgroups, differentiated and undifferentiated. TNM stages were determined according to the seventh edition of the UICC/AJCC manual. The extent of LN metastasis was classified as negative or positive carcinoma involvement. Patient survival was analyzed according to the numbers of retrieved LNs, using different combinations of cut-off brackets as follows: Group [1] 15 to 25, 26 to 35, 36 to 45, and >45, Group [2] 15 to 25 and >25, and Group [3] 15 to 39 and ≥40. Subgroup analysis of patient demographic, clinical, and pathologic factors was also performed according to the numbers of retrieved LNs.
Chi-squared tests and Student's t-tests were used to compare the relevant subgroup parameters. All deaths, including non-cancer-related mortalities, were regarded as events. Survival analyses were performed using the Kaplan-Meier method with the log-rank test. Multivariate analysis was performed using the Cox proportional hazards regression model to analyze the prognostic value of different variables. Statistical analyses were performed using the IBM Statistical Package for the Social Sciences software package version 20.0 (IBM Co., Armonk, NY, USA). Results were evaluated with a confidence interval of 95%, and P-values below 0.05 were considered statistically significant.
Results
Of the 369 gastric carcinoma patients enrolled, there were 253 (68.6%) men and 116 (31.4%) women. The mean patient follow-up period was 67.98±55.1 months (range, 2~221 months). Patient characteristics are listed in Table 1. The mean patient age at diagnosis was 59.1 years (men, 59.3 years and women, 58.7 years). The most common carcinoma location was the lower third of the stomach, and the mean tumor size was 5.45±3.1 cm. The most common tumor type and depth were Borrmann type III and T4a, respectively. The mean number of retrieved LNs per patient was 44.29±21.4 (42.97±19.9 in node-negative patients and 44.71±21.8 in node-positive patients).
Table 1.
Characteristics of patients with advanced gastric cancer
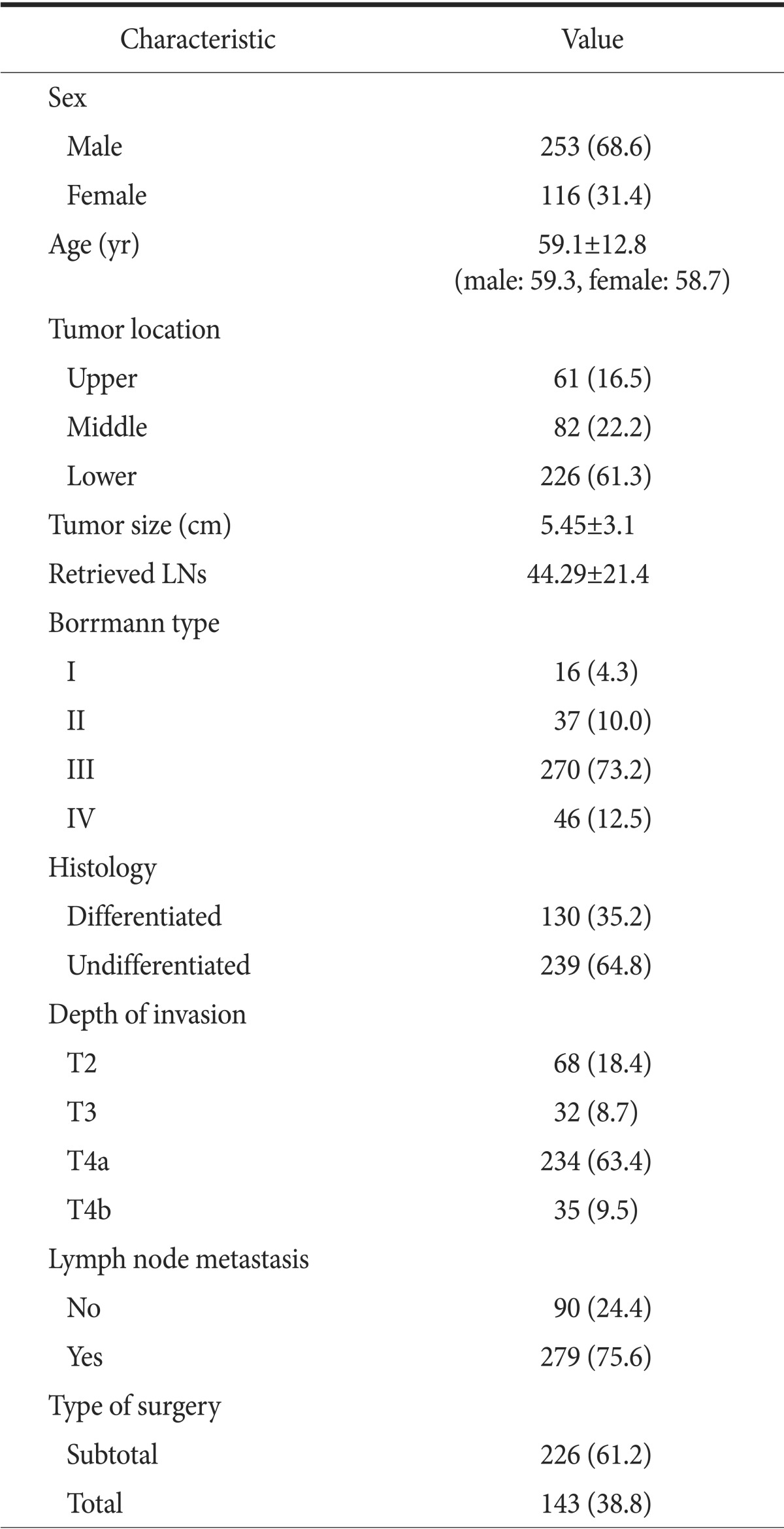
Values are presented as number (%) or mean±standard deviation.
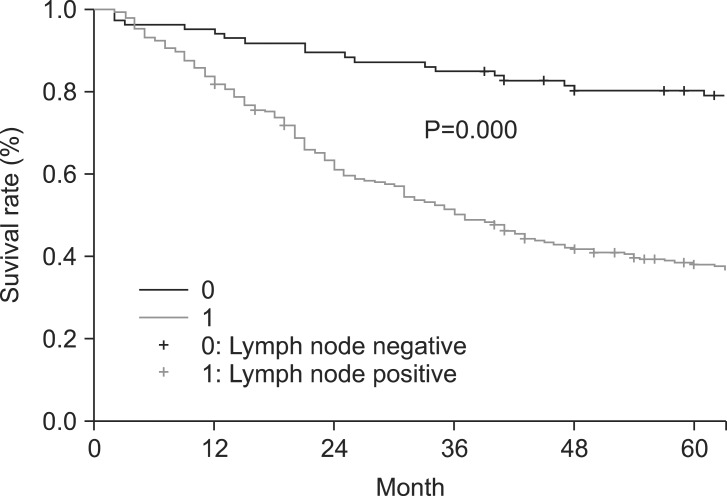
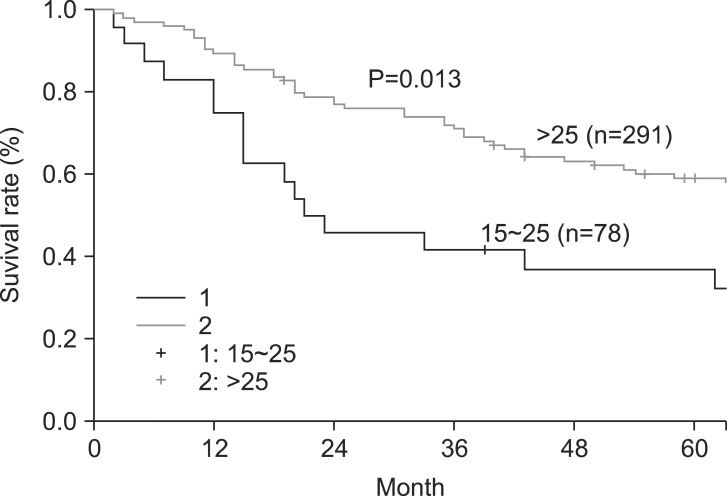
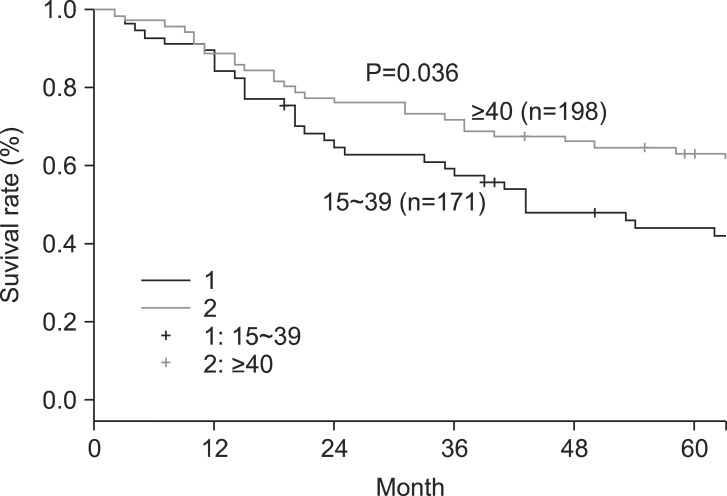
The overall 5- and 10-year survival rates were 45% and 33%, respectively. The 5-year survival rate of node-negative patients significantly differed from that of node-positive patients (74% and 36%, respectively) (Fig. 1). However, patient survival rates were not significantly associated with the numbers of LNs retrieved (Group [1] 15~25, 26~35, 36~45, and >45; Group [2] 15~25 and >25; Group [3] 15~39 and ≥40) (Table 2). Interestingly, in patients with differentiated carcinoma, the 5-year survival rate was significantly associated with the number of LNs retrieved (15~25 and >25) (Fig. 2). Similar results were also obtained when patients with differentiated carcinoma were stratified according to the numbers of retrieved LNs as 15~39 and ≥40 (Fig. 3). However, the 5-year survival rate was not significantly associated with the number of LNs retrieved from in patients with undifferentiated carcinoma. Although the number of LNs retrieved was associated with survival rate according to tumor histology, no significant differences were observed between the extent of LN positivity and the types of histologic differentiation.
Fig. 1.
Five-year survival rate according to the presence of lymph node metastasis.
Table 2.
Five- and 10-year SRs according to the numbers of retrieved LNs using the multiple cut-off values
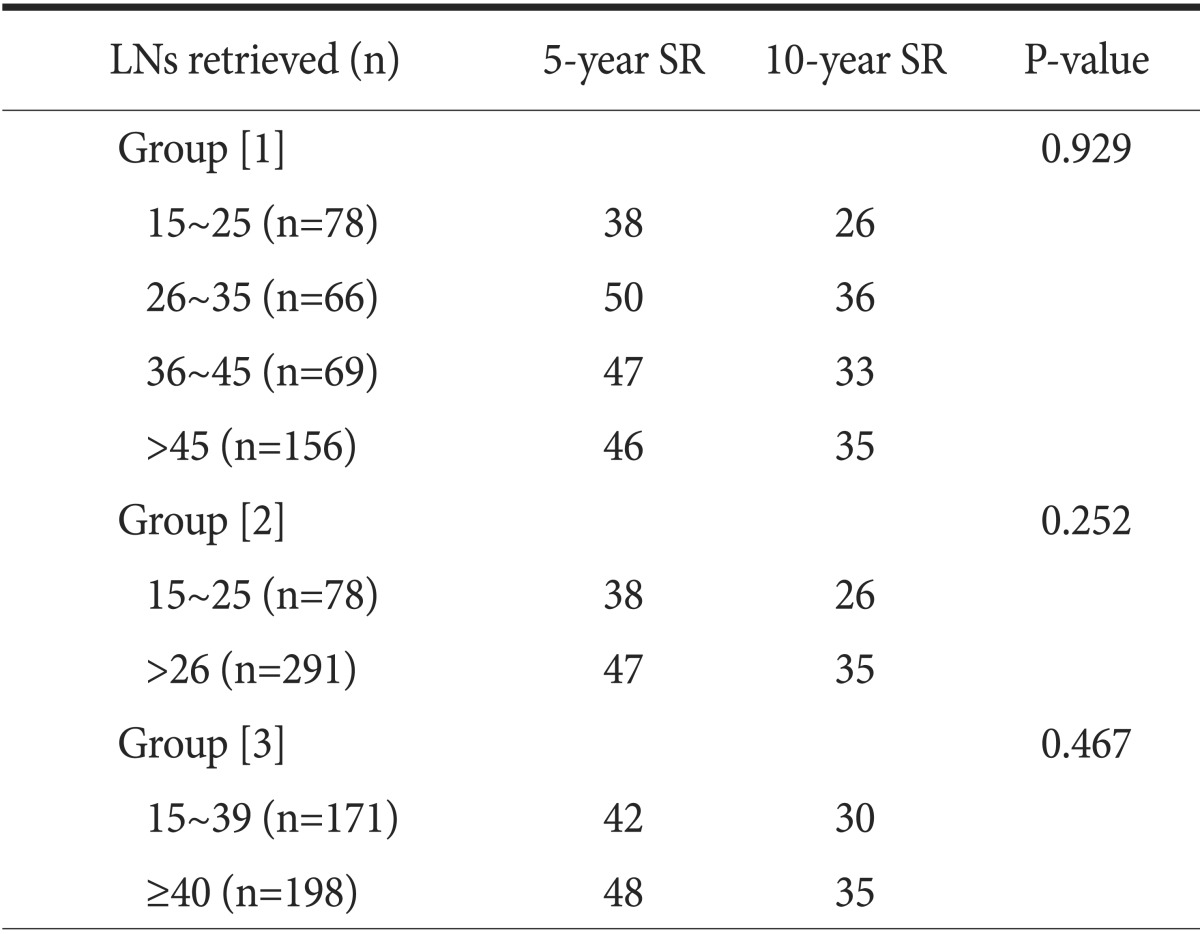
Values are presented as percent. LN = lymph node; SR = survival rate.
Fig. 2.
Five-year survival rate according to the numbers of lymph nodes retrieved (15~25 or >25) in patients with differentiated carcinoma.
Fig. 3.
Five-year survival rate according to the numbers of lymph nodes retrieved (15~39 or ≥40) in patients with differentiated carcinoma.
According to univariate analysis, tumor location, size, Borrmann type, depth of invasion, LN metastasis, and stage were all significantly correlated with overall survival. Multivariate regression analysis revealed that size (P=0.006), depth of invasion (P=0.000), LN metastasis (P=0.000), and stage (P=0.000) were independent variables with predictive value (Table 3).
Table 3.
Prognostic factors for patients with advanced gastric cancer
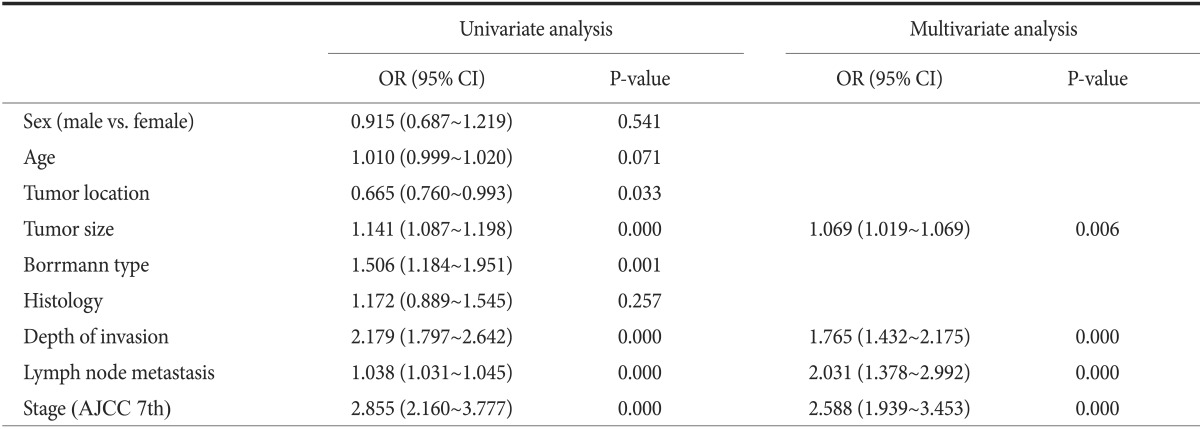
OR = odds ratio; CI = confidence interval; AJCC = American Joint Cancer Committee.
Discussion
The precise evaluation of the extent of LN metastasis allows a more accurate prediction of each patient's oncologic outcome. The anatomic definition of LN classification has not been used since the release of the fifth edition of the UICC/AJCC TNM classification manual. Furthermore, in 2010, the fourteenth edition of the JGCA staging system also began to use a numeric classification scheme, identical to the one recommended in the UICC/AJCC TNM system. As a result, the anatomic extent of metastatic LNs is no longer considered a prognostic factor. However, the precise number of LNs that need to be retrieved for curative treatment of gastric cancer is still under debate; moreover, the appropriate extent of curative LN dissection differs between Western and Eastern countries. Thus, the lack of a defined cut-off number of retrieved LNs for standard gastric carcinoma treatment remains an open question that complicates therapeutic strategies. In AGC patients, the retrieval of more than 25 LNs is reported to be associated with an overall survival advantage.3,4 Similarly, Smith et al.5 found that stage subgroup-specific survival was strongly associated with the total number of LNs examined and that the best survival was expected in cases of 40 or more LNs retrieved, which was strongly in favor of extended lymphadenectomy. Bouvier et al.10 suggested that staging was not reliable when fewer than 10 LNs were examined. Shen et al.11 could not determine an optimum cut-off value for accurate staging based on their large dataset. Although the minimum number of LNs necessary for accurate gastric cancer staging remains unclear, 15 is often considered sufficient according to the UICC system.9 The AJCC manual (seventh edition) suggested that a minimum of 16 regional nodes be assessed pathologically and that the pN0 status be assigned based on the actual number of nodes evaluated microscopically.12 Additionally, there are other factors that might influence the variability of LN retrieval. In many Asian centers, surgeons are responsible for individual LN sampling and submission for histological analysis, whereas Western surgeons often submit en-bloc resected specimens and rely upon pathologists to retrieve the individual LNs. The LN counts reported from Western centers therefore depend upon both the surgeon and pathologist.
In this study, the mean number of LNs retrieved per patient was 44.29±21.4 (42.97±19.9 in node-negative patients and 44.71±21.8 in node-positive patients), which was greater than that reported by other similar studies.3,9,13 We believe that in our case, the greater the number of LNs that were retrieved, the lower was the expected bias of stage migration; moreover, the analysis of these LNs was much less susceptible to reliability bias.14,15,16 In the present study, we performed survival analyses using multiple cut-off brackets for the numbers of retrieved LNs; however, we did not identify a fixed number that yielded superior survival outcomes among the groups, which were divided according to the number of LNs retrieved as follows: Group [1] 15 to 25, 26 to 35, 36 to 45, and >45; Group [2] 15 to 25 and >25; and Group [3] 15 to 39 and ≥40. However, in patients with differentiated carcinomas, the 5-year survival rate differed significantly according to the number of LNs retrieved using a cut-off value of 25 (15~25 and >25) (Fig. 2). Similar results were also obtained using a cut-off value of 40 (15~39 and ≥40) in these patients (Fig. 3). However, in patients with undifferentiated carcinomas, no such specific cut-off points were determined to be associated with a survival advantage. It is currently unclear why a higher number of retrieved LNs was associated with a better overall survival only in patients with differentiated carcinomas. Yasuda et al.17 and Kim et al.18 reported that tumor micrometastases were detected at a considerably higher rate in patients classified as having pN0 by routine histologic examination, ranging from 17% to 32%., Baiocchi et al.3 found that intestinal adenocarcinoma tumors were usually well to moderately differentiated, with nodal or hepatic recurrence, whereas diffuse cancers were frequently undifferentiated and exhibited peritoneal dissemination. These reports indicate that differentiated tumors may have a higher chance of LN micrometastasis than other types of carcinomas. Thus, we hypothesize that more extensive LN retrieval might more effectively remove micrometastatic LNs in patients with differentiated carcinomas, including those not identified as metastatic LNs in routine pathologic reports. However, it is worthy of note that a direct comparison of our data to those from previous studies is not strictly possible because of differences in study design.
Univariate analysis revealed that tumor size, Borrmann type, depth of invasion, LN metastasis, and stage were significantly associated with patient survival, whereas multivariate analysis identified size (P=0.006), depth of invasion (P=0.000), LN metastasis (P=0.000), and stage (P=0.001) as independent predictive factors affecting survival. These results are consistent with those from previous studies.10,19,20
Our study has several limitations. First, it was a retrospective analysis with a small sample size. Second, this analysis did not account for adjuvant chemotherapy treatments, which may have resulted in better survival in node-positive patients. Therefore, we could not completely exclude the bias of stage difference and LN positivity.
In conclusion, tumor size, depth of invasion, LN metastasis, and stage were found to be independent factors of patient survival. Moreover, the number of retrieved LNs was significantly associated with a long-term survival benefit in patients with differentiated carcinomas. Since it is impossible to determine the exact LN stage before surgery, the routine cut-off value of 15 LNs should be increased in patients with differentiated carcinomas before more curative surgeries are indicated. Moreover, extended LN dissection and careful pathologic examination should be performed in patients who may be serosa-positive. Additionally, larger randomized controlled studies should be conducted in the future to clarify these issues.
References
- 1.Sobin LH, Wittekind Ch, editors. TNM classification of malignant tumors. 5th ed. New York: Wileys-Liss; 1997. [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 3.Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, Bruno L, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252:70–73. doi: 10.1097/SLA.0b013e3181e4585e. [DOI] [PubMed] [Google Scholar]
- 4.Chen XZ, Yang K, Zhang B, Hu JK, Zhou C. Is retrieval of >25 lymph nodes a superior criterion for locally advanced gastric cancer surgery? Ann Surg. 2011;254:834–835. doi: 10.1097/SLA.0b013e318235dfda. [DOI] [PubMed] [Google Scholar]
- 5.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 6.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: fifth edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 7.Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, et al. National Comprehensive Cancer Network. Gastric cancer, version 2. 2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 8.Baxter NN, Tuttle TM. Inadequacy of lymph node staging in gastric cancer patients: a population-based study. Ann Surg Oncol. 2005;12:981–987. doi: 10.1245/ASO.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Witteking Ch, editors. TNM: classification of malignant tumor. 6th ed. New York: Wiley-Liss; 2002. [Google Scholar]
- 10.Bouvier AM, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer. 2002;94:2862–2866. doi: 10.1002/cncr.10550. [DOI] [PubMed] [Google Scholar]
- 11.Shen JY, Kim S, Cheong JH, Kim YI, Hyung WJ, Choi WH, et al. The impact of total retrieved lymph nodes on staging and survival of patients with pT3 gastric cancer. Cancer. 2007;110:745–751. doi: 10.1002/cncr.22837. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Compton C, Fritz A, Greene F, Trotti A, editors. AJCC Cancer Staging Handbook. 7th ed. New York: Springer; 2010. [Google Scholar]
- 13.Bunt AM, Hermans J, van de Velde CJ, Sasako M, Hoefsloot FA, Fleuren G, et al. Lymph node retrieval in a randomized trial on western-type versus Japanese-type surgery in gastric cancer. J Clin Oncol. 1996;14:2289–2294. doi: 10.1200/JCO.1996.14.8.2289. [DOI] [PubMed] [Google Scholar]
- 14.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Stage migration influences on stage-specific survival comparison between D1 and D3 gastric cancer surgeries. Eur J Surg Oncol. 2005;31:153–157. doi: 10.1016/j.ejso.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Roukos D, Lazaros A, Kappas A, Encke A. Extended lymph node dissection in gastric cancer induces substantial stage migration and increases stage-specific survival without improvement of overall survival. J Clin Oncol. 1996;14:2408–2410. doi: 10.1200/JCO.1996.14.8.2408. [DOI] [PubMed] [Google Scholar]
- 16.Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, et al. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897–905. doi: 10.1093/annonc/mdn707. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, Kitano S. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol. 2002;9:771–774. doi: 10.1007/BF02574499. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Park JM, Jung CW, Park SS, Kim SJ, Mok YJ, et al. The significances of lymph node micrometastasis and its correlation with E-cadherin expression in pT1-T3N0 gastric adenocarcinoma. J Surg Oncol. 2008;97:125–130. doi: 10.1002/jso.20937. [DOI] [PubMed] [Google Scholar]
- 19.Jeong JY, Kim MG, Ha TK, Kwon SJ. Prognostic factors on overall survival in lymph node negative gastric cancer patients who underwent curative resection. J Gastric Cancer. 2012;12:210–216. doi: 10.5230/jgc.2012.12.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho BC, Jeung HC, Choi HJ, Rha SY, Hyung WJ, Cheong JH, et al. Prognostic impact of resection margin involvement after extended (D2/D3) gastrectomy for advanced gastric cancer: a 15-year experience at a single institute. J Surg Oncol. 2007;95:461–468. doi: 10.1002/jso.20731. [DOI] [PubMed] [Google Scholar]