Abstract
It is well known that new-onset arrhythmias are common in septic patients. It is thought that hypophosphatemia in the early stages of sepsis may contribute to the development of new arrhythmias. In this study, we hypothesized that intravenous (IV) phosphorus replacement may reduce the incidence of arrhythmias in critically ill patients. 34 adult septic patients with hypophosphatemia admitted to the general intensive care unit were treated with IV phosphorus replacement per ICU protocol, and the incidence of new arrhythmias were compared with 16 patients from previously published data. IV phosphorus replacement was associated with a significantly reduced incidence of arrhythmias (38% vs. 63%, p=0.04). There were no differences in observed mortality between subgroups, which may be due to the small sample size. This study demonstrated that IV phosphorus replacement might be effective in reducing the incidence of new arrhythmias in septic patients.
Keywords: Hypophosphatemia, Cardiac arrhythmias, Phosphorus replacement, Critical care
Introduction
New-onset supraventricular arrhythmias are common in septic patients1,2,3). Kirpatrick and colleagues first described the clinical evidence and significance of cardiac arrhythmias as an early sign of sepsis in 19734). New-onset episodes of atrial fibrillation were shown to highly correlate with mortality in critically ill septic patients compared with non-septic patients5,6,7,8). Most cardiac arrhythmias in sepsis may be secondary to sepsis-induced myocardial dysfunction, preexisting cardiac comorbidities, autonomic dysfunction, or electrolyte disturbances1,2,3,4,5,6,7,8). Hypophosphatemia has been postulated as a cause of new cardiac arrhythmias in the intensive care population9,10). It is known that in the early stages of sepsis, there is a high prevalence of hypophosphatemia in critically ill patients11). Zazzo and colleagues12) demonstrated a strong correlation between hypophosphatemia and mortality in surgical intensive care patients. We previously demonstrated13) a high incidence of new cardiac arrhythmias in critically ill septic patients with low serum phosphate levels. We suspect that phosphorus replacement therapy in the early stages of sepsis may improve patient outcomes. In this study we investigated whether early hypophosphatemia correction resulted in a reduced incidence of arrhythmias in critically ill septic patients.
Patients and Methods
The Human Research and Ethics Committee at Soroka Medical Center in Beer-Sheva, Israel approved this study.
1. Sample
Clinical data were prospectively collected from all septic patients who presented with hypophosphatemia (normal range: 0.75-1.44mmol/L) and were admitted to the General Intensive Care Unit (GICU) of Soroka Medical Center between November 2009 and December 2010. The diagnostic criteria of Sepsis/SIRS utilized were defined by the International consensus of Survival Sepsis Campaign14). We also incorporated data that had been previously collected and published13) with septic patients who presented with hypophosphatemia and were admitted to the General Intensive Care Unit (GICU) of Soroka Medical Center between January 2002 and December 2002.
2. Inclusion criteria
All adult (age>18 years) septic patients who presented with hypophosphatemia, and were admitted to our GICU between November 2009 and December 2010, were included in the study.
3. Exclusion criteria
Patients with previously known coronary artery disease (history of myocardial infarction or ischemic congestive heart failure), previously documented cardiac arrhythmias, and patients treated with an inotropic agent (i.e. epinephrine, dopamine >5mcg/kg/min) were excluded from the study. Also, patients with other electrolyte disturbances (serum potassium level <3mmol/L or >5mmol/L, serum magnesium level <0.2mmol/L or >0.5mmol/L), calcium serum level <1.7mmol/L or >2.75mmol/L), severe hypoxemia (PaO2 <60mmHg) or severe acidosis (arterial blood pH <7.2) were excluded. Lastly, patients who developed acute renal failure during their ICU admission were excluded.
4. Variables and measures
Demographic data, reason for hospital admission, occurrence of new-onset arrhythmia, type of arrhythmia and antiarrhythmic therapy, length and dose of phosphorus replacement therapy, APACHE and SOFA score, and in-hospital mortality were collected and analyzed from patients' records. Laboratory data including serum blood phosphorus, potassium, sodium, calcium and magnesium concentrations, PaO2\FiO2 ratio, and blood and sputum, urine, skin and CSF cultures were drawn and analyzed during the first 6 hours after admission and at least twice daily.
5. Patients
All patients were allocated to one of two study groups according to the treatment of hypophosphatemia after admission to the GICU: Group 1 included 34 ICU patients treated with intravenous (IV) Phosphorus Replacement ICU Protocol and Group 2 included 16 ICU patients in whom hypophosphatemia was not treated (data extracted from a previously published study [13]). Group 2 patients in this study served as a control group. After the high incidence of new arrhythmias in septic patients with hypophosphatemia was demonstrated13), our ICU began routinely administering IV phosphorous as per the Phosphorus Replacement ICU Protocol. All patients were monitored for the presence of arrhythmias by the Apex Pro telemetry transmitters system. All telemetry clinical data were stored and recorded continuously by Clinical Information Center (CIC) GE Health Care. An intensive care staff physician examined all patients' ECG data every 24 hours for the detection of a new arrhythmia episode. The length and type of arrhythmias were assessed by two intensive care staff physicians blinded to the study groups.
6. The ICU intravenous Phosphorus Replacement Protocol
All septic patients with mild (0.67-0.77mmol/l), moderate (0.32-0.64mmol/l) or severe (<0.32mmol/l) hypophosphatemia were treated with IV sodium phosphate supplementation by Phocytan R solution (Sandoz (Canada)). The Phocytan R solution contains 0.3mmol/ml of sodium phosphate. For patients with mild or moderate hypophosphatemia, sodium phosphate was administered at a dose of 9-12 mmol (0.12mmol/kg) diluted in 150ml of normal saline (0.9%) over 6 hours. Severe hypophosphatemia was treated with 15-18mmol of IV sodium phosphate (0.25mmol/kg) at the same infusion rate. Repeat serum phosphorus levels were routinely measured 12 hours after administration of IV phosphorus. Doses of phosphorus were calculated according to ideal body weight.
7. Statistical analysis
Statistical comparisons of parametric data between the two study groups were done using the unpaired Student t-test. Non-parametric data were analyzed by the Mann-Whitney U test. P-values less than 0.05 were considered statistically significant.
Results
640 patients were admitted to our GICU during the study period. Of these, 95 patients were diagnosed with sepsis and 61 were excluded from the study based on the exclusion criteria defined above. Thirty-four septic patients who presented with hypophosphatemia were recruited in the study (Group 1) and treated according to the ICU IV Phosphorus Replacement Protocol. Previously published data13) with 44 septic patients admitted to our GICU in 2002 served as a control group. After applying exclusion criteria, twenty-eight patients were excluded from the study and sixteen patients were included in the non-treated hypophosphatemia group (Group 2). There were no differences in age, gender distribution, or mechanical ventilation support between the two study groups (Table 1). The etiologies of sepsis in patients were similarly distributed between the two groups (Table 1). There were no significant differences with regards to the microbial pathogens between both study groups (Table 1). The incidence of new arrhythmias were significantly higher in patients in the non-treated group compared with the groups treated with IV phosphorus replacement (38% vs 63%, p=0.04, Table 1).
Table 1.
Demographic data, etiology of sepsis and mechanical ventilation support
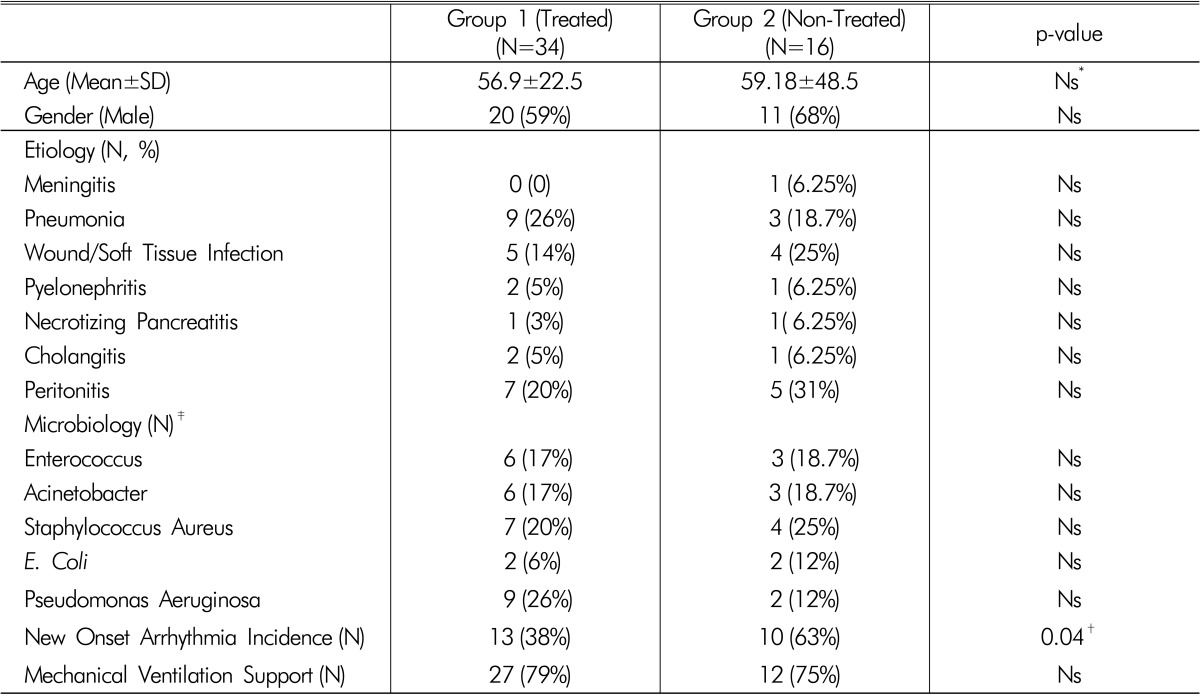
(Mean±SD, %).
*Ns p-value >0.05 (Considered No Significant)
†p-value <0.05 Was Defined As Statistically Significant
‡Presented From Microbiological Data Of Positive Sputum, Urine, Csf And Blood Cultures
Serum electrolyte levels, arterial blood pH, and oxygenation were similar between patients who developed new-onset arrhythmias and non-arrhythmic patients in both study groups (Table 2).
Table 2.
Laboratory data parameters
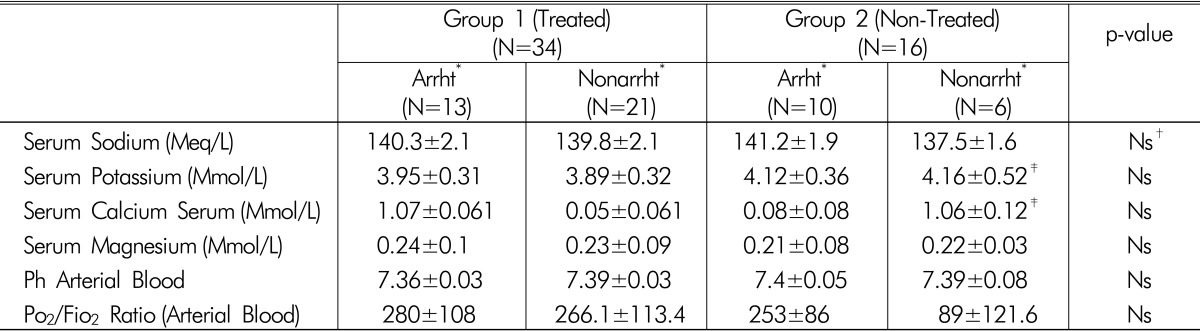
(Mean±SD)
*"Arrht" And "Nonarrht" - All Septic Patients With Hypophosphatemia Weresubdivided According To The Presence Of New-Onset Episode Of Arrhythmia Into Two Subgroups: Arrhythmic- "Arrht" And Non-Arrhythmic "Nonarrht".
†Ns-p value >0.05 (Considered No Significant)
‡Previously Published Clinical Data (From Schwartz Et Al.13)).
For patients in group 1, the total IV phosphorus replacement dose was higher in patients who did not develop any cardiac arrhythmias compared with septic patients with a new-onset arrhythmia (46.65±30.7 vs. 25.5±12.3, p=0.02, Table 3). However, there was no difference in the length of treatment between both subpopulations (p>0.05, Table 3).
Table 3.
Clinical data, serum phosphorus levels, and phosphorus replacement parameters of patients with and without new-onset cardiac arrhythmias
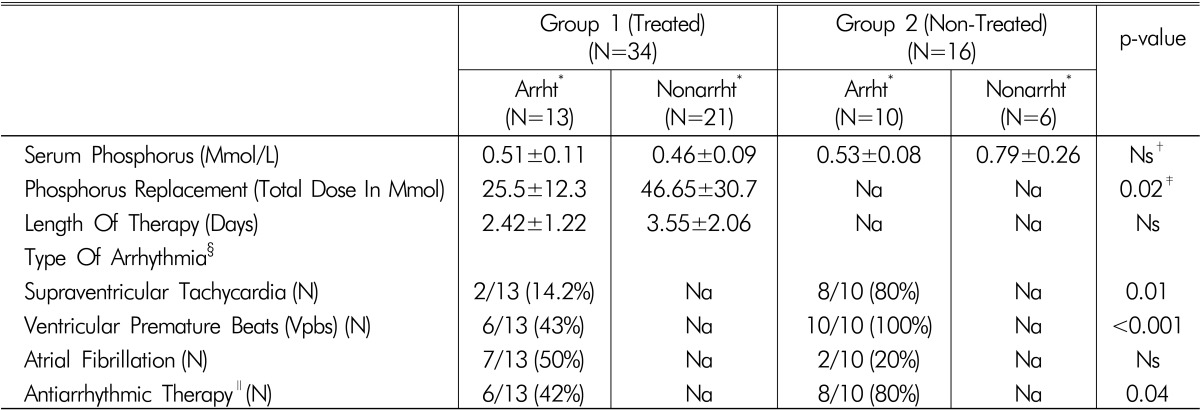
(Mean±SD)
*"Arrht" And "Nonarrht" - All Septic Patients With Hypophosphatemia Weresubdivided According To The Presence Of New-Onset Episode Of Arrhythmia Into Two Subgroups: Arrhythmic- "Arrht" And Non-Arrhythmic- "Nonarrht".
†Ns-p value >0.05 (Considered No Significant)
‡p value <0.05 Was Defined As Statistically Significant
§Most Patients Suffered From Different Types Of Arrhythmias
∥Antiarrhythmic Therapy Included Amiodarone (A Fib And Svt), Verapamil (Svt And Vpbs), Lidocaine (Svt And Vpbs) And Adenosine (Svt)
New-onset cardiac arrhythmias included supraventricular tachycardia (SVT), ventricular premature beats (VPBs) and atrial fibrillation (a-fib). It should be noted that there was a higher prevalence of a-fib in group 1 (Table 3) and a higher prevalence of SVT and VPBs in group 2. There was significantly less use of antiarrhythmic agents in patients in group 1 (Table 3).
APACHE and SOFA score values were similar in both study groups and unrelated to new cardiac arrhythmia events (Table 4). The survival rate in patients with new arrhythmias and patients without new arrhythmias were similar (Table 4).
Table 4.
Patients' clinical outcome values (APACHE score, SOFA score and mortality).
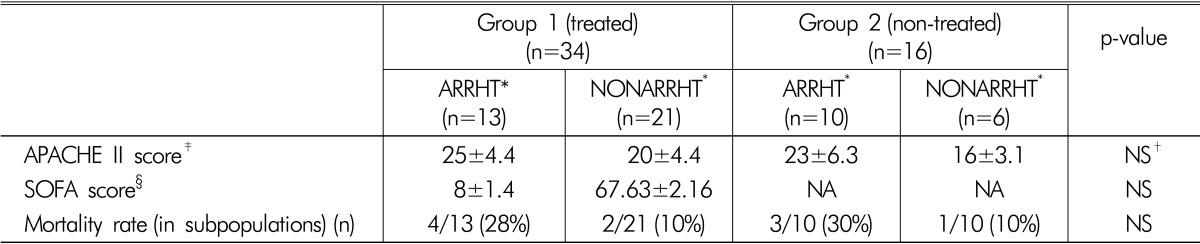
(Mean±SD, %)
*"ARRHT" and "NONARRHT" - All septic patients with hypophosphatemia weresubdivided according to the presence of new-onset episode of arrhythmia into two subgroups: arrhythmic- "ARRHT" and non-arrhythmic- "NONARRHT".
†NS-p value >0.05 (not significant)
‡APACHE (APACHE II- "Acute Physiology and Chronic Health Evaluation II")score is a severity-of-disease classification system and was estimated on the day of admission to the GICU21).
§SOFA (The Sequential Organ Failure Assessment score, or just SOFA score) is used to track a patient's status during the stay in an intensive care unit score and was estimated on the first day of hypophosphatemia22).
Discussion
Hypophosphatemia in the early stage of sepsis is common and is thought to be due to an acute and rapid intracellular shift of phosphorus15). Low phosphorus serum blood levels may worsen hemodynamic instability in severe sepsis by resulting in a decrease in myocardial contractility16), and are potentially associated with the development of new cardiac arrhythmias13). In this study we demonstrated that phosphorus replacement therapy in patients with hypophosphatemia (group 1) in the early stage of sepsis significantly reduced the incidence of arrhythmias (38% vs 63%, p=0.04). The prevalence of SVT and VPBs arrhythmias was higher in the non-treated group (Table 3). We demonstrated that routine phosphorus administration correlated with a lower percent of antiarrhythmic therapy in treated patients (Table 3). Our phosphorus replacement protocol management correlated well with previous data17). Moreover, for patients in group 1, the total dose of IV phosphorus was significantly higher in non-arrhythmic persons (46.6 vs. 25.5 mmol) than in patients who developed new arrhythmia (Table 3), despite a similar length of treatment (2.42 vs. 3.55 mean days) and serum blood phosphorus level (0.46 vs 0.51mmol/L) (Table 3). This may reflect a coincidental findings or some resistance to the treatment protocol. Importantly, regarding to phosphorus replacement therapy, Group 1 patients received less antiarrhythmic therapy compared to non-treated patients in Group 2 (42% vs 80%, p=0.04). Other electrolyte disturbances (hypokalemia, hypomagnesaemia, hypocalcaemia etc.) may be independent risk factors for the development of new cardiac arrhythmias18,19,20). In this study there were no significant differences in serum electrolyte levels, acid-base balance changes, or the incidence of hypoxemic events between patients in both study groups (see Table 2). Despite the statistically significant reduction in the incidence of new arrhythmias between both study groups, the overall mortality rate and length of hypophosphatemia treatment were similar (Table 3, 4). The lack of a difference observed in mortality and length of treatment may reflect the small sample size of the study.
A major limitation in our study was that we compared patients our treated group with previously published data13). Since it has been shown that hypophosphatemia in septic patients was associated with higher incidence of arrhythmias, phosphorus replacement protocol has became a standard of treatment hypophosphatemic septic patients in our department. Considering this fact, we could not recruit non-treated septic patients with hypophosphatemia for ethical considerations. The small sample size is another limitation of our study, which significantly restricts our conclusions regarding patients' clinical outcome.
In this study, phosphorus replacement therapy was shown to decrease the incidence of new-onset cardiac arrhythmias in septic patients. We demonstrated a trend of decreased antiarrhythmic therapy after aggressive hypophosphatemia correction. We believe that a future large, randomized control study should be done to better analyze potential mortality benefits and reduction in the incidence of new onset cardiac arrhythmias of critically ill septic patients after IV phosphorus replacement therapy and suggest correcting hypophosphatemia in septic patients.
References
- 1.Bender JS. Supraventricular tachycarrhythmias in the surgical intensive care unit: an under recognized event. Am Surg. 1996;62:73–75. [PubMed] [Google Scholar]
- 2.Goodman S, Weiss Y, Weissman C. A update on cardiac arrhythmias in the ICU. Curr Opin Crit Care. 2008;14:549–554. doi: 10.1097/MCC.0b013e32830a4c5d. [DOI] [PubMed] [Google Scholar]
- 3.Segun P, Signouret T, Laviolle B, Branger B, Mallédant Y. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. Crit Care Med. 2004;32:722–726. doi: 10.1097/01.ccm.0000114579.56430.e0. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick JR, Heilbrunn A, Sankaran S. Cardiac arrhythmias: an early sign of sepsis. Am Surg. 1973;39:380–382. [PubMed] [Google Scholar]
- 5.Christian SA, Schorr C, Ferchau L, Jarbrink ME, Parrillo JE, Gerber DRJ. Clinical characteristics and outcomes of septic patients with new-onset atrial fibrillation. J Crit Care. 2008;23:532–536. doi: 10.1016/j.jcrc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Meierhenrich R, Steinhilber E, Eggermann C, Weiss M, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. 2010;14:1–8. doi: 10.1186/cc9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salman S, Bajwa A, Gajic O, Afessa B. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med. 2008;23:178–183. doi: 10.1177/0885066608315838. [DOI] [PubMed] [Google Scholar]
- 8.Annane D, Sébille V, Duboc D, et al. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am J Respir Crit Care Med. 2008;178:20–25. doi: 10.1164/rccm.200701-031OC. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli SM, Goldfarb S. Hypophosphatemia; clinical consequences and management. J Am Soc Nephrol. 2007;18:1999–2003. doi: 10.1681/ASN.2007020143. [DOI] [PubMed] [Google Scholar]
- 10.Barak Y, Schwartz A, Kalichman I, Nisman B, Gurman G, Shoenfeld Y. Prevalence of hypophosphatemia in sepsis and infection; The role of cytokines. Am J Med. 1998;104:40–47. doi: 10.1016/s0002-9343(97)00275-1. [DOI] [PubMed] [Google Scholar]
- 11.Shoenfeld Y, Hager S, Berliner S, Gallant LA, Pinkas J. Hypophosphatemia as diagnostic aid in sepsis. N Y State J Med. 1982;82:163–165. [PubMed] [Google Scholar]
- 12.Zazzo JF, Troche G, Ruel P, Maintenant J. High incidence of hypophophatemia in surgical intensive care patients. Efficacity of phosphorus treatment on myocardial function. Intensive Care Med. 1995;21:826–831. doi: 10.1007/BF01700966. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz A, Gurman G, Cohen G, Gilutz H, Shenfeld Y. Association between hypophosphatemia and cardiac arrhythmias in the early stage of sepsis. Eur J Intern Med. 2002;13:434–438. doi: 10.1016/s0953-6205(02)00130-9. [DOI] [PubMed] [Google Scholar]
- 14.Dellinger RP, Levy HM, Carlet JM, Bion J, et al. International guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36:1394–1396. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 15.Bollaert PE, Levy B, Nace L, Laterre PF, Larcan A. Haemodynamic and metabolic effects of rapid correction of hypophosphatemia in patients with septic shock. Chest. 1995;107:1698–1701. doi: 10.1378/chest.107.6.1698. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor LR, Wheeler WS, Bethune JE. Effect of hypophosphatemia on myocardial performance in man. N Engl J Med. 1977;297:901–903. doi: 10.1056/NEJM197710272971702. [DOI] [PubMed] [Google Scholar]
- 17.Geerse D, Bindela A, Kuiper AM. Treatment of hypophosphatemia in the intensive care unit. Review. Chest. 2010;4:560–568. doi: 10.1186/cc9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18:233–245. [PubMed] [Google Scholar]
- 19.Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27:153–160. doi: 10.1016/j.jemermed.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Shechter M. Magnesium and cardiovascular system. Magnes Res. 2010;23:60–72. doi: 10.1684/mrh.2010.0202. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 22.Vincent JL, de Mendonça A, Cantraine F, et al. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]


