Abstract
Human-derived lactobacilli were isolated from fecal samples of healthy volunteers. Forty-six isolates from different volunteers were selected and investigated for their immunomodulatory properties. Conditioned medium from each isolate was assessed for its effect on tumor necrosis factor (TNF) production in lipopolysaccharide (LPS) - activated THP-1 monocytes. Of 46 Lactobacillus isolates, 12 significantly inhibited TNF production in varying magnitude. Lactobacillus strain TH58 displayed the most potent TNF - inhibitory activity (70% inhibition). In contrast, Lactobacillus strain TH14 exhibited immunostimulatory property by activating TNF production in THP-1 monocytes. Lactobacillus TH14 induced NF-κB activation in the absence of LPS stimulation, whereas Lactobacillus TH58 had no effect on NF-κB signaling, irrespective of LPS stimulation. Strain TH58 was identified as Lactobacillus saerimneri and strain TH14 as Lactobacillus ruminis by sequence analysis of the16S rRNA gene. This is the first report of a human isolate of L. saerimneri with TNF inhibitory activity and L. ruminis, an indigenous species to humans, with TNF stimulatory activity. Our data suggest the potential use of these two strains as immunoprobiotic candidates.
Keywords: Lactobacillus saerimneri, Lactobacillus ruminis, immunoprobiotics, anti-inflammatory, immunostimulatory, tumor necrosis factor
Introduction
Lactobacillus spp. represent sources of beneficial organisms indigenous in the human intestinal tracts and strains of this genus are used as probiotics. Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (FAO/WHO, 2001). Probiotics are attractive candidates for novel biological therapies because beneficial bacteria may be derived from commensal microorganisms and are generally recognized as safe (GRAS). Several lactobacilli of human origin are currently exploited commercially for various therapeutic applications.
Oral administration of probiotics may modulate cytokine profiles not only locally in the intestines, but also systemically (Solis-Pereyra et al., 1997; Meyer et al., 2007). Results pointing towards stimulation of both Th1 and Th2 responses have been reported in animals fed with probiotics (Tejada-Simon et al., 1999; Maassen et al., 2000; Cross, 2002; Perdigón et al., 2002). Ability to modulate cytokine network and innate immunity by probiotic bacteria is strain dependent. Certain probiotics stimulated the production of inflammatory cytokines such as tumor necrosis factor (TNF) (Miettinen et al., 1996; Solis-Pereyra et al., 1997; Morita et al., 2002; Cross et al., 2004) while others effectively inhibit TNF production (Pena & Versalovic, 2003; Pena et al., 2005; Chan Remillard & Ozimek, 2006; Lin et al., 2008).
The objective of this study was to isolate and characterize human-derived Lactobacillus strains for potential use as immunoprobiotics. For this purpose, Lactobacillus spp. were isolated from fecal samples of healthy human volunteers and investigated for their immunomodulatory properties.
Materials and methods
Subjects and sample collection
Fecal samples were collected from forty-six healthy human volunteers. The volunteers were students at the Thai Red Cross College of Nursing, Chulalongkorn University, which included both males and females in the age group of 17–19 years. No subject had consumed antibiotics or yogurt for at least two months prior to sample collection. Each individual gave informed consent. Fresh fecal samples were obtained by rectal swabbing with sterile cotton swabs, subsequently inserted into a modified Cary-Blair transport medium (Atlas & Snyder, 1995). The samples were processed immediately upon receipt.
Isolation of Lactobacillus
To isolate Lactobacillus, fecal swabs were suspended in normal saline solution (NSS), serially diluted and applied to deMan-Rogosa-Sharpe (MRS) agar (Becton Dickinson, Sparks, MD) and incubated anaerobically (10% CO2,10% H2 and 80% N2) at 37°C for 48–72 h in an anaerobic chamber (Concept Plus, Ruskinn Technology, UK). Each isolate was tested for catalase activity using 3% hydrogen peroxide solution and catalase-negative isolates were gram stained (Cappuccino & Sherman, 2005). All catalase-negative, gram-positive rods were tested for vancomycin (VA) resistance using a modified Kirby-Bauer disk diffusion test (Pena et al., 2004). Briefly, isolates were resuspended in NSS to a 0.5 McFarland standard and swabbed onto MRS agar. VA impregnated disks (5 μg; Becton Dickinson) were applied to the bacterial cultures and incubated anaerobically at 37°C for 24–48 h. Isolates displaying an inhibition zone of >15 mm were considered susceptible. All isolates that were gram-positive, regular rods or short rods, catalase-negative and vancomycin resistant were maintained as frozen cultures in MRS broth with 20% glycerol at −80°C for experimental use.
Preparation of Lactobacillus conditioned media
Lactobacillus conditioned media were prepared as previously described (Lin et al., 2008). Human-derived Lactobacillus isolates were cultured anaerobically in MRS medium at 37°C for 24 h. Overnight cultures were diluted to an OD600 of 0.1 in MRS media and incubated in anaerobic condition for an additional 24 h. Condition media were collected by centrifugation at 4000 x g for 10 min at 4°C, filter-sterilized using a 0.22 μm filter (Millipore, Bedford, MA) and concentrated by speed-vacuum drying (Speed vacuum DNA 110, Savant, NY). The residual pellet was resuspended in an equal volume of serum-free RPMI medium 1640 (Gibco-Invitrogen, Carlsbad, CA) and stored at −20°C.
THP-1 monocyte cell culture and bioassay for TNF activity
Cell culture and in vitro bioassays were performed as previously described (Lin et al., 2008) with modification. Briefly, THP-1 human monocytic cell lines (ATCC TIB- 202, Manassas, VA) were maintained in RPMI medium 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco-Invitrogen) in 96-well flat-bottomed tissue culture plates (Falcon; Becton Dickinson) and incubated at 37°C in a humidified 5% CO2 incubator. THP-1 cells (2.5×105 cells mL−1) were incubated with conditioned media (5% v/v) alone or in combination with purified LPS (100 ng mL−1) from Escherichia coli serotype O127:B8 (Sigma, St. Louis, MO) for 3.5 h at 37°C. The assay condition was previously optimized, i.e. 5% (v/v) of conditioned medium was used to minimize the confounding effects of MRS medium. Supernatants were collected from individual wells by centrifugation at 4°C and assayed for TNF.
Assay for cell viability and apoptosis
Cell viability was determined by Trypan Blue dye exclusion assay (Gibco-Invitrogen). Cell suspension was mixed with trypan blue which was excluded by viable cells but stained dead cells. The cells were counted on a hemocytometer under a phase contrast microscope. Percentage of viable cells was determined from the ratio of viable cells over total cells. In addition to trypan blue staining, TH58 conditioned medium treated cells and controls were stained with Annexin V-FITC and Propidium Iodide (PI) by using BD Pharmingen Annexin V-FITC Apoptosis detection kit I (BD Biosciences Pharmingen, San Diego, CA). Controls included THP-1 cells in RPMI and MRS treated cells. This staining method allowed the detection of viable, apoptotic and necrotic cells. Annexin V binds to cells that expose phosphatidylserine to the external cellular environment, a characteristic of early apoptotic cells. PI stains cells with loss of membrane integrity which accompanies the latest stage of cell death resulting from either apoptotic or necrotic processes. Viable cells were AnnexinV-FITC and PI negative. Early apoptotic cells were AnnexinV-FITC positive and PI negative. Cells that were in late apoptosis or already dead were both AnnexinV-FITC and PI positive. The percentage of viable cells was analyzed on FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) using the Cell Quest Pro software.
Assay for cell proliferation
An increase in the number of THP-1 cells was determined by microscopic count of cells after staining with trypan blue dye. The cells were counted in four 1-mm square grids on a hemocytometer. This assay was performed in TH14 conditioned medium treated cells and controls which included THP-1 cells in RPMI and MRS treated cells. The number of viable cells and total cells in each sample were calculated. The statistical differences were evaluated using the Student’s t test with a one-tailed distribution. A p value of 0.05 was considered statistically significant.
TNF measurement
TNF production in THP-1 cell culture supernatants was measured with human TNF-specific sandwich quantitative enzyme-linked immunosorbent assay (DuoSet, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Recombinant human TNF was used as standard. Absorbance was measured at 450 nm using a Spectramax 340PC (Molecular Devices Corporationvale, CA). Cytokine concentrations were quantified from standard curve and expressed as pg mL−1 of culture medium. Results were reported as mean value with standard deviations (SD) of three different experiments in triplicate. The statistical differences were evaluated using the Student’s t test with a one-tailed distribution. A p value of 0.05 was considered statistically significant.
Nuclear extraction from THP-1 monocytes and NF-κB measurement
THP-1 cells (2.5×105 cells mL−1) were incubated with conditioned media (5% v/v) in the presence or absence of purified LPS (100 ng mL−1) for 30 min. The THP-1 cells were collected and stored on ice before nuclear extraction. Nuclear extracts were isolated using the Nuclear Extract kit (Active Motif, Carlsbad CA, USA) according to the manufacturer’s instructions. Briefly, THP-1 cells were washed in ice-cold PBS, resuspended in 200 μL of hypotonic buffer and incubated on ice for 15 min. Detergent (25 μL) was added and each sample was vortexed. The nuclear pellets were collected by centrifugation and resuspended in ice-cold lysis buffer (20 μL) containing protease inhibitor. The samples were vortexed 8 times, each for 30 s, at highest setting and subjected to centrifugation at 14,000 x g at 4°C for 10 min. The nuclear extracts were stored at −80°C. The protein content of nuclear extracts were quantified using the microplate procedure of BCA™ Protein Assay kit (Pierce Biotechnology, Rockford, IL) as per the manufacturer’s instruction. The levels of nuclear factor NF-κB in THP-1 nuclear extracts were measured by ELISA with TransAM™ NF-κB p65 Transcription Factor Assay kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instruction.
16S rRNA gene sequencing
The species of selected bacterial isolates was determined using 16S rRNA gene sequencing as done previously (Pena et al., 2004). Genomic DNA was extracted from overnight cultures using the Wizard® Genomic DNA Purification kit (Promega Corporation Madison, WI, USA) following the manufacturer’s instructions. The 16S rRNA gene was amplified by PCR with primers 16S-8F (5′-AGA GTT TGA TCY TGG YTY AG-3′) and 16S-1541R (5′-AAG GAG GTG WTC CAR CC-3′) (Pena et al., 2004) in a reaction containing 200 ng DNA, 50 pmol of each primer, 1.25 U AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA), 2.5 mM dNTPs, 1X Amplitaq Reaction Buffer and 75 mM MgCl2. The following thermal cycling conditions were used: an initial denaturation of 94°C for 2 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 57°C for 30 s, extension at 72°C for 1 min; and a final extension at 72°C for 5 min. PCR products were purified by Qiagen MinElute PCR Purification kit (Qiagen Inc., Valencia, CA) and sequenced by SeqWright DNA Technology Services (Houston, TX) using primers 16S 8F and 16S 1541R. Approximately 1,400 nucleotides were analyzed by using the sequence match program at the Ribosomal Database Project –II (http://rdp.cme.msu.edu/).
Results
Isolation of Lactobacillus isolates
Bacterial colonies isolated from 46 healthy human volunteers were selected for genus Lactobacillus by presumptive tests including gram stain, catalase production and vancomycin susceptibility. Four hundred and thirty-seven isolates were gram-positive, catalase-negative and vancomycin resistant, making them candidates for Lactobacillus spp. Cell morphology in each isolate varied from long and slender rods, straight rods to bent rods, sometimes shot rods to coccobacilli; arranged in single, in pairs or short chains. Some isolates exhibited bipolar staining or internal granulations. The most frequently found colonies were small to medium sizes (1–2 mm) with white, circular, smooth and convex colonial morphologies. Most isolates grew well under anaerobic condition. Most Lactobacillus isolates were obligate anaerobes while some isolates were facultative anaerobes. One isolate from each volunteer was randomly selected and characterized further for immunomodulatory affects on THP-1 human monocytes.
Immunomodulatory effects of Lactobacillus isolates
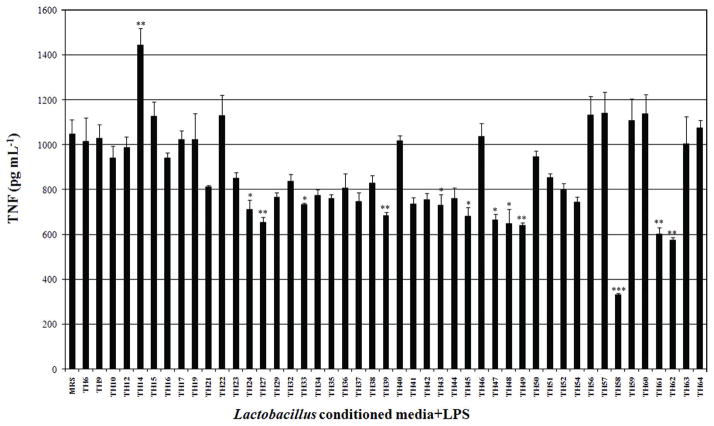
Forty-six Lactobacillus isolates were tested for their ability to modulate TNF production in LPS-activated THP-1 monocytic cells. Conditioned media from 12 Lactobacillus isolates significantly inhibited TNF production in LPS-activated THP-1 human monocytes with different magnitude. Lactobacillus TH58 showed the strongest TNF inhibitory activity of 70% (p <0.001) in LPS-activated THP-1 monocytes (Fig. 1). In contrast, conditioned medium from Lactobacillus TH14 stimulated TNF production (p <0.01) (Fig. 1). Ability of Lactobacillus TH 58 and TH14 to modulate TNF production in the presence or absence of LPS was investigated. The results confirmed the TNF inhibitory effect of Lactobacillus TH58 and TNF stimulatory effect of Lactobacillus TH14 conditioned medium (Fig. 2). Results also indicated that in the absence of LPS activation, Lactobacillus TH14 conditioned medium stimulated TNF production (p<0.001) in THP-1 human monocytes, while Lactobacillus TH58 conditioned medium did not (Fig. 2).
Fig. 1.
Effects of Lactobacillus conditioned media on TNF production in LPS-activated THP-1 cells. THP-1 cells were coincubated with Lactobacillus conditioned media and E. coli - derived lipopolysaccharide. TNF secretions were determined by human TNF ELISA. The results were from three independent experiments in triplicate. MRS: deMan-Rogosa-Sharpe, media control; Asterisks denote significantly different from media control; ***, p<0.001; **, p<0.01; *, p<0.05; Error bars indicate standard deviations.
Fig. 2.
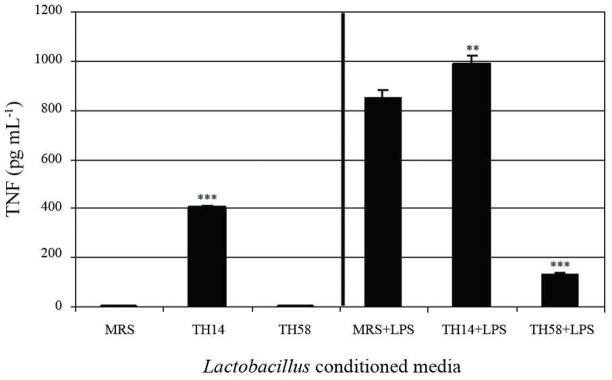
Immunomodulatory effects of Lactobacillus TH14 and TH58 conditioned media on TNF production in THP-1 cells. Bioassays for TNF secretion were performed without or with LPS activation. The results represent three independent experiments. MRS: deMan-Rogosa-Sharpe, media control; Asterisks denote significantly different from media control; ***, p<0.001; **, p<0.01; Error bars indicate standard deviations.
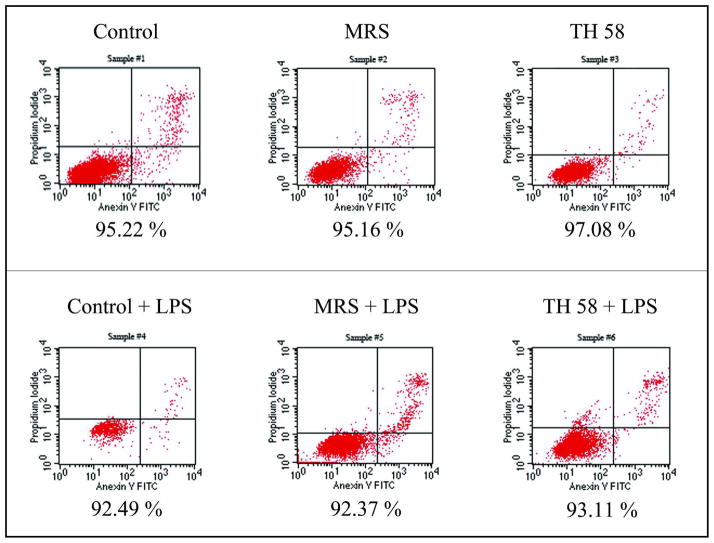
The inhibition of TNF production by TH58 conditioned medium did not have cytotoxic effects on the THP-1 cells as determined by Trypan Blue dye exclusion assay and Annexin V- Propidium Iodide staining and analysis by flow cytometer (Fig. 3). The percentage of viable cells in each sample was more than 90% when determined by both methods. The effect of TH14 conditioned medium on proliferation of THP-1 cells was examined and it was found that the stimulation of TNF production by TH14 conditioned medium did not result from the proliferation of THP-1 cells. The total number of TH14 conditioned medium treated cells was not significantly different from untreated controls and the percentage of viable cells in each sample was found to be more than 90%. As shown in Fig. 4, the number of viable cells in TH14 conditioned medium treated samples was not significantly different from the untreated controls.
Fig. 3.
The viability of THP-1 cells determined by staining with Annexin V-FITC and Propidium Iodide (PI) followed by flow cytometric analysis. Viable cells are negative for both Annexin V-FITC and PI. Cells that are in early apoptosis are Annexin V-FITC positive and PI negative. Cells that are in late apoptosis or already dead are both Annexin V-FITC and PI positive. The percentages of cell viability under each plot are representative of three experiments. Control: RPMI cell culture medium; MRS: deMan-Rogosa-Sharpe, bacterial media control; TH58: Lactobacillus TH58 conditioned medium.
Fig. 4.
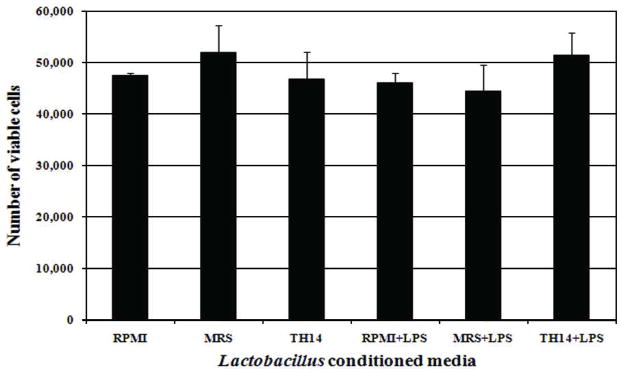
Effect of Lactobacillus TH 14 conditioned medium on the proliferation of THP-1 cells. THP-1 cells were incubated with TH14 conditioned medium in the absence or presence of LPS. Cell suspensions were stained with trypan blue, counted on a hemocytometer and the number of viable cells in each sample was calculated. RPMI: cell culture medium; MRS: deMan-Rogosa-Sharpe, bacterial media control; TH14: Lactobacillus TH14 conditioned medium. The number of viable cells is shown as mean value with standard deviation from three different experiments.
Pro-inflammatory mediators such as LPS induce TNF production via activation of the NF-κB signaling pathway (Ulvitch & Tobia, 1999; Aderem & Ulevitch, 2000). We speculated that Lactobacillus TH14 and TH58 mediated their pro- and anti-inflammatory effects by modulating the NF-κB signaling pathway. As expected, Lactobacillus TH14 conditioned medium stimulated phosphorylation of NF-κB in THP-1 cells in the absence of LPS stimulation (p<0.001) whereas Lactobacillus TH58 conditioned medium did not. However, Lactobacillus TH14 and TH58 conditioned media did not affect the phosphorylation of NF-κB in LPS-activated THP-1 cells (p >0.05) (Fig. 5).
Fig. 5.
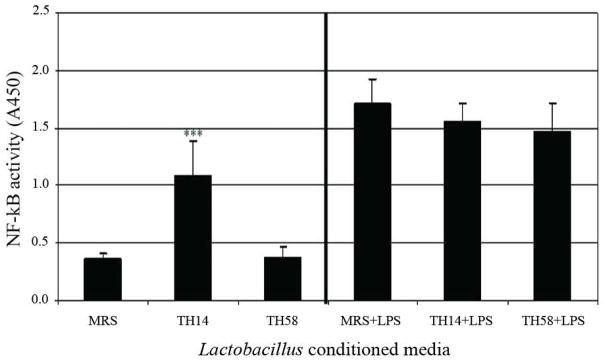
Effect on NF-κB activation by Lactobacillus TH14 and TH58 in THP-1 cells. THP-1 cells were incubated with TH14 or TH58 conditioned media in the absence or presence of LPS. Nuclear extracts were prepared and assayed for NF-κB levels. The results were from two independent experiments in triplicate. MRS: deMan-Rogosa-Sharpe, media control; Asterisk denotes significantly different from media control; ***, p<0.001; Error bars indicate standard deviations.
Genotypic identification of Lactobacillus isolates with immunomodulatory properties
Sequence analysis of the amplified 16S rRNA gene products allowed the identification of Lactobacillus isolates (Table 1). Seven out of 12 TNF-inhibitory isolates were identified as Lactobacullus plantarum or Lactobacillus pentosus, three as Lactobacillus plantarum, one as Lactobacillus salivarius and TH58 as Lactobacillus saerimneri. Lactobacillus TH14 was identified as Lactobacillus ruminis.
Table 1.
Identification of Lactobacillus isolates with immunomodulatory properties by 16 S rRNA gene sequencing
| Isolate | Match organism | Identity (%) |
|---|---|---|
| TH14 | Lactobacillus ruminis | 99.5 |
| TH24 | Lactobacillus plantarum or Lactobacillus pentosus | 100 |
| TH27 | Lactobacillus plantarum or Lactobacillus pentosus | 100 |
| TH33 | Lactobacillus salivarius | 100 |
| TH39 | Lactobacillus plantarum or Lactobacillus pentosus | 100 |
| TH43 | Lactobacillus plantarum or Lactobacillus pentosus | 100 |
| TH45 | Lactobacillus plantarum or Lactobacillus pentosus | 100 |
| TH47 | Lactobacillus plantarum | 100 |
| TH48 | Lactobacillus plantarum | 100 |
| TH49 | Lactobacillus plantarum | 100 |
| TH58 | Lactobacillus saerimneri | 98 |
| TH61 | Lactobacillus plantarum or Lactobacillus pentosus | 100 |
| TH62 | Lactobacillus plantarum or Lactobacillus pentosus | 99.5 |
Discussion
Lactobacillus isolates derived from humans are optimal candidates for exhibiting beneficial effects in human beings (FAO/WHO, 2001). Since probiotic strains have been reported to possess anti-inflammatory and immunostimulatory effects, an effort was made in this study to isolate human- derived Lactobacillus strains with these potential probiotic properties. Several Lactobacillus species produce immunoregulatory factors, known as immunomodulins, which may modulate cytokine production (Guarner et al., 2006). Among the inflammatory cytokines, TNF is important in mediating inflammation and immune function. In the current study, human-derived Lactobacillus isolates modulated TNF production via a contact- independent manner thereby suggesting the possibility that these isolates may secrete immunomodulins. Most of the immunomodulatory Lactobacillus isolated in this study inhibited TNF production in varying magnitude and Lactobacillus TH58 had the strongest TNF-inhibitory activity.
Similar results have been demonstrated in previous investigations. Report indicated that certain lactic acid bacteria secreted anti-inflammatory metabolites that inhibited TNF production and suppressed NF-κB activation in LPS-activated THP-1 cells (Menard et al., 2004). Conditioned media from Lactobacillus rhamnosus GG (Pena & Versalovic, 2003) and other murine lactobacilli significantly inhibited TNF production in LPS-activated murine macrophages (Pena et al., 2004). The supernatants of nine strains of probiotic Lactobacillus were able to inhibit TNF secretion in murine macrophages (Chan Remillard & Ozimek, 2006). Lactobacillus reuteri strain ATCC PTA 6475 was able to inhibit TNF production by LPS-activated THP-1 cells and human monocyte-derived macrophages from pediatric patients with Crohn’s disease by a contact-independent mechanism (Lin et al., 2008). In addition, the anti-inflammatory property of Lactobacillus reuteri ATCC PTA 6475 was shown to be strain-specific as the conditioned media of Lactobacillus reuteri strains CF48-3A and ATCC 55730 did not inhibit TNF production in those monocytic cells. Our data in a separate study of Panpetch, 2008 (Thesis, Graduate school, Chulalongkorn university) also supported the strain-specific nature of Lactobacillus sp. We found that condition media of some isolates of Lactobacillus gasseri and Lactobacillus salivarius cultivated from gastric biopsies of peptic ulcer patients could inhibit TNF production in LPS-activated THP-1 cells. On the contrary, some isolates of these species could not inhibit TNF production. These data supported the possibility of the secretion of immunomodulin(s) by Lactobacillus TH58 since it is unlikely that specific strains of Lactobacillus reuteri, some isolates of Lactobacillus gasseri and Lactobacillus salivarius metabolize MRS media to yield products that can inhibit TNF production, whereas specific strain or isolates of the same species do not metabolize MRS by the same pathway.
In this study, we also found that the anti-inflammatory activity (TNF inhibition) of Lactobacillus TH58 was most potent in conditioned media collected at 48 h (80% inhibition) compared to conditioned media collected at 24 h (70% inhibition) (data not shown). Based on growth characteristics of TH58, 24–28 h time point represented late logarithmic phase, > 28–40 h represented stationary phase and > 48 h time point represented early decline growth phase. It was possible that during the time point of stationary phase until the early decline phase, Lactobacillus TH58 increased secretion of putative immunomodulin(s) into the supernatant. Similar results by Chan Remillard and Ozimek indicated that supernatants from milk fermented by nine different strains of Lactobacillus showed increased TNF inhibitory activity over time (Chan Remillard & Ozimek, 2006).
Previous studies demonstrated that probiotic bacteria may stimulate the production of certain pro-inflammatory cytokines including TNF (Miettinen et al., 1996; Solis-Pereyra et al., 1997; Morita et al., 2002; Cross et al., 2004; Meyer et al., 2007). Results form the current study indicated that the conditioned medium of Lactobacillus TH14 exhibited immunostimulatory activity and stimulated TNF production in the absence of LPS. Similar immunostimulatory capabilities were also demonstrated in Lactobacillus reuteri strains CF48-3A and ATCC 55730 (Lin et al., 2008).
NF-κB transcription factor is known to regulate inflammatory mediators and cytokines (Bibiloni et al., 2005) including TNF, IL-12, IL-1, IL-6 and IL-8 (Aderem & Ulevitch, 2000; Egan & Toruner, 2006). Several reports indicated that certain Lactobacillus species diminish pro-inflammatory cytokine production via inhibiting NF-κB activation (Bai et al., 2004; Ma et al., 2004; Menard, 2004; Guarner et al., 2006; Iyer et al., 2008). As expected, Lactobacillus TH14 (TNF stimulatory strain) induced NF-κB activation in the absence of LPS stimulation. Whereas, Lactobacillus TH58 (TNF inhibitory strain) had no effect on NF-κB signaling pathway irrespective of LPS stimulation.
TNF production is regulated at different levels such as transcription, translation and secretion (Watkins et al., 1999; Papadakis & Targan, 2000). Kim et al. demonstrated that L. rhamnosus GG- and L. rhamnosus GR-1 suppressed LPS- induced TNF production in THP-1 cells via paracrine route involving granulocyte-colony stimulating factor (G-CSF). The suppression of TNF production by G-CSF was mediated through activation of signal transducer and activator of transcription (STAT)-3, subsequently inhibiting activation of c-Jun-N-terminal kinases (JNKs) in THP-1 cells (Kim et al., 2006). In another report, Lin et al. demonstrated the suppression of TNF in Mono-Mac-6 cells and THP-1 cells via down-regulation of activator protein-1 (AP-1) pathway without affecting NF-κB signaling (Lin et al., 2008). Results of these investigations suggested that Lactobacillus TH58-mediated suppression of TNF production may not be mediated via NF-κB signaling pathway. It is possible that Lactobacillus TH58 may mediate its anti-inflammatory effects via inhibition of c-Jun-N-terminal kinase (JNK) activation (Kim et al., 2006) or suppression of AP-1 transcription factor (Lin et al., 2008). However, these speculations require further investigation.
Lactobacillus TH58 (TNF-inhibitory strain) was identified as Lactobacillus saerimneri which was first isolated from a pig in 2004 (Pedersen & Roos, 2004), and until now not identified in humans. Lactobacillus TH14, TNF-stimulatory strain, was identified as Lactobacillus ruminis which is known to be a dominant indigenous species in the digestive tracts of humans and pigs (Reuter, 2001; Yin & Zheng, 2005).
In conclusion, the results of the present study demonstrated that Lactobacillus isolates exhibited different immunomodulatory properties. This is the first report of human-derived L. saerimneri strain with anti-inflammatory property and L. ruminis, the indigenous gut microbiota, with immunostimulatory property.
Acknowledgments
We thank Dr. Yea Ping Lin, Sara Jones and Carissa Thomas for advice and detailed protocols regarding the THP-1 bioassay and Supranee Buranapraditkun for technical assistance in flow cytometry. We also acknowledge Ruth Ann Luna, Kyle Menne, Sabeen Raza and Angela Major for their technical efforts and Tiffany Morgan for her administrative support. This work was supported from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0078/2546) and the Thai Government Research Budget to MT and ST, and JKS, CI and JV were supported by the National Institutes of Health (R01 DK065075).
References
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Atlas RM, Snyder JW. Handbook of media for clinical microbiology. CRC Press; Boca Raton, FL: 1995. p. 61. [Google Scholar]
- Bai AP, Quyang Q, Zhang W, Wang CH, Li SF. Probiotics inhibit TNF-α induced interleukin-8 secretion of HT29 cells. World J Gastroenterol. 2004;10:455–457. doi: 10.3748/wjg.v10.i3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibiloni R, Fedorak RN, Tannock GW, Madsen HL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- Cappuccino JG, Sherman N. Microbiology: a laboratory manual. 7. Pearson Benjamin Cummings; San Francisco, CA: 2005. pp. 71–74. [Google Scholar]
- Chan Remillard SKW, Ozimek L. Inhibition of tumour necrosis factor by lactic acid bacteria in mouse macrophages. DRTC Dairy Day. 2006:45–46. [Google Scholar]
- Cross ML. Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens. FEMS Immunol Med Microbiol. 2002;34:245–253. doi: 10.1111/j.1574-695X.2002.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Cross ML, Ganner A, Teilab D, Fray LM. Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunol Med Microbiol. 2004;42:173–180. doi: 10.1016/j.femsim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Egan LJ, Toruner M. NF-kB signaling: pros and cons of altering NF-κB as a therapeutic approach. Ann NY Acad Sci. 2006;1072:114–122. doi: 10.1196/annals.1326.009. [DOI] [PubMed] [Google Scholar]
- FAO/WHO. Evaluation of health and nutritional properties of probiotics in food, including powder milk with live lactic acid bacteria. Food and Agricultural Organization of United Nations and World Health Organization Expert Consultation Report. 2001 http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf.
- Guarner F, Bourdet-Sicard R, Brandtzaeg P, Gill HS, McGuirk P, van Eden W, Versalovic J, Weinstock JV, Rook GA. Mechanism of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastroenterol Hepatol. 2006;3:275–284. doi: 10.1038/ncpgasthep0471. [DOI] [PubMed] [Google Scholar]
- Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J. Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-κB and MAPK signalling. Cell Microbiol. 2008;10:1442–1452. doi: 10.1111/j.1462-5822.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- Kim SO, Sheikh HI, Ha SD, Martins A, Reid G. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus -induced suppression of TNF production in macrophages. Cell Microbiol. 2006;8:1958–1971. doi: 10.1111/j.1462-5822.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- Lin YP, Thibodeaux CH, Peña JA, Ferry GD, Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokine via c-Jun. Inflamm Bowel Dis. 2008;14:1068–1083. doi: 10.1002/ibd.20448. [DOI] [PubMed] [Google Scholar]
- Ma D, Forsythe P, Bienenstock J. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun. 2004;72:5308–5314. doi: 10.1128/IAI.72.9.5308-5314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen CBM, van Holten-Neelen C, Balk F, Heijne den Bak-Glashouwer M-J, Leer RJ, Laman JD, Boersma WJA, Claassen E. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine. 2000;18:2613–2623. doi: 10.1016/s0264-410x(99)00378-3. [DOI] [PubMed] [Google Scholar]
- Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secret metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53:821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AL, Elmadfa I, Herbacek I, Micksche M. Probiotic, as well as conventional yogurt, can enhance the stimulated production of proinflammatory cytokines. J Hum Nutr Diet. 2007;20:32–38. doi: 10.1111/j.1365-277X.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis factor alpha, interleukin-6 and interleukin-10 is induced by lactic acid bacteria. Infect Immun. 1996;64:5403–5405. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, He F, Fuse T, Ouwehand AC, Hashimoto H, Hosoda M, Mizumachi K, Kurisaki KI. Cytokine production by the murine macrophage cell line J774.1 after exposure to lactobacilli. Biosci Biotechnol Biochem. 2002;66:1963–1966. doi: 10.1271/bbb.66.1963. [DOI] [PubMed] [Google Scholar]
- Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology. 2000;119:1148–1157. doi: 10.1053/gast.2000.18160. [DOI] [PubMed] [Google Scholar]
- Pedersen C, Roos S. Lactobacillus saerimneri sp. nov., isolated from pig faeces. Int J Syst Evol Microbiol. 2004;54:1365–1368. doi: 10.1099/ijs.0.03057-0. [DOI] [PubMed] [Google Scholar]
- Pena JA, Versalovic J. Lactobacillus rhamnosus GG decreases TNF-α production in lipopolysacharide-activated murine macrophages by a contact independent mechanism. Cell Microbiol. 2003;5:277–285. doi: 10.1046/j.1462-5822.2003.t01-1-00275.x. [DOI] [PubMed] [Google Scholar]
- Pena JA, Li SY, Wilson PH, Thibodeau SA, Szary AJ, Versalovic J. Genotypic and phenotypic studies of murine intestinal lactobacilli: species differences in mice with and without colitis. Appl Environ Microbiol. 2004;70:558–568. doi: 10.1128/AEM.70.1.558-568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JA, Rogers AB, Ge Z, Ng V, Li SY, Fox JG, Versalovic J. Probiotic Lactobacillus spp diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect Immun. 2005;73:912–920. doi: 10.1128/IAI.73.2.912-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigón G, Galdeano CM, Valdez JC, Medici M. Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr. 2002;56:S21–S26. doi: 10.1038/sj.ejcn.1601658. [DOI] [PubMed] [Google Scholar]
- Reuter G. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol. 2001;2:43–53. [PubMed] [Google Scholar]
- Solis-Pereyra B, Aattouri N, Lemonnier D. Role of food in the stimulation of cytokine production. Am J Clin Nutr. 1997;66:521S–525S. doi: 10.1093/ajcn/66.2.421S. [DOI] [PubMed] [Google Scholar]
- Tejada-Simon MV, Lee JH, Ustunol Z, Pestka JJ. Ingestion of yogurt containing Lactobacillus acidophilus and Bifidobacterium to potentiate immunoglobulin A responses to cholera toxin in mice. J Dairy Sci. 1999;82:649–660. doi: 10.3168/jds.S0022-0302(99)75281-1. [DOI] [PubMed] [Google Scholar]
- Ulvitch RJ, Tobia PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hansen MK, Nguyne KT, Lee JE, Maier SF. Dynamic regulation of the proinflammatory cytokine, interleukin-1 beta. Life Sci. 1999;65:449–481. doi: 10.1016/s0024-3205(99)00095-8. [DOI] [PubMed] [Google Scholar]
- Yin Q, Zheng Q. Isolation and identification of the dominant Lactobacillus in gut and faeces of pig using carbohydrate fermentation and 16S rDNA analysis. J Biosci Bioengin. 2005;99:68–71. doi: 10.1263/jbb.99.68. [DOI] [PubMed] [Google Scholar]