Abstract
Background
Methylenetetrahydrofolate reductase (MTHFR), a key enzyme in folate metabolism, had significant effects on the homocysteine levels. The common functional MTHFR C677T polymorphism had been extensively researched. Several studies had evaluated the relationship between MTHFR C677T polymorphism and type 2 diabetes mellitus (T2DM), but the results were still controversial in the Chinese Han population. This meta-analysis was conducted to evaluate the relationship between MTHFR C677T polymorphism and T2DM in the Chinese Han population.
Methods
We searched the relevant studies in multiple electronic databases, which published up to December 2013. We reviewed and extracted data from all the included studies on the relationship between MTHFR C677T polymorphism and T2DM in the Chinese Han population. The odds ratios (ORs) and their 95% confidence intervals (95%CIs) were used to evaluate the relationship. Fixed-effects and random-effects meta-analysis were used to pool ORs by the heterogeneity. Publication bias and sensitivity analysis were also examined.
Results
29 studies were finally included in our meta-analysis, which contained 4656 individuals with T2DM and 2127 healthy controls. There was a significant relationship between MTHFR C677T polymorphism and T2DM under dominant (OR: 1.70, 95% CI: 1.42–2.02), recessive (OR: 1.48, 95% CI: 1.21–1.80), homozygous (OR: 1.89, 95% CI: 1.47–2.42), heterozygous (OR: 1.58, 95% CI: 1.33–1.87), and additive (OR: 1.46, 95% CI: 1.28–1.68) genetic model in a random-effects model. Subgroup analysis also reached similar results. Sensitivity analysis indicated that the overall result were dependable.
Conclusions
There was a significant relationship between MTHFR C677T polymorphism and T2DM in the Chinese Han population. The results of our meta-analysis suggested that MTHFR 677T allele might be a risk genetic factor of T2DM in the Chinese Han population.
Introduction
Type 2 diabetes mellitus (T2DM) is one of public health problems, seriously affects individual life quality, and increases individual economic burden. WHO estimates the number of people with diabetes will increase by 114% between 2000 and 2030, and China will become the major site of diabetes epidemic. In a systematic review of 22 studies on diabetes prevalence in China from 2000 to 2010, it increased from 2.6% to 9.7% during this decade [1]. It is estimated that China will have 380 million patients with T2DM by 2025 [2]. However, the pathogenesis of T2DM remains unclear [3]. Currently, the research on genetic polymorphisms is one of the most attention areas in the pathogenesis of T2DM, and some studies indicate that genetic polymorphisms have critical roles in the etiology of T2DM [4], [5].
Methylenetetrahydrofolate reductase (MTHFR) is a critical enzyme involved in folate metabolism, which converts 5, 10- methylene tetrahydrofolate to 5-methyl tetrahydrofolate. Mice deficient in MTHFR have reduced S-adenosylmethionine and increased S-adenosylhomocysteine, show hyperhomocysteinemia and global DNA hypomethylation [6]. The MTHFR C677T polymorphism is the most important genetic variation, which causes hyperhomocysteinemia [7]. The C677T polymorphism is a C to T transition at base pair 677, which will lead to the amino acid transition from Ala to Val and is associated with reduction of MTHFR activity. The variation of MTHFR C677T polymorphism may decrease enzyme activity by 65% and increase plasma total homocysteine levels particularly in the conditions of low dietary folate [8]. Some studies suggested that elevated plasma total homocysteine was associated with insulin resistance, which was the major cause of T2DM [3], [9], [10]. Homocysteine exposure can decline the viability of insulin-secreting cells, reduce glucokinase phosphorylating ability, and diminish insulin secretory responsiveness, lead to cell death [11]. Therefore, the MTHFR C677T polymorphism has been widely considered a genetic candidate for T2DM [12].
In recent years, numerous studies had demonstrated an association between MTHFR C677T polymorphism and T2DM. However, the results were not consistent [13]–[19]. A systematic review on Arab ethnicity found that MTHFR C677T polymorphism was significantly associated with T2DM [14], but another systematic review found that there was no association between MTHFR C677T polymorphism and T2DM around the world, similar results were repeated for ethnic group (Asian, Caucasian, African) [13]. Furthermore, previous studies also showed that the prevalence of MTHFR C677T polymorphism varies in different geographical regions and ethnic groups [20], and people from different ethnic groups had different genetic susceptibility with T2DM[21]. These findings suggested the study on the association between MTHFR C677T polymorphism and T2DM should be based on one single ethnical population to provide a precise estimation. Therefore, we conducted a meta-analysis to evaluate the association between MTHFR C677T polymorphism and T2DM specifically in Chinese Han population.
Materials and Methods
Search Strategy and Identification of Relevant Studies
A search strategy was carried out in multiple electronic databases (Cochrane, EMBASE, PubMed, CQVIP, CNKI (China National Knowledge Infrastructure), CBM (China Biological Medicine Database), and Wanfang databases) before December 2013. The following subject terms were used for searching by ‘methylenetetrahydrofolate reductase or MTHFR’, ‘gene or polymorphism or genetic polymorphism’, ‘Chinese or China’, and ‘diabetes or mellitus or diabetes mellitus or T2DM’. The papers were limited on humans and published in English or Chinese. In order to further identify any additional relevant data, we carefully searched the references in the selected studies.
Data Extraction
The data from all included studies were independently extracted by two authors (BZ and XW) according to a standard protocol. The third author (LL) resolved the disagreement between two authors. We excluded the studies that did not follow the inclusion criteria, that lacked of sufficient data, or that considered duplicated articles. If we found the same data in different studies, we used the data only one time. The following items were extracted from all included studies: the first author's name, year of publication, region (province), total number of study, gender, genotypic distribution, allele frequencies.
Inclusion Criteria
We set the inclusion criteria according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement[22]. a) Give information on the criteria and methods for selection. b) Describe laboratory methods, including source and storage of DNA, genotyping methods and platforms. c) Clearly define genetic variants using a widely used nomenclature system. d) State whether Hardy-Weinberg equilibrium was considered and, if so, how. e) Report numbers in each genotype category.
Statistical Analysis
STATA 11.0 software (StataCorp, College Station, TX, USA) was used to perform the meta-analysis. We used five genetic models, which included dominant (TT+CT vs. CC), recessive (TT vs. CC+CT), homozygous (TT vs. CC), heterozygous (CT vs. CC), and additive (T vs. C) models. The odds ratios (ORs) and their 95% confidence intervals (95%CIs) were used to evaluate the association between MTHFR C677T polymorphism and T2DM. We used Chi-square-based Q-tests to assess the heterogeneity between the individual studies [23]. If there was a significant heterogeneity among the individual studies, the random-effect model (DerSimonian and Laird method) was carried out to assess the pooled OR. Otherwise, the fixed-effect model (the Mantel–Haenszel method) was carried out.
We also conducted meta-regression and subgroup analysis to explore the sources of heterogeneity. To assess the reliability of the outcomes in the meta-analysis, a sensitivity analysis was performed by excluding one study at a time. Publication bias was assessed using the Egger's test [24]. We also conducted the Duval and Tweedie nonparametric “trim and fill” procedure to further assess the effect of publication bias in each genetic model [25]. Hardy-Weinbery equilibrium (HWE) in controls was assessed by the goodness-of-fit x2 test in each included study. The significance set at the P<0.05 in all analyses.
Results
Characteristics of Including Studies
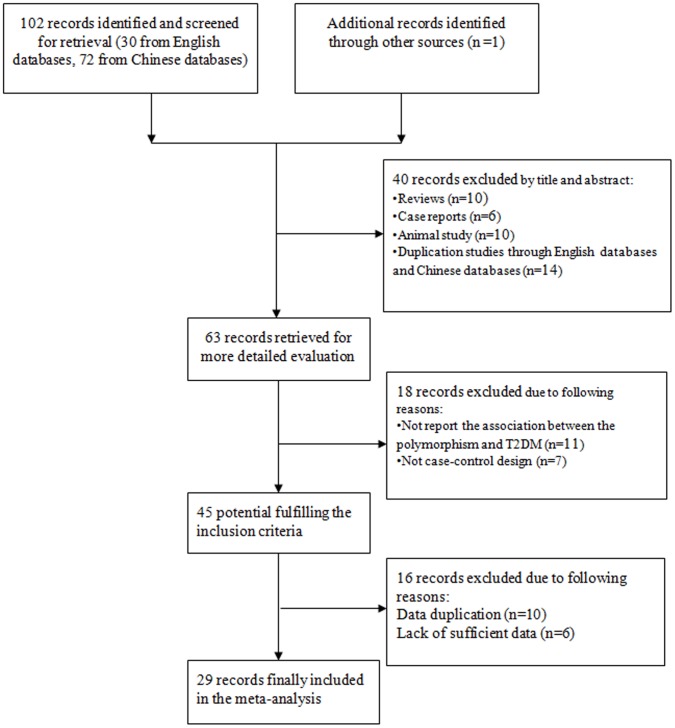
Figure 1 showed the procedure by which article was selected. A comprehensive search yielded 103 articles. After the removal of duplicated literatures and articles containing unspecific data that did not meet our criteria, a total of 29 studies was finally identified in our meta-analysis. Table 1 illustrated the characteristics of all the included studies in this meta-analysis. The data contained 4656 T2DM cases and 2127 healthy controls [15]–[17], [26]–[51]. The provinces of 29 studies included Heilongjiang, Beijing, Gansu, Shanxi, Zhejiang, Shanghai, Neimenggu, Guizhou, Tianjin, Guangdong, Hubei, Shandong, Jiangsu, Hebei, and Jilin. Except for 7 studies, the distribution of genotypes in the controls was consistent with HWE.
Figure 1. Flow diagram of included and excluded studies.
Table 1. Main characteristics of the 29 studies for meta-analysis.
| Number | Author | Year | Region | Total number of study | Male (%) | Genotypic distribution | Allele frequencies | HWE | ||||||||
| CC | CT | TT | C | T | ||||||||||||
| case | control | case | control | case | control | case | control | case | control | |||||||
| 1 | Sun, Lianga | 2013 | Beijing | 549 | 51.37 | 180 | 30 | 243 | 42 | 48 | 6 | 603 | 102 | 339 | 54 | Yes |
| 2 | Mei, Qingbu | 2012 | Heilongjiang | 215 | No | 17 | 17 | 51 | 70 | 23 | 37 | 85 | 104 | 97 | 144 | Yes |
| 3 | Dai, Hongshuanga | 2012 | Heilongjiang | 180 | 55.00 | 51 | 31 | 54 | 27 | 15 | 2 | 156 | 89 | 84 | 31 | Yes |
| 4 | Chen, Airong | 2010 | Gansu | 219 | 59.62 | 57 | 34 | 74 | 17 | 33 | 4 | 188 | 85 | 128 | 25 | Yes |
| 5 | Zhang, Qiaohuia,b,c | 2009 | Shanxi | 278 | 60.79 | 66 | 26 | 94 | 17 | 66 | 9 | 226 | 69 | 226 | 35 | Yes |
| 6 | Qiu, Yia,b | 2009 | Zhejiang | 299 | 54.85 | 83 | 53 | 68 | 29 | 48 | 18 | 234 | 135 | 164 | 65 | No |
| 7 | Hu, Linga,b | 2009 | Shanxi | 211 | 62.56 | 47 | 26 | 63 | 17 | 49 | 9 | 157 | 69 | 163 | 35 | Yes |
| 8 | Wen, Jie | 2008 | Shanghai | 211 | 52.13 | 43 | 27 | 82 | 25 | 29 | 5 | 168 | 79 | 140 | 35 | Yes |
| 9 | Luo, Dana,b | 2008 | Beijing | 226 | 47.79 | 59 | 43 | 63 | 31 | 19 | 11 | 181 | 117 | 101 | 53 | Yes |
| 10 | Chen, Ping | 2008 | Heilongjiang | 240 | No | 19 | 14 | 70 | 73 | 27 | 37 | 108 | 101 | 124 | 147 | No |
| 11 | Zhang, Chunyua,b,c | 2007 | Neimenggu | 141 | 51.77 | 28 | 34 | 29 | 19 | 19 | 12 | 85 | 87 | 67 | 43 | No |
| 12 | Luo, Dana,b | 2007 | Beijing | 274 | 52.64 | 55 | 42 | 102 | 35 | 26 | 14 | 222 | 119 | 154 | 63 | Yes |
| 13 | Yue, Honga,b,c | 2006 | Shanxi | 282 | 57.09 | 66 | 17 | 131 | 11 | 55 | 2 | 263 | 45 | 241 | 15 | Yes |
| 14 | Xiao, Yana,b | 2006 | Guizhou | 146 | No | 16 | 47 | 53 | 25 | 4 | 1 | 85 | 119 | 61 | 27 | Yes |
| 15 | Sun, Yinga,b,c | 2006 | Tianjin | 355 | 60 | 113 | 47 | 85 | 25 | 68 | 17 | 311 | 119 | 221 | 49 | No |
| 16 | Shi, Chengjun | 2006 | Guangdong | 295 | No | 108 | 68 | 60 | 34 | 18 | 7 | 276 | 170 | 96 | 48 | Yes |
| 17 | Liang, Wenchang | 2005 | Zhejiang | 122 | No | 33 | 17 | 34 | 18 | 15 | 5 | 100 | 52 | 64 | 28 | Yes |
| 18 | Guo, Lixina,b | 2005 | Beijing | 288 | 57.29 | 60 | 58 | 51 | 34 | 50 | 35 | 171 | 150 | 151 | 104 | No |
| 19 | Sun, Jiazhong. | 2005 | Hubei | 342 | 67.25 | 101 | 63 | 78 | 31 | 49 | 20 | 280 | 57 | 176 | 71 | No |
| 20 | Zhou, Juna,b,c | 2004 | Heilongjiang | 208 | No | 16 | 8 | 78 | 31 | 45 | 30 | 110 | 47 | 168 | 91 | Yes |
| 21 | Sun, Leia,b,c | 2004 | Shandong | 155 | 47.44 | 27 | 29 | 52 | 18 | 27 | 2 | 106 | 76 | 106 | 24 | Yes |
| 22 | Mao, Lia,b,c | 2004 | Jiangsu | 122 | 46.92 | 35 | 18 | 37 | 18 | 11 | 3 | 107 | 70 | 59 | 24 | Yes |
| 23 | Chen, Aironga,b,c | 2004 | Gansu | 126 | 64.29 | 24 | 21 | 45 | 9 | 22 | 5 | 93 | 51 | 89 | 19 | No |
| 24 | Xu, Jinshenga,b,c | 2003 | Hebei | 175 | 45.14 | 30 | 7 | 54 | 25 | 39 | 20 | 114 | 39 | 132 | 65 | Yes |
| 25 | Zhang, Guodong | 2002 | Shanghai | 298 | No | 56 | 40 | 108 | 49 | 34 | 11 | 220 | 129 | 176 | 71 | Yes |
| 26 | Shi, Jieping | 2002 | Jilin | 106 | No | 12 | 22 | 31 | 29 | 7 | 5 | 55 | 55 | 45 | 45 | Yes |
| 27 | Yang, Guoqinga,c | 2001 | Beijing | 288 | 53.61 | 57 | 26 | 113 | 28 | 56 | 8 | 227 | 80 | 225 | 44 | Yes |
| 28 | Wang, Longqinga | 2001 | Guangdong | 264 | 52.27 | 65 | 37 | 75 | 38 | 39 | 10 | 205 | 112 | 153 | 58 | Yes |
| 29 | Hu, Shenga,b,c | 2001 | Hubei | 168 | 55.36 | 49 | 30 | 48 | 24 | 16 | 1 | 146 | 84 | 80 | 26 | Yes |
HWE: Hardy-Weinbery equilibrium; a: The distribution of gender between case and control group is in balance; b: The distribution of age between case and control group is in balance; c: The distribution of BMI between case and control group is in balance.
Results of the Overall Meta-Analysis
Table 2 showed the ORs with their 95% CIs for the association between MTHFR C677T polymorphism and T2DM in the recessive, dominant, homozygous, heterozygous, and additive genetic model. There was a significant association between MTHFR C677T polymorphism and T2DM under dominant (OR: 1.70, 95% CI: 1.42–2.02), recessive (OR: 1.48, 95% CI: 1.21–1.80), homozygous (OR: 1.89, 95% CI: 1.47–2.42), heterozygous (OR: 1.58, 95% CI: 1.33–1.87), and additive (OR: 1.46, 95% CI: 1.28–1.68) genetic model in a random-effects model.
Table 2. The overall and stratified analysis for the association between MTHFR and T2DM.
| Genetic Model | Subgroup | Model for meta-analysis | OR(95% CI) | P for heterogeneity | I2 (%) | P for Egger's test |
| Dominant | overall | R | 1.70(1.42–2.02) | 0.00 | 56.9 | 0.45 |
| Region | ||||||
| Southern China | R | 1.71(1.32,2.21) | 0.04 | 49.6 | ||
| Northern China | R | 1.68(1.32,2.14) | 0.00 | 61.9 | ||
| HWE | ||||||
| Yes | R | 1.73(1.39,2.15) | 0.00 | 60.5 | ||
| No | F | 1.57(1.28,1.93) | 0.07 | 47.8 | ||
| Recessive | overall | R | 1.48(1.21–1.80) | 0.02 | 37.7 | 0.00 |
| Region | ||||||
| Southern China | F | 1.70(1.29–2.23) | 0.81 | 0.00 | ||
| Northern China | R | 1.39(1.07–1.81) | 0.01 | 50.4 | ||
| HWE | ||||||
| Yes | R | 1.61(1.23–2.09) | 0.01 | 44.3 | ||
| No | F | 1.28(1.00–1.63) | 0.34 | 11.6 | ||
| Homozygous | overall | R | 1.89(1.47–2.42) | 0.00 | 50.0 | 0.01 |
| Region | ||||||
| Southern China | F | 2.07(1.56,2.76) | 0.60 | 0.00 | ||
| Northern China | R | 1.81(1.28,2.56) | 0.00 | 62.1 | ||
| HWE | ||||||
| Yes | R | 2.13(1.53,2.95) | 0.00 | 53.2 | ||
| No | F | 1.51(1.16,1.96) | 0.18 | 32.6 | ||
| Heterozygous | overall | R | 1.58(1.33–1.87) | 0.00 | 46.4 | 0.33 |
| Region | ||||||
| Southern China | R | 1.57(1.18,2.08) | 0.03 | 52.3 | ||
| Northern China | R | 1.58(1.28,1.97) | 0.02 | 46.0 | ||
| HWE | ||||||
| Yes | R | 1.59(1.30,1.95) | 0.00 | 51.1 | ||
| No | F | 1.52(1.20,1.92) | 0.16 | 35.1 | ||
| Additive | overall | R | 1.46(1.28–1.68) | 0.00 | 64.5 | 0.01 |
| Region | ||||||
| Southern China | F | 1.53(1.34,1.75) | 0.29 | 16.6 | ||
| Northern China | R | 1.42(1.17,1.72) | 0.00 | 72.7 | ||
| HWE | ||||||
| Yes | R | 1.48(1.26,1.75) | 0.00 | 66.9 | ||
| No | R | 1.41(1.11,1.78) | 0.02 | 64.5 |
OR: odds ratio; R: random-effects model; F: fix-effects model. HWE: Hardy-Weinbery equilibrium
Meta-Regression and Stratified Analysis
There was a significant heterogeneity in each genetic model (Table 2). we used meta-regression to explore the sources of heterogeneity in each genetic model separately. Similarly, heterogeneity can be explained by the number of the control group in each genetic model (Table 3).
Table 3. The results of meta-regression in the five genetic models.
| Genetic Model | Variables | P for meta-regression |
| Dominant | year | 0.521 |
| total number of study | 0.175 | |
| number of control | 0.008 | |
| number of case | 0.504 | |
| male (%) | 0.152 | |
| Recessive | year | 0.534 |
| total number of study | 0.738 | |
| number of control | 0.013 | |
| number of case | 0.530 | |
| male (%) | 0.396 | |
| Homozygous | year | 0.479 |
| total number of study | 0.373 | |
| number of control | 0.003 | |
| number of case | 0.995 | |
| male (%) | 0.347 | |
| Heterozygous | year | 0.580 |
| total number of study | 0.150 | |
| number of control | 0.028 | |
| number of case | 0.367 | |
| male (%) | 0.152 | |
| Additive | year | 0.683 |
| total number of study | 0.419 | |
| number of control | 0.008 | |
| number of case | 0.952 | |
| male (%) | 0.116 |
In the subgroup analysis based on region, we divided the included studies into two major group, the northern and the southern [20]. The northern group included Beijing, Gansu, Heilongjiang, Hebei, Tianjin, Jilin, Neimenggu, Shandong, Shanxi, and the southern group included Hubei, Jiangsu, Shanghai, Guizhou, Zhejiang, and Guangdong. There was a significant association between MTHFR C677T polymorphism and T2DM under each genetic model in both groups. Likewise, we performed subgroup analysis on studies in which the MTHFR alleles in the control group were in HWE and on studies in which they were not in HWE, there was a significant association between MTHFR C677T polymorphism and T2DM under each genetic model in both groups (Table 2).
Sensitivity Analysis
Table 4 showed the pooled ORs and their 95%CIs of sensitivity analysis by excluding one study at a time in each genetic model, the results in the five genetic models indicated that the overall result was dependable.
Table 4. Sensitivity analysis by removing each study in each model.
| Study Removed | Dominant | Recessive | Homozygous | Heterozygous | additive |
| OR(95% CI) | OR(95% CI) | OR(95% CI) | OR(95% CI) | OR(95% CI) | |
| Sun, Liang | 1.73(1.45,2.07) | 1.49(1.31,1.82) | 1.92(1.49,2.48) | 1.61(1.36,1.91) | 1.48(1.29,1.71) |
| Mei, Qingbu | 1.74(1.47,2.07) | 1.52(1.25,1.85) | 1.97(1.54,2.51) | 1.61(1.36,1.91) | 1.49(1.31,1.71) |
| Dai, Hongshuang | 1.71(1.42,2.04) | 1.45(1.19,1.76) | 1.86(1.45,2.39) | 1.59(1.34,1.90) | 1.46(1.27,1.68) |
| Chen, Airong | 1.66(1.39,1.98) | 1.44(1.18,1.76) | 1.83(1.43,2.35) | 1.55(1.31,1.84) | 1.44(1.26,1.65) |
| Zhang, Qiaohui | 1.67(1.40,2.00) | 1.46(1.19,1.79) | 1.86(1.44,2.40) | 1.56(1.31,1.85) | 1.45(1.26,1.66) |
| Qiu, Yi | 1.70(1.42,2.04) | 1.49(1.21,1.83) | 1.91(1.47,2.48) | 1.58(1.33,1.89) | 1.47(1.27,1.69) |
| Hu, Ling | 1.68(1.40,2.00) | 1.46(1.19,1.78) | 1.86(1.44,2.40) | 1.56(1.31,1.86) | 1.45(1.26,1.66) |
| Wen, Jie | 1.68(1.40,2.01) | 1.46(1.19,1.78) | 1.85(1.44,2.38) | 1.56(1.31,1.86) | 1.45(1.26,1.67) |
| Luo, Dan | 1.71(1.42,2.05) | 1.50(1.22,1.84) | 1.93(1.49,2.49) | 1.58(1.33,1.89) | 1.47(1.28,1.70) |
| Chen, Ping | 1.74(1.47,2.07) | 1.52(1.25,1.85) | 1.97(1.55,2.50) | 1.61(1.37,1.91) | 1.50(1.31,1.71) |
| Zhang, Chunyu | 1.69(1.41,2.02) | 1.48(1.21,1.82) | 1.90(1.46,2.45) | 1.57(1.32,1.87) | 1.46(1.27,1.68) |
| Luo, Dan | 1.70(1.41,2.04) | 1.51(1.24,1.85) | 1.92(1.48,2.49) | 1.57(1.31,1.87) | 1.47(1.28,1.69) |
| Yue, Hong | 1.66(1.39,1.97) | 1.45(1.19,1.76) | 1.83(1.43,2.35) | 1.55(1.31,1.83) | 1.44(1.26,1.65) |
| Xiao, Yan | 1.63(1.38,1.91) | 1.46(1.20,1.79) | 1.85(1.45,2.37) | 1.50(1.30,1.74) | 1.43(1.25,1.63) |
| Sun, Ying | 1.70(1.41,2.04) | 1.45(1.19,1.78) | 1.91(1.47,2.49) | 1.59(1.33,1.89) | 1.45(1.26,1.67) |
| Shi, Chengjun | 1.72(1.44,2.06) | 1.48(1.21,1.81) | 1.91(1.48,2.47) | 1.60(1.35,1.91) | 1.47(1.28,1.70) |
| Liang, Wenchang | 1.72(1.44,2.05) | 1.48(1.21,1.81) | 1.91(1.48,2.46) | 1.60(1.35,1.90) | 1.47(1.28,1.69) |
| Guo, Lixin | 1.71(1.42,2.05) | 1.51(1.22,1.85) | 1.93(1.49,2.51) | 1.58(1.33,1.89) | 1.47(1.28,1.70) |
| Sun, Jiazhong. | 1.70(1.42,2.05) | 1.50(1.22,1.84) | 1.92(1.48,2.50) | 1.58(1.32,1.88) | 1.47(1.27,1.69) |
| Zhou, Jun | 1.72(1.44,2.05) | 1.52(1.26,1.84) | 1.95(1.52,2.50) | 1.59(1.33,1.88) | 1.49(1.31,1.71) |
| Sun, Lei | 1.65(1.39,1.96) | 1.42(1.18,1.72) | 1.80(1.42,2.28) | 1.54(1.30,1.83) | 1.43(1.25,1.63) |
| Mao, Li | 1.70(1.42,2.03) | 1.47(1.20,1.79) | 1.90(1.47,2.44) | 1.58(1.33,1.88) | 1.46(1.27,1.68) |
| Chen, Airong | 1.65(1.39,1.97) | 1.47(1.20,1.80) | 1.85(1.44,2.38) | 1.54(1.30,1.81) | 1.44(1.26,1.65) |
| Xu, Jinsheng | 1.74(1.47,2.07) | 1.52(1.25,1.85) | 1.97(1.55,2.50) | 1.61(1.30,1.90) | 1.50(1.31,1.71) |
| Zhang, Guodong | 1.70(1.41,2.04) | 1.47(1.20,1.81) | 1.88(1.46,2.44) | 1.58(1.32,1.88) | 1.47(1.27,1.69) |
| Shi, Jieping | 1.69(1.41,2.02) | 1.48(1.21,1.81) | 1.88(1.46,2.42) | 1.57(1.32,1.86) | 1.48(1.29,1.70) |
| Yang, Guoqing | 1.68(1.40,2.01) | 1.45(1.19,1.77) | 1.85(1.44,2.39) | 1.57(1.32,1.87) | 1.45(1.26,1.67) |
| Wang, Longqing | 1.71(1.43,2.05) | 1.46(1.19,1.78) | 1.88(1.46,2.44) | 1.60(1.35,1.90) | 1.47(1.27,1.69) |
| Hu, Sheng | 1.70(1.42,2.04) | 1.44(1.19,1.75) | 1.85(1.45,2.36) | 1.59(1.34,1.89) | 1.46(1.27,1.67) |
Assessment of Publication Bias
As shown in table 2, Egger's test suggested no publication bias in dominant and heterozygous, but not in recessive, homozygous and additive genetic model. Because of this, we used the trim and fill method, the pooled analysis incorporating the hypothetical studies continued to show a statistically significant association between MTHFR C677T polymorphism and T2DM under recessive (OR: 1.26, 95% CI: 1.02–1.54), homozygous (OR: 1.60, 95% CI: 1.23–2.08) and additive (OR: 1.29, 95% CI: 1.12–1.49) genetic model.
Discussion
This current study, to our knowledge, was the first to use a meta-analysis to evaluate the association between MTHFR C677T polymorphism and T2DM specifically in China. There was a significant relationship between MTHFR C677T polymorphism and T2DM in each genetic model. The prevalence of MTHFR C677T polymorphism varies in the different regions in China [20], so we separated northern group from southern group, and still got similar results, which compared to the overall results. According to whether HWE in control, we also found that there was a significant association between MTHFR C677T polymorphism and T2DM in each genetic model. Sensitivity analysis indicated there was no significant change on the overall results by removing one study in each turn. Egger's test suggested publication bias in recessive, homozygous and additive genetic model. The trim and fill analysis did not change the general results in the three genetic models (although the strength of the association was slightly attenuated), suggesting that the results of our analysis were credible. Based on the results of our meta-analysis, we can speculate that MTHFR 677T allele might increase the risk of T2DM in the Chinese Han population.
As an essential intermediate, homocysteine plays an important role between floate and activated methyl cycle, which is involved in the transfer of activated methyl groups from tetrahydrofolate to S-adenosylmethionine [52]. The methyl cycle has effects on global and gene promoter-specific DNA methylation in regulating gene expression [53], [54]. Some studies suggested that homocysteine exposure had adverse effects on beta cell glucose metabolism and cell viability, and impaired insulin secretory function [55]. There was a significant association between homocysteine level and insulin resistance [9], [56]. Due to its biological relevance and its association with metabolic disorders, homocysteine metabolism is an important candidate pathway for T2DM. The C677T variant of MTHFR plays an important role on homocysteine metabolism [57]. The homozygous 677TT and heterozygous 677CT genotypes have decreased 70% and 35% in the enzyme activity of MTHFR respectively, compared to the 677CC genotype [58]. Individuals with the homozygous 677TT genotype have higher plasma homocysteine and lower plasma folate levels than those with 677CC genotype [59]. MTHFR C677T polymorphism has also been reported to be associated with type 2 diabetes, and its complications [17], [30], .
The variation of MTHFR A1298C polymorphism, which was an A to C transition at base pair 1298 resulting in the amino acid transition from Glu to Ala, could also decrease enzyme activity, and lead to hyperhomocysteinemia. The A1298C variation was located in the C-terminal regulatory domain of the MTHFR gene, while the C677T variation was located in the gene catalytic domain [61]. The A1298C variation had lower impact on the enzyme activity, compared with the C677T variation [61]. So the majority of studies on T2DM mainly paid attention to the C677T variation, not the A1298C variation, and we only collected one study on the relationship between MTHFR A1298C polymorphism and T2DM in Chinese Han population before December 2013 [37]. Therefore, in our meta-analysis, we only considered the studies on the relationship between MTHFR C677T polymorphism and T2DM.
In 2013, Khalid et al. found that there was a significant association between MTHFR C677T polymorphism and T2DM in Arab population [14], and Zhong et al. also conducted a meta-analysis of the relationship between MTHFR C677T polymorphism and T2DM, and concluded that there was no association between MTHFR C677T polymorphism and T2DM, regardless of the ethnicity of the patient or the presence of serious DM-related complications [13]. Our meta-analysis showed a significant relationship between MTHFR C677T polymorphism and T2DM under five genetic models in Chinese Han population. The results in our meta-analysis were similar to Khalid's study, and different from Zhong's study. There are several reasons for this difference. First, Zhong et al. conducted the meta-analysis all over the world, only loosely classified the study population as African, Asian, or Caucasian. Because MTHFR C677T polymorphism distribution varies among different ethnic groups, the relationship between MTHFR C677T polymorphism and T2DM should be studied on a single ethnic group. Therefore, our study focused on the Chinese Han population to derive an accurate evaluation. Second, more than a third of included studies focused on the Chinese Han population in Zhong's study, but he just conducted subgroup analysis in Asian population, did not further analyze the association in Chinese Han population. Third, Zhong's study only included 16 studies on the Chinese Han population, while our study included 29 studies. We think the number of included studies for Zhong' meta-analysis was inadequate, for example Sun et al. [15], Qiu et al. [28], Chen et al. [32] and so on. The findings suggested that we need to further analyze the association between MTHFR C677T polymorphism and T2DM in the Chinese Han population.
There are several limitations in our meta-analysis. First of all, the subjects in the included studies were too small, so more large-scale studies were needed to assess the association between MTHFR C677T polymorphism and T2DM. And due to lack of necessary personal information in the included studies, we were unable to further perform subgroup analysis for the relevant influential factors (gender, age, BMI and so on). Second, all included studies were cross-sectional design and all the subjects came from hospitals, their results were not adjusted by the relevant influential factors. They could not infer cause-effect relationship. Third, the development of T2DM was affected by the multiple genes, and our meta-analysis only focuses on MTHFR C677T polymorphism, so the influence of MTHFR C677T polymorphism on T2DM may be affected with other gene polymorphism. Some studies had suggested that ACE insertion/deletion (I/D) polymorphism may act synergistically with MTHFR C677T polymorphism to increase the risk of T2DM [62].
In conclusion, our meta-analysis suggested there was a significant association between MTHFR C677T polymorphism and T2DM in the Chinese Han population, and indicated that MTHFR 677T allele might be a risk genetic factor in developing T2DM. Because of the limitations in our study, more large-scale studies need consider the relevant influential factors and other gene polymorphism to verify the results of our study.
Supporting Information
PRISMA Checklist of this systematic review.
(DOC)
Meta-analysis on Genetic Association Studies Checklist.
(DOCX)
Funding Statement
The authors have no support or funding to report.
References
- 1. Li H, Oldenburg B, Chamberlain C, O'Neil A, Xue B, et al. (2012) Diabetes prevalence and determinants in adults in China mainland from 2000 to 2010: A systematic review. Diabetes Research and Clinical Practice 98: 226–235. [DOI] [PubMed] [Google Scholar]
- 2. Beulens JW, Grobbee DE, Nealb B (2010) The global burden of diabetes and its complications: an emerging pandemic. European Journal of Cardiovascular Prevention & Rehabilitation 17: s3–s8. [DOI] [PubMed] [Google Scholar]
- 3. Guo S (2014) Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol 220: T1–T23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flores-Martinez S, Islas-Andrade S, Machorro-Lazo M, Revilla M, Juarez R, et al. (2004) DNA polymorphism analysis of candidate genes for type 2 diabetes mellitus in a Mexican ethnic group. Elsevier. pp. 339–348. [DOI] [PubMed]
- 5. Radha V, Mohan V (2007) Genetic predisposition to type 2 diabetes among Asian Indians. The Indian journal of medical research 125: 259–274. [PubMed] [Google Scholar]
- 6. Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, et al. (2001) Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet 10: 433–443. [DOI] [PubMed] [Google Scholar]
- 7. Sibani S, Christensen B, O'Ferrall E, Saadi I, Hiou-Tim F, et al. (2000) Characterization of six novel mutations in the methylenetetrahydrofolate reductase (MTHFR) gene in patients with homocystinuria. Hum Mutat 15: 280–287. [DOI] [PubMed] [Google Scholar]
- 8. Rozen R (1997) Genetic predisposition to hyperhomocysteinemia: deficiency of methylenetetrahydrofolate reductase (MTHFR). Thromb Haemost 78: 523–526. [PubMed] [Google Scholar]
- 9. Hemati T, Moghadami-Tabrizi N, Davari-Tanha F, Salmanian B, Javadian P (2011) High plasma homocysteine and insulin resistance in patients with polycystic ovarian syndrome. Iranian Journal of Reproductive Medicine 9: 223–228. [PMC free article] [PubMed] [Google Scholar]
- 10. Schachter M, Raziel A, Friedler S, Strassburger D, Bern O, et al. (2003) Insulin resistance in patients with polycystic ovary syndrome is associated with elevated plasma homocysteine. Human Reproduction 18: 721–727. [DOI] [PubMed] [Google Scholar]
- 11. Scullion SM, Gurgul-Convey E, Elsner M, Lenzen S, Flatt PR, et al. (2012) Enhancement of homocysteine toxicity to insulin-secreting BRIN-BD11 cells in combination with alloxan. J Endocrinol 214: 233–238. [DOI] [PubMed] [Google Scholar]
- 12. Tavakkoly Bazzaz J, Shojapoor M, Nazem H, Amiri P, Fakhrzadeh H, et al. (2010) Methylenetetrahydrofolate reductase gene polymorphism in diabetes and obesity. Mol Biol Rep 37: 105–109. [DOI] [PubMed] [Google Scholar]
- 13. Zhong JH, Rodriguez AC, Yang NN, Li LQ (2013) Methylenetetrahydrofolate reductase gene polymorphism and risk of type 2 diabetes mellitus. PLoS One 8: e74521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al-Rubeaan K, Siddiqui K, Saeb AT, Nazir N, Al-Naqeb D, et al. (2013) ACE I/D and MTHFR C677T polymorphisms are significantly associated with type 2 diabetes in Arab ethnicity: a meta-analysis. Gene 520: 166–177. [DOI] [PubMed] [Google Scholar]
- 15. Sun L, Wang S, Shi X, Yang Z (2013) Interactions between APOE and MTHFR Mutations is Associated with the Risk for Type 2 Diabetic Nephropathy. Journal of Medical Molecular Biology 10: 95–99. [Google Scholar]
- 16. Mei Q, Chen P, Zheng L (2012) Correlation study between gene polymorphism of methylene tetrahydrofolate reductase and type 2 diabetes. China Medical Herald 09: 162–163. [Google Scholar]
- 17. Hongshuang D, Yu Z (2012) An Association Study of MTHFR and eNOS Genes Polymorphism with Diabetic Nephropathy. Chinese Journal of Trauma and Disability Medicine 20: 4–6. [Google Scholar]
- 18. Abbas S, Raza ST, Ahmed F, Ahmad A, Rizvi S, et al. (2013) Association of Genetic polymorphism of PPARγ-2, ACE, MTHFR, FABP-2 and FTO genes in risk prediction of type 2 diabetes mellitus. Journal of biomedical science 20: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu HC, Chiou JF, Wang YH, Chen CH, Mau SY, et al. (2013) Folate deficiency triggers an oxidative-nitrosative stress-mediated apoptotic cell death and impedes insulin biosynthesis in RINm5F pancreatic islet beta-cells: relevant to the pathogenesis of diabetes. PLoS One 8: e77931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang B, Liu Y, Li Y, Fan S, Zhi X, et al. (2013) Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: findings from 15357 adults of Han nationality. PLoS One 8: e57917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, et al. (2013) Ethnic Differences in the Relationship Between Insulin Sensitivity and Insulin Response A systematic review and meta-analysis. Diabetes care 36: 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Little J, Higgins J, Ioannidis J, Moher D, Gagnon F, et al. (2009) STrengthening the REporting of Genetic Association studies (STREGA)–an extension of the STROBE statement. European Journal of Clinical Investigation 39: 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 26. Chen A, Zhang H, Wang Z, Fu S, Yang P, et al. (2010) C-reactive protein, vitamin B12 and C677T polymorphism of N-5,10-methylenetetrahydrofolate reductase gene are related to insulin resistance and risk factors for metabolic syndrome in Chinese population. Clin Invest Med 33: E290–297. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Q, Liu J (2009) The relationship of carotid intima-medla thickness with C677T polymorphism of MTHFR and plasma homocysteine level in type 2 diabetes. Chinese Journal of Diabetes 17: 356–358. [Google Scholar]
- 28. Qiu Y (2009) C677T polymorphisrn of MTHFR gene in type 2 diabetes with carotid artherosclerosis. Zhe Jiang Medical Journal 31: 426–428. [Google Scholar]
- 29. Hu L, Zhang Q, Fang M, Qin J, Liu J (2009) Association of non-alcoholic fatty liver with plasma homocysteine and methylenetetrahydrofloate reductase gene polymorphism in patients of type 2 diabetes mellitus in Shanxi. Chinese Journal of General Pratitioners 08: 385–388. [Google Scholar]
- 30. Wen J, Lu Z, Li X, Wu D, Zhang Y, et al. (2008) Correlation of MTHFR gene polymorphism and plasma homocysteine with microalbuminuria in type 2 diabetes. Shanghai Medical Journal 31: 47–51. [Google Scholar]
- 31. Luo D, Yan S, Ma H, Cheng Y, Song Y (2008) Relationship between homocysteine methylene tetrahyrdrofolate reductase polymorphism and coronary heart disease in Chinese type 2 diabetes mellitus. Journal of China-Japan Friendship Hospiatal 22: 24–27. [Google Scholar]
- 32. Chen P, Pan Y, Sun D, Bai J, Fu S (2008) The Relationship between Methylenetetrahydrofolate reductase gene C677T polymorphism and type 2 diabetes mellitus. Journal of Qiqihar Medical College 29: 672–673. [Google Scholar]
- 33. Zhang C, Li Z, Liu G, Hu R (2007) MTHFR, eNOS gene polymorphisms connecting research of the patients with T2DM complicating cerebral infarction. Journal of Clinical Internal Medine 24: 458–460. [Google Scholar]
- 34. Luo D, Yan S, Li J, Cheng Y, Song Y (2007) The Association between Methylenetetrahydrofolate reductase gene C677T polymorphism and coronary heart disease in type 2 diabetes mellitus. Chinese Journal of Clinical Laboratory Science 25: 114–116. [Google Scholar]
- 35. Yue H, Liu J, Kang W, Hu L, Qin J, et al. (2006) Relationship between plasma level of homocysteine and urine microalbumin in incipient type 2 diabetic nephropathy. Chinese Journal of General Pratitioners 5: 725–729. [Google Scholar]
- 36. Xiao Y, Hu Z, Shan K, Guan Z, Ren X (2006) An Investigation on the Relationship Between the Polymorphism of MTHFR Gene and Type 2 Diabetic Cardiovascular Disease. Journal of Guiyang Medical College 31: 317–319. [Google Scholar]
- 37.Sun Y, Liu D, Fan X (2006) The Study on detection of homocysteine in Diabetes Mellitus and its vascular complication The Third Laboratory Medicine Conference in three provinces and two cities of Northern in China. Chengde. pp. 126–131.
- 38. Shi C, He Y, Cheng G, Wang W, Liu J, et al. (2006) Detection of the 677 C-T variant of MTHFR gene in Chinese diabetic patients with fluorescent MGB probe real-time PCR Chinese Journal of Diabetes. 14: 258–260. [Google Scholar]
- 39. Sun J, Xu Y, Xue J, Zhu Y, Lu H (2005) Methylenetetrahydrofolate reductase polymorphism associated with susceptibility to coronary heart disease in Chinese type 2 diabetic patients. Mol Cell Endocrinol 229: 95–101. [DOI] [PubMed] [Google Scholar]
- 40.Liang W (2005) The study on MTHFR gene polymorphism, insulin resistance and high sensitivity C-reactive protein level in patients with type 2 diabetes [Master]: Zhejiang University.
- 41. Guo L, Pan Q, Chu M, Guo F, Sun M, et al. (2005) Relationship between genetic polymorphisms of methylenetetrahydrofolate reductase and macrovascular diseases in type 2 diabetes. Journal of Clinical Internal Medicine 22: 468–470. [Google Scholar]
- 42. Zhou J, Li X, Zhang J (2004) Relationship between the C677T polymorphism in the methylenetetrahydrofolate reductase gene and cerebral infarction complicated type 2 diabetes. Journal of Apoplexy and Nervous Diseases 21: 136–138. [Google Scholar]
- 43. Sun L, Chen L, Ren J, Wang D, Zheng X, et al. (2004) Relationship of plasma homocysteine and gene polymorphism of homocysteine metabolism related enzyme with diabetic peripheral neuropathy Chinese Journal of Endocrinology and Metabolism. 20: 536–537. [Google Scholar]
- 44.Mao L, Gao Y, Qin W, Shi H (2004) The Association of Methlenetetrahydrofolate Reductase Gene Polymorphism With Cerebral Infarction in Type 2 Diabetes Mellitus. Academiae Medicinae Nantong 24: : 146–147,150. [Google Scholar]
- 45. Chen A, Ning Y, Zhu X, Li L, Shi H (2004) Study on the Relationship between Gene Polymorphisms of N5,10-methylenetetrahydrofolate Reductase and Nephropathy in type 2 Diabetes Mellitus in Gansu Han Chinese of China. Chinese Journal of Prevention and Control of Chronic Non-communication Diseases 12: 195–197. [Google Scholar]
- 46. Xu J, Zhang J, Shan B, Ma H (2003) Relationship between methylenetetrahydrofolate reductase gene polymorphism and diabetic nephropathy in type 2 diabetes mellitus in the Hans of Hebei Province. Clinical Focus 18: 787–789. [Google Scholar]
- 47. Zhang G, Xiang K, Weng Q, Li J (2002) Association between 677C/T polymorphism of methylenetetrahydrofolate reductase gene and type 2 diabetes with macrovascular complications in Shanghai. Chinese Journal of Endocrinology and Metabolism 18: 362–365. [Google Scholar]
- 48. Shi J, Li B, Yu Y, Chen Y, Tao R, et al. (2002) The relationship between the polymorphysm of MTHFR gene and type 2 diabetes mellitus. Journal of Jilin University(Medicine Edition) 28: 371–374. [Google Scholar]
- 49. Yang G, Lu J, Pan C (2001) Study on the relation between N5,10-methylenetetrahydrofolate reductase gene polymorphism and the susceptibility to microangiopathy in type 2 diabetes mellitus. Chinese Journal of Endocrinology and Metabolism 17: 224–227. [Google Scholar]
- 50. Wang L, Wang J, Xue Y, Cheng Y, Zhou H, et al. (2001) Relation between methylenetetrahydrofolate reductase gene polymorphism and diabetic retinopathy. Chinese Journal of Ocular Fundus Diseases 17: 198–200. [Google Scholar]
- 51. Hu S, Gan P, Li J, Bi H (2001) The Relation between the mutation of methylenetetrahydrofolate reductase gene 677C-T and the diabetic microangiopathy. Chinese Journal of Medical Genetics 18: 118–121. [PubMed] [Google Scholar]
- 52. Medina MÁ, Urdiales JL, Amores-Sánchez MI (2001) Roles of homocysteine in cell metabolism. European Journal of Biochemistry 268: 3871–3882. [DOI] [PubMed] [Google Scholar]
- 53. Thaler R, Agsten M, Spitzer S, Paschalis EP, Karlic H, et al. (2011) Homocysteine suppresses the expression of the collagen cross-linker lysyl oxidase involving IL-6, Fli1, and epigenetic DNA methylation. J Biol Chem 286: 5578–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bleich S, Lenz B, Ziegenbein M, Beutler S, Frieling H, et al. (2006) Epigenetic DNA hypermethylation of the HERP gene promoter induces down-regulation of its mRNA expression in patients with alcohol dependence. Alcohol Clin Exp Res 30: 587–591. [DOI] [PubMed] [Google Scholar]
- 55. Patterson S, Flatt PR, Brennan L, Newsholme P, McClenaghan NH (2006) Detrimental actions of metabolic syndrome risk factor, homocysteine, on pancreatic beta-cell glucose metabolism and insulin secretion. J Endocrinol 189: 301–310. [DOI] [PubMed] [Google Scholar]
- 56. Nafiye Y, Sevtap K, Muammer D, Emre O, Senol K, et al. (2010) The effect of serum and intrafollicular insulin resistance parameters and homocysteine levels of nonobese, nonhyperandrogenemic polycystic ovary syndrome patients on in vitro fertilization outcome. Fertil Steril 93: 1864–1869. [DOI] [PubMed] [Google Scholar]
- 57. Pare G, Chasman DI, Parker AN, Zee RR, Malarstig A, et al. (2009) Novel associations of CPS1, MUT, NOX4, and DPEP1 with plasma homocysteine in a healthy population: a genome-wide evaluation of 13 974 participants in the Women's Genome Health Study. Circ Cardiovasc Genet 2: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 59. Wang W, Wang Y, Gong F, Zhu W, Fu S (2013) MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. PLoS One 8: e58041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qin X, Li J, Zhang Y, Ma W, Fan F, et al. (2012) Prevalence and associated factors of diabetes and impaired fasting glucose in Chinese hypertensive adults aged 45 to 75 years. PLoS One 7: e42538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Friedman G, Goldschmidt N, Friedlander Y, Ben-Yehuda A, Selhub J, et al. (1999) A common mutation A1298C in human methylenetetrahydrofolate reductase gene: association with plasma total homocysteine and folate concentrations. J Nutr 129: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 62. Mehri S, Koubaa N, Nakbi A, Hammami S, Chaaba R, et al. (2010) Relationship between genetic polymorphisms of angiotensin-converting enzyme and methylenetetrahydrofolate reductase as risk factors for type 2 diabetes in Tunisian patients. Clin Biochem 43: 259–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist of this systematic review.
(DOC)
Meta-analysis on Genetic Association Studies Checklist.
(DOCX)