Abstract
Background and objective
There are inconsistent data of the cytochrome P450 1A1 (CYP1A1) Ile462Val (rs1048943) single nuclear polymorphism (SNP) as a genetic susceptibility factor for cervical cancer in various populations. Moreover, little is known about the interaction of this SNP with other risk factors, including contraceptive use, postmenopausal status, parity, and tobacco smoking.
Methods
Polymerase chain reaction-restriction fragment length polymorphism was used to study the prevalence of the CYP1A1 Ile462Val SNP in women with cervical cancer (n = 456) and controls (n = 495).
Results
Logistic regression analysis adjusting for age, parity, oral contraceptive use, tobacco smoking, and menopausal status demonstrated that that the CYP1A1 Ile/Val polymorphism was not associated with an increased risk of cervical cancer in all patients. The adjusted odds ratio (OR) for patients with the Ile/Val genotype vs. Ile/Ile genotype was 1.539 (95 % confidence interval [CI] 0.932–2.541, p = 0.091). However, an increase in cervical cancer risk was seen among patients with a positive history of tobacco smoking and parity. The adjusted OR for positive history of tobacco smoking with the Ile/Val vs. Ile/Ile genotypes was 2.978 (95 % CI 1.382–6.418, p = 0.0052). The adjusted OR for parity with the Ile/Val vs. Ile/Ile genotype was 1.739 (95 % CI 1.006–3.009, p = 0.0472).
Conclusion
Our genetic study suggests that the CYP1A1 Ile462Val SNP may be a risk factor for cervical cancer among patients with a positive history of tobacco smoking and parity.
Introduction
Cervical cancer is the third most common type of cancer in women worldwide [1]. This cancer develops slowly; starting from a precancerous dysplasia designated cervical intraepithelial neoplasia (CIN) that may further develop to invasive cervical carcinoma [2, 3]. Almost all cervical cancers are caused by the human papilloma virus (HPV), which is considered the primary etiologic factor of this cancer [3, 4]. Some oncogenic HPV oncoproteins, including E6 and E7, cause a defect in the host’s innate and adaptive immunity and change the apoptotic pathway, leading to immortalization of normal cervical epithelial cells [2, 4]. Apart from HPV, multiparity, oral contraceptive use, tobacco smoking, and environmental insults have been recognized as secondary risk factors [5].
Cytochrome P450 1A1 (CYP1A1) belongs to the CYP1 family and is involved in the metabolism of endogenous molecules and xenobiotics [6]. The actions of CYP1A1 include aryl hydrocarbon hydroxylase activity, which is the first step in the metabolism of tobacco polycyclic aromatic hydrocarbons, leading to their carcinogenic activation [7]. CYP1A1 also participates in the metabolism of estrogen [8]. Therefore, the activity of CYP1A1 may influence cells via at least two distinct pathways, i.e., smoking and estrogen, on cervical carcinogenesis [5].
Two single nucleotide polymorphisms (SNPs) in the CYP1A1 gene have been studied as risk factors of cervical carcinogenesis: T3801C (rs4646903) and A2455G (rs1048943) [9]. The T3801C SNP is located in the 3′ untranslated region and A2455G corresponds to the substitution Ile462Val in exon 7. These two SNPs have been suggested as being in linkage disequilibrium (LD) in Caucasian populations [9]. The role of both of these SNPs in CYP1A1 as risk factors in cervical cancer development in different ethnicities is still disputable [10–13]. Moreover, little is known about the interaction of these SNPs with the other known risk factors of cervical cancer. We evaluated the CYP1A1 Ile462Val genotype and allele frequencies in patients with cervical cancer (n = 456) and controls (n = 495) in the Polish population, stratified based on contraceptive use, postmenopausal status, parity, and tobacco smoking.
Patients and Methods
Patients and Controls
The patients included 456 women with histologically determined cervical carcinoma according to the International Federation of Gynecology and Obstetrics. All women were enrolled between April 2007 and December 2012 at the Department of Radiotherapy, Greater Poland Cancer Center in Poznan, Poland (Table 1). The control group included 285 unrelated healthy female volunteers who were matched by age to the patients. The controls were enrolled during medical examination at the University Hospital, Clinic of Gynecological Surgery at Poznan University of Medical Science (Table 1). Data about parity, oral contraceptive use, tobacco smoking, and menopausal status were obtained during the clinical interview. Patients and controls were Caucasian, enrolled from the Wielkopolska (Greater Poland) area of Poland. Patients and controls provided written informed consent. The study was approved by the Local Ethical Committee of Poznan University of Medical Sciences.
Table 1.
Clinical and demographic characteristics of patients and controls
| Characteristic | Patients (n = 456) | Controls (n = 495) |
|---|---|---|
| Mean agea (years) ± SD | 52.4 ± 9.8 | 51.9 ± 10.2 |
| Tumor stage, n (%) | ||
| IA | 61 (13.4) | |
| IB | 63 (13.8) | |
| IIA | 59 (12.9) | |
| IIB | 55 (12.1) | |
| IIIA | 148 (32.5) | |
| IIIB | 53 (11.6) | |
| IVA | 9 (2.0) | |
| IVB | 8 (1.7) | |
| Histologic grade, n (%) | ||
| G1 | 87 (19.1) | |
| G2 | 145 (31.8) | |
| G3 | 98 (21.5) | |
| Gx | 126 (27.6) | |
| Histologic type, n (%) | ||
| Squamous cell carcinoma | 384 (84.2) | |
| Adenocarcinoma | 56 (12.3) | |
| Other | 16 (3.5) | |
| Parity , n (%) | ||
| Never | 51 (11.2) | 56 (11.31) |
| Ever | 405 (88.8) | 439 (88.6) |
| Oral contraceptive pill use, n (%) | ||
| Never | 248 (54.4) | 278 (56.2) |
| Ever | 208 (45.6) | 217 (43.8) |
| Tobacco smoking, n (%) | ||
| Never | 295 (64.7) | 331 (66.9) |
| Ever | 161 (35.3) | 164 (33.1) |
| Menopausal status, n (%) | ||
| Premenopausal | 159 (34.9) | 190 (38.4) |
| Postmenopausal | 297 (65.1) | 305 (61.6) |
| HPV genotypesb, n (%) | ||
| 16 and 18 | 311 (68.2) | |
| 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 | 352 (77.2) | |
aAge at first diagnosis
bHPV genotypes were determined by Cobas® HPV Test Roche Molecular Systems, Inc. (Alameda, CA, USA)
HPV human papilloma virus, SD standard deviation
Genotyping
DNA was obtained from peripheral leukocytes employing a salting-out procedure. Identification of the CYP1A1 Ile462Val (rs1048943) polymorphic variant was performed by polymerase chain reaction-restriction fragment length polymorphism. The CYP1A1 Ile462Val DNA fragment was amplified employing the primers 5′ ACCCATCTGAGTTCCTACC 3′ and 5′ TCCACCTTCACGCCCAGT 3′. The PCR-amplified fragments of CYP1A1 that were 199 bp in length were isolated and digested with endonuclease HpyCH4III (5′ ACNGT 3′) from the New England BioLabs (Ipswich, USA). The CYP1A1 Val allele was cleaved into 116-bp and 83-bp fragments, whereas the CYP1A1 Ile allele remained uncut. Presence of the CYP1A1 Ile462Val polymorphism was verified by commercial sequencing analysis of 10 % randomly selected samples.
Statistical Analysis
The differences in genotypic and allelic distribution between patients and controls and their genotype deviation from the Hardy–Weinberg (HW) equilibrium were evaluated by the Chi-squared test. Moreover, the odds ratio (OR) and 95 % confidence intervals (95 % CI) were calculated. Unconditional logistic regression analysis was used to adjust for the effect of confounders such as age, parity, oral contraceptive use, tobacco smoking, and menopausal status. A p-value of <0.05 was considered statistically significant.
Results
Distribution of the CYP1A1 Ile462Val Polymorphism in Women with Cervical Cancer
Prevalence of the CYP1A1 Ile462Val genotypes did not display significant differences from the HW equilibrium between patients and controls. The distribution and adjusted analyses of the CYP1A1 Ile462Val genotypes in cases and controls are presented in Table 2. The CYP1A1 Ile/Val heterozygous genotype frequency was 1.5-fold greater in women with cervical cancer compared with controls and was 0.09 and 0.06, respectively. There were no individuals, either patients or controls, with the homozygous CYP1A1 Val/Val genotype. The CYP1A1 Val allele frequency was slightly increased in patients as compared with controls and was 0.04 and 0.03, respectively. Logistic regression analysis demonstrated that the CYP1A1 Ile/Val polymorphism was not associated with an increased risk of cervical cancer. The adjusted OR for patients with the Ile/Val genotype vs. Ile/Ile genotype was 1.539 (95 % CI 0.932–2.541, p = 0.091). However, stratification of the patients based on the histologic type of cancer also did not show a contribution of the CYP1A1 Ile/Val polymorphism with squamous cell carcinoma, adenocarcinoma, or with tumor stage and histologic grade (data not shown).
Table 2.
Association of the CYP1A1 Ile462Val (rs1048943) polymorphism with cervical cancer
| Genotype | Patients (frequency) | Controls (frequency) | OR (95 % CI) | p-Valuea | Adjusted OR (95 % CI)b | p-Valuea |
|---|---|---|---|---|---|---|
| Ile/Ile | 415 (0.91) | 466 (0.94) | Referent | – | Referent | |
| Ile/Val | 41 (0.09) | 29 (0.06) | 1.588 (0.9689–2.416) | 0.0646 | 1.539 (0.932–2.541) | 0.091 |
| Val/Val | 0 (0.00) | 0 (0.00) | ||||
| MAF | 0.04 | 0.03 |
aChi-square analysis
bORs were adjusted by age, parity, oral contraceptive use, tobacco smoking, and menopausal status
CI confidence interval, OR odds ratio
Stratified Analyses Between the CYP1A1 Ile462Val Polymorphism and Cervical Cancer Risks
The age-adjusted assessment of the CYP1A1 Ile462Val polymorphism and cervical cancer risk stratified by parity, oral contraceptive use, tobacco smoking, and menopausal status are listed in Table 3 and Fig. 1. An increase in cervical cancer risk was seen among patients with a positive history of tobacco smoking and parity. In patients with a positive history of tobacco smoking, the adjusted OR for the Ile/Val vs. Ile/Ile genotype was 2.978 (95 % CI 1.382–6.418, p = 0.0052). The adjusted OR for parity in patients with the Ile/Val vs. Ile/Ile genotype was 1.739 (95 % CI 1.006–3.009, p = 0.0472). However, no significant association was seen between CYP1A1 Ile462Val and patients with a positive history of oral contraceptive use and menopausal women (Table 3; Fig. 1). Moreover, we did not find an association between CYP1A1 Ile462Val and tumor stage, histologic grade, and type (Table 4; Fig. 2).
Table 3.
Stratified analyses between the distribution of CYP1A1 Ile462Val genotypes and cervical cancer risks: pregnancy, oral contraceptive use, tobacco smoking, and menopausal status
| High-risk exposure | Patients | Controls | Adjusted OR (95 % CI)a | p-Valueb | ||
|---|---|---|---|---|---|---|
| Genotype | Ile/Ile | Ile /Val | Ile/Ile | Ile /Val | ||
| Parity | ||||||
| Ever | 370 | 35 | 416 | 23 | 1.739 (1.006–3.009) | 0.0472 |
| Never | 45 | 6 | 50 | 6 | 1.596 (0.441–5.773) | 0.4709 |
| Oral contraceptive use | ||||||
| Ever | 187 | 21 | 205 | 12 | 1.923 (0.918–4,030) | 0.0821 |
| Never | 228 | 20 | 261 | 17 | 1.370 (0.697–2.692) | 0.3597 |
| Smoking | ||||||
| Ever | 134 | 27 | 154 | 10 | 2.978 (1.382–6.418) | 0.0052 |
| Never | 281 | 14 | 312 | 19 | 1.338 (0.575–3.113) | 0.4983 |
| Menopausal status | ||||||
| Premenopausal | 149 | 10 | 178 | 12 | 0.939 (0.391–2.257) | 0.8885 |
| Postmenopausal | 266 | 31 | 288 | 17 | 1.857 (0.997–3.460) | 0.0507 |
a(Ile /Val vs. Ile/Ile)
bChi square analysis
All p-values were adjusted by age. Significant results are highlighted in bold font
CI confidence interval, OR odds ratio
Fig. 1.
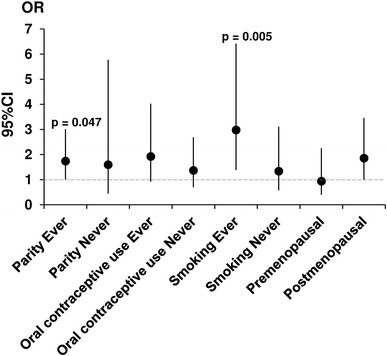
Adjusted odds ratio (OR) plot for genotyping frequencies of the CYP1A1 Ile462Val polymorphism for patients stratified based on parity, oral contraceptive use, tobacco smoking, and menopausal status. Each OR value is represented by the corresponding black dot with arms representing 95 % confidence intervals (95 % CI)
Table 4.
Stratified analyses between the distribution of CYP1A1 Ile462Val genotypes and clinical characteristics: stage, histologic grade, and type
| Genotype | Ile/Ile | Ile/Val | OR (95 % CI)a | p-Valueb |
|---|---|---|---|---|
| Controls | 466 | 29 | ||
| Patients | ||||
| Clinical stage | ||||
| IA | 55 | 6 | 1.753 (0.697–4.410) | 0.2275 |
| IB | 57 | 6 | 1.691 (0.673–4.250) | 0.2584 |
| IIA | 53 | 6 | 1.819 (0.722–4.583) | 0.1982 |
| IIB | 49 | 6 | 1.968 (0.779–4.973) | 0.1455 |
| IIIA | 139 | 9 | 1.040 (0.481–2.251) | 0.9200 |
| IIIB | 48 | 5 | 1.674 (0.619–4.526) | 0.3051 |
| IVA | 7 | 2 | 4.591 (0.912–23.107) | 0.1006c |
| IVB | 7 | 1 | 2.296 (0.273–19.300) | 0.3907c |
| Histologic grade | ||||
| G1 | 80 | 7 | 1.406 (0.596–3.319) | 0.4347 |
| G2 | 133 | 12 | 1.450 (0.720–2.920) | 0.2958 |
| G3 | 91 | 7 | 1.236 (0.525–2.908) | 0.6267 |
| Histologic type | ||||
| Squamous cell carcinoma | 353 | 31 | 1.407 (0.832–2.379) | 0.2003 |
| Adenocarcinoma | 48 | 6 | 2.009 (0.794–5.081) | 0.1336 |
ORs for patients with histologic grade Gx and unknown histologic type of cervical cancer were not calculated
a(Ile/Val vs. Ile/Ile)
bChi square analysis
cFisher exact test
CI confidence interval, OR odds ratio
Fig. 2.
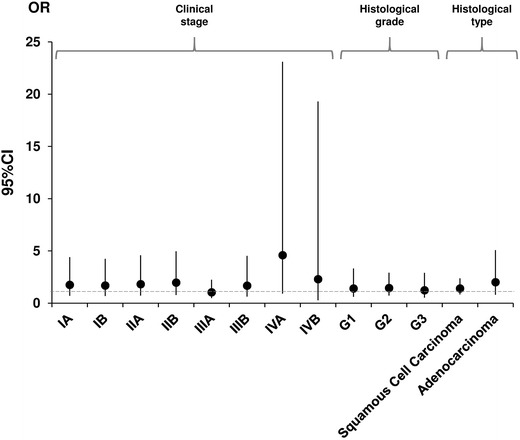
Adjusted odds ratio (OR) plot for genotyping frequencies of the CYP1A1 Ile462Val polymorphism for patients with cervical cancer stratified based on clinical characteristics: stage, histologic grade, and type. Each OR value is represented by the corresponding black dot with arms representing 95 % confidence intervals (95 % CI). ORs for patients with histologic grade Gx and unknown histological type of cervical cancer were not calculated
Discussion
The CYP1A1 Ile462Val polymorphism has been shown to be a risk factor for many types of cancer and hematopoietic malignancies [14–21]. To date, the CYP1A1 Ile462Val SNP has been shown to be a risk factor in the development of pharyngeal, prostate, lung, oral, ovary, bladder, and colorectal cancers [14–20]. In addition to these findings, the CYP1A1 Ile462Val SNP has been suggested to be a low-penetrant risk factor for acute leukemia [21]. There are also studies suggesting that the CYP1A1 Ile462Val SNP may be a risk factor for cervical cancer development [11, 12]. However, we did not observe, in our study, the CYP1A1 Ile462Val polymorphism to be a risk factor of cervical cancer in our patient group. Our results are similar to those of Gutman et al. [10], who did not find an increased risk for cervical cancer in the Jewish Israeli population. Moreover, there was no significant association between the CYP1A1 Ile462Val SNP and cervical cancer in the Japanese population [13]. In contrast, the study conducted by Taskiran et al. [11] in the Turkish population demonstrated the CYP1A1 Val gene variant as a significant risk factor of CIN 1 and 2 and for cervical adenocarcinoma and squamous cell carcinoma. Joseph et al. [12] observed a significant contribution of the CYP1A1 Ile462Val SNP to cervical cancer, when adjusted for HPV status in Indian populations. Moreover, the CYP1A1 Ile462Val polymorphism has been inconsistently shown to be a risk factor of cervical cancer in the Chinese population [22–27]. In addition to these findings, two recently conducted meta-analyses have indicated that the Val gene variant may be a risk factor in cervical cancer development in Caucasian but not Asian populations [28, 29].
The use of tobacco has been recognized as a factor that can increase the risk of numerous carcinomas, including lung, larynx, mouth, esophagus, kidneys, urinary tract, cervix, and others [30]. In our study, we found that the CYP1A1 Ile462Val SNP increased the risk of cervical cancer in women with a positive history of tobacco smoking. Gutman et al. [10] demonstrated that tobacco smoking is an independent risk factor for cervical cancer. The human CYP1A1 enzyme is present in many epithelial tissues and conducts oxidative reactions involved in the bioactivation of tobacco’s aromatic hydrocarbons, aromatic amines, and nitrosamines to carcinogens [7, 31]. These substances interact with DNA leading to the formation of DNA adducts, which make the carrier prone to carcinogenesis [7, 31]. It has been demonstrated that tobacco consumers bearing the CYP1A1 462Val variant possess more polycyclic aromatic hydrocarbon-DNA adducts in white blood cells as compared with smokers without that variant [32]. Although the CYP1A1 Ile462Val substitution situated in the heme-binding region of the enzyme did not change the enzyme’s activity in vitro, this substitution is accompanied by a twofold increase in microsomal enzyme activity [33, 34]. The CYP1A1 Ile462Val SNP has been proposed to be in complete LD with the CYP1A1 T3801C (rs4646903) polymorphism, which experimentally exhibited up-regulation of enzymatic activity [9].
Moreover, we found a borderline risk for cervical cancer in women with the Ile462Val SNP that had a positive history of parity, which is in agreement with other studies demonstrating parity as a risk factor for cervical cancer [35]. It has been suggested that high parity might increase the risk of cervical carcinoma owing to the many years of transformation of the exocervical zone, leading this area to become more susceptible to direct exposure to HPV and carcinogens [35].
Our genetic evaluation is the first to demonstrate that the CYP1A1 Ile462Val polymorphism can be a risk factor for cervical cancer in women with a positive history of tobacco smoking and parity, therefore this study should be replicated in other independent cohorts.
Acknowledgments
Supported by Grant No. 502-01-01124182-07474, Poznań University of Medical Sciences. None of the authors indicate a conflict of interest.
References
- 1.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Georgieva S, Iordanov V, Sergieva S. Nature of cervical cancer and other HPV-associated cancers. J BUON. 2009;14(3):391–398. [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother. 2012;18(6):807–815. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 5.Castellsague X, Munoz N. Chapter 3: cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31:20–28. doi: 10.1093/oxfordjournals.jncimonographs.a003477. [DOI] [PubMed] [Google Scholar]
- 6.Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013;368(1612):20120431. doi: 10.1098/rstb.2012.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McManus ME, Burgess WM, Veronese ME, et al. Metabolism of 2-acetylaminofluorene and benzo(a)pyrene and activation of food-derived heterocyclic amine mutagens by human cytochrome P-450. Cancer Res. 1990;50:3367–3376. [PubMed] [Google Scholar]
- 8.Monostory K, Dvorak Z. Steroid regulation of drug-metabolizing cytochromes P450. Curr Drug Metab. 2011;12(2):154–172. doi: 10.2174/138920011795016854. [DOI] [PubMed] [Google Scholar]
- 9.Petersen DD, McKinney CE, Ikeya K, et al. Human CYP1A1 gene: cosegregation of the enzyme inducibility phenotype and an RFLP. Am J Hum Genet. 1991;48(4):720–725. [PMC free article] [PubMed] [Google Scholar]
- 10.Gutman G, Morad T, Peleg B, et al. Cyp1a1 and cyp2d6 gene polymorphisms in Israeli Jewish women. Int J Gynecol Cancer. 2009;19(8):1300–1302. doi: 10.1111/IGC.0b013e3181b9fa5d. [DOI] [PubMed] [Google Scholar]
- 11.Taskiran C, Aktas D, Yigit-Celik N, et al. CYP1A1 gene polymorphism as a risk factor for cervical intraepithelial neoplasia and invasive cervical cancer. Gynecol Oncol. 2006;101(3):503–506. doi: 10.1016/j.ygyno.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Joseph T, Chacko P, Wesley R, et al. Germline genetic polymorphisms of cyp1a1, gstm1 and gstt1 genes in Indian cervical cancer: associations with tumor progression, age and human papillomavirus infection. Gynecol Oncol. 2006;101(3):411–417. doi: 10.1016/j.ygyno.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Sugawara T, Nomura E, Sagawa T, et al. Cyp1a1 polymorphism and risk of gynecological malignancy in Japan. Int J Gynecol Cancer. 2003;13(6):785–790. doi: 10.1111/j.1525-1438.2003.13607.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Wu G, Xue F, et al. Functional CYP1A1 genetic variants, alone and in combination with smoking, contribute to development of head and neck cancers. Eur J Cancer. 2013. [DOI] [PubMed]
- 15.Han G, Ma Y, Liu P, et al. Quantitative synthesis of the association between the cytochrome P450 1A1 Ile462Val polymorphism and prostate cancer risk. Tumour Biol. 2013;34(3):1511–1516. doi: 10.1007/s13277-013-0676-4. [DOI] [PubMed] [Google Scholar]
- 16.Ji YN, Wang Q, Suo LJ. CYP1A1 Ile462Val polymorphism contributes to lung cancer susceptibility among lung squamous carcinoma and smokers: a meta-analysis. PLoS ONE. 2012;7(8):e43397. doi: 10.1371/journal.pone.0043397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sergentanis TN, Economopoulos KP, Choussein S, et al. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and ovarian cancer risk: a meta-analysis. Mol Biol Rep. 2012;39(11):9921–9930. doi: 10.1007/s11033-012-1860-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhuo X, Zhao H, Chang A, et al. Cytochrome P450 1A1 Ile462Val polymorphism and oral carcinoma risk: an updated meta-analysis including 1,515 cases and 2,233 controls. Tumour Biol. 2012;33(6):2079–2089. doi: 10.1007/s13277-012-0467-3. [DOI] [PubMed] [Google Scholar]
- 19.Öztürk T, Kahraman ÖT, Toptaş B, et al. The effect of CYP1A1 and GSTM1 gene polymorphisms in bladder cancer development in a Turkish population. In Vivo. 2011;25(4):663–668. [PubMed] [Google Scholar]
- 20.Jin JQ, Hu YY, Niu YM, et al. CYP1A1 Ile462Val polymorphism contributes to colorectal cancer risk: a meta-analysis. World J Gastroenterol. 2011;17(2):260–266. doi: 10.3748/wjg.v17.i2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuo W, Zhang L, Zhu B, et al. Association between CYP1A1 Ile462Val variation and acute leukemia risk: meta-analyses including 2164 cases and 4160 controls. PLoS ONE. 2012;7(10):e46974. doi: 10.1371/journal.pone.0046974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding FY, Ma GF, Song XH, Shi WH, Lan JY, Yu HY. Relationship between cyp1a1 gene polymorphism and genetic susceptibility of cervical carcinoma. Jiangsu Med J. 2011;37:2562. [Google Scholar]
- 23.Geng J, Shi YR, Wang H, Qin R. Research of cytochrome p450 i a1 lle/val polymorphism and genetic susceptibility in cervical cancer. J Bengbu Med Coll. 2010;35:762. [Google Scholar]
- 24.Shi YR, Geng J, Cheng LQ, Wang H, Zhang Y. Association of cytochrome p450 1a1 gene polymorphisms with cervical cancer. Fudan Univ J Med Sci. 2011;38(05):428–431. [Google Scholar]
- 25.Zhang SH, Kong AR. Polymorphisms of cyp1a1 gene and hpv infection of cervical squamous carcinoma: Master’s thesis of Taishan Medical University; 2009.
- 26.Zhang X. P450 1a1 gene polymorphism and cervical cancer level correlation. Jilin Med J. 2011;32:419. [Google Scholar]
- 27.Huang YK, Hsieh HC, Sun JA, et al. Genetic polymorphisms of phase I and phase II xenobiotic enzymes in human papillomavirus related lesion and cancer of the uterine cervix. Tzu Chi Med J. 2006;18(4):267–274. [Google Scholar]
- 28.Yang S, Jia C, Zhu H, et al. CYP1A1 Ile462Val polymorphism and cervical cancer: evidence from a meta-analysis. Tumour Biol. 2012;33(6):2265–2272. doi: 10.1007/s13277-012-0488-y. [DOI] [PubMed] [Google Scholar]
- 29.Sergentanis TN, Economopoulos KP, Choussein S, et al. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and cervical cancer risk: a meta-analysis. Mol Biol Rep. 2012;39(6):6647–6654. doi: 10.1007/s11033-012-1470-x. [DOI] [PubMed] [Google Scholar]
- 30.Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene. 2002;21(48):7307–7325. doi: 10.1038/sj.onc.1205807. [DOI] [PubMed] [Google Scholar]
- 31.Landi MT, Bertazzi PA, Shields PG, et al. Association between CYP1A1 genotype, mRNA expression and enzymatic activity in humans. Pharmacogenetics. 1994;4(5):242–246. doi: 10.1097/00008571-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Mooney LA, Bell DA, Santella RM, et al. Contribution of genetic and nutritional factors to DNA damage in heavy smokers. Carcinogenesis (Lond.) 1997;18:503–509. doi: 10.1093/carcin/18.3.503. [DOI] [PubMed] [Google Scholar]
- 33.Zhang ZY, Fasco MJ, Huang L, et al. Characterization of purified human recombinant cytochrome P4501A1-lle462 and Val462: assessment of a role for the rare allele in carcinogenesis. Cancer Res. 1996;56:3926–3933. [PubMed] [Google Scholar]
- 34.Persson I, Johansson I, Ingelman-Sundberg M. In vitro kinetics of two human CYP1A1 variant enzymes suggested to be associated with interindividual differences in cancer susceptibility. Biochem Biophys Res Commun. 1997;231:227–230. doi: 10.1006/bbrc.1997.6051. [DOI] [PubMed] [Google Scholar]
- 35.Muñoz N, Franceschi S, Bosetti C, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Multicentric Cervical Cancer Study Group. Lancet. 2002;359(9312):1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]


