Abstract
Background
Transient receptor potential vanilloid type 1 (TRPV1) is a non-selective cation channel widely expressed in skin tissues, and peripheral sensory nerve fibres. Activation of TRPV1 releases neuropeptides; the resulting neurogenic inflammation is believed to contribute to the development of pruritus. A TRPV1 antagonist has the potential to perform as an anti-pruritic agent. SB705498 is a TRPV1 antagonist that has demonstrated in vitro activity against cloned TRPV1 human receptors and when orally administered has demonstrated pharmacodynamic activity in animal models and clinical studies.
Objectives
To select a topical dose of SB705498 using the TRPV1 agonist capsaicin; to confirm engagement of the TRPV1 antagonistic action of SB705498 and assess whether the dose selected has an effect on itch induced by two challenge agents.
Methods
A clinical study was conducted in 16 healthy volunteers to assess the effects of 3 doses of SB705498 on skin flare induced by capsaicin. Subjects with a robust capsaicin response were chosen to determine if the selected topical formulation of SB705498 had an effect on challenge agent induced itch.
Results
Following capsaicin challenge the greatest average reduction in area of flare was seen for the 3% formulation. This dose was selected for further investigation. Itch intensity induced by two challenge agents (cowhage and histamine) was assessed on the Computerised Visual Analogue Scale. The difference in average itch intensity (Weighted Mean Over 15 Mins) between the 3% dose of SB705498 and placebo for the cowhage challenge was −0.64, whilst the histamine challenge showed on average a −4.65 point change.
Conclusions
The 3% topical formulation of SB705498 cream was clinically well tolerated and had target specific pharmacodynamic activity. However there were no clinically significant differences on pruritus induced by either challenge agent in comparison to placebo. SB705498 is unlikely to be of symptomatic benefit for histaminergic or non-histaminergic induced itch.
Trial Registration
ClinicalTrials.gov NCT01673529
Introduction
Pruritus (itching) is a common symptom of skin disease and can best be defined as an unpleasant cutaneous sensation that leads to a desire to scratch [1], [2]. It can also be a common symptom in systemic disease and psychiatric disorders. All human beings experience pruritus in the course of their lifetime.
Chronic itch, which lasts for longer than 6 weeks, has a profound impact on quality of life, including detrimental effects on sleep, attention, and sexual function. At present, there is no universally accepted effective therapy for itch.
Historically, the neuronal pathways for itch have been principally characterised by responses to histamine. Intracutaneous application of histamine produces intense itch and a large area of axon-reflexive vasodilation (“flare”) around the application site. Both phenomena are thought to be mediated through neuronal activity in itch-specific, mechanoinsensitive C-fibre afferents(CMi). However, mechanical and electrical stimuli that do not activate CMi fibres can cause the sensation of itch, and itch may occur without flare, suggesting that other neuronal itch pathways exist [3]. There are many direct mediators of itch and there may be redundant systems. Numerous publications have identified the Transient Receptor Potential (TRP) channels (e.g. TRPV1, TRPV3, TRPA1, TRPM8) as having a key role in pruritus (for review see [4]–[8]) and Atopic Dermatitis (AD). TRPV1 has been shown to be up-regulated in AD-skin lesions, and the activation of TRPV1 causes the release of proinflammatory and pruritic mediators [7], [9].Ultimately these channels are key in depolarizing itch sensing neurons independent of upstream (redundant) pathways. Blocking these channels has the potential to block the itch sensation
The TRPV1 receptor can be activated by the TRPV1 agonist capsaicin or endogenous inflammatory mediators. The TRPV1 receptor is expressed in skin tissue including keratinocytes and peripheral sensory nerve fibres (C and Aδ).
SB705498 is a selective potent TRPV1 antagonist [10] that has demonstrated in vitro antagonist activity against cloned human TRPV1 receptors and when orally administered has shown pharmacodynamic activity in animal models and in clinical studies of pain and nasal secretion. [11]–[14].
Two challenge agents (Histamine and Cowhage) were selected as they induce pruritus by different mechanisms and hence would allow exploration of the therapeutic potential of SB705498. Histamine is thought to initiate pruritus through activation of sensory neurons predominantly C-fibers and via activation of phospholipase A2 and 12-lipoxygenase [15] and is the key puritogen in urticarial skin diseases in which antihistamines are most effective [16]. However several skin disorders including atopic dermatitis are resistant to antihistamine therapies [17]; [18]. Cowhage spicules (Mucuna pruriens), act through a histamine independent pruriceptive neuronal pathway releasing a cysteine protease (mucunanin) that activates proteinase-activated receptor 2 (PAR2) and PAR4 [19] in nerve fibers and keratinocytes. PAR2 activation has been reported to modulate the expression and ion channel activity of TRPV1. [20]; [21]. Costa et al. [22] suggested trypsin injection could activate PAR-2 receptors, stimulating the release of several mast cell mediators which in turn may sensitise TRPV1 receptors on sensory nerves, transmitting itch sensations to the CNS. More recently Belghiti et al. [23] showed that activation of PAR2 signalling sensitizes nociceptors by augmenting the expression and activity of neuronal TRPV1 channels contributing to the persistence of a puritogenic state in a rat model of liver disease. PAR2 receptors and their ligands, serine proteases, have previously been demonstrated to have a significant role in the itch associated with AD [7]. The potential role for an anti-pruritic effect of a TrpV1 antagonist (PAC14028) has been assessed and found to have a positive impact on a PAR-2 mediated murine atopic dermatitis and itching models [24].
Objectives
The study was designed as a two part study in order to address the role of TRPV1 in pruritus and investigate the therapeutic potential of SB705498.
Part A was designed to assess whether any of three doses (1%, 3% and 5%) of SB705498 were able to adequately reach the target activated by the TRPV1 agonist capsaicin and to evaluate the safety and tolerability of SB705498 compared to placebo. Only if Part A was positive was Part B of the study initiated, with the dose of SB705498 producing the largest and/or most consistent average reduction in flare in Part A. Part B was designed to assess the affect of SB705498 on itch intensity and duration of itch induced by challenge agents (cowhage and histamine) compared to placebo.
Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; See Checklist S1 and Protocol S1.
Study Overview
The study was conducted as a single site, two part randomised, double-blind, placebo controlled trial. The study took place at GSK's Clinical Unit in Addenbrookes Hospital, Cambridge, United Kingdom, between July and October 2012. The clinical research was reviewed and approved by GSKs internal review panels and Independent Local Research Ethics Committee located in Brent, London, United Kingdom. Written informed consent was obtained prior to study recruitment and the investigations were conducted according to the principles expressed in the Declaration of Helsinki.
This study was open to adult (≥18 years old) male or female healthy volunteers. Screening involved review of medical history, physical examination and laboratory screening tests. The use of recreational drugs, alcohol and nicotine products, was restricted or prohibited. Participants were required to avoid UV exposure for 7 days prior to screening, during study conduct and for 7 days after the last dose. Subjects were not eligible for inclusion if they presented with any skin infection or inflammation on the forearm or suffered from any acute or chronic dermatological problems. In each treatment session subjects were screened for drugs of abuse and alcohol, vital signs, and ECG prior to all other assessments. All eligible participants were required to show a flare response to capsaicin and to have pruritus induced by both challenge agents (cowhage and histamine) prior to enrolment in the study. Details of the capsaicin and cowhage/histamine challenge are detailed under Screening.
The clinical study staff were blinded to the treatment until the study was completed, however specified members of the team had details of treatment allocation for safety purposes and also to allow for selection of subjects for further study (Double Blind (sponsor un-blind)).
Sample Size
Part A was designed to randomise 16 subjects in a complete block 4-period crossover design. Sixteen subjects would ensure that the 95% confidence interval for the ratio between active and placebo for the area of flare as induced by capsaicin would be no wider than 37.7%, assuming a within subject coefficient of variation of 0.45.
Part B of the study was powered to detect a 20 point difference between the chosen dose of SB705498 and placebo on the 0–100 Computerized Visual Analogue Scale (COVAS, Medoc, Ramat-Yishai, Israel), assuming a within subject standard deviation of 11.5 with a two-sided type 1 error rate of 5%. Ten subjects were required for the crossover design.
Randomisation Details
The centre based randomisation schedule for part A was created adopting a Williams design of a generalised Latin square, for a 4×4 crossover period design using a block size of 8. The four sequences were 1 2 4 3, 2 3 1 4, 3 4 2 1 or 4 1 3 2, where 1 was placebo and 2 to 4 were assigned to each increase in dose, and each treatment was assigned using an equal randomisation ratio.
The centre based randomisation schedule for part B, was an incomplete block crossover design using a block size of 8, as 8 sequences were created. An equal randomisation ratio was allocated between the sequences but given the number of subjects not all sequences were utilised twice. The sequences utilised were: E F E F, F E F E, E F F E or F E E F in a 1∶1∶1∶1 ratio where one of these were placebo and the other was SB705498.
Allocation/Implementation
A randomisation sequence was generated by the GSK statistician and the randomisation schedule was sent to the clinical site. Based on treatment allocation detailed in the randomisation schedule, doses of the active cream or vehicle of the cream (placebo) was prepared by the pharmacy staff at the Clinical site.
Participants were enrolled into the study by recruitment staff at the site according to their standard procedure for healthy volunteer studies. Participants were identified via the site's healthy volunteer database. Once the participants passed screening they were assigned to the allocated interventions by the principal investigator.
The site staff and the participants were blinded to the treatment with the exception of the site pharmacy staff, who did the packaging and releasing of the cream or vehicle of the cream (placebo).
Screening
At screening all eligible participants were required to show a flare response to capsaicin and to have pruritus induced by both challenge agents (cowhage and histamine) - scoring greater than 40 on the 0–100 COVAS. The COVAS allows rating of itch intensity on a 100 mm scale that ranges from ‘no itch’ at one end to ‘unbearable itch’ at the other [25]. All subjects were familiarised with study assessments, including any pharmaco-dynamic tests at the screening visit.
Thirty days were allowed between screening and Part A of the study and a maximum of 60 days between screening and Part B of the study. No subjects were required to re-screened prior to the start of Part B.
Capsaicin Challenge
Approximately 0.5 mL of capsaicin cream (Axsain, 0.075% capsaicin w/w) was applied to a 3×3 cm square area on the volar aspect of one arm. The cream was left on the skin for 30 minutes and then gently wiped off. Assessments of skin blood flow were performed before and after capsaicin application by monitoring cutaneous blood flow using Laser Doppler imaging (LDI) (LDI-2, Moor Instruments Ltd., Devon, UK). A suitable area of approximately 16×8 cm around the stimulation site was scanned. The flare area (in cm2) was calculated from all pixels around the stimulation site in which flux values exceeded the 95% percentile (mean +2 SD) of the baseline distribution. The mean blood flow in the area of flare was also calculated using relative flux (arbitrary units).
Cowhage
Approximately 70–100 cowhage spicules/fragments were counted for each application under a microscope to ensure between 30–35 spicules of sufficient quality for itch induction were available. These spicules were transferred from the microscope to folded paper troughs using disposable gel-loading tips and taken to the clinic on the paper trough in Petri dishes. The spicules were then transferred directly from the paper to a predefined 3x3 cm area on the volar aspect of the forearm by holding the paper vertically and tapping gently with a suitable implement e.g. a pen (being careful not to inadvertently flick the paper and disperse spicules).
The spicules were gently rubbed with a gloved finger for 45 seconds onto the subject's skin with a circular motion to facilitate contact. Approximately 1–2 minutes before contact with the cowhage spicules subjects were asked to start rating their itch intensity using the 0–100 COVAS. The itch intensity was recorded for 15 minutes or until an itch score of 0 (baseline) was recorded for 60 continuous seconds. Recording of pruritus did not exceed 15 minutes.
Histamine
A 1% solution of histamine was applied using the skin prick method. [26]. This is a widely accepted method used in allergy clinics to test for histamine sensitivity; causing pruritus, skin flare and a skin wheal. The formulation and dose of histamine was based on methodology used in similar studies of itch [25]. The skin prick method involves placing a drop of 1% histamine solution onto the skin and using a lancet to gently pierce the superficial layer of the skin. The excess histamine solution was then wiped away. Itch intensity was rated using the COVAS as previously described.
Design of Part A
16 subjects were recruited in Part A and were required to participate in a capsaicin challenge.
Subjects received individual applications of one of three doses of 1%, 3% and 5% of SB705498 as well as a placebo in a randomised order on four discrete 3×3 cm square patches on the volar surface of both forearms over 2 days (Two applications were applied each day). The topical application of SB705498 or placebo was left on the arm for 1 hour. Following the procedures described under screening the capsaicin was applied and assessments of skin blood flow (flare) were performed using LDI. A baseline LDI scan was performed prior to the application of any cream (SB705498 or Placebo) and again after 1 hr once the excess cream had been wiped away. A third scan occurred 5 minutes after the capsaicin challenge (once excess capsaicin has been wiped away). A final scan took place 2 hours post application of the SB705498/Placebo. A reduction in flare compared to placebo was considered a positive study outcome for Part A.
The decision to progress to Part B of the study was made by the unblinded members of the study team after reviewing the data from Part A. A reduction in area of flare for subjects receiving SB705498 compared with placebo was required to be observed on at least one of the dose strengths studied. The dose level deemed to have the largest and most consistent effect was the 3% cream and this was the dose strength studied in Part B.
Design of Part B
Of the sixteen subjects randomised to participate in Part A, ten subjects who passed a second round of screening and who showed the most optimal treatment response during the capsaicin challenge (Part A) compared to placebo were asked to participate in Part B. Each volunteer was randomised to receive the placebo and the 3% SB705498 cream for both the cowhage and histamine challenge. Either the placebo or active cream was applied 1 hour before treatment with the challenge agent. Between itch inductions a break was taken to allow previous itch sensations to completely subside.
Each subject participated in both a cowhage and a histamine challenge. Challenge agents were applied on consecutive days with both treatment arms in a crossover fashion. Full details of the application process for the cowhage and histamine are described under screening.
Statistical Analysis
Part A
The primary endpoint, area of flare, was log transformed prior to analysis and analysed via a mixed effects model, fitting treatment and period as fixed effects and subject as a random effect, calculating pair wise comparison for each active dose against placebo. Ratios to placebo and 95% confidence intervals of the ratio of the difference between each active dose strength and placebo were calculated.
Part B
The primary endpoint of average itch was calculated over the 15 minute assessment period, as a weighted mean, weighting the itch scores depending on the amount of time between each itch intensity assessments. This was analysed using a mixed effects model, fitting terms for treatment and period as fixed effects and subject as a random effect. A separate model was performed for each stimulant. No baseline was fitted in the model since no itch was present until the stimulant was added. Point estimates and 95% percent confidence intervals for the difference between and placebo were constructed.
Upon inspection of the data it was decided to perform an additional analysis of the average itch over the itch period only, as determined by the time of itch onset until the time to zero itch or the end of the challenge period. This was due to less itch than expected being reported over the 15 minute assessment period.
Descriptive statistics were used to describe all endpoints in Part B, including time to onset, time for itch to return to zero, duration of itch, time to peak itch, peak itch intensity and duration of peak itch.
Results
Enrolment and baseline Characteristics
Between July and October 2012 a total of 45 healthy volunteers (≥18 years old) were screened and 16 white male subjects were recruited into Part A of the study. Whilst male and female subjects were eligible to participate only male subjects were recruited into this study. Only volunteers that were sensitive to capsaicin i.e. develop flare on application of 0.5 ml of Axsain and had a score of ≥40 on the COVAS for both histamine and cowhage were enrolled.
Ten of the best available responders were identified and subsequently invited to participate in Part B (See Figure 1). Demographic data for the cohort is provided in Table 1.
Figure 1. CONSORT diagram.
Table 1. Demographics and Baseline Characteristics.
| Part A | Part B | |
| Demographics | ||
| Age in Years, Mean (Range) | 39.4 (30,52) | 39.9 (30,52) |
| Sex, n | ||
| Female: | 0 | 0 |
| Male: | 16 | 10 |
| BMI (kg/m2), Mean (Range) | 26.36 (22.9,30.7) | 25.97 (23.3,30.7) |
| Height (cm), Mean (Range) | 177.6 (162,189) | 178.7 (162,189) |
| Weight (kg), Mean (Range) | 83.1 (69,100) | 82.8 (71,100) |
| Ethnicity, n (%) | ||
| Hispanic or Latino: | 0 | 0 |
| Not Hispanic or Latino: | 16 (100%) | 10 (100%) |
| Race, n (%) | ||
| White – White/Caucasian/European Heritage | 16 (100%) | 10 (100%) |
Pharmacodynamic Results
Plasma/serum analysis revealed no quantifiable drug above the detection limit of the assay (0.5 ng/mL) and as such no PK analysis could be conducted. No clinically significant drug related AE's or any SAE's were reported from either part of the study.
Part A
Area of Flare
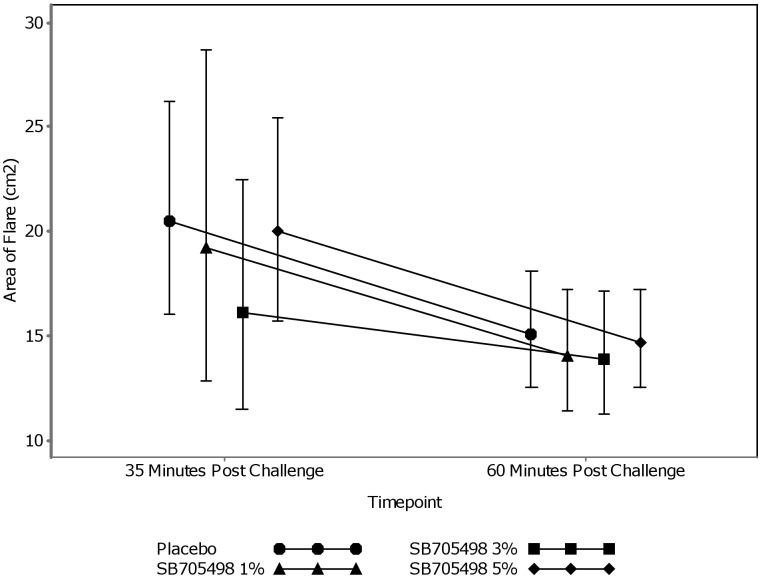
No clear dose response was observed at either of the post challenge timepoints for the three doses of SB705498 and placebo and therefore conclusions were drawn from the mixed effects model. Figure 2 shows the geometric mean profile with 95% C.I.of area of flare for placebo and the 1%, 3% and 5% doses of SB705498.
Figure 2. Geometric Mean Profile Plot of Area of Flare.
Adjusted geometric means, produced from the statistical analysis, for each treatment arm and for both timepoints are shown in Table 2. For the 35 minute post capsaicin challenge timepoint the biggest difference observed between the active doses and the placebo was seen on the 3% cream. Table 3 shows the adjusted ratio of the treatment difference was 0.78 (95% C.I. (0.52, 1.18) indicating a 22% average reduction in area of flare for 3% compared with placebo. Based on the data, the probability there was any treatment effect for this dose strength (ratio<1) was 88%. The 1% and 5% cream showed a small beneficial effect when compared with the placebo cream.
Table 2. Adjusted Geometric Means from Statistical Analysis of Area of flare.
| Challenge | Treatment | N | n | Adjusted means (SE Logs) | 95% Confidence Interval |
| 35 Mins Post Challenge | Placebo | 16 | 16 | 20.49 (0.148) | (15.24, 27.56) |
| SB705498 1% | 16 | 16 | 19.18 (0.148) | (14.26, 25.80) | |
| SB705498 3% | 16 | 16 | 16.07 (0.148) | (11.95, 21.61) | |
| SB705498 5% | 16 | 16 | 19.98 (0.148) | (14.86, 26.87) | |
| 60 Mins Post Challenge | Placebo | 16 | 16 | 15.06 (0.089) | (12.60, 17.99) |
| SB705498 1% | 16 | 16 | 14.04 (0.089) | (11.75, 16.78) | |
| SB705498 3% | 16 | 16 | 13.90 (0.089) | (11.63, 16.62) | |
| SB705498 5% | 16 | 16 | 14.70 (0.089) | (12.30, 17.57) |
Table 3. Treatment Comparisons from Statistical Analysis of Area of Flare.
| Challenge | Comparison | Adjusted Ratio(SE Logs) | 95% Confidence Interval | Probability Ratio1 | ||
| <1 | <0.9 | <0.7 | ||||
| 35 Mins Post Challenge | SB705498 1% - Placebo | 0.94 (0.205) | (0.62, 1.41) | 0.63 | 0.42 | 0.22 |
| SB705498 3% - Placebo | 0.78 (0.205) | (0.52, 1.18) | 0.88 | 0.75 | 0.54 | |
| SB705498 5% - Placebo | 0.98 (0.205) | (0.65, 1.47) | 0.55 | 0.35 | 0.17 | |
| 60 Mins Post Challenge | SB705498 1% - Placebo | 0.93 (0.119) | (0.73, 1.19) | 0.72 | 0.38 | 0.10 |
| SB705498 3% - Placebo | 0.92 (0.119) | (0.73, 1.17) | 0.75 | 0.41 | 0.12 | |
| SB705498 5% - Placebo | 0.98 (0.119) | (0.77, 1.24) | 0.58 | 0.25 | 0.05 | |
Posterior Probability the treatment ratio is less than the stated number. This was calculated assuming non-informative priors.
For the 60 minute post capsaicin challenge timepoint the beneficial treatment effect for the 3% cream reduced to a comparable level with the 1% and 5% cream. All 3 doses showed minor benefit when compared to the placebo.
Given these results it was decided to progress the 3% cream through to Part B of the study.
Part B
Itch Intensity (Weighted Mean over 15 Mins)
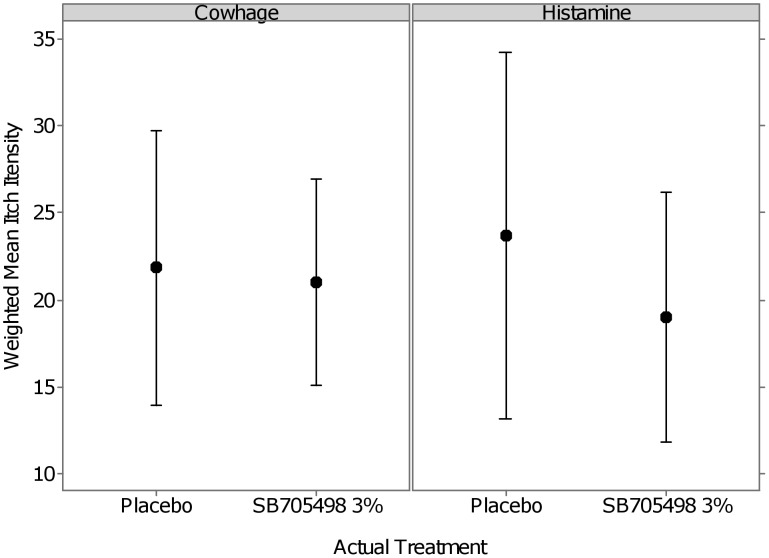
The mean average itch intensity as defined as the weighted itch scores over the 15 min assessment window (95% C.I.) to both the cowhage and histamine challenge can be seen in Figure 3. The mean responses for the cowhage challenge appear to be very similar across treatment arms however with slightly less variability for SB705498 3% compared to placebo.
Figure 3. Mean profile plot of average itch intensity (weighted mean over 15 minutes).
The adjusted means for each treatment arm for each challenge from the mixed effects model for the average itch intensity (Weighted Mean Over 15 Mins) can be seen in Table 4 and the comparisons for each compared to placebo can be seen in Table 5.
Table 4. Adjusted Means for Average Itch Intensity.
| Challenge | Treatment | N | n | Adjusted means (Std Err) | 95% Confidence Interval |
| Cowhage | Placebo | 10 | 9 | 21.62 (2.838) | (15.32, 27.93) |
| SB705498 3% | 10 | 10 | 20.99 (2.805) | (14.72, 27.25) | |
| Histamine | Placebo | 10 | 10 | 23.67 (3.999) | (14.86, 32.48) |
| SB705498 3% | 10 | 10 | 19.02 (3.999) | (10.21, 27.83) |
Table 5. Treatment Comparison for Average Itch Intensity.
| Challenge | Comparison | Adjusted Mean Difference (std Err) | 95% Confidence Interval | Probability Difference1 | |||
| <0 | <−5 | <−10 | |||||
| Weighted mean over 15 mins | Cowhage | SB705498 3% - Placebo | −0.64 (1.307) | (−3.71, 2.44) | 0.68 | <0.01 | <0.01 |
| Histamine | SB705498 3%- Placebo | −4.65 (2.514) | (−10.44, 1.15) | 0.95 | 0.45 | 0.03 | |
Posterior Probability the treatment difference is less than the stated number. This was calculated assuming non-informative priors.
The average difference between SB705498 3% and placebo for the cowhage challenge was -0.64 (95% C.I. (−3.71, 2.44)) indicating a small average reduction in average itch intensity for those on SB705498. The probability based on the observed data that the true difference is less than zero on the VAS, i.e. any beneficial effect compared with placebo, was 68% and the probability for a 5 point improvement for SB705498 3% is less than 1%.
The histamine challenge showed on average a −4.65 (95% C.I. (−10.44, 1.15)) reduction in average itch intensity (Weighted Mean Over 15 Mins) for SB705498 3% compared to placebo. For histamine the probability based on the observed data that the true difference is less than zero on the VAS, i.e. any beneficial effect compared with placebo, was 95% and the probability for a 5 point improvement for SB705498 3% is 45%.
Itch Intensity (Weighted Mean over Itch Period)
Itch Intensity over the itchy period, defined as the time from itch onset until time to zero itch, or the end of the 15 min period was calculated. The average difference between SB705498 3% and placebo for the cowhage challenge over the itch period was −2.08 (95% C.I. (−6.21, 2.04)) again indicating a small reduction in average itch intensity for those on SB705498 (See Table 6). The probability based on the observed data that the true difference is less than zero on the VAS, i.e. any beneficial effect compared with placebo, was 86% and the probability for a 5 point improvement for SB705498 3% was 7%. There was a small statistical difference on the histamine data as this showed a point change of −4.71 (95% C.I.−9.12, −0.31) however was not deemed clinically significant (See Table 7).
Table 6. Adjusted Means for Weighted Mean over Itch Period.
| Challenge | Treatment | N | n | Adjusted means (Std Err) | 95% Confidence Interval |
| Cowhage | Placebo | 10 | 9 | 35.54 (1.639) | (32.05, 39.03) |
| SB705498 3% | 10 | 10 | 33.45 (1.546) | (30.15, 36.76) | |
| Histamine | Placebo | 10 | 10 | 31.02 (3.265) | (23.81, 38.24) |
| SB705498 3% | 10 | 10 | 26.31 (3.265) | (19.09, 33.52) |
Table 7. Treatment Comparisons for Weighted Mean over Itch Period.
| Challenge | Comparison | Adjusted Mean Difference (std Err) | 95% Confidence Interval | Probability Difference1 | |||
| <0 | <−5 | <−10 | |||||
| Weighted mean over itch period | Cowhage | SB705498 3% - Placebo | −2.08 (1.786) | (−6.21, 2.04) | 0.86 | 0.07 | <0.01 |
| Histamine | SB705498 3% - Placebo | −4.71 (1.910) | (−9.12, −0.31) | 0.98 | 0.44 | 0.01 | |
Posterior Probability the treatment difference is less than the stated number. This was calculated assuming non-informative priors.
Secondary Endpoints
Table 8 shows the results of the summary statistics calculated for each secondary endpoint over the 15 minute period during which itch was recorded. Consistent results were seen across all endpoints.
Table 8. Summary Statistics for Secondary Endpoints.
| Challenge | Treatment | N | n1 | Mean | Standard Deviation | Median | 95% Confidence Interval | |
| Time to Itch onset (seconds) | Cowhage | Placebo | 10 | 9 | 136.2 | 78.65 | 169.7 | 75.8, 196.7 |
| SB705498 3% | 10 | 10 | 176.1 | 23.44 | 185.0 | 159.3, 192.8 | ||
| Histamine | Placebo | 10 | 10 | 175.5 | 18.78 | 169.0 | 162.1, 188.9 | |
| SB705498 3% | 10 | 10 | 180.1 | 25.40 | 172.5 | 162.0, 198.3 | ||
| Time to zero Itch (seconds) | Cowhage | Placebo | 10 | 7 | 518.8 | 138.15 | 483.0 | 391.1, 646.6 |
| SB705498 3% | 10 | 8 | 548.0 | 122.18 | 500.4 | 445.9, 650.2 | ||
| Histamine | Placebo | 10 | 8 | 657.7 | 134.71 | 658.0 | 545.0, 770.3 | |
| SB705498 3% | 10 | 8 | 644.0 | 165.97 | 620.9 | 505.3, 782.2 | ||
| Duration of Itch (Seconds) | Cowhage | Placebo | 10 | 7 | 343.7 | 147.44 | 307.8 | 207.3, 480.1 |
| SB705498 3% | 10 | 8 | 369.0 | 113.60 | 330.9 | 274.0, 464.0 | ||
| Histamine | Placebo | 10 | 8 | 486.7 | 132.72 | 479.4 | 375.7, 597.6 | |
| SB705498 3% | 10 | 8 | 461.1 | 166.07 | 440.7 | 322.2, 599.9 | ||
| Time to peak Itch (seconds) | Cowhage | Placebo | 10 | 9 | 277.9 | 103.61 | 216.3 | 198.2, 357.5 |
| SB705498 3% | 10 | 10 | 234.2 | 33.81 | 230.2 | 210.0, 258.4 | ||
| Histamine | Placebo | 10 | 10 | 336.0 | 257.05 | 223.5 | 152.1, 519.9 | |
| SB705498 3% | 10 | 10 | 277.7 | 109.62 | 218.9 | 199.2, 356.1 | ||
| Peak Itch Intensity | Cowhage | Placebo | 10 | 9 | 88.8 | 8.81 | 86.0 | 82.0, 95.6 |
| SB705498 3% | 10 | 10 | 87.2 | 14.05 | 87.5 | 77.1, 97.3 | ||
| Histamine | Placebo | 10 | 10 | 72.3 | 19.39 | 78.9 | 58.4, 86.2 | |
| SB705498 3% | 10 | 10 | 65.4 | 16.51 | 60.0 | 53.6, 77.2 | ||
| Duration of Peak Itch (Milliseconds) | Cowhage | Placebo | 10 | 9 | 172.9 | 141.32 | 118.5 | 64.3, 281.6 |
| SB705498 3% | 10 | 10 | 204.2 | 175.64 | 164.2 | 78.5, 329.8 | ||
| Histamine | Placebo | 10 | 10 | 154.0 | 211.41 | 44.7 | 2.8, 305.2 | |
| SB705498 3% | 10 | 10 | 166.8 | 192.71 | 105.2 | 29.0, 304.7 |
Some of these values are not 100% of the population because time to zero itch, for example, was only recorded if the subject returned to zero itch. Likewise duration of itch was only recorded if time to zero itch had occurred.
Discussion
No clinically significant drug related AE's or any SAE's were reported from either Part A or Part B of the study.
Part A
Capsaicin is a selective and potent exogenous agonist for the TRPV1 receptor, application of which will produce a flare on the skin. We were able to see a reduction in flare with all three doses of the TRPV1 antagonist SB705498 cream indicating TRPV1 receptor engagement was achieved.
There was no clear dose response observed for each of the three doses at either timepoint for the capsaicin challenge, however the 3% dose performed better - showing on average a 20% better response then the 5% cream at 35 mins post challenge (p-values = 0.2927). The difference between the 3 and 5% dose was likely due to saturation with the 3% dose having the greatest amount of drug in solution.
The 3% SB705498 cream produced the largest reduction in area of flare in comparison to the other two doses at the 35 minute time point and therefore was selected to take forward to Part B. All of the doses, including the placebo had smaller areas of flare at 60 minutes. This may have been a result of the decrease in effect of the capsaicin cream at that time point, which has been observed in other studies [27].
The main objective, to show engagement of the TRPV1 receptor mechanism was achieved.
Part B
Based on the available literature a clinically effective treatment would be expected to be associated with a 20 point change in the itch COVAS score compared with placebo.
TRPV1 has a proven role in itch and in particularly histamine induced itch. As histamine induces itch by activating the TRPV1 signalling pathway and certain pruritogens, including ATP, lipoxygenase products, acids and prostaglandins, are known to potentiate TRPV1 activity on sensory neurons. Therefore a topical TRPV 1 receptor antagonist that can block the TRPV 1 receptors located at keratinocytes and intraepidermal nerve fibres would be a good candidate drug for itch. However no differentiation was observed in the average itch intensity between the 3% cream and placebo following cowhage challenge (0.64 difference compared to placebo with 95% confidence intervals). The 3% SB705498 treatment in some subjects showed individually notable responses with respect to reduction in pruritus caused by histamine but as a cohort there was no clinically significant difference in the results compared to placebo (4.65 for histamine with 95% confidence intervals).
The time to itch onset data showed an increase in the mean time to itch onset of 39.9 seconds for the cowhage challenge and 4.6 seconds for the histamine challenge. This delay in time to itch onset is not thought to be enough to have any significant impact on the itch scratch cycle in AD.
Though the results were conclusive there were possible limitations to the study. Only male subjects were included in the study. The itch recordings on the COVAS score were subjective and subject to variation. Part B looked at the 10 best responders and hence the results should have been skewed towards those individuals most likely to show a response rather than representative of the general population. SB705498 is relatively insoluble and hence the study may have been limited by the formulation. Though a reduction in flare was observable indicating TRPV1 antagonism had taken place we may not have achieved the maximum effect on TRPV1 receptors.
The TRPV1 antagonist PAC-14028 has been shown to be effective in the attenuation of inflammation and pruritus associated with atopic dermatitis in mice (for summary see [28], [29]). PAC-14028 belongs to a novel class of non-vanilloid TRPV1 antagonists with a cinnamoyl background and hence the properties of the molecule may make it suitable for an anti-pruritic treatment.
Conclusions
Overall the data shows that a topical formulation of 3% SB705498 cream was clinically well tolerated, with no clinically significant drug related AE's or any SAE's reported from either Part A or Part B of the study. As demonstrated by the reduction in flare following capsaicin challenge engagement of the mechanism and target specific pharmacodynamic activity in humans was seen. However engagement of the mechanism did not translate into what is believed to be a clinically significant effect on pruritus induced by either cowhage or histamine in comparison to placebo. An effect was noted with regard to the histamine challenge but this was not felt to be clinically significant. It may be possible that application of histamine by the skin prick method might have lead to recruitment of nerve endings not reached by SB705498 and hence if a formulation with a greater skin penetrance was used a clinically significant impact on the itch induced by histamine may have been seen.
The biology of itch is regulated by a number of highly complex pathways, and information was obtained during the experimental medicine study which indicated that TRPV1 receptor appears to have a minor effect on histamine-mediated pruritus. These findings indicate there are other mechanisms, yet to be elucidated, involved in the initiation and relief of histamine-independent itch induction [30].
The implications of these results for further development of SB705498 as an effective treatment for AD indicate that whilst we can see that the topical formulation of SB705498 does engage the mechanism and has shown some reduction in histaminergic itch, this reduction is not thought to be significant enough to likely elicit a beneficial treatment for dermatitis type diseases that involve significant pruritus.
Supporting Information
VRD115246_Published Protocol Amendment 1.
(PDF)
Consort Checklist TrpV1 Clinical Paper.
(DOC)
Acknowledgments
The authors wish to acknowledge Dr Ethan Lerner from the Cutaneous Biology Research Center, Massachusetts General Hospital, Charleston, MA for generously supplying the cowhage spicules for this study. The authors also wish to acknowledge the GSK Topical SB705498 project team and members of the GSK Clinical Unit at Addenbrookes Hospital, Cambridge, UK who supported this study. Kevin Smart and Rob Stubbs for PK assistance; Mark Schilling for sourcing and ensuring all necessary approvals were in place for the use of the challenge agents, and Simon McHugh/Cornelia Gewert of GSK Clinical Unit at Addenbrookes Hospital, Cambridge for method development with the challenge agents.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study NCT01673529 was funded by GlaxoSmithKline Research and Development. The following authors are all employees of GlaxoSmithKline - Rachel A. Gibson, Jon Robertson, Harshna Mistry, Stewart McCallum, Disala Fernando and Melody Wyres. The funder had a role in study design, data collection and analysis, decision to publish, and preparation of the manuscript.
References
- 1. Patel T, Yosipovitch G (2010) Therapy of Pruritus. Expert Opin Pharmacother 11: 1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yosipovitch G, Goon A, Wee J (2000) The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol 143: 969–973. [DOI] [PubMed] [Google Scholar]
- 3. Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, et al. (2007) Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci 27: 7490–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valdes-Rodriguez R, Kaushik SB, Yosipovitch G (2013) Transient receptor potential channels and dermatological disorders. Curr Top Med Chem 13: 335–343. [DOI] [PubMed] [Google Scholar]
- 5. Bíró T, Tóth BI, Marincsák R, Dobrosi N, Géczy T, et al. (2007) TRP channels as novel players in the pathogenesis and therapy of itch. Biochim. Biophys. Acta – Molecular Basis of Disease 1772: 1004–1021. [DOI] [PubMed] [Google Scholar]
- 6. Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, et al. (2009) TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA 106: 11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinhoff S, Neisius U, Ikoma A, Fartasch M, Heyer G, et al. (2003) Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J. Neurosci 23: 6176–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valdes-Rodriguez R, Kaushik SB, Yosipovitch G (2013) Transient receptor potential channels and dermatological disorders. Curr Top Med Chem 13: 335–343. [DOI] [PubMed] [Google Scholar]
- 9. Hutter MM, Wick EC, Day AL, Maa J, Zerega EC, et al. (2005) Transient receptor potential vanilloid (TRPV-1) promotes neurogenic inflammation in the pancreas via activation of the neurokinin-1 receptor (NK-1R). Pancreas 30: 260–265. [DOI] [PubMed] [Google Scholar]
- 10. Gunthorpe MJ, Hannan S, Smart D, Jerman J, Arpino S, et al. (2007) Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-,acid-, and heat-mediated activation of the receptor. J Pharmacol Exp Ther 312: 1183–1192. [DOI] [PubMed] [Google Scholar]
- 11. Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, et al. (2007) The effects of the TRPV1 antagonist on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 132: 132–141. [DOI] [PubMed] [Google Scholar]
- 12. Changani K, Hotee S, Campbell S, Pindoria K, Dinnewell L, et al. (2013) Effect of the TRPV1 antagonist SB-705498 on the nasal parasympathetic reflex response in the ovalbumin sensitized guinea pig. British Journal of Pharmacology 169: 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alenmyr L, Greiff L, Andersson M, Sterner O, Zygmunt PM, et al. (2012) Effect of mucosal TRPV1 inhibition in allergic rhinitis. Basic Clin Pharmacol Toxicol 110: 264–268. [DOI] [PubMed] [Google Scholar]
- 14.Davis JB, Rami HK, Stevens AJ (2005) SB-705498, a clinical candidate with antagonist activity at TRPV1 and efficacy in a wide range of preclinical pain models. Presentation No. 364.2 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. Online. Accessed 2014 Jun 5. [Google Scholar]
- 15. Shim WS, Tak MH, Lee MH, Kim M, Kim M, et al. (2007) TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 27: 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ortonne J-P (2012) Urticaria and its subtypes: The role of second-generation antihistamines European Journal of Internal Medicine. 23: 26–30. [DOI] [PubMed] [Google Scholar]
- 17. Yosipovitch G, Papoiu AD (2008) What causes itch in atopic dermatitis? Curr. Allergy Asthma Rep 8: 306–311. [DOI] [PubMed] [Google Scholar]
- 18. Xiao B, Patapoutian A (2011) Scratching the surface: a role of painsensing TRPA1 in itch. Nat Neurosci 14: 540–542. [DOI] [PubMed] [Google Scholar]
- 19. Reddy B (2008) Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 28: 4331–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, et al. (2004) Protease-Activated Receptor 2 Sensitizes the Capsaicin Receptor Transient Receptor Potential Vanilloid Receptor 1 to Induce Hyperalgesia J Neurosci. 24: 4300–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, et al. (2004) Proteinase-Activated Receptor 2-Mediated Potentiation of Transient Receptor Potential Vanilloid Subfamily 1 Activity Reveals a Mechanism for Proteinase-Induced Inflammatory Pain J Neurosci. 24: 4293–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costa R, Marotta DM, Manjavachi MN, Fernandes ES, Lima-Garcia JF, et al. (2008) Evidence for the role of neurogenic inflammation components in trypsin- elicited scratching behaviour in mice Br J Pharmacol. 154: 1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Belghiti M, Estévez-Herrera J, Giménez-Garzó C, González-Usano A, Montoliu C, et al. (2013) Potentiation of the transient receptor potential vanilloid 1 channel contributes to pruritogenesis in a rat model of liver disease J. Biol Chem. 288: 9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yun JW, Seo JA, Jang WH, Koh HJ, Bae IH, et al. (2011) Antipruritic Effects of TRPV1 Antagonist in Murine Atopic Dermatitis and Itching Models J Invest Dermatol. 131: 1576–1579. [DOI] [PubMed] [Google Scholar]
- 25. Papoiu ADP, Tey HL, Coghill RC, Wang H, Yosipovitch G (2011) Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch. PLOS ONE 6(3): e17786 doi:10.1371/journal.pone. 0017786. PubMed: 21423808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kofler L, Ulmer H, Kofler H (2011) 50-Skin-Prick Test: A Tool to Diagnose Histamine Intolerance. Scholarly Research Network ISRN Allergy. Article ID 353045, 5 pages doi:10.5402/2011/353045. [DOI] [PMC free article] [PubMed]
- 27. Francke K (2009) A study to validate a capsaicin model of experimental pain in healthy human volunteers. Clinical Pharmacology and Therapeutics 85 SUPPL. 1(S62).. [Google Scholar]
- 28. Lim KM, Park YH (2012) Development of PAC-14028, a novel transient receptor potential vanilloid type 1 (TRPV1) channel antagonist as a new drug for refractory skin diseases. Arch Pharm Res 35: 393–396. [DOI] [PubMed] [Google Scholar]
- 29. Yun JW, Seo JA, Jeong YS, Bae IH, Jang WH, et al. (2011) TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery J Dermatol Sci. 62: 8–15. [DOI] [PubMed] [Google Scholar]
- 30. Roberson DP, Gudes S, Sprague JM, Patoski HAW, Robson VK, et al. (2013) Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons Nature Neuroscience. 16: 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VRD115246_Published Protocol Amendment 1.
(PDF)
Consort Checklist TrpV1 Clinical Paper.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.