Summary
To determine the long-term health and function of transplanted dopamine neurons in Parkinson’s disease (PD) patients, the expression of dopamine transporters (DAT) and mitochondrial morphology was examined in human fetal midbrain cellular transplants. DAT was robustly expressed in transplanted dopamine neuron terminals in the reinnervated host putamen and caudate, for at least 14 years after transplantation. The transplanted dopamine neurons showed a healthy and non-atrophied morphology at all time points. Labeling of the mitochondrial outer membrane protein Tom20 and alpha-synuclein showed typical cellular pathology in the patients’ own substantia nigra, which was not observed in transplanted dopamine neurons. These results show that the vast majority of transplanted neurons remain healthy long-term in PD patients, consistent with the clinically maintained function of fetal dopamine neuron transplants for up to 15–18 years in patients. These findings are critically important for the rational development of stem cell-based dopamine neuronal replacement therapies for PD.
Introduction
There is a need to understand how transplanted neurons can survive despite ongoing disease processes in the Parkinson’s patient brain. There is come current controversy surrounding the neural transplantation field and neuroscience research regarding the interaction between potentially pathological toxic proteins to cause neurodegeneration, and the concept of “disease spread” from cell to cell (Desplats et al., 2009; Isacson and Mendez, 2010). The accumulation of Lewy-body-like inclusions in some transplanted fetal dopamine neurons after long-term survival (over a decade) in the Parkinson’s disease (PD) brain, has been described (Cooper et al., 2009; Kordower et al., 2008; Kurowska et al., 2011; Li et al., 2008). Such pathology is a rare occurrence, with only a very low frequency (~1%) of grafted neuromelanin-containing neurons in cell suspension grafts exhibiting signs of alpha-synuclein pathology even after 22 years after grafting (Kurowska et al., 2011). These isolated cell inclusions are not observed in all patients (Mendez et al., 2008), are usually found in less than 1–5% of transplanted neurons depending on transplantation methodology, and clinical and post-mortem data indicate that this rare pathology does not affect overall graft function (Cooper et al., 2009; Isacson and Mendez, 2010). There have been suggestions that such Lewy body-like pathology is a product of protein transfer from the parkinsonian host brain to the transplanted fetal cells (Kurowska et al., 2011). However, alpha-synuclein pathology is not definitive of PD and incidental alpha-synuclein pathology is also reported in the normal aging brain, with the frequency between 8–22.5% in normal aging, and up to 34.8% in centenarians (Ding et al., 2006; Klos et al., 2006; Mikolaenko et al., 2005; Saito et al., 2004; Wakisaka et al., 2003). Experimental paradigms of oxidative stress (e.g. rotenone exposure) or neuroinflammation can also induce alpha-synuclein accumulation in dopamine neurons (Gao et al., 2008; Sherer et al., 2003).
Recent reports of post-mortem examination of fetal ventral mesencephalic grafts in PD patients have suggested that dopamine transporters (DAT) are down-regulated in the transplanted dopamine neurons (Kordower et al., 2008; Kurowska et al., 2011), and that such changes (that also include reduction of the dopamine neuron phenotypic marker, tyrosine hydroxylase) are indicative of neuronal dysfunction and PD pathophysiological changes in the transplanted neurons. Since a cell therapy approach holds considerable promise as a therapeutic strategy in PD (Freed et al., 2013; Kefalopoulou et al., 2013; Ma et al., 2010; Mendez et al., 2005; Politis et al., 2012; Politis et al., 2010) it is important to address the status of transplanted fetal dopamine cells in more detail. We have previously reported the surgical, clinical and histopathological data from 5 patients with advanced idiopathic PD that had received intracerebral transplantation of fetal dopaminergic cell suspension grafts 4–14 years earlier (Cooper et al., 2009; Mendez et al., 2005; Mendez et al., 2008). In those studies, therapeutic improvements were seen without clinical side-effects, such as off-period dyskinesias. Post-mortem examinations demonstrated that grafted dopaminergic neurons survived for up to 14 years post-transplantation. In the current study we have examined DAT expression as a measure of neuronal function, and also the mitochondrial marker Tom20 for examination of mitochondrial morphology, to further understand the long-term phenotypical characteristics of the transplanted dopamine neurons and potential effects of the aging of transplants.
Results
Dopamine transporter localization and expression in transplanted fetal dopamine neurons
In the present study we have assessed DAT immunostaining in 4–14 year old grafts in 5 patients from our previously published series (Mendez et al., 2005; Mendez et al., 2008), in order to further understand the long-term phenotypical characteristics of the transplanted dopamine neurons and potential effects of the aging of transplants. We used immunofluorescence staining for DAT using a monoclonal antibody recognising the N-terminus of DAT (Miller et al., 1997), and performed colabeling with a TH antibody to label dopaminergic neurons and fibers. General assessment of the integrity of the grafted TH-immunoreactive neurons in all patients, revealed cells with a healthy appearance, including a robust cell soma and absence of signs of atrophy (Fig. 1a–c, f–h, k–m, p–r). In two independent patients at 4 years post-transplantation (Fig. 1a–j), examination of DAT/TH immunostaining at low magnification (Fig. 1a and f) showed dense DAT and TH expression in the reinnervated putamen and caudate in areas both near to and further away from the graft. Although DAT was also expressed in the grafted cell soma, the intense punctate staining pattern in the reinnervated areas was most striking (Fig. 1b and g). This expression, consistent with that of synaptic proteins, was easily observed at high magnification (Fig. 1c–d and h–i) where DAT was localized along TH-immunoreactive fibers.
Figure 1. Dopamine transporter expression in the reinnervated putamen 4–14 years post-transplantation.
Double immunolabeling for dopamine transporter (DAT) (green) and tyrosine hydroxylase (TH) (red) in Subjects 1 (panels A–E, graft survival of 4 years), 2 (panels F–J, graft survival of 4 years), 4 (panels K–O, graft survival of 9 years), and 5 (panels P–T, graft survival of 14 years). Panels A, F, K, and P show a low magnification composite for each subject to illustrate the grafted cell bodies and adjacent reinnervated putamen. Successively higher magnification images are illustrated in panels B–D, G–I, L–N and Q–S. Boxed areas represent the image shown in the subsequent panel. Panels E, J, O and T show a low magnification composite of the patient’s putamen and adjacent external segment of the globus pallidus. DAT immunostaining shows a robust punctate localization along dopaminergic (TH-immunoreactive) fibers in the reinnervated putamen and caudate in all grafts, even up to 14 years post-transplantation. In addition, the transplanted dopamine neurons show a healthy and non-atrophied morphology. Parallel control immunostainings, where the primary antibodies were omitted, showed no immunoreactivity of DAT or TH (data not shown). To further confirm specificity of the DAT labeling observed in the reinnervated putamen and caudate, we also examined DAT immunolabeling in adjacent anatomical regions on the same tissue sections. In the lateral and medial globus pallidi, which are regions that receive comparatively little dopaminergic innervation and normally exhibit little DAT expression in the human brain (Ciliax et al., 1999), we, as expected, observed weak DAT immunolabeling and a sharp boundary from high to low DAT and TH expression (panels E, J, O, T). We also performed immunostaining in the substantia nigra of each patient and confirmed the loss of both DAT and TH immunoreactivity (data not shown). g = graft, h = host, GPe = globus pallidus externa. Scale bars in A, E, F, J, K, O, P, T = 400μm; B, G, L, Q = 100μm; C, H, M, R = 50μm; D, I, N, S = 20μm. See also Figure S1.
We next determined whether DAT expression was maintained long-term and thus examined DAT immunolabeling in transplanted neurons at 9 years post-transplantation, and at 14 years post-transplantation (Fig. 1k–t). As observed at the younger time point, robust punctate expression in the reinnervated striatum was observed in all patients (Fig. 1k–m and p–r), and higher magnification imaging verified coexpression of DAT puncta along TH- immunoreactive dopaminergic fibers (Fig. 1, panels n and s). The intensity of DAT immunofluorescence was quantified in the reinnervated putamen at 4–14 years after transplantation (Supplementary Figure 1), and compared to DAT labeling intensity in the contralateral (non-transplanted) putamen from Subject 2. As expected, very low levels of DAT labeling in the non-transplanted parkinsonian putamen were observed, consistent with the severe loss of DAT expression in the putamen in PD (Miller et al., 1997). In contrast, at 4, 9 and 14 years after transplantation, DAT expression was significantly increased within the grafted putamen.
Parallel control immunostainings, where the primary antibodies were omitted, showed no immunoreactivity of DAT or TH (data not shown). To further confirm specificity of the DAT labeling observed in the reinnervated putamen and caudate, we also examined DAT immunolabeling in adjacent anatomical regions on the same tissue sections (see Fig. 1). In the lateral and medial globi pallidi, which are regions that receive comparatively little dopaminergic innervation and normally exhibit little DAT expression in the human brain (Ciliax et al., 1999), we, as expected, observed weak DAT immunolabeling and a sharp boundary from high to low DAT and TH expression (Fig. 1, panels e, j, o, t).
Mitochondrial localization and expression in transplanted fetal dopamine neurons
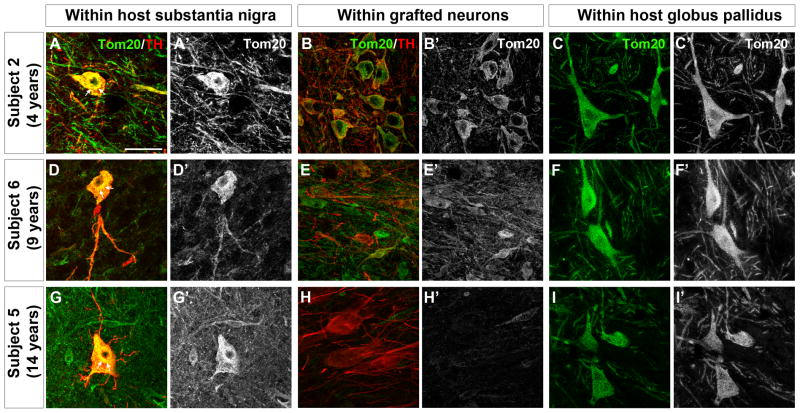
Tom20 (translocase of outer mitochondrial membrane 20 kDa) was used to label mitochondria in grafted neurons and also in the host substantia nigra and globus pallidus. In remaining substantia nigra TH-immunoreactive neurons from Parkinson’s disease patients (Subjects 2, 5, 6) (Fig. 2a, a′, d, d′, g, g′), Tom20 labeling often appeared intensely labeled in the cell soma with accumulation in the perinuclear area and little immunostaining in the axon and processes. In neurons co-stained with Tom20 and alpha-synuclein, the host patient’s substantia nigra showed Lewy bodies and variable or reduced distribution of Tom20 stained mitochondria (Fig. 3A). In grafted TH-immunoreactive neurons at 4 years post-transplantation (Fig. 2b, b′) Tom 20 immunostaining was robust in the perikarya and neuronal processes, similar to that observed in the normal brain. At 9 and 14 years post-transplantation, Tom20 labeling was generally less intense in the grafted TH-ir neurons (Fig. 2e, e′, h, h′) compared to the Tom20 staining pattern observed in Subject 2 at 4 years post-transplantation, however, there was no abnormal accumulation of mitochondria in the cell soma as was observed in the host substantia nigra. The localization of Tom20 in neurons within the host medial globus pallidus (Fig. 2c, f, i) exhibited a homogenous localization throughout the cell soma and processes, and showed no evidence of abnormal perinuclear accumulation as was observed in the patients’ own substantia nigra. In neurons within the transplants co-stained with Tom20 and alpha-synuclein, normal distribution of Tom20 staining was observed in the absence of Lewy bodies (Fig. 3B–D).
Figure 2. Mitochondrial phenotype in transplanted fetal dopamine neurons 4–14 years post-transplantation.
Double immunolabeling for translocase of outer mitochondrial membranes 20kDa (Tom20) (green) and tyrosine hydroxylase (TH) (red) in the host substantia nigra (left panels) and grafted dopamine neurons (middle panels) and in the host globus pallidus (right panels) in Subject 2 (panels A, A′, B, B′, C, C′, graft survival of 4 years), Subject 6 (panels D, D′, E, E′, F, F′, graft survival of 9 years) and Subject 5 (panels G, G′, H, H′, I, I′, graft survival of 14 years). Panels A′-I′ show single channel Tom20 labeling from corresponding panels A-I. In dopamine (TH-immunoreactive) neurons from the patients’ own substantia nigra, Tom20 labeling often appeared intensely labeled in the cell soma with accumulation in the perinuclear area (arrows) and little immunostaining in the dopaminergic axons and processes (panels A, A′, D, D′, G, G′). In grafted TH-immunoreactive neurons at 4 years post-transplantation Tom20 labeling was robust in the perikarya and neuronal processes (panels B, B′). At 9 and 14 years post-transplantation, Tom20 labeling was generally less intense in the grafted TH-immunoreactive neurons (panels E, E′, H, H′) compared to the Tom20 staining pattern observed in Subject 2 at 4 years post-transplantation, however, there was no abnormal accumulation of mitochondria in the cell soma as was observed in the host substantia nigra. No perinuclear accumulation or fragmentation of Tom20-labeled mitochondria was observed in the host globus pallidus (panels C, C′, F, F′, I, I′). Scale bar = 50μm
Figure 3. Mitochondria abnormalities occur independently of Lewy body pathology in host neurons and neither Lewy body pathology or mitochondrial abnormalities were found in transplanted neurons.
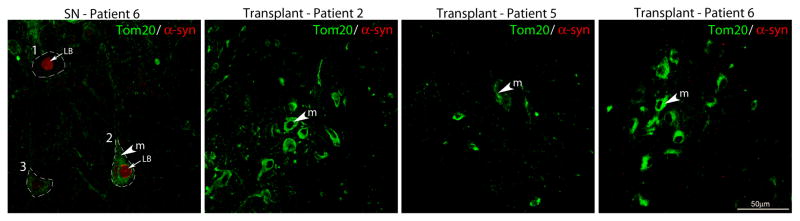
Co-immunofluorescence labeling of a-synuclein (red) and the mitochondrial import receptor subunit, Tom20, (green) was observed in neurons within the substantia nigra of PD patient 6 (Panel A). Mitochondria in neurons containing accumulated a-synuclein in the host were either diffusely distributed throughout the cytoplasm (Cell 1) or were dense in appearance (Cell 2), (Panel A). Mitochondria were also diffusely distributed in the cytoplasm of cells without Lewy bodies (Cell 3), (Panel A). No Lewy body pathology was found in the transplanted neurons of PD patients 2 (Panel B), 5 (Panel C) and 6 (Panel D) and mitochondria were dense throughout the cytoplasm. Annotations: m = mitochondria and LB = Lewy body. Scale bar =50μm.
Discussion
Efficacious fetal ventral mesencephalic grafts can reduce both PD motor symptoms and levodopa-induced dyskinesia for many years, and can reduce or negate the requirement for dopamine replacement therapy. Months to years are required for the newly replaced dopaminergic neurons to mature, integrate into the host brain, and function (Barker et al., 2013), and most fetal ventral mesencephalic cell transplantation provides improvement in PD motor symptoms starting at ~1 year after transplantation (Evans et al., 2012). However, successful transplants can survive and function for many years and in fact the most recently published reports from Politis and colleagues (Kefalopoulou et al., 2013; Politis et al., 2012; Politis et al., 2010) describe two patients who are still improving (as shown by PET neuroimaging of dopamine uptake and reduction of UPDRS score) more than 18 years after receiving transplantation of fetal ventral mesencephalic cells.
This report shows long-term graft survival in PD patients with maintained DAT localization along TH-immunoreactive axons in the reinnervated striatum, indicating functional dopaminergic neurons. Abnormalities in mitochondrial localization, as indicated by accumulation in the cell soma in dopaminergic neurons in the host substantia nigra, are not observed in grafted neurons. These data are consistent with clinical and neuroimaging data showing stable dopamine cell survival and function over 18 years after surgery (Kefalopoulou et al., 2013; Politis et al., 2012; Politis et al., 2010).
DAT is a plasma membrane protein located on presynaptic dopamine nerve terminals where it is responsible for the termination of dopamine transmission and reuptake of dopamine released into the synaptic cleft back into the presynaptic dopamine neurons (Nirenberg et al., 1997). DAT is highly concentrated in the striatum in nigrostriatal dopaminergic projections and localized to the plasma membranes of axonal varicosities and terminals containing synaptic vesicles (Nirenberg et al., 1997), consistent with it’s involvement in dopaminergic synaptic transmission in the striatum. It has recently been suggested that in human PD patients, DAT expression in transplanted fetal dopamine ventral mesencephalic neurons is downregulated over time, and that this is indicative of PD disease processes within the graft and loss of function of the grafted dopamine neurons (Kordower et al., 2008; Kurowska et al., 2011). In these studies, the authors used immunolabeling for DAT and subsequent light microscopy. Surprisingly, within older (4 and 14 year-old) grafts these studies only described the expression of DAT in the grafted cell soma, with no reported analysis or discussion of the expression and localization of DAT in the dopamine neuron fibers reinnervating the putamen. In the current study we also noted a qualitative reduction in the intensity of the DAT signal in the cell soma of the grafted dopamine neurons over time when comparing grafts at 4, 9 and 14 years post-transplantation, but it was striking to us that the punctate DAT expression in the reinnervated putamen and caudate was maintained, even over a decade post-transplantation. We have previously shown that the extent of DAT labeling in the caudate and putamen of MPTP-lesioned monkeys, as detected by [11C]CFT PET neuroimaging, is congruent with the number of surviving dopaminergic fibers (Hantraye et al., 1992). DAT is a marker of mature dopamine synapse function, and thus our findings of robust DAT labeling in the putamen of patients analyzed in our series are indicative of the long-term, continued health and function of transplanted dopamine neurons. We therefore based on DAT expression, do not find evidence of PD pathophysiological processes in grafted dopaminergic neurons. Clearly, using the current transplant methodology, fetal grafts result in clinically functional dopamine neurons for long-term (Barker et al., 2013; Mendez et al., 2005). A previously described analysis of α-synuclein pathology in the same transplant cases reported in the current manuscript (Cooper et al., 2009; Mendez et al., 2008), have observed only one (1) single Lewy body in a neuron of a fetal graft in one of these cases (Cooper et al., 2009). In fetal ventral mesencephalic transplant cases from other transplant method series, in which more frequent Lewy body and α-synuclein pathology has been observed also by us (although still less than 5% of grafted neurons), the overall morphology of surviving transplanted dopaminergic neurons is also unchanged (Cooper et al., 2009; Kordower et al., 2008; Kurowska et al., 2011). Indeed, it has also been reported that there is no alteration in the expression of VMAT2, another marker of dopamine presynaptic nerve terminals, in grafted dopamine neurons over time (Kordower et al., 2008). The current study provides important, additional evidence against the relevance of a postulated prion-like alpha-synuclein mechanism for disease propagation. An alternative interpretation to the proposed α-synuclein spreading from host to graft to cause dysfunction may actually be the opposite; such that the healthy transplanted cells provide a clearance mechanism of exogenous unfolded proteins. Our present manuscript unequivocally demonstrates positive markers of dopamine neuron function. This is an essential demonstration to this field by providing further rationale for clinical cell therapy proposals for PD using stem cell-derived dopamine neuron equivalents.
We also examined the localization and expression of mitochondria in grafted dopaminergic neurons as a possible indicator of increased aging and readout of neuronal function. Mitochondrial dynamics including fission, fusion, and transport are crucial for the maintenance of bioenergetic function and prevention of apoptosis (Detmer and Chan, 2007). Mitochondrial dysfunction is a prominent feature of PD and dopaminergic substantia nigra neurons are particularly vulnerable to mitochondrial dysfunction due to their high metabolic rate dependence (Exner et al., 2012). We frequently observed that remaining dopaminergic neurons in the host SNc contained Tom20-labeled mitochondria that were accumulated in the cell soma, with very little staining or showing a fragmented staining pattern in neurites. Such a redistribution of mitochondria has also been described in pyramidal neurons in AD post-mortem brain, using antibodies against COX I, and mitochondria fusion/fission proteins (Wang et al., 2009). Intracellular mitochondrial distribution is critical to the functioning of neurons and mitochondrial fragmentation and a reduced localization of mitochondria in neurites may reflect disruptions in axonal transport and/or disrupted mitochondrial fusion/fission. In contrast, in grafted TH-immunoreactive neurons at 4 years post-transplantation, the Tom20-labeled mitochondria displayed a more uniform distribution of throughout the cell soma and neurites, with no indication of fragmentation, or accumulation in the cell soma. The overall reduced expression of Tom20-labeled mitochondria that we observed in grafted dopamine neurons at 9 and 14 years post-transplantation may reflect an overall reduced mitochondrial biogenesis or increased mitophagy, as a function of accelerated aging in these neurons. The distribution we describe of the mitochondrial network by all current cell biological accounts is likely more informative than a total mitochondrial count, which is practically difficult, if not impossible to obtain in regular postmortem material. Furthermore, even if such a method existed, a similar mitochondrial mass or number between populations of neurons do not necessarily equate with physiologically functional mitochondria because of heteroplasmia, which is the coexistence of faulty and functional mitochondria in the same cell of its mitochondrial populations (Kraytsberg et al., 2006; Larsson, 2010; Sanders et al., 2014).
The observations of continued DAT expression and normal mitochondrial cellular distribution at 4–14 years post-transplantation is consistent with the clinical data and show that implanted dopamine neurons remain healthy and functional for decades (Freed et al., 2013; Kefalopoulou et al., 2013; Politis et al., 2012; Politis et al., 2010). In summary, our post-mortem examination of tissue from PD patients, who have received fetal ventral mesencephalic cells transplanted as a cell suspension shows that following long-term graft survival, DAT localization along TH-immunoreactive axons and fibers is maintained in the reinnervated striatum, indicating functional dopaminergic neurons. Abnormalities in mitochondrial localization, as indicated by accumulation in the cell soma in dopaminergic neurons in the host substantia nigra, are not observed in grafted neurons. Our data is not consistent with suggestions that the grafts degenerate over time, and instead show that there is no cell biological evidence for such assertions. These data support clinical and neuroimaging findings of the long-lasting functional benefit of dopamine neuron transplantation in PD patients (Freed et al., 2013; Kefalopoulou et al., 2013; Ma et al., 2010; Mendez et al., 2005; Politis et al., 2012; Politis et al., 2010), and are an important foundation for the development of future fetal and stem cell-derived dopamine replacement therapies for PD.
Experimental Procedures
Patient Selection
Caudate putamen, globus pallidus and substantia nigra tissues from five patients (referred to as subjects 1, 2, 4, 5 and 6) with advanced idiopathic PD, who received fetal tissue transplantation for 4–14 years, were examined in this study. We have previously reported the surgical procedures, neuroimaging data, clinical outcome, and post-mortem histological assessments of phenotypical characteristics and PD pathophysiological markers in transplanted neurons, in this series of patients (Cooper et al., 2009; Mendez et al., 2005; Mendez et al., 2008).
Tissue preparation
At postmortem (delay 3–4 hours), the brains were infused with 2 liters of cold 0.1M phosphate buffer (pH 7.4), followed by 2 liters of ice-cold 4% paraformaldehyde in 0.1M phosphate buffer. The brains were subsequently blocked in the coronal plane in 3 cm thick slabs. The slabs were cryoprotected in 30% sucrose in phosphate-buffered saline (PBS) at 4°C.
Immunohistochemistry
Sections (40 μm) were stained using immunofluorescence techniques. In order to maintain consistency of DAT or TOM20 labeling between subjects, immunostainings were performed at the same time and using identical reagents. DAT labeling was enhanced using a fluorescent Streptavidin conjugate, as described below. Sections were rinsed for 3 times 10 min in PBS, incubated in 10% normal donkey serum (Vector Laboratories) and 0.3% Triton X-100 in PBS for 60 min and then incubated with gentle agitation for 48 hours at 4°C in primary antibody (rat anti-DAT, 1:200 [MAB369, Millipore, MA, USA]; sheep anti-TH, 1:300 [P60101-0, Pel-Freez, AK, USA]; rabbit anti-Tom20, 1:200 [sc-11415, Santa-Cruz, CA, USA]. For alpha-synuclein staining, the LB509 antibody was used at 1:500 dilution [Invitrogen/Life Technologies, MD, USA]. After an additional three 10-min rinses in PBS the sections were incubated in fluorescent dye-conjugated secondary antibodies (for detection of TH and Tom20 and alpha-synuclein antibodies) (Alexa Fluor donkey anti-rabbit/sheep 488/568/660; 1:500 [Molecular Probes, OR, USA] and alpha-synuclein secondary donkey anti-mouse 1:300 [Jackson Labs, PA, USA]) in PBS for 60 min at room temperature. For detection of DAT, sections were incubated in a biotinylated secondary antibody (donkey anti-rat; 1:250, Vector Laboratories, CA, USA) in PBS for 60 min at room temperature, followed by rinsing for 3 times 10 min in PBS, and incubation in Streptavidin Alexa Fluor 488 (1:500, Molecular Probes, OR, USA) for 60 minutes. After rinsing in PBS (3 times 10 min), sections were mounted onto Superfrost Plus slides and an autofluorescence eliminator reagent was applied (Millipore) prior to coverslipping in Mowiol mounting media. Specificity of DAT, Tom20, TH and alpha-synuclein labeling was confirmed using stainings in parallel tissue sections from each subject in which the primary antibody was omitted.
Confocal Microscopy
Immunofluorescence staining was examined using a confocal microscope (LSM510 Meta; Carl Zeiss, Thornwood, NY, USA) at 10, 25, or 100x magnification. Single or z-stack images were acquired using a sequential scanning mode with a frame size of 1024×1024 pixels, and averaging of four frames. The laser intensity, confocal aperture, photomultiplier voltage, scan speed, image size, filter and zoom were kept identical whilst all images were acquired. For quantification of DAT labeling, confocal images at 25x magnification (at least 5 per subject) were obtained in the reinnervated putamen, or in the denervated, non-transplanted putamen. The average optical density of DAT immunofluorescence intensity in each subject was analyzed using Image J software (NIH Image J 1.44i, National Institutes of Health, USA).
Supplementary Material
Highlights.
DAT expression is maintained for 4–14 years after transplantation in the PD brain.
No abnormal mitochondrial localization is observed in transplanted dopamine neurons.
Grafted human fetal dopamine neurons remain healthy and functional long-term.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke P50 (Grant NS39793) (OI), the Harvard Stem Cell Institute Translational Neuroscience Fund (OI), the Canadian Institute of Health Research, Queen Elizabeth II Health Sciences Centre (IM), The Consolidated Anti-Aging Foundation (OI), The Poul Hansen Family (OI) and the Harold and Ronna Cooper Family (OI). We thank Dr. Gaynor Smith for helpful discussions and Eduardo Perez-Torres and David Ahmadi for technical assistance.
Footnotes
Author contributions: PH designed research, performed research, analyzed and interpreted data, and wrote the paper. OC designed research and analyzed data. DS performed research. HR evaluated data and wrote the paper. IM designed research, performed research, evaluated data and wrote the paper. OI designed research, performed research, analyzed and interpreted data, and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cooper O, Astradsson A, Hallett P, Robertson H, Mendez I, Isacson O. Lack of functional relevance of isolated cell damage in transplants of Parkinson’s disease patients. J Neurol. 2009;256(Suppl 3):310–316. doi: 10.1007/s00415-009-5242-z. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Ding ZT, Wang Y, Jiang YP, Hashizume Y, Yoshida M, Mimuro M, Inagaki T, Iwase T. Characteristics of alpha-synucleinopathy in centenarians. Acta Neuropathol. 2006;111:450–458. doi: 10.1007/s00401-005-0015-y. [DOI] [PubMed] [Google Scholar]
- Evans JR, Mason SL, Barker RA. Current status of clinical trials of neural transplantation in Parkinson’s disease. Prog Brain Res. 2012;200:169–198. doi: 10.1016/B978-0-444-59575-1.00008-9. [DOI] [PubMed] [Google Scholar]
- Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31:3038–3062. doi: 10.1038/emboj.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed CR, Breeze RE, Zawada WM, Fahn S, Eidelberg D, Jones S, Zhou W. Human embryonic dopamine neurons transplanted into putamen of Parkinson patients survive for at least 22 years without immunosuppression. Paper presented at: Society for Neuroscience.2013. [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantraye P, Brownell AL, Elmaleh D, Spealman RD, Wullner U, Brownell GL, Madras BK, Isacson O. Dopamine fiber detection by [11C]-CFT and PET in a primate model of parkinsonism. Neuroreport. 1992;3:265–268. doi: 10.1097/00001756-199203000-00013. [DOI] [PubMed] [Google Scholar]
- Isacson O, Mendez I. Being too inclusive about synuclein inclusions. Nature Med. 2010;16:960–961. doi: 10.1038/nm0910-960b. [DOI] [PubMed] [Google Scholar]
- Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, Widner H, Rehncrona S, Brundin P, Bjorklund A, et al. Long-term Clinical Outcome of Fetal Cell Transplantation for Parkinson Disease: Two Case Reports. JAMA Neurology. 2013;71:83–87. doi: 10.1001/jamaneurol.2013.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nature Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Kurowska Z, Englund E, Widner H, Lindvall O, Li JY, Brundin P. Signs of Degeneration in 12–22-Year Old Grafts of Mesencephalic Dopamine Neurons in Patients with Parkinson’s Disease. Journal of Parkinson’s Disease. 2011;1:83–92. doi: 10.3233/JPD-2011-11004. [DOI] [PubMed] [Google Scholar]
- Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nature Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, Freed C, Dhawan V, Eidelberg D. Dopamine cell implantation in Parkinson’s disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med. 2010;51:7–15. doi: 10.2967/jnumed.109.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, Dagher A, Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nature Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolaenko I, Pletnikova O, Kawas CH, O’Brien R, Resnick SM, Crain B, Troncoso JC. Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA) J Neuropathol Exp Neurol. 2005;64:156–162. doi: 10.1093/jnen/64.2.156. [DOI] [PubMed] [Google Scholar]
- Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, Levey AI. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurol. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Immunogold localization of the dopamine transporter: an ultrastructural study of the rat ventral tegmental area. J Neurosci. 1997;17:5255–5262. doi: 10.1523/JNEUROSCI.17-14-05255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Oertel WH, Bjorklund A, Lindvall O, Piccini P. Serotonin neuron loss and nonmotor symptoms continue in Parkinson’s patients treated with dopamine grafts. Sci Transl Med. 2012;4:128ra141. doi: 10.1126/scitranslmed.3003391. [DOI] [PubMed] [Google Scholar]
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med. 2010;2:38ra46. doi: 10.1126/scitranslmed.3000976. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, Yamanouchi H, Murayama S. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol. 2004;63:742–749. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- Sanders LH, Laganiere J, Cooper O, Mak SK, Vu BJ, Huang YA, Paschon DE, Vangipuram M, Sundararajan R, Urnov FD, et al. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson’s disease patients: reversal by gene correction. Neurobiol Dis. 2014;62:381–386. doi: 10.1016/j.nbd.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T. Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol. 2003;106:374–382. doi: 10.1007/s00401-003-0750-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;109(Suppl 1):153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.