Abstract
Background
Asthma is a chronic respiratory disease that afflicts millions of people and accounts for substantial utilization of healthcare resources in most industrialized countries, including in the United States. However, the exact cost and utilization of anti-asthma medications in Medicaid in the past 2 decades have not been well studied. Considering the safety issues surrounding the long-acting beta-agonists, guideline updates, and the increase in asthma prevalence, understanding anti-asthma medication prescribing trends is important to payers and patients.
Goal
The purpose of this study was to analyze the utilization and spending trends for anti-asthmatic agents in the US Medicaid program over the past 2 decades.
Methods
This study was based on a retrospective, descriptive analysis of trends in utilization of and spending on anti-asthma medications, including short-acting beta-agonists, inhaled corticosteroids, long-acting beta-agonists, and inhaled corticosteroid/long-acting beta-agonist combinations. Quarterly utilization and expenditure data were obtained from the national Medicaid pharmacy files provided by the Centers for Medicare & Medicaid Services from quarter 1 of 1991 through quarter 2 of 2010. Average reimbursement per prescription was calculated each quarter as a proxy for drug price.
Results
The total number of prescriptions for the studied anti-asthma medications rose from 8.9 million in 1991 to 15.6 million in 2009, peaking at 20.8 million in 2005, the year before Medicare and Medicaid dual-eligible beneficiaries were moved to Medicare Part D. From 1991 to 2009, Medicaid spending on anti-asthma medications overall rose from $180.7 million to $1.3 billion, and spending on inhaled corticosteroid/long-acting beta-agonist combinations rose from $52.8 million in 2001—their first year on the market—to $411.7 million in 2009. The average price per prescription has risen in all the anti-asthma drug classes: overall, spending per prescription has increased 4-fold between 1991 and 2009, significantly faster than the consumer price index (57.5%) over the same period. In quarter 2 of 2010, Medicaid spent more on the combination medication fluticasone-salmeterol—$60 million—than on any other anti-asthma medication.
Conclusion
Anti-asthma medications are a major and growing expense for state Medicaid programs and can be expected to be the same for Medicare Part D in the future. Increased disease prevalence has in part contributed to the rise in pharmacotherapy cost. Nevertheless, drug therapy is crucial for managing asthma and asthma exacerbations.
Asthma is a chronic respiratory disease that affects anywhere from 1% to 18% of the world population.1–4 In the United States, the prevalence rate is estimated at 11%, which represents approximately 36 million individuals.5 Because asthma is known to occur concomitantly with allergic rhinitis and is often associated with other comorbidities and risk factors,6–9 symptoms reported by patients are multifaceted and have consequences that are inimical to work productivity and health.10–12
In the United States, asthma accounts for more than 10 million missed work days for adults annually and for approximately 13 million missed school days.13 The economic burden of asthma includes the direct costs of health services utilized for prevention and treatment, as well as indirect costs of lost productivity, reduced work performance, and premature mortality.14,15 In 2009, annual total costs for asthma in the United States were estimated at more than $20 billion.16 Environmental changes, continued exposure to allergens, and infections are purported culprits underlying allergic events and account for the increasing prevalence of asthma, as well as the inability to control asthma symptoms in certain populations.17–19
Pharmacotherapy is essential for asthma management; according to the 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines, drug therapy should be based on stepwise treatment for different levels of asthma severity—intermittent, mild persistent, moderate persistent, and severe persistent.20 Before initiating drug therapy, the most important aspect of treatment is to ensure that patients undergo appropriate evaluation and diagnostic testing to determine the etiology underlying asthma or allergic-induced asthma symptoms; 70% of patients with allergies also have asthma.21
Asthma medications are broadly divided into 2 categories. Rescue medications or relievers, consisting of short-acting beta-agonists (SABAs), are utilized for immediate relief of asthma symptoms and are recommended for all patients with asthma, including those with intermittent disease. Controllers or maintenance medications, such as long-acting beta-agonists (LABAs) or inhaled corticosteroids (ICSs), are recommended for those with persistent asthma.22–25
Although SABAs provide immediate relief for acute bronchospasm, the guidelines for asthma therapy state that SABAs that are used more than twice weekly, with the exception of use for exercise-induced bronchospasm, generally indicate inadequate control of symptoms.20 Given the safety concerns surrounding LABA side effects, a patient whose persistent asthma symptoms are not controlled adequately with an ICS alone can be managed by increasing the ICS dose or adding a LABA to the ICS.26–29 Furthermore, findings from some studies indicated that using a LABA as monotherapy is not efficient for a significant reduction of asthma symptoms. Consequently, for patients with severe or persistent asthma, a LABA should be combined with an ICS to increase the control of asthma symptoms.30–32
KEY POINTS
-
▸
Approximately 36 million Americans are affected by asthma.
-
▸
Asthma accounts for approximately 10 million missed work days and 13 million missed school days annually.
-
▸
The prevalence and severity of asthma in the United States have been increasing in the past 2 decades, especially among children in low-income households.
-
▸
From 1991 to 2009, Medicaid spending on anti-asthma medications rose by 595%, from $180.7 million to $1.3 billion; the total annual cost of asthma was estimated at $20 billion in 2009.
-
▸
The average price of an anti-asthma medication in the past 2 decades increased by 295%, as a result of a tremendous rise in demand for these medications by patients and payers; this rise in price greatly exceeded the 57.5% increase in the consumer price index over the same period.
-
▸
Despite these significant cost trends, pharmacotherapy is key to controlling the serious nature of asthma and its associated exacerbations, which often lead to hospitalizations and even greater costs and healthcare utilization.
Previous studies and several comprehensive meta-analyses have addressed issues related to safety and efficacy of SABAs, LABAs, and ICSs. However, only a few studies have examined market share and prescribing trends in the asthma medication market.33–35 Considering the safety issues surrounding LABA use, guideline updates, and worldwide increase in asthma prevalence, understanding medication prescribing trends would seem to be important. The objectives of this study were to describe trends in reimbursement, utilization, and prices of individual medications and classes of medications (SABAs, LABAs, ICSs, and ICS/LABA combination drugs) for the US Medicaid population over the past 2 decades. We realize that these medications are indicated for other diseases besides asthma, such as LABAs for chronic obstructive pulmonary disease (COPD). The trends that we identify will be for overall drug spending and utilization regardless of indication treated.
Methods
This study was a retrospective, descriptive analysis of trends in utilization of and spending for SABAs, LABAs, ICSs, and ICS/LABA combination drugs. Pharmacy utilization and expenditure data were obtained from the national Medicaid pharmacy files provided by the Centers for Medicare & Medicaid Services (CMS) from quarter 1 of 1991 through quarter 2 of 2010. These files contained the number of outpatient prescriptions and amount of payments made for all National Drug Code (NDC) drug forms by each of 49 states (all except Arizona) plus the District of Columbia.36 This large database was compiled across all states and contains some reporting errors. If data for a particular drug for a specific quarter were considered unreliable, data were imputed from values from previous and/or later quarters.
Table 1 lists the anti-asthma drugs studied, as well as the manufacturers, approval dates, and patent expiration dates for those drugs. For each quarter, drug utilization was determined by summing the number of prescriptions across NDC codes associated with that drug. Similarly, reimbursement was calculated by summing across the reimbursements for individual NDCs. If a particular drug was produced by both branded and generic drug manufacturers, generic and branded data were both collected.
Table 1.
Study Drugs
| Brand name | Generic name | FDA approval date | Manufacturer | Patent expiration |
|---|---|---|---|---|
| Short-acting beta-agonists | ||||
| Alupent | Metaproterenol sulfate | 01/01/1982 | Boehringer Ingelheim | NA |
| Brethine | Terbutaline sulfate | 01/01/1982 | Novartis | NA |
| Maxair | Pirbuterol acetate | 12/30/1986 | 3M | 05/12/2004 |
| ProAir HFA | Albuterol | 10/29/2004 | Teva Global | 02/25/2014 |
| Proventil | Albuterol | 01/01/1982 | Schering | NA |
| Proventil HFA | Albuterol | 08/15/1996 | 3M | 07/06/2010 |
| Ventolin | Albuterol | 04/19/2001 | GlaxoSmithKline | 10/14/2015 |
| Ventolin HFA | Albuterol | 04/19/2001 | GlaxoSmithKline | 10/14/2015 |
| Xopenex | Levalbuterol hydrochloride | 03/25/1999 | Sepracor | 01/05/2010 |
| Xopenex HFA | Levalbuterol tartrate | 03/11/2005 | Sepracor | 07/06/2010 |
| Inhaled corticosteroids | ||||
| Aerobid | Flunisolide | 08/17/1984 | Roche Palo | 06/12/2007 |
| Asmanex | Mometasone furoate | 03/30/2005 | Schering | 07/27/2014 |
| Azmacort | Triamcinolone acetonide | 04/23/1982 | Abbott | 12/31/2007 |
| Flovent | Fluticasone propionate | 05/14/2004 | GlaxoSmithKline | 08/19/2014 |
| Flovent HFA | Fluticasone propionate | 05/14/2004 | GlaxoSmithKline | 08/19/2014 |
| Pulmicort | Budesonide | 06/24/1997 | AstraZeneca | 08/27/2006 |
| Pulmicort Flexhaler | Budesonide | 07/12/2006 | AstraZeneca | 01/09/2018 |
| Qvar | Beclomethasone dipropionate | 09/15/2000 | Ivax Res | 11/28/2009 |
| Vancenase | Beclomethasone dipropionate | 01/01/1982 | Schering | 12/21/1999 |
| Vanceril | Beclomethasone dipropionate | 01/01/1982 | Schering | NA |
| Long-acting beta-agonists | ||||
| Foradil | Formoterol fumarate | 02/16/2001 | Novartis | 03/08/2019 |
| Serevent | Salmeterol xinafoate | 02/04/1994 | GlaxoSmithKline | 02/12/2008 |
| Inhaled corticosteroid + long-acting beta-agonist combinations | ||||
| Advair Diskus | Fluticasone propionate + salmeterol xinafoate | 08/24/2000 | GlaxoSmithKline | 08/12/2008 |
| Advair HFA | Fluticasone propionate + salmeterol xinafoate | 06/08/2006 | GlaxoSmithKline | 08/12/2008 |
| Symbicort | Budesonide + formoterol fumarate dihydrate | 07/21/2006 | AstraZeneca | 09/23/2012 |
FDA indicates US Food and Drug Administration; NA, not applicable.
In 2005, the US Food and Drug Administration (FDA) mandated that the propellant chlorofluorocarbon used in inhalers be replaced with the more environmentally friendly hydrofluoroalkane (HFA). Several HFA brands, including Xopenex (levalbuterol), Ventolin (albuterol sulfate), and Proventil (albuterol sulfate), entered the market during the study period, and (for purposes of this study) were combined with their non-HFA counterparts.
By dividing the total reimbursement by the number of prescriptions, a prescription price was determined for each drug. This price is prerebate, because we do not have access to mandated or supplemental rebate data. The price does include reimbursement for pharmacy dispensing.
The per-prescription cost was not adjusted by medication strength (eg, 40 mg or 80 mg). Because most medications in these drug classes are prescribed using standard protocols and exhibit similar trends in utilization, concerns for bias were minimal.37,38
Both SAS Version 9.1 (SAS Institute Inc, Cary, NC) and Microsoft Excel 2007 were used for the analysis.
Results
Increased Overall Utilization, Cost
Table 2 (page 144) shows annual utilization in Medicaid by anti-asthma drug class. The total number of prescriptions rose from 8.9 million in 1991 to 15.6 million in 2009, peaking at 20.8 million in 2005. Whereas the trend in SABA prescriptions has remained relatively flat, the number of prescriptions for combination ICS/LABA inhalers increased sharply from 0.4 million in 2001—their first year of market entry—to 3.9 million in 2005, and then dropped to 2.0 million in 2009, after the movement of Medicare and Medicaid dual-eligible beneficiaries to Medicare Part D in 2006. Prescriptions for ICSs and LABAs were 4.6 million and 0.1 million, respectively, in 2009.
Table 2.
Medicaid Annual Spending on SABAs, ICSs, LABAs, and ICS/LABA Combinations, 1991–2010
| Year | Utilization, millions of prescriptions | Spending, $ million | Spending/prescription, $ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SABA | ICS | LABA | ICS/LABA | Total | SABA | ICS | LABA | ICS/LABA | Total | SABA | ICS | LABA | ICS/LABA | |
| 1991 | 8.9 | 7.0 | 1.9 | 180.7 | 144.3 | 36.4 | 20.39 | 20.72 | 19.19 | ||||||
| 1992 | 11.6 | 8.9 | 2.7 | 253.4 | 192.9 | 60.5 | 21.93 | 21.75 | 22.53 | ||||||
| 1993 | 13.3 | 10.0 | 3.3 | 307.0 | 223.6 | 83.4 | 23.01 | 22.31 | 25.15 | ||||||
| 1994 | 13.1 | 9.5 | 3.6 | 0.0 | 320.7 | 222.2 | 98.2 | 0.3 | 24.48 | 23.44 | 27.15 | 45.82 | |||
| 1995 | 13.0 | 9.4 | 3.5 | 0.1 | 338.5 | 226.2 | 108.3 | 3.9 | 26.07 | 24.09 | 30.84 | 50.03 | |||
| 1996 | 12.1 | 7.9 | 4.1 | 0.1 | 328.9 | 187.5 | 135.8 | 5.5 | 27.19 | 23.76 | 33.16 | 51.13 | |||
| 1997 | 11.6 | 7.1 | 4.4 | 0.1 | 334.8 | 164.4 | 164.0 | 6.5 | 28.75 | 23.03 | 37.33 | 54.88 | |||
| 1998 | 10.3 | 6.8 | 3.5 | 0.0 | 286.5 | 136.3 | 147.7 | 2.5 | 27.76 | 20.17 | 42.00 | 55.24 | |||
| 1999 | 11.9 | 8.1 | 3.8 | 0.1 | 338.2 | 158.6 | 174.2 | 5.4 | 28.33 | 19.65 | 46.11 | 62.58 | |||
| 2000 | 12.9 | 8.7 | 4.1 | 0.1 | 392.3 | 173.5 | 210.2 | 8.6 | 30.35 | 19.94 | 51.30 | 67.44 | |||
| 2001 | 14.9 | 9.5 | 4.8 | 0.2 | 0.4 | 610.6 | 269.3 | 277.6 | 10.9 | 52.8 | 40.88 | 28.29 | 57.58 | 71.17 | 119.60 |
| 2002 | 16.1 | 10.0 | 4.6 | 0.1 | 1.4 | 783.3 | 285.3 | 304.8 | 10.5 | 182.7 | 48.52 | 28.55 | 66.60 | 71.69 | 127.97 |
| 2003 | 18.5 | 11.0 | 4.6 | 0.4 | 2.5 | 1003.5 | 298.0 | 332.7 | 31.4 | 341.5 | 54.38 | 27.16 | 71.83 | 80.87 | 138.71 |
| 2004 | 19.4 | 10.8 | 4.7 | 0.6 | 3.4 | 1189.6 | 289.1 | 354.9 | 49.8 | 495.8 | 61.46 | 26.85 | 76.25 | 89.69 | 146.65 |
| 2005 | 20.8 | 11.6 | 4.9 | 0.4 | 3.9 | 1307.2 | 271.0 | 389.6 | 39.7 | 606.8 | 62.79 | 23.37 | 79.27 | 96.52 | 155.87 |
| 2006 | 14.0 | 8.4 | 3.8 | 0.1 | 1.8 | 1051.6 | 335.6 | 370.1 | 10.2 | 335.7 | 75.24 | 40.16 | 98.61 | 118.02 | 188.39 |
| 2007 | 14.0 | 8.4 | 3.8 | 0.1 | 1.8 | 1054.2 | 335.6 | 370.1 | 10.2 | 338.3 | 75.34 | 40.16 | 98.61 | 118.02 | 188.18 |
| 2008 | 14.6 | 8.5 | 4.0 | 0.1 | 2.0 | 1176.7 | 375.1 | 401.0 | 9.1 | 391.4 | 80.53 | 43.92 | 99.04 | 137.53 | 200.13 |
| 2009 | 15.6 | 8.9 | 4.6 | 0.1 | 2.0 | 1256.5 | 403.4 | 433.4 | 8.0 | 411.7 | 80.63 | 45.25 | 93.34 | 150.92 | 208.77 |
ICSs indicates inhaled corticosteroids; LABAs, long-acting beta-agonists; SABAs, short-acting beta-agonists.
From 1991 to 2009, Medicaid spending on anti-asthma medications rose from $180.7 million to $1.3 billion annually, an increase of 595%. Spending on ICS/LABA combination drugs rose from $52.8 million in 2001 to $411.7 million in 2009, an increase of 680% in just 9 years. Average price per prescription has risen in all the anti-asthma drug classes. Overall spending per prescription has increased 4-fold between 1991 and 2009, significantly faster than the 57.5% increase of the consumer price index over the same period.39 The average price for an ICS/LABA combination prescription was $208.77 in 2009 compared with an average price of $119.60 in 2001.
Individual Drug Utilization, Cost: SABAs
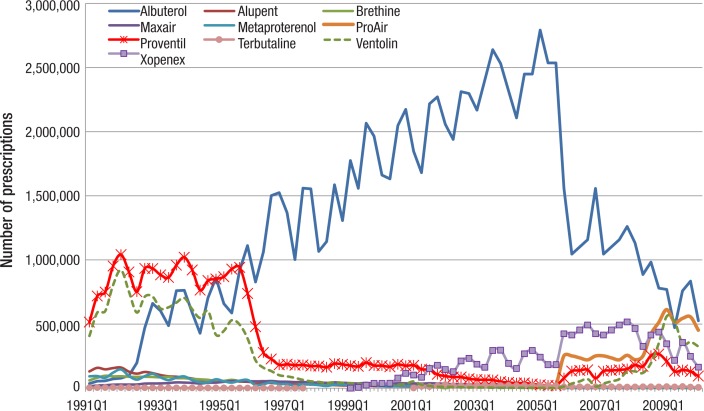
Figure 1 (page 145) presents the utilization of individual SABAs. It is clear that albuterol dominated the market during most of the study period, with a maximum of approximately 2.5 million prescriptions reimbursed by Medicaid each quarter in 2004 and 2005. In quarter 2 of 2010, the last quarter in the study, approximately 0.5 million prescriptions for albuterol were reimbursed. In the early 1990s, Proventil was the market leader, with utilization of approximately 800,000 to 1 million prescriptions per quarter. After 1995, the utilization of Proventil decreased dramatically, to less than 200,000 prescriptions per quarter, and its utilization kept decreasing through quarter 4 of 2005, although it rebounded after 2005.
Figure 1. Quarterly Utilization of Individual SABAs by Medicaid: 1991–2010.
SABAs indicates short-acting beta-agonists.
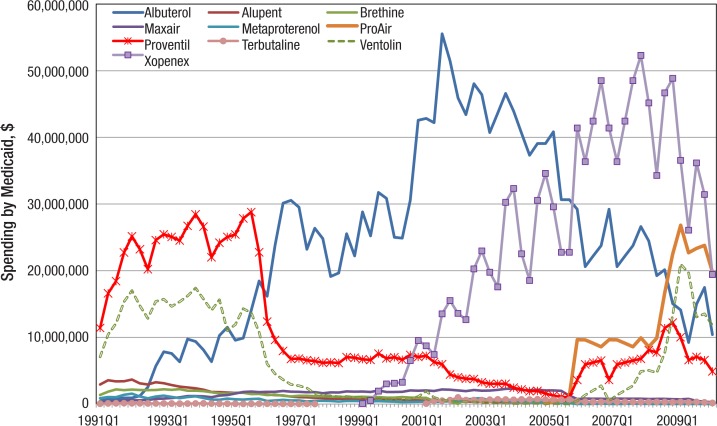
Ventolin was also widely prescribed in the early 1990s, with 500,000 to 850,000 prescriptions per quarter. Xopenex entered the anti-asthma medication market in 1999, and since 2001 has sold 100,000 to 500,000 or so prescriptions per quarter to Medicaid. As seen in Figure 2 (page 145), in the early 1990s Medicaid spent the most on the SABAs Proventil and Ventolin, but later, it spent the most on albuterol and Xopenex. In quarter 4 of 2001, Medicaid spent $55.5 million on albuterol and $51.5 million the next quarter.
Figure 2. Quarterly Spending on Individual SABAs by Medicaid, 1991–2010.
SABAs indicates short-acting beta-agonists.
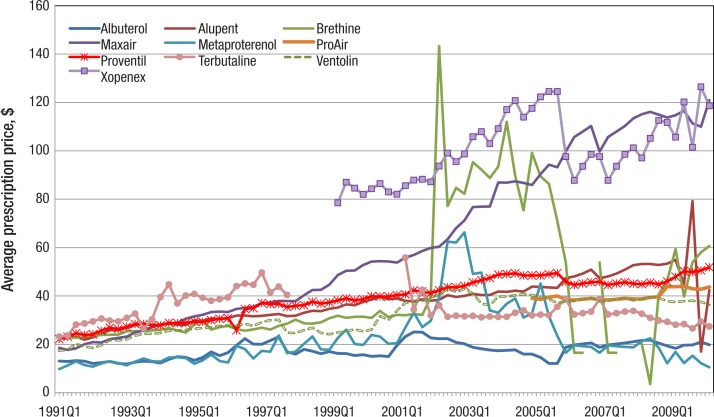
SABA prices have generally increased over time. The price of Xopenex rose from $78.52 in quarter 2 of 1999, its quarter of market entry, to $118.62 in quarter 2 of 2010 (Figure 3, page 145). The price of Maxair (pirbuterol acetate) increased impressively from $18.51 in quarter 1 of 1991 to $50.53 in quarter 4 of 1999, and then to $120.97 in quarter 2 of 2010. Although the price of Brethine (terbutaline) mimicked that for Xopenex for several years, reaching a maximum of $143.26 in quarter 2 of 2002, its price decreased quickly after that, and, indeed, was down to $60.63 in quarter 2 of 2010, about half the price of Xopenex. Prices for Proventil and Ventolin showed more modest increases from $22.18 and $17.43, respectively, in quarter 1 of 1991, to $51.88 and $36.34 in quarter 3 of 2010, although still considerably outpacing the rate of inflation. A prescription for albuterol cost Medicaid $19.81 in quarter 2 of 2010, an increase from $13.10 in quarter 1 of 1991.
Figure 3. Quarterly Per-Prescription Spending on Individual SABAs by Medicaid, 1991–2010.
SABAs indicates short-acting beta-agonists.
Individual Drug Utilization, Cost: LABAs
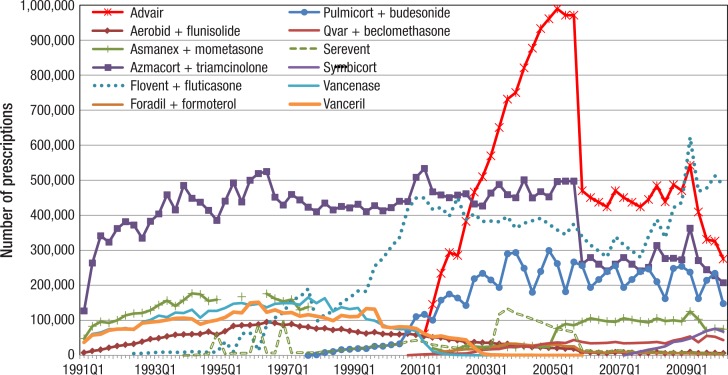
As seen in Figure 1 for rescue medications, Figure 4 (page 146) depicts the prescription trends for individual maintenance medications, including ICSs, LABAs, and ICS/LABA combinations. As is evident from Figure 4, Advair (fluticasone and salmeterol), an ICS/LABA combination drug in a single inhaler, was the most prescribed maintenance medication between quarter 4 of 2002 and quarter 1 of 2009. The quarterly Advair prescription count reached almost 1.0 million in quarter 4 of 2005.
Figure 4. Quarterly Utilization of Individual ICSs, LABAs, and ICS/LABA Combinations by Medicaid, 1991–2010.
ICSs indicates inhaled corticosteroids; LABAs, long-acting beta-agonists.
Since quarter 1 of 2009, the prescriptions for Flovent (fluticasone propionate) have exceeded those for Advair. Utilization of Pulmicort (budesonide) was steady at 200,000 to 300,000 prescriptions per quarter at the end of the study period.
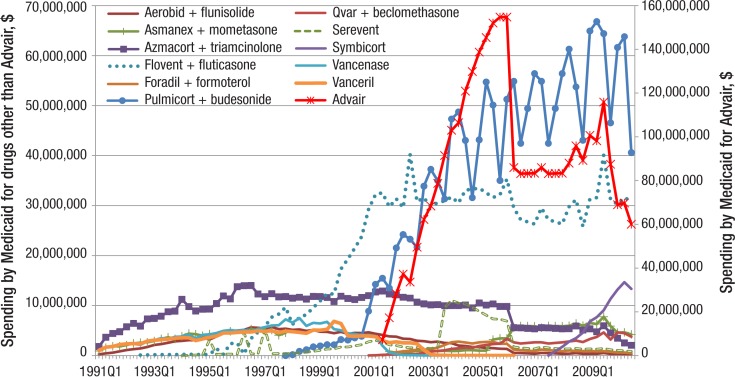
In terms of Medicaid expenditures on maintenance medications, Advair has dominated the market (Figure 5, page 146). Medicaid spent more than $100 million each quarter from quarter 4 of 2003 through quarter 4 of 2005 on this drug alone. The second vertical axis in Figure 5 emphasizes the dominance of Advair in Medicaid spending.
Figure 5. Quarterly Spending on Individual ICSs, LABAs, and ICS/LABA Combinations by Medicaid, 1991–2010.
ICSs indicates inhaled corticosteroids; LABAs, long-acting beta-agonists.
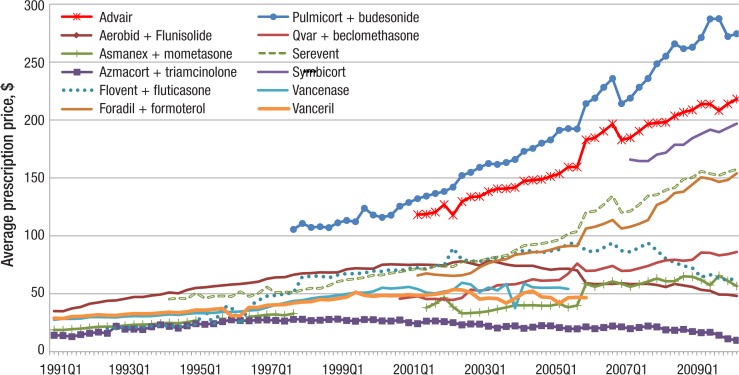
Some of the maintenance medications have seen steeply rising prices (Figure 6, page 146). The price of Advair rose steadily, from $118.27 in 2001 quarter 2 to $218.07 in 2010 quarter 2. The price of Symbicort, the other FDA-approved ICS/LABA combination drug, was $196.79 in quarter 2 of 2010. The per-prescription price of Pulmicort more than doubled from $105.52 in quarter 4 of 1997 to $274.44 in quarter 2 of 2010.
Figure 6. Quarterly Per-Prescription Spending on Individual ICSs, LABAs, and ICS/LABA Combinations by Medicaid, 1991–2010.
ICSs indicates inhaled corticosteroids; LABAs, long-acting beta-agonists.
Discussion
Increasing Disease Prevalence, and Severity
The rise in utilization of asthma medications by Medicaid beneficiaries from 1991 to 2010 is not surprising. First, the prevalence rate of asthma has increased over time in the United States, from 3.5% in 1982 to 5.5% in 1996, and then from 7.3% in 2001 to 7.7% in 2007.40 The increase has been even sharper for children, who make up a fairly large percentage of the Medicaid population.13
Moreover, asthma disproportionately impacts households of a lower socioeconomic status. Complicating the treatment of asthma in Medicaid beneficiaries is the substantial exposure they have to asthma triggers, such as dust and dust reservoirs, dust mites, roaches, rodents, molds, and tobacco smoke that could exacerbate their condition.41
Meanwhile, the number of Medicaid beneficiaries has more than doubled over the past 2 decades. The average monthly enrollment in Medicaid in the year 1990 was 22.9 million.42 In 2009, that figure reached 50.7 million.42 In 2010, average monthly Medicaid enrollment was 53.6 million (more than one sixth of the US population),42 after the worst economic recession (December 2007-June 2009) in a quarter century. In fact, in October 2009, the national unemployment rate reached 10.1%.43
This cyclical component (more Medicaid beneficiaries in times of recession and declining numbers in expansions) may also explain some of the downward movement in medication use in the late 1990s, when the US economy was quite strong. With the implementation of Medicare Part D in 2006, dual-eligibles (those eligible for Medicaid and Medicare) were moved to Medicare Part D, explaining the decrease in utilization that year.
As the prevalence rate for asthma has been increasing over time, its severity level has been rising as well.40 Because all patients with persistent asthma are encouraged to supplement their rescue medication with a maintenance medication, it is not surprising to see a rise in the utilization of LABAs, ICSs, and ICS/LABA combinations accompanying rising severity.
The Introduction of Long-Acting Beta-Agonists
The introduction of LABAs was considered a major advance in bronchodilator therapy, with evidence that their use led to improved lung function and quality of life.44–46 Because inhaled SABAs were effective for only 4 to 6 hours, they required frequent daily administration and were often ineffective in preventing common nighttime asthma episodes. Moreover, the SABAs Proventil and Ventolin, both of which contained a 50:50 racemic mixture of albuterol sulfate, appeared to produce unwanted side effects, such as bronchoconstriction and inflammatory responses in some patients with asthma.47
This undesired activity was attributed to (S)-albuterol. When Xopenex, containing levalbuterol, entered the market in 1999, it was hoped that the newer drug could control symptoms at lower doses, reduce hospital admissions, decrease length of hospital stays, reduce side effects, and reduce the need for multiple medications.48,49 Although there is ongoing discussion regarding medication preference,50 these potential benefits to levalbuterol could account for the increasing market share for Xopenex since 2000.
LABAs have also been plagued by safety issues; physicians have been reluctant to prescribe a LABA without concomitant use of an ICS, a combination that seems to mitigate some of the safety concerns.51 For this reason, and the convenience to the patient of having both medications in a single inhaler, it makes sense to see the extreme positive market reaction to the ICS/LABA combination drugs. In addition, the 2007 NHLBI guidelines recommend the use of LABA therapy as a supplemental treatment to SABAs and ICSs to achieve favorable long-term asthma control in children and adults with moderate-to-severe persistent asthma.20
Increased Medication Price
Besides increasing utilization of anti-asthma medications, the increase in the average per-prescription price has certainly played a role in the increased Medicaid spending for SABAs, LABAs, ICSs, and ICS/LABA combination drugs. There has been a 295% increase in average price across all the medications studied between 1991 and 2009. The tremendous increase in demand for anti-asthma medications by patients and payers has exerted upward pressure on price. New entry of branded medications has had no effect on the price dynamics of other drugs in the market. This lack of effect of new entry on price trends of drugs already in the market has been found for other drug classes as well, for example, antipsychotics and anti-ulcer medications.37,52,53
Without generic medication entry, there is not much that Medicaid can do to cut its spending on prescription asthma medications. A number of previous studies have shown that the only significant remedy for skyrocketing drug costs is the entry of generic-manufactured drugs.37,52,53 Because some of the anti-asthma medications have been only fairly recently approved by the FDA, their patent protection will last a number of years. It is more than likely that when branded-drug patents do expire, state Medicaid programs will take advantage of available generic options.54
Pharmacotherapy Is Cost-Effective
Despite the high and increasing cost of both rescue and maintenance therapy, to the extent that medication reduces the number of serious asthma exacerbations resulting in emergency department visits, hospitalizations, and intubations, spending on pharmaceuticals may be cost-effective.
Increasing numbers of children are being hospitalized for asthma because of an increase in asthma severity, poor disease management, and rising poverty. In the United States, asthma is the most common reason for hospitalizations among 3- to 12-year-old children and the second most common reason for hospitalizations among all children, accounting for 7.4% of all pediatric hospitalizations.55 It is suspected that medication adherence is less than optimal especially among low-income, publicly insured children; therefore, some of the hospitalizations could potentially be avoidable.56 However, because of the dynamic nature of asthma, children may be hospitalized in any case, an unavoidable consequence of rising disease severity.
Limitations
This study has a number of limitations. First, patient-specific information was not available in the Medicaid pharmacy claims database; thus, it was not possible to determine the exact indication or overlapping indications for medication use. Although it is likely that most of the patients taking the study drugs had asthma, others might have had COPD or allergy problems. Because indications were not available, it was not possible to compare the use of these medications with prevalence rates provided by other studies. Nor have we studied an exhaustive list of drugs, which would include, for instance, leukotriene modifiers, mast-cell stabilizers, theophylline, and oral and intravenous steroids used in the treatment of asthma.
Second, reimbursement per prescription is but a proxy for drug price. It does not account for manufacturer rebates. In addition, because we summarize reimbursement and prescriptions over all NDC codes for each drug, this does not allow for changes over time in the product mix (ie, varying strengths). Summing also means that some of the NDCs corresponding to generic drug formulations (especially mometasone and fluticasone) that are included in the study are not necessarily used in the treatment of asthma.
Third, the CMS data contain possible reporting errors. To ensure reliability, some of the values in this study were imputed. Fourth, the results are specific to the Medicaid population and, hence, do not necessarily represent utilization and expenditure trends in other US markets.
Finally, the CMS database includes pharmacy claims administered by the state Medicaid programs. Because there has been a significant trend toward Medicaid managed care, some of the pharmacy claims may be administered by managed care rather than through pharmacy carve-out plans. Our database does not allow us to determine the degree of managed care participation in pharmacy claim administration.
Conclusion
Anti-asthma medications, including SABAs, LABAs, ICSs, and ICS/LABA combination drugs, are a major expense for US Medicaid programs. Moreover, rising asthma prevalence and worsening severity suggest a high expenditure future as far as these drugs are concerned. Although the rising prevalence is worse for children than for adults and is particularly severe among low-income households, Medicare is also predicted to experience a rising expenditure trend in the future, because this program covers prescription drugs for the elderly. Despite its cost, drug therapy is of paramount importance to control the serious events associated with asthma and its exacerbations.
Stakeholder Perspective
Medicaid Spending on Asthma Medications
PAYERS: Given the severe budget shortfalls currently experienced by Medicaid programs across the country, it is now more important than ever to scrutinize trends within pharmacy spending to ensure that increasingly precious dollars find their way to efficient use of effective medications. In evaluating those trends for asthma agents, the authors of the present article point out that the introduction of new technology, most notably the long-acting beta-agonists, is a significant contributor to the upward trend in the utilization of asthma medication class. Payers are now left to do what they can to drive the effective use of these newer medications, ensuring that factors such as the timing of onset do not lead to inappropriate utilization and nonadherence. These issues are often compounded in the Medicaid population, where health literacy levels are likely to be lower than in the general population, which often affects medication adherence.
As the authors note, the lack of transparency in arriving at Medicaid's “real” cost of medications makes this sort of analysis difficult. The Medicaid Drug Rebate program results in the offset of a substantial portion of Medicaid drug spending, with the system of rebate rate determination structured in such a way that the price benefit of generics is often minimized, and sometimes eliminated completely. In addition, because the rebate rate of brand agents is adjusted by Medicaid in an attempt to negate price increases over the consumer price index, the results of drug price inflation are seen in the initial drug spending but are partially counterbalanced by a corresponding increase in the rebate amount. These circumstances may result in a drastically different relative cost picture when evaluating initial pharmacy reimbursement costs than when considering costs after these rebates.
PATIENTS: Patients are currently faced with a potentially more complicated regimen of therapy for the treatment of asthma than was the case in previous decades, requiring patients to have a better understanding of their pharmacotherapy. The pharmacology of controller medications, often not having an effect that is immediately noticeable, increases the potential that patients may forgo controller medications and rely on rescue inhalers that produce an immediate beneficial result.
For the value of these newer controller medications to be realized, it is critical that patient adherence to the entire medication regimen be encouraged at every possible interaction, whether with the prescriber, the pharmacy, or the payer.
Bryan Amick, PharmD, MBA
Director, Clinical Services, Magellan Medicaid Administration, Columbia, SC
Biographies

Shih-Feng Chiu

Christina M.L. Kelton
Author Disclosure Statement
The authors did not receive any funding for this study. Dr Guo receives honoraria from AstraZeneca; is a consultant to Baxter Healthcare; and receives grant support from Bristol-Myers Squibb, Eli Lilly, Genentech, Janssen Ortho-McNeil, and Novartis. Dr Kelton receives grant support from Eli Lilly, Janssen Ortho-McNeil, and Novartis. Dr Lin receives research support from Hospira and Rite Aid. Dr Wigle receives grant support from Novartis. Mr Chiu and Dr Szeinbach have reported no conflicts of interest.
Contributor Information
Shih-Feng Chiu, PhD candidate, James L. Winkle College of Pharmacy, University of Cincinnati Medical Center, Ohio.
Christina M.L. Kelton, Professor of Economics, College of Business, University of Cincinnati, Ohio.
Jeff Jianfei Guo, Associate Professor of Pharmacoeconomics & Pharmacoepidemiology, James L. Winkle College of Pharmacy, University of Cincinnati Medical Center, Ohio.
Patricia R. Wigle, Associate Professor of Pharmacy Practice, James L. Winkle College of Pharmacy, University of Cincinnati Medical Center, Ohio.
Alex C. Lin, Assistant Professor of Pharmacy Systems and Administration, James L. Winkle College of Pharmacy, University of Cincinnati, Ohio.
Sheryl L. Szeinbach, Professor, Division of Pharmacy Practice and Administration, College of Pharmacy, Ohio State University, Columbus, Ohio.
References
- 1.Klomp H, Lawson JA, Cockcroft DW, et al. Examining asthma quality of care using a population-based approach. CMAJ. 2008; 178: 1013–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2009. www.ginasthma.com/download.asp?intId=411 Accessed March 9, 2011.
- 3.Mannino DM, Homa DM, Pertowski CA, et al. Surveillance for asthma—United States, 1960–1995. MMWR CDC Surveill Summ. 1998; 47: 1–27 [PubMed] [Google Scholar]
- 4.Senthilselvan A. Prevalence of physician-diagnosed asthma in Saskatchewan, 1981 to 1990. Chest. 1998; 114: 388–392 [DOI] [PubMed] [Google Scholar]
- 5.Masoli M, Fabian D, Holt S, Beasley R; for the Global Initiative for Asthma (GINA) program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59: 469–478 [DOI] [PubMed] [Google Scholar]
- 6.Gaugris S, Sazonov-Kocevar V, Thomas M. Burden of concomitant allergic rhinitis in adults with asthma. J Asthma. 2006; 43: 1–7 [DOI] [PubMed] [Google Scholar]
- 7.Celikel S, Isik SR, Demir AU, et al. Risk factors for asthma and other allergic diseases in seasonal rhinitis. J Asthma. 2008; 45: 710–714 [DOI] [PubMed] [Google Scholar]
- 8.Boulet LP. Influence of comorbid conditions on asthma. Eur Respir J. 2009; 33: 897–906 [DOI] [PubMed] [Google Scholar]
- 9.Szeinbach SL, Rodriguez-Monguio R, Baran RW, Williams PB. Sleep disorders and chronic constipation: relation to other co-morbidities? Open Allergy J. 2010; 3: 29–34 [Google Scholar]
- 10.Dean BB, Calimlim BM, Kindermann SL, et al. The impact of uncontrolled asthma on absenteeism and health-related quality of life. J Asthma. 2009; 46: 861–866 [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF. Quality-of-life considerations in the treatment of asthma. Pharmacoeconomics. 1995; 8: 123–138 [DOI] [PubMed] [Google Scholar]
- 12.Bousquet J, Knani J, Dhivert H, et al. Quality of life in asthma. I. Internal consistency and validity of the SF-36 questionnaire. Am J Respir Crit Care Med. 1994; 149: 371–375 [DOI] [PubMed] [Google Scholar]
- 13.Akinbami L. Asthma prevalence, health care use and mortality: United States, 2003–2005. CDC National Center for Health Statistics, 2006. www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm Accessed March 9, 2011. [PubMed] [Google Scholar]
- 14.Weiss KB, Sullivan SD. The economic costs of asthma: a review and conceptual model. Pharmacoeconomics. 1993; 4: 14–30 [DOI] [PubMed] [Google Scholar]
- 15.Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009; 9: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Heart, Lung, and Blood Institute. Morbidity & Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed March 9, 2011.
- 17.Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol. 2010; 126: 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006; 355: 2226–2235 [DOI] [PubMed] [Google Scholar]
- 19.Sawicki GS, Vilk Y, Schatz M, et al. Uncontrolled asthma in a commercially insured population from 2002 to 2007: trends, predictors, and costs. J Asthma. 2010; 47: 574–580 [DOI] [PubMed] [Google Scholar]
- 20.National Heart, Lung, and Blood Institute. Expert panel report 3: guidelines for the diagnosis and management of asthma. 2007. www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf Accessed February 28, 2011.
- 21.World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. 2007. www.who.int/gard/publications/GARD_Manual/en/index.html Accessed March 9, 2011.
- 22.Sears MR, Taylor DR, Print CG, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990; 336: 1391–1396 [DOI] [PubMed] [Google Scholar]
- 23.Mcivor RA, Pizzichini E, Turner MO, et al. Potential masking effects of salmeterol on airway inflammation in asthma. Am J Respir Crit Care Med. 1998; 158: 924–930 [DOI] [PubMed] [Google Scholar]
- 24.Salpeter SR, Ormiston TM, Salpeter EE. Meta-analysis: respiratory tolerance to regular beta2-agonist use in patients with asthma. Ann Intern Med. 2004; 140: 802–813 [DOI] [PubMed] [Google Scholar]
- 25.Hasford J, Virchow JC. Excess mortality in patients with asthma on long-acting beta2-agonists. Eur Respir J. 2006; 28: 900–902 [DOI] [PubMed] [Google Scholar]
- 26.Price AH, Clissold SP. Salbutamol in the 1980s: a reappraisal of its clinical efficacy. Drugs. 1989;38: 77–122 [DOI] [PubMed] [Google Scholar]
- 27.Spitzer WO, Suissa S, Ernst P, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992; 326: 501–506 [DOI] [PubMed] [Google Scholar]
- 28.Pearce N, Beasley R, Crane J, et al. End of the New Zealand asthma mortality epidemic. Lancet. 1995; 345: 41–44 [DOI] [PubMed] [Google Scholar]
- 29.Rider NL, Craig TJ. A safety review of long-acting beta2-agonists in patients with asthma. J Am Osteopath Assoc. 2006; 106: 562–567 [PubMed] [Google Scholar]
- 30.Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001; 285: 2583–2593 [DOI] [PubMed] [Google Scholar]
- 31.Mann M, Chowdhury B, Sullivan E, et al. Serious asthma exacerbations in asthmatics treated with high-dose formoterol. Chest. 2003; 124: 70–74 [DOI] [PubMed] [Google Scholar]
- 32.Lemanske RF, Jr, Sorkness CA, Mauger EA, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001; 285: 2594–2603 [DOI] [PubMed] [Google Scholar]
- 33.Simpson CR, Sheikh A. Trends in the epidemiology of asthma in England: a national study of 333,294 patients. J Soc Med. 2010; 103: 98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008; 134: 14–19 [DOI] [PubMed] [Google Scholar]
- 35.Broder MS, Gutierrez B, Chang E, et al. Ratio of controller to total asthma medications: determinants of the measure. Am J Manag Care. 2010; 16: 170–178 [PubMed] [Google Scholar]
- 36.Centers for Medicare & Medicaid Services. State drug utilization data. www.cms.hhs.gov/MedicaidDrugRebateProgram/SDUD/list.asp#TopOfPage Accessed March 9, 2011.
- 37.Chen Y, Kelton CML, Jing Y, et al. Utilization, price, and spending trends for antidepressants in the US Medicaid program. Res Soc Adm Pharm. 2008; 4: 244–257 [DOI] [PubMed] [Google Scholar]
- 38.Jing Y, Klein P, Kelton CML, et al. Utilization and spending trends for antiretroviral medications in the US Medicaid program from 1991 to 2005. AIDS Res Ther. 2007; 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bureau of Labor Statistics. Consumer price index-all urban consumers. US city average. All items. www.bls.gov/cpi Accessed March 6, 2011.
- 40.American Lung Association. Trends in asthma morbidity and mortality. February 2010. www.lungusa.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf Accessed on March 9, 2011.
- 41.Krieger JW, Song L, Takaro TK, Stout J. Asthma and the home environment of low-income urban children: preliminary findings from the Seattle-King County healthy homes project. J Urban Health. 2000; 77: 50–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Medicare & Medicaid Services. Data compendium, 2010 edition. www.cms.gov/DataCompendium/14_2010_Data_Compendium.asp#TopOfPage Accessed March 9, 2011.
- 43.National unemployment rate. http://data.bls.gov/timeseries/LNS14000000 Accessed March 6, 2011.
- 44.Kemp JP, Bierman CW, Cocchetto DM. Dose-response study of inhaled salmeterol in asthmatic patients with 24-hour spirometry and Holter monitoring. Ann Allergy. 1993; 70: 316–322 [PubMed] [Google Scholar]
- 45.Derom EY, Pauwels RA, Van der Straeten ME. The effect of inhaled salmeterol on methacholine responsiveness in patients with asthma up to 12 hours. J Allergy Clin Immunol. 1992; 89: 811–815 [DOI] [PubMed] [Google Scholar]
- 46.Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997; 10: 815–821 [PubMed] [Google Scholar]
- 47.Ameredes BT, Calhoun WJ. Levalbuterol versus albuterol. Curr Allergy Asthma Rep. 2009; 9: 401–409 [DOI] [PubMed] [Google Scholar]
- 48.Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003; 123: 128–135 [DOI] [PubMed] [Google Scholar]
- 49.Gawchik SM, Saccar CL, Noonan M, et al. The safety and efficacy of nebulized levalbuterol compared with racemic albuterol and placebo in the treatment of asthma in pediatric patients. J Allergy Clin Immunol. 1999; 103: 615–621 [DOI] [PubMed] [Google Scholar]
- 50.Crader M, Borkowski J. Nebulized albuterol versus levalbuterol in pediatric and adult patients: a review. Formulary. 2009; 44: 108–118 [Google Scholar]
- 51.Jaeschke R, O'Byrne PM, Mejza F, et al. The safety of long-acting beta-agonists among patients with asthma using inhaled corticosteroids: systematic review and metaanalysis. Am J Respir Crit Care Med. 2008; 178: 1009–1016 [DOI] [PubMed] [Google Scholar]
- 52.Jing Y, Kelton CML, Guo JJ, et al. Price, utilization, and spending for antipsychotic medications in the Medicaid program. Drug Benefit Trends. 2007; 19: 27–41 [Google Scholar]
- 53.Guo JJ, Kelton CML, Pasquale MK, et al. Price and market-share competition of anti-ulcer gastric medications in the Ohio Medicaid market. Int J Pharm Med. 2004; 18: 271–282 [Google Scholar]
- 54.Kaiser Commission on Medicaid and the Uninsured. State Medicaid outpatient prescription drug policies: findings from a national survey, 2005 update. www.kff.org/medicaid/upload/State-Medicaid-Outpatient-Prescription-Drug-Policies-Findings-from-a-National-Survey-2005-Update-report.pdf Accessed March 9, 2011.
- 55.Owens PL, Thompson J, Elixhauser A, Ryan K. Care of children and adolescents in U.S. hospitals, 2003. HCUP Fact Book No. 4, publication No. 04-0004. Rockville, MD: Agency for Healthcare Research and Quality; 2003. www.ahrq.gov/data/hcup/factbk4/factbk4.pdf Accessed April 25, 2011. [Google Scholar]
- 56.Berger WE. Levalbuterol: pharmacologic properties and use in the treatment of pediatric and adult asthma. Ann Allergy Asthma Immunol. 2003; 90: 583–592 [DOI] [PubMed] [Google Scholar]