Abstract
Perturbing the circadian system by electrolytically lesioning the suprachiasmatic nucleus (SCN) or varying the environmental light:dark schedule impairs memory, suggesting that memory depends on the circadian system. We used a genetic approach to evaluate the role of the molecular clock in memory. Bmal1−/− mice, which are arrhythmic under constant conditions, were examined for hippocampus-dependent memory, LTP at the Schaffer-collateral synapse, and signal transduction activity in the hippocampus. Bmal1−/− mice exhibit impaired contextual fear and spatial memory. Furthermore, LTP in hippocampal slices from Bmal1−/− mice is also significantly decreased relative to that from wild-type mice. Activation of Erk1,2 MAP kinase (MAPK) during training for contextual fear memory and diurnal oscillation of MAPK activity and cAMP in the hippocampus is also lost in Bmal1−/− mice, suggesting that the memory defects are due to reduction of the memory consolidation pathway in the hippocampus. We conclude that critical signaling events in the hippocampus required for memory depend on BMAL1.
Hippocampus-dependent learning and memory is initiated by a calcium influx mediated through NMDA receptors (Morris et al. 1986; Tsien et al. 1996) and leads to the activation of several signaling pathways, including the cAMP and Erk1,2 MAP kinase (MAPK) pathways (Adams and Sweatt 2002; Wang and Storm 2003). Activation of CaM-stimulated adenylyl cyclases and MAPK causes stimulation of CRE-mediated transcription (Impey et al. 1998; Athos et al. 2002; Sindreu et al. 2007). Perturbing any of these signaling events disrupts memory consolidation (Bourtchuladze et al. 1994; Atkins et al. 1998; Wong et al. 1999; Athos et al. 2002; Pittenger et al. 2002).
Despite the potential for memories to persist over the lifetime of an individual, the proteins that underlie strengthened synaptic connections degrade. Recurring rounds of transcription and protein synthesis may contribute to the persistence of memories (Bekinschtein et al. 2007; Eckel-Mahan et al. 2008). Specifically, Eckel-Mahan et al. (2008) showed that the cAMP/MAPK/CREB transcriptional pathway undergoes a circadian oscillation in the hippocampus that, when blocked, inhibits the maintenance of contextual fear memory in mice. The mammalian circadian system may provide the signal to coordinate returning rounds of transcription important for memory maintenance.
Previous research has established that time of day can influence learning and memory of fear conditioning under both light:dark entrained and free-running conditions (Chaudhury and Colwell 2002; Eckel-Mahan et al. 2008). Hippocampal LTP also depends on time of day under light:dark entrained conditions (Chaudhury et al. 2005). This time-of-day influence on memory and LTP suggests involvement of the circadian system. Perturbing the circadian system through environmental disruption of lighting conditions or lesioning the master circadian regulator can impair memory (Tapp and Holloway 1981; Devan et al. 2001; Lyons et al. 2006; Ruby et al. 2008; Loh et al. 2010; Phan et al. 2011).
The suprachiasmatic nucleus (SCN) is the master regulatory clock in mammals. The SCN receives light input from the environment via the retinohypothalamic pathway and synchronizes peripheral oscillators via synaptic connection or diffusible factors (Antle and Silver 2005). A molecular clock comprised of positively and negatively regulated transcriptional/translational loops drives the oscillations in central and peripheral sites with a period of about 24 h (Ko and Takahashi 2006). BMAL1 and CLOCK heterodimerize and bind to E-box elements to promote the transcription of circadian-regulated genes, including the heterodimer's negative regulators, Period and Cryptochrome. PERIOD and CRYPTOCHROME dimerize and translocate to the nucleus to inhibit the activity of the BMAL1:CLOCK heterodimer. A second loop involving the competitive binding of REV-ERBα and RORα at RORE sites in the Bmal1 promoter regulates BMAL1 levels.
Only the Bmal1−/− mouse is completely arrhythmic in constant conditions and exhibits impaired entrainment to a light:dark cycle (Bunger et al. 2000). Other molecular clock knockout mouse strains retain some rhythmicity due to compensatory functional isoforms and present with subtle or no memory deficits (Garcia et al. 2000; Debruyne et al. 2006; Zueger et al. 2006; Van der Zee et al. 2008). Bmal1−/− mice provide an intriguing model to genetically address the role of the molecular clock in memory.
We hypothesize that the molecular clock is required for the diurnal oscillation of signaling activity in the hippocampus and hippocampus-dependent memory. Here, we report that Bmal1−/− mice have impaired contextual fear memory, defects in learning and spatial memory, impaired LTP at the Schaffer-collateral synapse, as well as no diurnal change in cAMP and MAPK activity.
Results
Bmal1−/− mice exhibit impaired hippocampus-dependent learning and memory
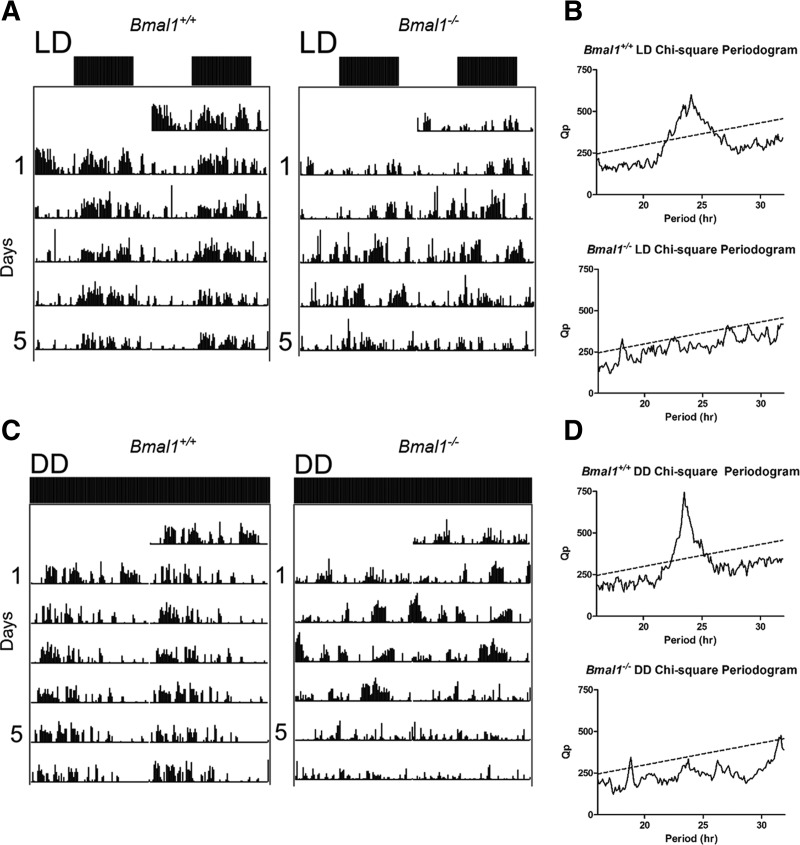
Consistent with previous reports (Bunger et al. 2000), Bmal1−/− mice are arrhythmic under dark:dark (DD) conditions while Bmal1+/+ littermates exhibit an expected τ close to 24 h (Fig. 1C,D). Bmal1−/− mice also exhibit difficulties entraining to a 12-h light:dark (LD) cycle (Fig. 1A,B), thus all experiments were performed under diurnal conditions.
Figure 1.
Bmal1−/− mice locomotor activity. Mice were monitored in 12-h LD conditions for 1 wk before exposed to DD conditions for 2 wk. Analysis of DD conditions is restricted to the second week of monitoring. (A) Representative Bmal1+/+ and Bmal1−/− double-plotted actograms in 12-h LD conditions. Each line represents 48 h. (B) Bmal1+/+ and Bmal1−/− χ-square periodograms. Bmal1+/+ periodogram reveals τ = 1445 min, or 24.1 h. Bmal1−/− periodogram does not reveal a period of 24 h. For the one peak that does cross the line of significance, τ = 1085 min, or 18.1 h. (C) Representative Bmal1+/+ and Bmal1−/− double-plotted actograms in DD conditions. (D) Bmal1+/+ periodogram reveals τ = 1410 min, or 23.5 h. Bmal1−/− periodogram does not reveal a period of 24 h. For the one peak that does cross the line of significance, τ = 1130 min, or 18.8 h.
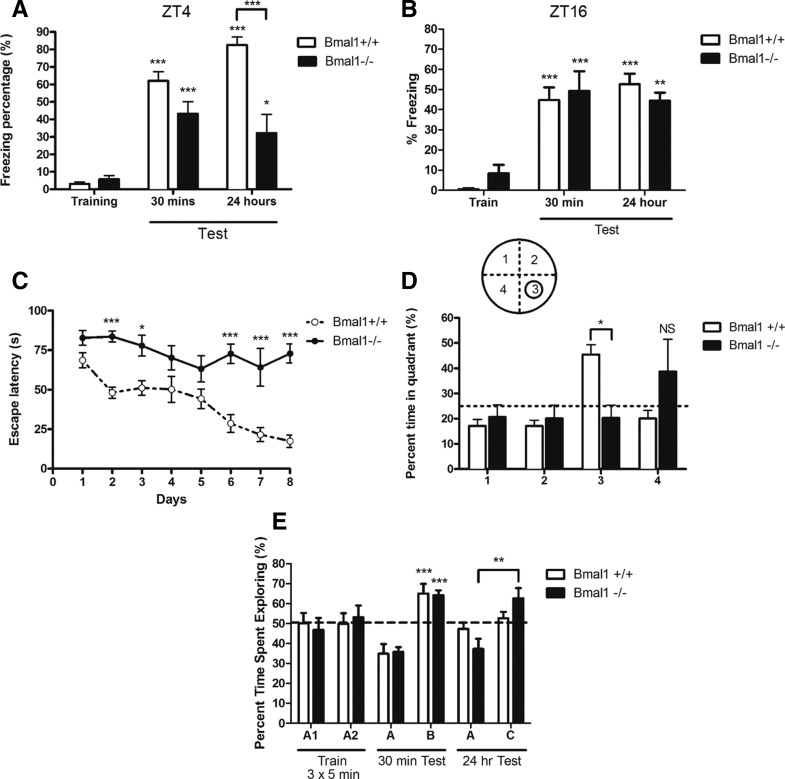
Bmal1+/+ and Bmal1−/− mice were trained and tested for contextual fear conditioning from ZT3 (3 h after light onset) to ZT5, the peak of MAPK activity and fear memory (Chaudhury et al. 2002; Eckel-Mahan et al. 2008), and under red light from ZT15 to ZT17, the trough of MAPK activity and fear memory. Mice explored a novel context for 2 min before receiving a 0.7-mA foot shock. Bmal1−/− mice froze significantly less than their wild-type littermates when they were tested 24 h after training (F(5,45) = 31.44, P < 0.0001, one-way ANOVA, Tukey post-hoc test) (Fig. 2A). While Bmal1−/− mice did learn to associate the novel context with a foot shock (P < 0.0001 and P < 0.05, Tukey post-hoc test) (Fig. 2A), their long-term memory for contextual fear conditioning was not as strong as that of Bmal1+/+ controls. Bmal1−/− mice contextual fear memory was comparable to that of Bmal1+/+ controls when trained and tested during the night (Fig. 2B). Both groups freeze more 30 min and 24 h after training (F(5,41) = 17.49, P < 0.0001, one-way ANOVA, Tukey post-hoc test). Thus, while Bmal1−/− mice are able to learn and remember contextual fear conditioning, the strength of their memory is comparable to Bmal1+/+ freezing levels during the trough of fear memory.
Figure 2.
Bmal1−/− mice demonstrate impaired learning and memory in hippocampus-dependent tasks. (A) Contextual fear freezing behavior at ZT4. Bmal1−/− mice freeze significantly less 24 h (F(5,45) = 31.44, P < 0.0001) after contextual fear conditioning training with a 0.7-mA foot shock. One-way ANOVA with Tukey post-hoc test. (B) Contextual fear freezing behavior at ZT16. Both groups demonstrate significant freezing behavior 30 min and 24 h after training (F(5,41) = 17.49, P < 0.0001, one-way ANOVA, Tukey post-hoc test). (C) Morris water maze training curve. Bmal1−/− consistently took longer to find the platform for four trials of training per day over 8 d. There is a significant effect of genotype (F(1,98) = 34.25, P < 0.001) and day of training (F(7,98) = 9.043, P < 0.001). Two-way ANOVA with Bonferroni post-hoc test. (D) Morris water maze probe trial. Bmal1+/+ mice spend significantly more time in quadrant 3, where the platform used to be, compared to other quadrants and Bmal1−/− mice (F(3,52) = 4.080, P < 0.01). Two-way ANOVA with Bonferroni post-hoc test. (E) Novel object recognition. Thirty minutes after training to object A, both Bmal1−/− and Bmal1+/+ mice spend significantly more time exploring novel object B (F(3,22) = 18.12, P < 0.001). Bmal1−/− mice spent more time interacting with novel object C 24 h after exposure to object A (P < 0.001). One-way ANOVA, Tukey post-hoc test. All values represent mean ± SEM, n = 6–10.
In the Morris water maze, Bmal1+/+ and Bmal1−/− mice were trained to navigate to a hidden platform for 8 d from ZT4 to ZT8. Throughout this training, Bmal1−/− mice consistently took longer to find the hidden platform compared to their wildtype littermates (Bonferroni post-hoc test) (Fig. 2C). Two-way ANOVA revealed a significant effect of genotype (F(1,98) = 34.25, P < 0.001) and day of training (F(7,98) = 9.043, P < 0.001). When the platform was removed during the probe trial, Bmal1+/+ littermates spent significantly more time in the quadrant where the platform was during training compared to Bmal1−/− mice, 45.4 ± 3.9% vs. 20.3 ± 5.0% of probe trial (F(3,52) = 4.080, P < 0.01, two-way ANOVA with Bonferroni post-hoc test) (Fig. 2D). Furthermore, Bmal1+/+ mice crossed the platform's previous location more frequently than Bmal1−/− mice, 3.9 ± 0.8 vs. 0.8 ± 0.4 crossings (t(13) = 2.964, P = 0.0110, two-tailed unpaired t-test).
While Bmal1−/− mice showed impairment in contextual fear conditioning and the Morris water maze, they demonstrated no deficit in the novel object recognition task. Mice were exposed to object A for 5 min, three times. Both Bmal1+/+ and Bmal1−/− mice spent more time exploring novel object B 30 min after training (F(3,22) = 18.12, P < 0.001, one-way ANOVA, Tukey post-hoc test) (Fig. 2E). In fact, Bmal1−/− mice even spent more time interacting with a novel object 24 h after exploring the original object (P < 0.001, Tukey post-hoc test) (Fig. 2E).
Bmal1−/− mice have impaired LTP
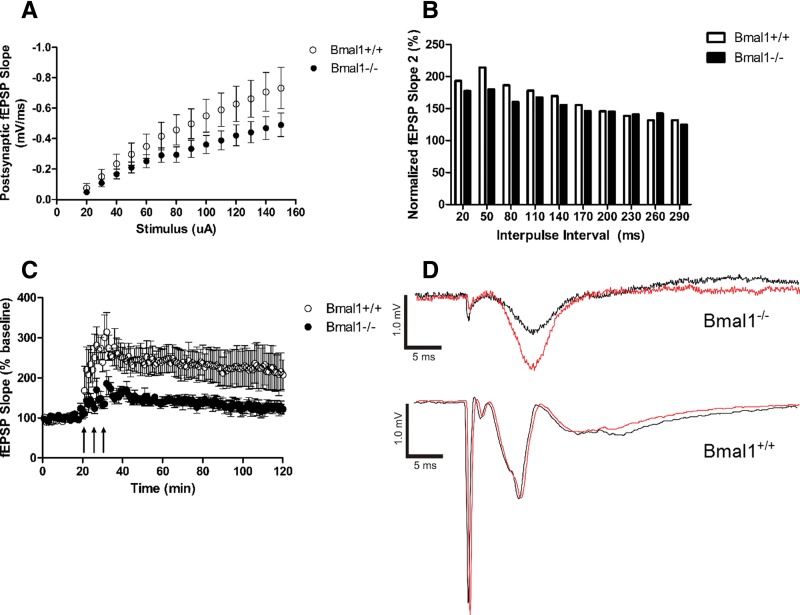
The Schaffer collateral efferent fibers in the stratum radiatum of hippocampal area CA1 were stimulated in the acute brain slice and the resulting fEPSPs recorded. Before inducing LTP, the input/output (I/O) curve and paired-pulse facilitation were characterized. There was no significant difference between the Bmal1+/+ and Bmal1−/− I/O values at each stimulus (Tukey's multiple comparison test) (Fig. 3A). There was also no significant difference between the normalized slopes of the second fEPSP at each interpulse interval for paired-pulse facilitation (Tukey's multiple comparison test) (Fig. 3B). To induce LTP, three tetanic stimuli separated by 5 min were delivered to the acute slice and fEPSPs monitored for 2 h (Fig. 3C). For the first 30 min following LTP induction, the Bmal1+/+ hippocampus mean fEPSP slope response was 251.50 ± 3.43% of baseline, while the Bmal1−/− hippocampus demonstrated significantly reduced LTP with a mean fEPSP slope response of 152.20 ± 2.51% of baseline (U = 0.0, P < 0.0001, r = 1.21, Mann–Whitney). The Bmal1−/− hippocampus continued to demonstrate a significantly reduced response (228.7 ± 1.2% of baseline vs. 133.3 ± 1.1% of baseline) 60–90 min following induction (U = 0.0, P < 0.0001, r = 1.22, Mann–Whitney). These results are consistent with behavioral data, suggesting a reduction in long-term synaptic plasticity.
Figure 3.
LTP at the Schaffer-collateral synapse is impaired in the Bmal1−/− hippocampus. (A) Input/output (I/O) relationship between stimulus (µA) and the slope of the postsynaptic evoked fEPSP (mV/ms). Stimulus ranged from 20 to 150 µA. There is no difference between the Bmal1+/+ and Bmal1−/− I/O values at each stimulus. Tukey's multiple comparison test. n = 10–12. (B) Paired-pulse facilitation with interpulse interval ranging from 20 to 290 msec. There was no difference between the normalized slopes of the second fEPSP at each interpulse interval. Tukey's multiple comparison test. n = 7–9. (C) Averaged LTP response of the Schaffer-collateral fibers, represented by fEPSP slope and normalized to pretetanic baseline. To induce LTP, three tetanic stimuli (1-sec, 100-Hz train with 0.1-msec pulse length) separated by 5 min were delivered. Bmal1−/− hippocampus demonstrated significantly reduced LTP with a mean fEPSP slope response within first 30 min following induction (U = 0.0, P < 0.0001, r = 1.21, Mann–Whitney). The Bmal1−/− hippocampus continued to demonstrate a significantly reduced response 60–90 min following induction (U = 0.0, P < 0.0001, r = 1.22, Mann–Whitney). (D) Representative LTP traces from individual slices. Black is 5 min after tetanic stimulations. Red is 60 min after tetanic stimulations. All values represent mean ± SEM.
The hippocampus of Bmal1−/− mice does not demonstrate diurnal oscillation of MAPK activity or cAMP
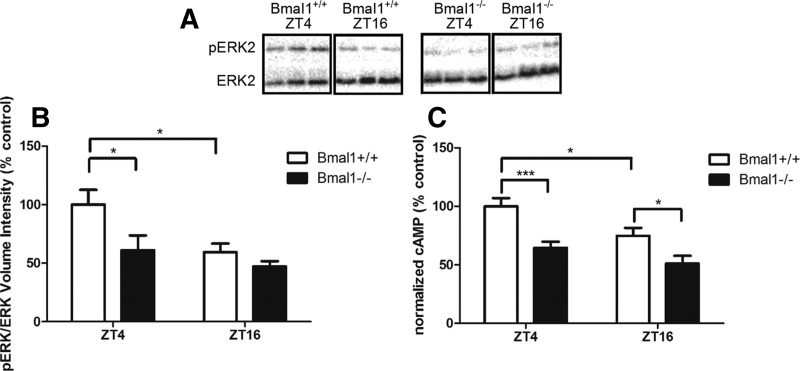
Previous studies have shown that wild-type mice exhibit a diurnal oscillation in MAPK activity and cAMP that is required for long-term hippocampus-dependent memory (Eckel-Mahan et al. 2008). Therefore, Bmal1+/+ and Bmal1−/− hippocampi were collected at ZT4 and ZT16, the peak and trough of the MAPK oscillation, and analyzed for MAPK activity. Two-way ANOVA revealed significant effects of genotype (F(1,26) = 6.085, P < 0.05) and time of day (F(1,26) = 6.875) on diurnal phospho-MAPK levels. Bmal1+/+ hippocampi demonstrated increased levels of phospho-MAPK at ZT4 compared to ZT16 (P < 0.05, Bonferroni post-hoc test) (Fig. 4B). Bmal1−/− hippocampi showed no diurnal change in phospho-MAPK at ZT4 and exhibited lower levels of phospho-MAPK at ZT4 compared to Bmal1+/+ hippocampi (P < 0.05, Bonferroni post-hoc test) (Fig. 4B). Similarly, two-way ANOVA revealed a significant effect of genotype (F(1,54) = 21.56, P < 0.0001) and time of day (F(1,54) = 9.101, P = 0.0039) on diurnal cAMP levels. Bmal1+/+ hippocampi demonstrated increased levels of cAMP at ZT4 compared to ZT16 (P < 0.05, Bonferroni post-hoc test) (Fig. 4C). Bmal1−/− hippocampi showed no diurnal change in cAMP at ZT4 and exhibited lower levels of cAMP at ZT4 compared to Bmal1+/+ hippocampi (P < 0.001, Bonferroni post-hoc test) (Fig. 4C).
Figure 4.
There is no diurnal oscillation of ERK activity or cAMP in Bmal1−/− hippocampi. (A) Western blot image depicts representative phosphorylated ERK and ERK intensities from isolated hippocampi of individual mice at ZT4 or ZT16. (B) Ratio of pERK to ERK volume intensities normalized to control Bmal1+/+ ZT4 levels. There is a significant effect of genotype (F(1,26) = 6.085, P < 0.05) and time of day (F(1,26) = 6.875) on diurnal phospho-MAPK levels. For Bmal1+/+ hippocampi, phospho-MAPK is higher at ZT4 compared to ZT16 (P < 0.05). Bmal1−/− hippocampi showed no change in phospho-MAPK activity between ZT4 and ZT16 and exhibited lower levels of phospho-MAPK at ZT4 compared to Bmal1+/+ hippocampi (P < 0.05). Two-way ANOVA with Bonferroni post-hoc test. All values represent mean ± SEM, n = 6–9. (C) Ratio of cAMP concentration (pmol) to protein (mg) normalized to Bmal1+/+ ZT4 group. There are significant effects of genotype (F(1,54) = 21.56, P < 0.0001) and time of day (F(1,54) = 9.101, P = 0.0039) on diurnal cAMP levels. Bmal1+/+ hippocampi demonstrated increased levels of cAMP at ZT4 compared to ZT16 (P < 0.05). Bmal1−/− hippocampi showed no diurnal change in cAMP at ZT4 and exhibited lower levels of cAMP at ZT4 compared to Bmal1+/+ hippocampi (P < 0.001). Two-way ANOVAs with Bonferroni post-hoc test. All values represent mean ± SEM, n = 12–16.
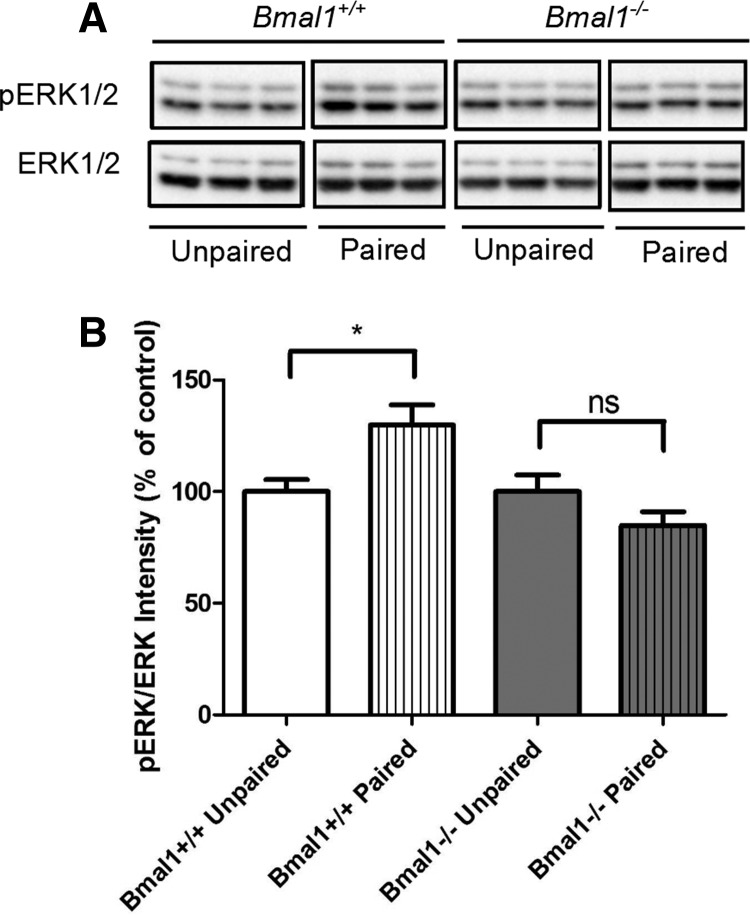
The Bmal1−/− hippocampus shows no training-induced activation of MAPK
When wild-type mice are trained for contextual fear memory, there is a measurable increase in MAPK activity in area CA1 of the hippocampus (Sindreu et al. 2007). Therefore, Bmal1+/+ and Bmal1−/− mice were exposed to a novel context (ZT4 to ZT6) and either immediately received a 0.7-mA foot shock or were first allowed to explore for 2 min. Bmal1+/+ mice that experienced the paired association between the novel context and foot shock showed an increase in phospho-MAPK in the hippocampus 30 min after training compared to unpaired controls which were shocked immediately (Bmal1+/+ hippocampi [t(16) = 2, P = 0.0121, two-tailed unpaired t-test]) (Fig. 5). There was no difference between Bmal1−/− paired and unpaired hippocampi (t(15) = 1.585, P = 0.1339, two-tailed unpaired t-test) (Fig. 5), indicating that MAPK activity did not increase when Bmal1−/− mice were trained for contextual fear memory.
Figure 5.
There is no increase in phosphorylated ERK activity in Bmal1−/− hippocampi 30 min after contextual fear conditioning. (A) Western blot image depicts phosphorylated ERK and ERK intensities from isolated hippocampi of individual mice 30 min after contextual fear conditioning. Unpaired animals immediately received a 0.7-mA foot shock after placement in a novel cage. Paired animals explored the novel context for 2 min before receiving a foot shock. (B) Ratio of pERK to ERK intensities normalized to unpaired group for each genotype. pERK is higher for Bmal1+/+ paired hippocampi compared to Bmal1+/+ unpaired hippocampi (t(16) = 2, P = 0.0121). There is no change in pERK between the unpaired and paired Bmal1−/− hippocampi (t(15) = 1.585, P = 0.1339). Two-tailed unpaired t-tests. All values represent mean ± SEM, n = 8–9.
Discussion
Bmal1−/− mice offer the opportunity to investigate the relationship between a functional molecular clock and memory. We discovered that Bmal1−/− mice are impaired in hippocampus-dependent memory tasks. At 24 h after training for contextual fear conditioning, Bmal1−/− mice freeze less than wild-type littermates when trained and tested during the day. While Bmal1−/− mice do learn and exhibit fear memory for the foot shock, their memory for contextual fear is similar to that of wild-type littermates during the night. Bmal1−/− mice are also impaired in spatial learning and memory, as measured by the Morris water maze.
Consistent with these memory deficits, LTP at the Schaffer-collateral pathway is reduced in the Bmal1−/− hippocampus. The Bmal1−/− hippocampus also does not demonstrate diurnal changes in cAMP or MAPK activity in the hippocampus, which Eckel-Mahan et al. (2008) demonstrated are necessary for the maintenance of contextual fear learning. Furthermore, the Bmal1−/− hippocampus exhibits impaired training-induced activation of MAPK, which is normally seen when wild-type mice are trained for contextual fear conditioning (Sindreu et al. 2007). The hippocampal memory deficits exhibited by Bmal1−/− mice are most likely due to deficiencies in key signaling events required for formation and maintenance of hippocampus-dependent memory, including activation of MAPK and diurnal oscillation of cAMP and MAPK activity. The dampened level of MAPK activity in the Bmal1−/− hippocampus may still be sufficient to permit learning and memory of contextual fear conditioning. Alternatively, other signaling pathways leading to the phosphorylation of CREB and CRE-mediated transcription could compensate for the reduction of MAPK activity (Johannessen et al. 2004).
We were surprised to find that Bmal1−/− mice demonstrate no deficits in novel object recognition, and even enhancement 24 h later compared to wild-type littermates. Though it is clear that the hippocampus is required for spatial memory, there exists debate as to the extent of the hippocampus’ involvement for recognition memory (Clark et al. 2000; Broadbent et al. 2004; Winters et al. 2004; Good et al. 2007). Phan et al. (2011) similarly find that SCN lesioned mice demonstrate no deficit in novel object recognition, despite the impairment to both contextual fear and Morris water maze memory. Kondratova et al. (2010) report that Bmal1−/− mice exhibit altered exploratory activity, which may explain their behavior in the novel object recognition task. Specifically, Bmal1−/− mice are hyperactive when exposed to novelty, as assessed by open field activity.
Given that sleep is also strongly associated with hippocampus-dependent memory (Diekelmann and Born 2010) and Bmal1−/− mice have an impaired sleep architecture and diurnal distribution, even in LD conditions (Laposky et al. 2005), sleep disruption could contribute to impaired hippocampus-dependent function in Bmal1−/− mice. Bmal1−/− mice spend more time in REM and NREM sleep, but this increase in sleep is not concentrated during the day as it is with wildtype mice. Eckel-Mahan et al. (2008) find that the peak in MAPK signaling activity in the hippocampus occurs during the day in wild-type mice, when mice spend the most time sleeping. Luo et al. (2013) further demonstrate that the cAMP/MAPK/CREB transcriptional pathway is higher specifically during REM sleep compared to NREM and wake stages when evaluated at ZT4–ZT8 under 12-h LD conditions. Perturbing sleep can disrupt hippocampus-dependent memory, synaptic plasticity, and molecular signaling. In rats, sleep deprivation negatively effects consolidation of contextual fear conditioning, but not cued fear conditioning (Graves et al. 2003), and decreases LTP, synaptic transmission, and MAPK activation in the hippocampus (Ravassard et al. 2009). Thus, alterations in sleep may contribute to the reduction of diurnal, hippocampal cAMP/MAPK signaling activity and impairments in hippocampus-dependent memory in Bmal1−/− mice.
Our data are consistent with the observation that lesioning the SCN impairs memory persistence and inhibits the diurnal oscillation of MAPK activity and cAMP in the hippocampus (Phan et al. 2011). There is no direct synaptic link between the SCN and the hippocampus (Watts et al. 1987; Morin et al. 1994) thereby implicating indirect synaptic input and/or diffusible factors in entraining hippocampal rhythms. The SCN indirectly projects to the limbic system (including the amygdala and hippocampus) through the paraventricular nucleus, the lateral septum, and the bed nucleus of the stria terminalis (Morin et al. 1994). Potential diffusible candidates include glucocorticoids (Girotti et al. 2009; Dickmeis et al. 2013) and melatonin (Stehle et al. 2013) as they are susceptible to circadian influence from the SCN and also act on the hippocampus (Reul and de Kloet 1985; Musshoff et al. 2002). For example, melatonin is a night cue for mammals which inhibits the activity of type 1 adenylyl cyclase and compromises LTP (Wang et al. 2005). However, C57BL/6J mice, the most background in transgenic mouse strains, including the global Bmal1−/− mouse, have a natural melatonin knockdown due to a mutation in arylalkylamine-N-acetyltransferase (AANAT) (Roseboom et al. 1998). As C57BL/6J mice maintain circadian oscillations outside of the SCN (Stehle et al. 2002) despite dramatically reduced melatonin signaling, melatonin is not essential. Similar to melatonin, glucocorticoids are not essential in entraining peripheral circadian rhythms. In adrenalectomized Per1:luciferase rats, only some peripheral tissues exhibited a phase shift of activity (Pezuk et al. 2012). The SCN likely contributes to the molecular oscillations of signaling activity in the hippocampus through redundant inputs rather than relying on a single mechanism. The connection between the SCN and signaling oscillations in the hippocampus warrants further investigation.
We find that genetically abolishing the molecular clock compromises hippocampus-dependent learning and memory tasks. This report extends our understanding of the relationship between diurnal rhythms and the hippocampus and is consistent with substantial literature implicating the circadian system in memory (Gerstner and Yin 2010).
Materials and Methods
Mice
Bmal1+/− breeders in the C57/Bl6 background were generously provided by Dr. Christopher Bradfield from the University of Wisconsin Medical School, Madison, WI. Mice were genotyped with PCR as previously described (Bunger et al. 2000). Bmal1+/+ littermates were used as controls. All experiments were performed on adult, male mice aged 3–5 mo. Food and water were provided ab libitum and animals were maintained on a 12-h light:dark (LD) cycle, with light onset ZT0 at 8 a.m., unless otherwise specified. Experiments were in accordance with the Institutional Animal Care and Use Committee's recommendations at the University of Washington.
Activity monitoring
Mice were individually housed and their voluntary activity data were acquired using QA-4 activity input modules coupled to infrared motion detectors and the VitalView Data Acquisition System (Mini Mitter, version 4.1). Actograms and periodograms were generated with the ImageJ plug-in ActogramJ (University of Wuerzburg). Mice were monitored for 7 d in 12-h LD conditions and 14 d in DD conditions. Only the last 7 d in DD conditions were included in depicted actograms and periodogram analysis.
Contextual fear conditioning
Mice were handled for at least 5 d before all behavioral testing. For contextual fear training, mice were exposed to a 10 (W) × 10 (D) × 16 (H)-inch Plexiglas chamber with a metal shock grid (Colbourn Instruments). Between each subject, the chamber was wiped with diluted acetic acid. Mice were allowed to explore the novel context for 2 min before receiving a 0.7-mA foot shock. They were returned to their home cage after a 1-min recovery. Behavior during testing was video recorded and the percentage of freezing activity was scored by a blinded investigator. Freezing was scored as all four paws on the ground and no movement other than respiration. Mice were trained and tested from ZT3 to ZT5, during the light phase, or under red light from ZT15 to ZT17, during the night phase.
Morris water maze
Mice were placed in a 122-cm-diameter pool filled with room temperature water (22°C) made opaque with nontoxic, white, tempera paint. They were trained for 8 d, four times a day, to locate the hidden and fixed platform (circular, 5-cm diameter) using visual cues to navigate. Mice were placed in randomized locations of the pool for each trial. During training, the mice were given 90 sec to find the platform and guided to the platform when they did not find it. They sat on the platform for 30 sec before removal. Their memory was tested using a probe trial, where the platform is removed, lasting 90 sec. The probe trial was video recorded and their swim behavior was analyzed using Ethovision (Noldus). Mice were trained and tested from ZT3 to ZT8, during the light phase.
Novel object recognition
Mice were habituated to a 17 (W) × 8.5 (D) × 6 (H) cage overnight. After habituation, they explored two identical plastic objects (A) for 5 min, three times, in the habituated cage. Thirty minutes after training, they were presented with object A and a new object B for 5 min. Twenty-four hours after training, they were presented with object A and a new object C for 5 min. Objects were sprayed with ethanol in between animal subjects. Investigators video recorded each trial and later scored exploration time with each object. Exploration was defined as anytime a mouse's head was oriented toward the object and their nose was within 1 cm of the object. Time spent climbing on objects was eliminated from exploration time. Mice were trained and tested from ZT3 to ZT6, during the light phase.
Electrophysiology
Hippocampal LTP was compared between Bmal1+/+ and Bmal1−/− male mice at ages 2–3 mo. After dissection at ZT2, brains were put in ice-cold cutting aCSF (12.5 mM NaCl, 0.25 mM KCl, 0.6 mM MgCl2, 0.05 mM CaCl2, 0.125 mM NaH2PO4, 10 mM glucose, 25 mM NaHCO3) containing APV (40 µM, obtained from Tocris) and then cut into 400-µm coronal sections using a vibratome (Leica VT1000S, Leica Microsystems). Slices recovered for 2 h in recording aCSF (12.5 mM NaCl, 0.25 mM KCl, 0.1 mM MgCl2, 0.25 mM CaCl2, 0.125 mM NaH2PO4, 10 mM glucose, 25 mM NaHCO3). Slices were continuously oxygenated with a biological mix (95% oxygen, 5% carbon dioxide). After recovery, the Schaffer collateral efferent fibers in the stratum radiatum of hippocampal area CA1 were stimulated and the resulting fEPSPs recorded from ZT4 to ZT10. The slice was stimulated with the S88 Stimulator (Astro-Med Inc., Grass Instrument Division) using a concentric bipolar electrode (Frederick Haer & Co). Electric responses were amplified by the Axopatch 200B (Axon Instruments) and digitized by the Digidata 1440A (Axon CNS). Before inducing LTP, the basal field responsiveness was characterized by generating an input/output (I/O) curve. The stimulus was varied from 10 to 150 µA in 10-µA increments. Paired pulse facilitation was also measured while varying the interpulse interval from 20 msec to 310 msec in steps of 30 msec. To induce LTP, three tetanic stimuli separated by 5 min were delivered. Each stimulus consisted of a 1-sec, 100-Hz train with a pulse length of 0.1 msec. fEPSPs were monitored for 2 h following LTP induction.
Western analysis
Mice were cervically dislocated under dim, red light at ZT4 and ZT16. Both lobes of the hippocampus were dissected out and immediately frozen on liquid nitrogen. The hippocampi were homogenized in buffer containing 10 mM Tris base, pH 7.5, 100 mM NaCl, 1 mM EDTA, 1% sodium deoxycholate, 10% glycerol, 1% NP-40, 100 mM NaF, 1 mM Na3VO4, 1 mM PMSF, phosphatase inhibitor I and III diluted 1:100 (Sigma), and Complete Mini protease inhibitor tablet (Roche). Homogenates were centrifuged at 14,000g for 10 min. The supernatant was isolated and an equal volume of Laemmli Sample Buffer (BioRad) with β-mercaptoethanol (1:20) was added. The samples were sonicated for 10 sec. Samples were boiled and run on a 12.5% Tris-HCl polyacrylamide gel (BioRad). Proteins were transferred to a PVDF membrane (Millipore) and blocked with 5% milk in a phosphate-buffered saline (PBS) solution with 0.05% Tween. Proteins were detected with rabbit polyclonal antibody to phospho-p44/42 MAPK (Thr202/Tyr204, 1:1000, Cell Signaling, RRID: AB_331775), mouse polyclonal antibody to pan-ERK (1:1000, BD Transduction Laboratories, RRID: AB_397447), and mouse antibody to actin (1:2000, Millipore, RRID:AB_94235). Blots were probed with alkaline phosphatase-conjugated goat to rabbit IgG (Sigma) and alkaline phosphatase-conjugated goat to mouse IgG (Sigma). Immunoreactivity was developed with the CDP-star western alkaline phosphatase detection system (Tropix). Blots were imaged with ChemiDoc XRS+ and analyzed with Image Lab (BioRad). pERK intensity was normalized to ERK intensity, which served as a loading control.
cAMP ELISA
The ELISA-based cAMP Biotrak Enzyme immunoassay System protocol (Amersham Biosciences) was used with some minor deviations. Hippocampal tissue was collected and homogenized as described in the Western methods but with the addition of 1 mM of the PDE inhibitor IBMX. Ice-cold ethanol (100%) was added to the homogenate. Homogenates were spun for 2 min at a speed of 1000g at 4°C. Resulting supernatants were evaporated in a heat block at 55°C and precipitates were resuspended in 100 µL assay buffer. Competition binding was carried out according to the Enzyme Immunoassay System instructions. Extracts were diluted 1:30 to achieve concentrations in the linear range of the assay. cAMP levels were normalized to protein concentrations.
Statistical analysis
Statistical tests were performed in GraphPad Prism. α value was set at 0.05. The specific tests used are reported with results.
Acknowledgments
This work was supported by National Institutes of Health Grants NS 20498 and MH 073601.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.035451.114.
References
- Adams JP, Sweatt JD 2002. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42: 135–163 [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R 2005. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci 28: 145–151 [DOI] [PubMed] [Google Scholar]
- Athos J, Impey S, Pineda VV, Chen X, Storm DR 2002. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat Neurosci 5: 1119–1120 [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraits JJ, Trzaskos JM, Sweatt JD 1998. The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1: 602–609 [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH 2007. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron 53: 261–277 [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59–68 [DOI] [PubMed] [Google Scholar]
- Broadbent N, Squire L, Clark R 2004. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci 40: 14515–14520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA 2000. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS 2002. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res 133: 95–108 [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS 2005. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms 20: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Zola S, Squire L 2000. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20: 8853–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM 2006. A clock shock: Mouse CLOCK is not required for circadian oscillator function. Neuron 50: 465–477 [DOI] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL, Antoniadis EA, Hong NS, Ko CH, Leblanc L, Lebovic SS, Lo Q, Ralph MR, et al. 2001. Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol Learn Mem 75: 51–62 [DOI] [PubMed] [Google Scholar]
- Dickmeis T, Weger BD, Weger M 2013. The circadian clock and glucocorticoids—interactions across many time scales. Mol Cell Endocrinol 380: 2–15 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J 2010. The memory function of sleep. Nat Rev Neurosci 11: 114–126 [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR 2008. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci 11: 1074–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, Reick M, Scott K, Diaz-Arrastia R, McKnight SL 2000. Impaired cued and contextual memory in NPAS2-deficient mice. Science 288: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Gerstner JR, Yin JC 2010. Circadian rhythms and memory formation. Nat Rev Neurosci 11: 577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Weinberg MS, Spencer RL 2009. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab 296: E888–E897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good MA, Barnes P, Staal V, McGregor A, Honey RC 2007. Context- but not familiarity-dependent forms of object recognition are impaired following excitotoxic hippocampal lesions in rats. Behav Neurosci 121: 218–223 [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T 2003. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem 10: 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR 1998. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci 1: 595–601 [DOI] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U 2004. What turns CREB on? Cell Signal 16: 1211–1217 [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS 2006. Molecular components of the mammalian circadian clock. Hum Mol Genet 15: 271–277 [DOI] [PubMed] [Google Scholar]
- Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV 2010. Circadian clock proteins control adaptation to novel environment and memory formation. Aging 2: 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F 2005. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 28: 395–409 [DOI] [PubMed] [Google Scholar]
- Loh DH, Navarro J, Hagopian A, Wang LM, Deboer T, Colwell CS 2010. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS One 5: e12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Phan T, Yang Y, Garelick MG, Storm DR 2013. Increases in cAMP, MAPK activity, and CREB phosphorylation during REM sleep: implications for REM sleep and memory consolidation. J Neurosci 33: 6460–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons LC, Sol Collado M, Khabour O, Green CL, Eskin A 2006. The circadian clock modulates core steps in long-term memory formation in Aplysia. J Neurosci 26: 8662–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Goodless-Sanchez N, Smale L, Moore RY 1994. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience 61: 391–410 [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS 1986. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319: 774–776 [DOI] [PubMed] [Google Scholar]
- Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ 2002. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus 12: 165–173 [DOI] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Wang LA, Menaker M 2012. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology 153: 4775–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TX, Chan GC, Sindreu CB, Eckel-Mahan KL, Storm DR 2011. The diurnal oscillation of MAP (mitogen-activated protein) kinase and adenylyl cyclase activities in the hippocampus depends on the suprachiasmatic nucleus. J Neurosci 31: 10640–10647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, Kandel ER 2002. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron 34: 447–462 [DOI] [PubMed] [Google Scholar]
- Ravassard P, Pachoud B, Comte J, Mejia-Perez C, Scoté-Blachon C, Gay N, Claustrat B, Touret M, Luppi P, Salin PA 2009. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in dorsal hippocampus. Sleep 32: 227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER 1985. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117: 2505–2511 [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Aryan Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC 1998. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Mol Brain Res 63: 189–197 [DOI] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC 2008. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci 105: 15593–15598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR 2007. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron 53: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle JH, von Gall C, Korf HW 2002. Organisation of the circadian system in melatonin-proficient CH3 and melatonin-deficient C57BL mice: a comparative investigation. Cell Tissue Res 309: 173–182 [DOI] [PubMed] [Google Scholar]
- Stehle JH, von Gall C, Korf HW 2013. Melatonin: a clock-output, a clock-input. J Endocrinol 15: 383–389 [DOI] [PubMed] [Google Scholar]
- Tapp WN, Holloway FA 1981. Phase shifting circadian rhythms produces retrograde amnesia. Science 211: 1056–1058 [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S 1996. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87: 1327–1338 [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Havekes R, Barf RP, Hut RA, Nijholt IM, Jacobs EH, Gerkema MP 2008. Circadian time-place learning in mice depends on Cry genes. Curr Biol 18: 844–848 [DOI] [PubMed] [Google Scholar]
- Wang H, Storm DR 2003. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol Pharmacol 63: 463–468 [DOI] [PubMed] [Google Scholar]
- Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS 2005. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci 22: 2231–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G 1987. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol 258: 204–229 [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ 2004. Double disassociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function in the temporal lobe. J Neurosci 24: 5901–5908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR 1999. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 23: 787–798 [DOI] [PubMed] [Google Scholar]
- Zueger M, Urani A, Chourbaji S, Zacher C, Lipp HP, Albrecht U, Spanagel R, Wolfer DP, Gass P 2006. mPer1 and mPer2 mutant mice show regular spatial and contextual learning in standardized tests for hippocampus-dependent learning. J Neural Transm 113: 347–356 [DOI] [PubMed] [Google Scholar]