Abstract
Background
Understanding the economic implications of oral anticoagulation therapy requires careful consideration of the risks and costs of stroke and major hemorrhage. The majority of patients with atrial fibrillation (AF) are aged ≥65 years, so focusing on the Medicare population is reasonable when discussing the risk for stroke.
Objective
To examine the relative economic burden associated with stroke and major hemorrhage among Medicare beneficiaries who are newly diagnosed with nonvalvular atrial fibrillation (NVAF).
Methods
This study was a retrospective analysis of a 5% sample of Medicare claims data for patients with NVAF from 2006 to 2008. Patients with NVAF without any claims of AF during the 12 months before the first (index) claim for AF in 2007 (baseline period) were identified and were classified into 4 cohorts during a 12-month follow-up period after the index date. These cohorts included (1) no claims for ischemic stroke or major hemorrhage (without stroke or hemorrhage); (2) no claims for ischemic stroke and ≥1 claims for major hemorrhage (hemorrhage only); (3) ≥1 claims for ischemic stroke and no major hemorrhage claims (stroke only); and (4) ≥1 claims each for ischemic stroke and for major hemorrhage (stroke and hemorrhage). The 1-year mean postindex total all-cause healthcare costs adjusted by the Centers for Medicare & Medicaid Services Hierarchical Condition Categories (HCC) score were compared among the study cohorts. Results: Of the 9455 eligible patients included in this study, 3% (N = 261) of the patients had ischemic stroke claims only, 3% (N = 276) had hemorrhage claims only, and <1% (N = 13) had both during the follow-up period. The unadjusted follow-up healthcare costs were $63,781 and $64,596 per patient for the ischemic stroke only and the hemorrhage only cohorts, respectively, compared with $35,474 per patient for those without hemorrhage or stroke claims. After adjustment for HCC risk score, the mean incremental costs for patients with stroke claims only and hemorrhage claims only, relative to those without stroke or hemorrhage claims, were $26,776 (95% confidence interval [CI], $20,785-$32,767; P <.001) and $26,168 (95% CI, $20,375-$31,961; P <.001), respectively.
Conclusion
The economic burden of managing patients with NVAF who experience ischemic stroke and hemorrhage were similarly significant during the first year after a diagnosis of NVAF. The burden of major bleeding complications on patients, clinicians, and payers should not be overlooked, and these complications should be considered in conjunction with the cost-savings associated with ischemic stroke risk reduction in future cost-benefit evaluations of oral anticoagulation therapy.
Atrial fibrillation (AF) is the most common form of sustained cardiac arrhythmia.1,2 The most recent estimates (published in 2013) of the prevalence of AF in the United States are for 2010 and range from 2.7 million to 6.1 million.3,4 The prevalence of AF doubles with each decade of life after the age of 60 years and occurs in approximately 10% of the US population aged ≥80 years.5–7 A recent study estimates that the number of patients with AF in the United States could potentially reach 12.1 million by 20303; other estimates range from 5.6 million to 12 million patients with AF by 2050.4
Patients with AF have an approximate 5-fold increased risk for stroke compared with patients in normal sinus rhythm.4 Furthermore, the percentage of strokes that can be attributed to AF increases steeply with age, with rates of 1.5% in patients aged 50 to 59 years and 23.5% in those aged 80 to 89 years.4 The term “nonvalvular atrial fibrillation” (NVAF) is used to describe cases of AF that occur in the absence of rheumatic mitral valve disease, mitral valve repair, or a prosthetic heart valve.8 NVAF affects approximately 85% of the overall population with AF and is a substantial medical burden for Medicare beneficiaries (aged ≥65 years) in the United States.9,10
The current evidence-based clinical guidelines recommend the use of oral anticoagulation in patients with NVAF who are at an intermediate to high risk for stroke.8,11 Although the efficacy of oral anticoagulation therapy to prevent stroke in patients with NVAF is well-established, it is also associated with a risk of bleeding.12–15 Understanding the relative economic burdens of stroke and major hemorrhage is important when considering the costs and benefits of anticoagulation therapy.
Several studies have reported the incremental costs associated with stroke alone or with hemorrhage alone using different NVAF payer populations (ie, commercial or Medicare), and a few recent studies have provided incremental cost data for stroke and hemorrhage for the Medicare population, reporting significant incremental costs in the year after the stroke or hemorrhage index dates.7,16–20 Other studies have analyzed the incremental costs associated with stroke alone or with hemorrhage alone, or have analyzed these costs for a commercial population with NVAF.21–24 Most of these studies were done in separate patient populations and different time periods, making assessment of the relative economic burden of stroke versus bleeding difficult. By contrast, our study provides the cost estimates for these 2 conditions simultaneously based on the same patient population, which allows a more appropriate comparison of the economic implication of these 2 major consequences of oral anticoagulation therapy for the prevention of stroke among patients with NVAF.
The prespecified objective in our study was to assess the relative economic burden (including Medicare Part D costs) associated with ischemic stroke and with major hemorrhagic events (ie, intracranial and gastrointestinal [GI] bleeding) among Medicare beneficiaries with newly diagnosed NVAF in the 12 months after the NVAF index diagnosis.
Methods
Data Source
This study was a retrospective analysis of claims data between 2006 and 2008 from the Medicare 5% sample research identifiable file (RIF) provided by the Research Data Assistance Center, a Centers for Medicare & Medicaid Services (CMS) contractor that assists researchers in obtaining CMS data. Requests for RIF data files, which contain beneficiary-level protected health information, are reviewed by the CMS Privacy Board to ensure that beneficiary privacy is appropriately protected and that the need for such data is justified. The RIF data used in our study included de-identified enrollment data and Medicare Part A, Part B, and Part D claims from Medicare beneficiaries who were identified to have chronic AF by the CMS Chronic Conditions Data Warehouse in 2006 or 2007.
KEY POINTS
-
▸
The prevalence of atrial fibrillation is high among patients aged ≥65 years and continues to grow.
-
▸
Nonvalvular atrial fibrillation (NVAF) increases the risk for ischemic stroke or bleeding, which carry a substantial economic burden.
-
▸
Based on this new analysis of Medicate data, the costs associated with stroke and major hemorrhage in patients with newly diagnosed NVAF are almost doubled that of Medicare beneficiaries without NVAF.
-
▸
The healthcare costs were $63,781 per patient with ischemic stroke only and $64,596 with hemorrhage only versus $35,474 for those without these conditions.
-
▸
A large contributor to the incremental cost differences was inpatient utilization for stroke and bleeding.
-
▸
The median number of hospital admissions was tripled in patients with ischemic stroke or major hemorrhage.
-
▸
From an economic perspective, minimizing the risk for major bleeding is as important as reducing the risk for stroke.
-
▸
Bleeding complications should be considered in conjunction with cost-savings associated with ischemic stroke risk reduction in the context of oral anticoagulation therapy management.
Patient Inclusion and Exclusion Criteria
Patients included in the analysis had to have at least 2 inpatient, emergency department/observation, or physician evaluation and management claims associated with an International Classification of Diseases, Ninth Revision (ICD-9) code of AF (ie, 427.31) during 2007 to confirm the presence of AF. The 2 claims had to occur at least 30 days apart, and 1 of the claims had to be for care in an outpatient setting to confirm that the patients included in this study had chronic AF.
Patients were excluded from the analysis if they were (1) aged ≤18 years; (2) without 12 months of Medicare Parts A and B eligibility before the index date; (3) without 12 months of Medicare Part A, B, and D eligibility after the index date for reasons other than death; (4) identified as having mitral and/or aortic valvular disease (repair or replacement), transient preoperative AF, or hyperthyroidism in the 12 months before the index date; or (5) identified as having a major hemorrhage or ischemic stroke in the 12 months before the AF diagnosis index date. The analysis was further limited to patients with newly diagnosed NVAF, which included those who had no claims with an ICD-9 diagnosis code of 427.31 in any position of the claim in the 12 months before the AF index date.
Patient Cohorts
Patients with NVAF were assigned to 1 of 4 cohorts based on the presence of ischemic stroke and hemorrhage claims during the 12 months after the AF index date. Cohort 1 included patients with no claims for ischemic stroke, intracranial hemorrhage, or GI hemorrhage (without stroke or hemorrhage); cohort 2 included patients with no claims for ischemic stroke and ≥1 claims for intracranial hemorrhage or GI hemorrhage (hemorrhage only); cohort 3 included patients with ≥1 claims for ischemic stroke and no claims for intracranial hemorrhage or GI hemorrhage (stroke only); and cohort 4 included patients with ≥1 claims for ischemic stroke and ≥1 claims for intracranial hemorrhage or GI hemorrhage (stroke and hemorrhage).
Ischemic stroke was identified by the presence of an inpatient or emergency department claim associated with an ICD-9 code for a primary diagnosis of ischemic stroke, and major hemorrhage was identified by an inpatient claim associated with a primary diagnosis of major hemorrhage (Appendix).
For 50% of patients with a stroke in the 12 months after their AF index date, their first stroke occurred on or 1 day after the AF index date. By contrast, only 25% of patients with major hemorrhage in the 12 months after the AF index date had their first major hemorrhage on or 1 day after their AF index date. To eliminate a bias toward a longer cost follow-up opportunity for stroke events, all patients who had an ischemic stroke or a major hemorrhage on or 1 day after their index date were excluded from the analysis to ensure there is sufficient length of a follow-up period for the cost assessment. After excluding such patients, the distributions of time to first event (ie, first ischemic stroke or major hemorrhage) were similar across the cohorts.
Patient Cohort Analysis
Descriptive statistics were used to assess the differences in baseline characteristics among the study cohorts. Healthcare resource use, including inpatient admissions, emergency department visits, and outpatient visits during the 12 months after the AF index date, was assessed in each cohort. The mean total all-cause healthcare costs during the 12-month follow-up period were compared among the study cohorts using ordinary least squares, adjusting for CMS Hierarchical Condition Categories (HCCs). All costs represent the Medicare allowed costs.
The CMS-HCC model, developed in 2004 to adjust Medicare capitation payments to private healthcare plans for the health expenditure risk of their enrollees, generates a risk score for each Medicare patient based on age, sex, original reason for Medicare entitlement, dual-eligibility status, and comorbidities during a 12-month baseline period. The risk score is designed and has been validated to be a credible predictor for healthcare costs incurred during the following 12-month period.25 We used the 2012 HCC definitions and weights to calculate a prospective risk score for each patient using claims from the 12 months before the AF index date.
Linear regression was used to determine the predictive relationship between the HCC score and the healthcare costs in the 12 months after the index date within each of the patient cohorts. The incremental cost difference between the cohorts was calculated after controlling for the differences in expected healthcare costs in the 12 months after the index date as predicted by HCC score. P values were also calculated using a 1-sided t-test to statistically compare the difference in mean incremental cost between the patients in the cohort without stroke or hemorrhage and the patients in each of the other 3 cohorts.
Results
Study Population
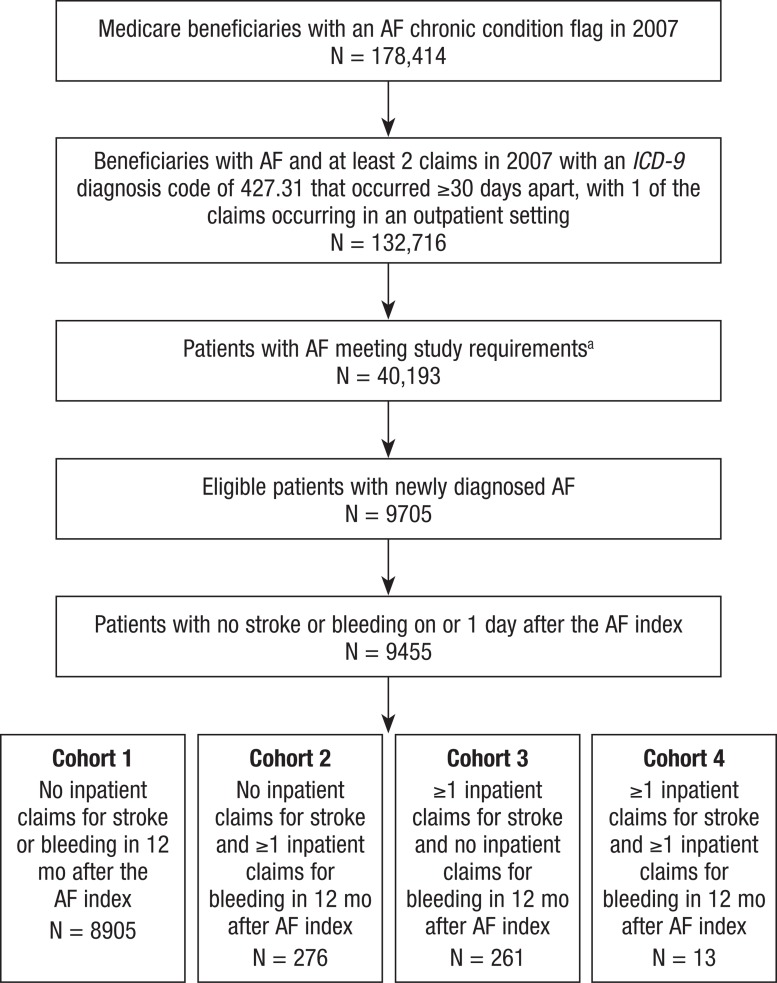
Data from 178,414 Medicare beneficiaries with chronic AF that were flagged in 2007 were available for analysis (Figure). Of these, 132,716 (74.4%) beneficiaries had at least 2 claims with an ICD-9 diagnosis code of 427.31 that occurred ≥30 days apart, with 1 of the claims occurring in an outpatient setting in 2007. After applying the additional exclusion criteria and including only patients with newly diagnosed NVAF, a total of 9705 patients were eligible to be included in the analysis. The newly diagnosed NVAF population was further reduced to 9455 patients, to include only those who did not have a stroke or a major hemorrhage on or 1 day after the NVAF index date.
Figure. Population Attrition.
aStudy requirements: Having Medicare Part A and Part B coverage 12 months before and after index month and age ≥18 years; having Medicare Part D coverage 12 months after AF index month; not having valvular disease, hyperthyroidism, or transient AF in 12 months before AF index date; not having a stroke in 12 months before index date; not having an intracranial hemorrhage in 12 months before index date; not having a gastrointestinal hemorrhage in 12 months before index date.
AF indicates atrial fibrillation; ICD-9, International Classification of Diseases, Ninth Revision.
Cohort Distribution
The study sample (mean age, 79 years; 35% male) was divided into 4 cohorts based on the occurrence of a stroke or a major hemorrhage during the 12 months after the NVAF index date (Figure). Table 1 provides demographics and baseline characteristics of the patients in the 4 cohorts. The mean age of the cohorts ranged from 78.6 years among patients with ischemic stroke and major hemorrhage claims to 83.5 years among patients with an ischemic stroke claim and no major hemorrhage claims. Patients with either a major hemorrhage claim or an ischemic stroke claim, but not both, were less likely to be male (28.3% and 26.4%, respectively) than patients without ischemic stroke or major hemorrhage claims.
Table 1.
Descriptive Summary of the 4 Study Cohorts
| Patient characteristic | Study sample (N = 9455) | Cohort 1 (n = 8905) | Cohort 2 (N = 276) | Cohort 3 (N = 261) | Cohort 4 (N = 13) |
|---|---|---|---|---|---|
| Mean age, yrs (SD) | 79 (9.5) | 78.8 (9.5) | 80.9 (9.1) | 83.5 (8) | 78.6 (9.5) |
| Age range, yrs (median) | 27–106 (80) | 27–106 (79) | 36–102 (81) | 55–99 (84) | 60–91 (77) |
| Males, % | 35 | 35 | 28.3 | 26.4 | 38.5 |
| ≥1 warfarin prescriptions in 90 days after AF index, %a | 49.3 | 49.3 | 45.7 | 30.3 | 46.2 |
| Mean all-cause allowed costs in 12 months before AF index, $ (SD) | 13,073 (21,020) | 12,949 (20,548) | 15,773 (25,491) | 14,605 (29,880) | 9920 (17,847) |
| Mean HCC score (SD) | 1.84 (1.55) | 1.83 (1.54) | 2.17 (1.72) | 2.00 (1.70) | 1.91 (1.72) |
Excluded patients who received warfarin after having a stroke.
AF indicates atrial fibrillation; HCC, Hierarchical Condition Categories; SD, standard deviation.
Overall, approximately 49% of patients had ≥1 prescriptions for warfarin filled within the 90 days after the index date; the proportion of patients filling warfarin prescriptions was highest among patients who did not have an ischemic stroke or hemorrhage claim (49.3%) and was lowest among patients with an ischemic stroke claim but no hemorrhage claim (30.3%).
The healthcare costs in the 12 months before the AF index ranged from $9920 among patients with ischemic stroke and major hemorrhage claims to $15,773 in patients with a major hemorrhage claim but no ischemic stroke claim. The overall mean HCC score was 1.84, ranging from 1.83 in patients without ischemic stroke or major hemorrhage claims to 2.17 in patients with a major hemorrhage claim but no ischemic stroke claim.
Events in the 12 Months after the Index Date Claim
The average incidence of ischemic stroke and of major hemorrhage in the overall study cohort of patients with AF was 3.3% and 3.4%, respectively. Among patients with major hemorrhage and no ischemic stroke, the mean number of major hemorrhagic events per member in the 12 months after the index NVAF date was 1.09 (standard deviation [SD], 0.36), with 15% associated with an intracranial major hemorrhage ICD-9 diagnosis. The median allowed cost per major hemorrhage event was $6328 (interquartile range [IQR], $3400). Among patients with ischemic stroke and no major hemorrhage claims, the mean number of strokes per member was 1.14 (SD, 0.38), with a median allowed cost per event of $7242 (IQR, $3550). Among patients with both ischemic stroke and major hemorrhage, the mean number of strokes and major hemorrhagic events were 1.15 (SD, 0.38) and 1.31 (SD, 0.63), respectively, with 47% of major hemorrhage claims associated with a diagnosis of intracranial bleeding. The median allowed costs were $6211 (IQR, $6959) per stroke event and $7077 (IQR, $8628) per major hemorrhage event.
Total Healthcare Costs in the 12 Months after the Index Date Claim
The total allowed healthcare costs per member in each cohort in the 12 months after the index NVAF date were calculated and were grouped into 7 cost categories: inpatient facility, skilled nursing facility, home health, emergency department, outpatient facility, professional services, and pharmaceutical (Table 2). The mean numbers of inpatient admissions and emergency department/office visits per member are also shown in Table 2. Overall, the mean total allowed healthcare cost per member was $37,157, with approximately 50% of the cost attributed to inpatient care.
Table 2.
Healthcare Costs and Utilization in the 12 Months after the AF Index
| Cost component | Study sample (N = 9455) | Cohort 1 (n = 8905) | Cohort 2 (N = 276) | Cohort 3 (N = 261) | Cohort 4 (N = 13) |
|---|---|---|---|---|---|
| Mean (SD) healthcare cost per member in the 12 mo after the AF index | |||||
| Total cost, $ | 37,157 (42,920) | 35,474 (41,875) | 64,596 (51,594) | 63,781 (48,422) | 72,984 (46,418) |
| Inpatient facility, $ | 18,592 (30,159) | 17,628 (29,742) | 34,919 (31,291) | 33,242 (33,908) | 38,152 (31,217) |
| Skilled nursing facility, $ | 5269 (11,659) | 4806 (11,014) | 10,791 (16,800) | 14,621 (18,141) | 17,796 (23,515) |
| Home health, $ | 2 (44) | 2 (44) | 0 (0) | 4 (42) | 35 (92) |
| Emergency department, $ | 402 (539) | 385 (527) | 676(716) | 683 (554) | 692 (499) |
| Outpatient facility, $ | 2782 (6096) | 2765 (6068) | 2840 (5878) | 3292 (7274) | 2927 (3908) |
| Professional services, $ | 7031 (8487) | 6819 (8311) | 11,647 (12,440) | 9215 (7678) | 10,451 (6110) |
| Pharmaceutical, $ | 3080 (3450) | 3070 (3378) | 3723 (5734) | 2724 (2459) | 2931 (3163) |
| Site-of-care utilization | |||||
| Mean inpatient admissions per member, N (SD) | 1.7 (1.8) | 1.6 (1.7) | 3.7 (2.4) | 3.4 (2.3) | 4.5 (2.5) |
| Median inpatient admissions per member, N | 1 | 1 | 3 | 3 | 5 |
| Mean emergency department visits per member, N (SD) | 0.8 (1.4) | 0.8 (1.4) | 1.1 (1.8) | 1.1 (1.4) | 1.4 (2.1) |
| Median emergency department visits per member, N | 0 | 0 | 1 | 1 | 1 |
| Mean office visits per member, N (SD) | 12.7 (10.7) | 12.8 (10.8) | 12.8 (10.8) | 10 (9.2) | 9.3 (8) |
| Median office visits per member, N | 11 | 11 | 11 | 8 | 8 |
AF indicates atrial fibrillation; SD, standard deviation.
The mean total allowed healthcare costs per patient by cohort were $35,474 in the patients without stroke or major hemorrhage; $64,596 with major hemorrhage only; $63,781 with stroke only; and $72,984 in patients with stroke and major hemorrhage (Table 2).
Incremental Cost of the Cohorts
The unadjusted mean postindex total all-cause healthcare costs in the 12 months after the index date were lowest among patients without stroke or major hemorrhage and highest among patients with both stroke and major hemorrhage (Table 3). After adjusting for the HCC risk score, the adjusted mean cost for patients with major hemorrhage and no ischemic stroke was $26,168 higher (P <.001) than the adjusted mean cost for patients with no major hemorrhage or ischemic stroke.
Table 3.
Incremental Cost of the 4 Study Cohorts
| Cohort | Members, N | Average HCC score | Total allowed costs per member in the 12 months after AF index | ||||
|---|---|---|---|---|---|---|---|
| Unadjusted total all-cause postindex mean cost, $ | Difference in mean cost vs patients with no stroke or bleeding claims after adjusting for HCC | ||||||
| Mean estimate, $ | 95% CI, lower bound, $ | 95% CI, upper bound, $ | Probability that cohort 1 is more expensive | ||||
| 1 | 8905 | 1.83 | 35,474 | ||||
| 2 | 276 | 2.17 | 64,596 | 26,168 | 20,375 | 31,961 | <.001 |
| 3 | 261 | 2.00 | 63,781 | 26,776 | 20,785 | 32,767 | <.001 |
| 4 | 13 | 1.91 | 72,984 | 36,723 | 10,715 | 62,731 | .005 |
AF indicates atrial fibrillation; CI, confidence interval; HCC, Hierarchical Condition Categories.
Patients with ischemic stroke and no major hemorrhage had an adjusted mean cost that was $26,776 higher (P <.001) than patients with no major hemorrhage or ischemic stroke. Patients with both ischemic stroke and major hemorrhage had an adjusted mean cost that was $36,723 higher (P = .005) than patients with no major hemorrhage or ischemic stroke.
Discussion
A large contributor to the incremental cost difference between patients with stroke and/or bleeding and the cohort without stroke or bleeding was primarily driven by inpatient utilization. The median number of hospital admissions was 3 times higher in patients experiencing ischemic stroke or major hemorrhage than in patients who experienced neither adverse event, whereas the utilization of emergency department services was similar. The use of a skilled nursing facility was significantly higher for patients with stroke and/or bleeding as were professional claims.
Furthermore, the incremental costs of ischemic stroke and major hemorrhage during the 1-year follow-up period were similar, suggesting that minimizing the risk for major hemorrhage is no less important than reducing the risk for stroke, from an economic perspective. By considering the relative economic burdens of ischemic stroke and major hemorrhage, these data provide important information for future cost-effectiveness evaluations of alternative oral anticoagulant medications with different efficacy and safety profiles.
Although the 12-month incidence rate of ischemic stroke observed in this study was 2.9%, which is within the range of 2.2% to 6% noted for patients with AF receiving warfarin or placebo in previous clinical trials,12 the incidence rate of major hemorrhage in our study sample was higher (3.06%) than the rates observed in clinical trials of patients receiving warfarin versus placebo (0.69%-1.27%)12 or in a more recent retrospective claims study of patients with AF who received warfarin (1.5%).26 This difference may be attributed to limiting the current study to Medicare beneficiaries, because it is believed that older age is a major risk factor for bleeding while receiving oral anticoagulation therapy.27,28
A scatterplot was constructed for each cohort using member HCC scores (x-axis) and allowed costs in the 12 months after the NVAF index date (y-axis) after which linear regression was performed to obtain a best-fit line. In patients without ischemic stroke or major hemorrhage, the modeled line fit the data points with a high R2 value (0.9265), thereby showing a clear and credible linear relationship between HCC score and cost. (Note: The linear relationship in this study is assessed by R2 rather than by t-test.) In patients with major hemorrhage and no ischemic stroke, there is evidence of a meaningful linear relationship between the HCC score and cost; however, the modeled line does not fit extremely well, as shown by a lower R2 value (0.4899), and its intercept is higher than that of the modeled line for patients without ischemic stroke or major hemorrhage.
These findings indicate that baseline costs are higher in patients with major hemorrhage and no ischemic stroke, regardless of risk. The modeled lines for patients with ischemic stroke and no major hemorrhage and patients with both ischemic stroke and major hemorrhage are poorly fitting with very low R2 values (0.0161 and 0.1638, respectively), suggesting the lack of a linear relationship between HCC score and cost. Among patients with ischemic stroke and no major hemorrhage, the findings indicate that stroke costs did not increase significantly in patients with higher HCC scores. The small sample sizes for patients with both ischemic stroke and major hemorrhage likely affect the findings for that cohort.
Although our study design compared costs for the 12 months after patients were newly diagnosed with NVAF, whereas other studies compared costs from the day of the adverse event (stroke or hemorrhage) for patients with newly diagnosed and those with existing NVAF, our incremental cost findings corroborate those of previous studies. In a retrospective observational cohort study investigating the costs (in 2004 US dollars) associated with the onset of chronic NVAF, the use of anticoagulants, and the presence of cerebrovascular events in a commercially insured population, Boccuzzi and colleagues reported that the total direct healthcare costs (per member per month [PMPM] before and after the onset of AF in 3891 patients with incidence chronic NVAF were $412 and $1235, respectively ($494 and $1480, respectively, in 2011 US dollars).19,20 Of the 448 patients (12%) who had a cerebrovascular event (ie, transient ischemic attack, ischemic stroke, or major bleeding), the PMPM costs ranged from $2235 to $3135 ($2679-$3758, respectively, in 2011 US dollars) based on stroke risk status and on exposure to anticoagulation.19,20 The total cohort PMPM costs before and after the event were $3446.91 and $4262.12, respectively ($4132 and $5109, respectively, in 2011 US dollars), which represents an increase of 24%.19,20
Data from a study conducted by Mercaldi and colleagues assessing the cost-effectiveness of anticoagulation therapy on medical costs in 119,764 Medicare beneficiaries with NVAF showed that the average annual total healthcare cost was $15,718 (in 2006 US dollars) in patients with no major events during follow-up compared with $43,937; $60,123; and $39,943 in patients who had an ischemic stroke, hemorrhagic stroke, or major bleeding, respectively, at any time during the study.7 The incremental cost relative to the average annual costs of patients with no events was $34,201 for ischemic stroke; $44,716 for hemorrhagic stroke; and $29,965 for major bleeding.7 Of note, these incremental costs are higher than the costs that we report, even though these authors' calculations are based on 2006 US dollars.
Mercaldi and colleagues also recently conducted a retrospective cohort study to quantify long-term costs in 25,465 Medicare beneficiaries with newly diagnosed NVAF who subsequently developed ischemic stroke, major bleeding, or intracranial hemorrhage.16 Using Medicare medical and enrollment data (1999–2009), patients with NVAF and events were matched with patients with NVAF without events to separate the event-related costs from the general NVAF-related healthcare costs.16 The total incremental costs of events were calculated as the cost difference between patients with and without events for up to 3 years.16 The investigators reported that the average incremental costs in the first year after the event were $32,900; $23,414; and $47,640 for ischemic stroke, major bleeding, and intracranial hemorrhage, respectively, and that the costs remained higher, by $3156 to $5400 annually, at 3 years postevent.16
Limitations
We acknowledge several potential study limitations. First, although risk adjustment based on HCC was performed, unobserved confounding variables that could affect healthcare costs (eg, patient socioeconomic status and race) are not available in the database, and therefore were not included for adjustment. We acknowledge that, in general, risk adjustment is most effective in predicting costs across large populations and may not be as predictive with smaller cohorts.
Second, as a result of data limitations and study design, we are only able to observe 12 months of claims before a patient's AF index date. As a result, our definition of “newly diagnosed” (ie, no claim with an ICD-9 diagnosis code of 427.31 in any position in the 12 months before the index date) could potentially have included some patients with AF who had infrequent contact with a healthcare provider. We are not able to adjust for major hemorrhage or ischemic stroke occurring more than 12 months before the AF index.
Third, all the major hemorrhagic events occurred after the NVAF index diagnosis in this study; however, we could not confirm if these bleeding events were caused by oral anticoagulant treatment.
Finally, our analysis was based on Medicare CMS Chronic Conditions Data Warehouse RIF data, which may not be generalizable to a commercial patient population.
Conclusion
NVAF poses a substantial economic burden in Medicare beneficiaries as a result of an increased risk for ischemic stroke and hemorrhagic events. Although oral anticoagulation has been shown to reduce the risk of stroke in patients with NVAF, it remains underused in clinical practice, possibly because of concerns over the risk for bleeding.29–31 The economic burden of managing patients with AF experiencing ischemic stroke or major hemorrhage was similar during the first year after a diagnosis of AF. The burden of bleeding complications to patients, clinicians, and payers should not be overlooked, and these complications should be considered in conjunction with cost-savings associated with ischemic stroke risk reduction in the future cost-benefit evaluation of oral anticoagulation therapy. Understanding the economic burden of clinical events is an important aspect of health economics and outcomes research that, in addition to providing a basis for decision-making, also provides the fundamental data input for a cost-benefit analysis. A future cost-benefit analysis of this topic is recommended as a future area of research to provide further information for decision makers.
Stakeholder Perspective
The Clinical Toll of Nonvalvular Atrial Fibrillation Poses Significant Economic Burden
By Raymond L. Singer, MD, MMM, CPE
Chief, Division of Cardiothoracic Surgery, Vice Chair, Quality and Patient Safety, Department of Surgery, Lehigh Valley Health Network, Philadelphia, PA
PATIENTS: Atrial fibrillation (AF) is the most common arrhythmia in patients aged ≥65 years and is associated with a significant risk for thromboembolic stroke. Nonvalvular AF (NVAF) increases the risk for stroke by 5 times, whereas AF in the setting of valvular heart disease increases stroke risk by 20 times versus patients who have a normal sinus rhythm.1
Many patients with AF are completely asymptomatic, thus underscoring the importance of routine physical examinations for senior patients. Common presentations include palpitations and a fast, irregular heartbeat. More serious presentations include congestive heart failure resulting from ischemia, valvular heart disease, or AF-arrhythmia–induced cardiomyopathy.
Because of the ineffective contractions of the atria, blood stasis occurs, resulting in clot formation. Typically, clotting may be found in the left atrial appendage. The greatest risk for stroke occurs with paroxysmal AF (PAF). The clot forms in the atrial appendage during periods of PAF and may be ejected as an embolus during a subsequent normal sinus contraction. The appropriate use of antithrombotic medications substantially reduces the risk of stroke in patients with AF and PAF.
Warfarin is the most frequently prescribed antithrombotic medication. Warfarin is inexpensive and is well-tolerated; however, its use is associated with an increased risk for bleeding complications. Moreover, patients taking warfarin must watch their diets, because many foods and medications interact with warfarin, either decreasing or even enhancing its anticoagulation potential.2
Patients also need monthly blood testing to ensure the appropriate anticoagulation level by measuring prothrombin time, which is reported as an international normalized ratio (INR). Unlike most medications that are administered as a fixed dose, warfarin dosing is adjusted according to the INR blood test results.2 Therefore, it is important that patients are compliant with their follow-up testing. A patient with an abnormally high INR level is at an increased risk for bleeding complications. Conversely, if the INR is subtherapeutic, the patient is at an increased risk for thromboembolic events. Of note, if the patient needs urgent warfarin reversal (eg, as a result of trauma or to undergo an invasive test or surgery), the effect of warfarin can be reversed by administering vitamin K and/or fresh frozen plasma transfusions.2
Recently, safe and effective alternatives to warfarin have been introduced, including dabigatran, rivaroxaban, and apixaban. These drugs are direct thrombin and/or factor Xa inhibitors that do not require anticoagulation monitoring and are not associated with food or medication interactions.3 Early randomized, double-blind clinical trials suggest that these drugs may be more effective than warfarin for the prevention of stroke in patients with NVAF.4,5 In addition, apixaban may also be associated with a lower risk for bleeding complications compared with warfarin.6 However, these drugs are also substantially more expensive and may not be covered by insurance plans, which is a particular concern for patients with Medicare or Medicaid coverage. In addition, although the half-life of these drugs can be as little as 5 to 14 hours, patients need to understand that there are no antidotes for these drugs. Patients may be apprehensive about taking a drug that cannot be reversed even in an emergency setting.
Patients must weigh the benefits and risks of antithrombotic medication for the treatment of NVAF. In their retrospective claims analysis, Fitch and colleagues show that stroke and bleeding complications significantly increase the clinical and economic burdens on patients during the first year after a diagnosis of NVAF. To improve the safety of antithrombotic therapy, the associated risks for stroke (eg, hypertension and hypercholesterolemia) and for bleeding (eg, liver disease and alcohol intake) must be assessed before the initiation of the drug regimen. Patient compliance, along with consistent, timely, and accurate anticoagulation monitoring, is paramount.
PAYERS: As Fitch and colleagues have shown, NVAF, along with its associated complications, pose a significant economic burden. Even more concerning is that the incidence and prevalence of AF continue to increase as the population ages.7 It is expected that the incidence of AF will double from 1.2 million cases in 2010 to 2.6 million cases in 2030. Given this increase in incidence, the prevalence of AF is projected to increase from 5.2 million in 2010 to 12.1 million cases in 2030.7
More outcomes research is needed to investigate the benefits and risks of antithrombotic medications for NVAF. Although the newer agents are easier to prescribe and to monitor, the costs of these drugs remain high (Table). Payers will need to balance the cost of these drugs with their safety profiles, efficacy, cost of monitoring, and most of all, the evidence (or lack thereof) of clinical superiority.
Table.
Monthly Costs of Select Antithrombotic Medications
| Antithrombotic medication | Cost of monthly supply, $ |
|---|---|
| Warfarin 5 mg | 17.25 |
| Dabigatran | 349 |
| Rivaroxaban | 343.62 |
| Apixaban | 349.99 |
Source: Health Spectrum Pharmacy, Lehigh Valley Health Network, Allentown, PA.
PROVIDERS: Providers must also balance the benefits and risks of selecting an antithrombotic regimen for patients with NVAF. Risk stratification schemes (eg, CHADS2, CHA2DS2-VASc, and HAS-BLED) are beyond the scope of this perspective, but they represent scoring guidelines that have been validated in multiple cohorts with NVAF. Based on this score, a patient can be stratified as low, intermediate, or high risk for a thromboembolic event, and therefore be properly evaluated for the initiation of antithrombotic medication. In patients aged ≥65 years, it would be rare to have a CHADS2 or CHA2DS2-VASc of zero, and therefore the majority of patients will be treated. Furthermore, studies indicate that all women aged ≥65 years with NVAF should be treated, even with a score of zero, because of their observed increased risk for stroke.1,8
To reduce the risk for bleeding, providers must offer proper education and timely, consistent, and accurate monitoring to patients who are receiving antithrombotic medications. The education should include the importance of drug compliance, dietary restrictions, medication interactions, and lifestyle changes. There must be a defined process in place for prothrombin/INR testing. Equally important is to establish a defined process for communicating the testing results to the patient and for any adjusted medication dosing. Many thromboembolic and bleeding complications occur as a result of inconsistent monitoring and poor communication. In addition to the quality and safety concerns discussed herein, the mismanagement of warfarin is a common source of medical malpractice litigation.
Because of the simplicity of monitoring, many providers prefer to use the newer antithrombotic agents as first-line therapy for patients with nonvalvular AF. However, as previously noted here, the cost of these newer agents can be ≥5 times as expensive as warfarin and may not be covered by the patient's insurance plan. The lack of an antidote, again, may be a concern for the patient, but should also give some caution to the provider.
Although many patients have few symptoms from NVAF, providers should nonetheless make every effort to convert the patient back to a normal sinus rhythm. Many antiarrhythmic medications are available today. If the patient cannot be converted to a sinus rhythm using medications alone, transesophageal echocardiography cardioversion and ablation procedures should be considered. Surgical options using radiofrequency ablation and left atrial appendage occlusion are also showing great promise.
I wish to thank Courtney E. Bennett, DO, for her assistance in preparing this perspective.
- 1.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014. Mar 28. [Epub ahead of print]. [DOI] [PubMed]
- 2.Fiumara K, Goldhaber SZ. A patient's guide to taking coumadin/warfarin. Circulation. 2009; 119: e220–e222 [DOI] [PubMed] [Google Scholar]
- 3.Spinler SA, Shafir V. New oral anticoagulants for atrial fibrillation. Circulation. 2012; 126: 133–137 [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361: 1139–1151 [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365: 883–891 [DOI] [PubMed] [Google Scholar]
- 6.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365: 981–992 [DOI] [PubMed] [Google Scholar]
- 7.Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013; 112: 1142–1147 [DOI] [PubMed] [Google Scholar]
- 8.Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients wih non-valvular atrial fibrillation: executive summary. Eur Heart J. 2013; 34: 2094–2106 [DOI] [PubMed] [Google Scholar]
Acknowledgments
We would like to thank Lauren Lee for her contribution to the study design and data analysis, as well as Xin Ye for his assistance in the preparation of this manuscript.
Biography

Kathryn Fitch
Appendix. ICD-9 Codes Used to Identify Strokes and Hemorrhages
| ICD-9 codes used to identify ischemic strokes; any inpatient or emergency department claim coded with any of the following diagnosis codes in the primary position of the claim: 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91 |
ICD-9 codes used to identify major gastrointestinal hemorrhage; any inpatient admission coded with the following diagnosis codes in the primary position of the claim: 455.2, 455.5, 455.8, 456.0, 456.20, 530.82, 531.0x, 531.2x, 531.4x, 532.0, 532.2, 532.4, 532.6, 531.6x, 533.0x, 533.2x, 533.4x, 533.6x, 534.0x, 534.2x, 534.4x, 534.6x, 535.x1, 537.83, 562.02, 562.03, 562.12, 562.13, 569.3, 578.xx |
|
ICD-9 codes used to identify intracranial hemorrhage; any inpatient admission coded with the following diagnosis codes in the primary position of the claim: 430.x, 431.x, 432.xx |
ICD-9 indicates International Classification of Diseases, Ninth Revision.
Funding Source
This study was funded by Daiichi Sankyo, Inc.
Author Disclosure Statement
Ms Fitch is an employee of Milliman, Inc, and Mr Broulette was formerly an employee of Milliman, Inc, which consults with several pharmaceutical and medical device companies, including Daiichi Sankyo, Inc. Dr Kwong is an employee of Daiichi Sankyo, Inc.
Contributor Information
Kathryn Fitch, Principal and Healthcare Consultant, Milliman, Inc, New York, NY.
Jonah Broulette, Former Associate Actuary, Milliman, Inc, New York, NY.
Winghan Jacqueline Kwong, Senior Director, Health Economics and Outcomes Research, Daiichi Sankyo, Inc, Parsippany, NJ.
References
- 1.Ali A, Bailey C, Abdelhafiz AH. Stroke prophylaxis with warfarin or dabigatran for patients with non-valvular atrial fibrillation—cost analysis. Age Ageing. 2012; 41: 681–684 [DOI] [PubMed] [Google Scholar]
- 2.Andrew NE, Thrift AG, Cadilhac DA. The prevalence, impact and economic implications of atrial fibrillation in stroke: what progress has been made? Neuroepidemiology. 2013; 40: 227–239 [DOI] [PubMed] [Google Scholar]
- 3.Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013; 112: 1142–1147 [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, et al. for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013; 127: e6–e245 Erratum in: Circulation. 2013; 127: e841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali A, Bailey C, Abdelhafiz AH. Stroke prevention with oral anticoagulation in older people with atrial fibrillation—a pragmatic approach. Aging Dis. 2012; 3: 339–351 [PMC free article] [PubMed] [Google Scholar]
- 6.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008; 92: 17–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercaldi CJ, Ciarametaro M, Hahn B, et al. Cost efficiency of anticoagulation with warfarin to prevent stroke in Medicare beneficiaries with nonvalvular atrial fibrillation. Stroke. 2011; 42: 112–118 [DOI] [PubMed] [Google Scholar]
- 8.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011; 57: e101–e198 [DOI] [PubMed] [Google Scholar]
- 9.Lévy S, Maarek M, Coumel P, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999; 99: 3028–3035 [DOI] [PubMed] [Google Scholar]
- 10.Fitch K, Iwasaki K, Pyenson B. Non-valvular atrial fibrillation and anticoagulation therapy: an actuarial study of the Medicare population. August 2010. http://publications.milliman.com/research/health-rr/pdfs/non-valvular-atrial-fibrillation.pdf Accessed February 3, 2014.
- 11.You JJ, Singer DE, Howard PA, et al. for the American College of Chest Physicians. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141 (2 suppl): e531S–e575S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146: 857–867 [DOI] [PubMed] [Google Scholar]
- 13.Connolly SJ, Ezekowitz MD, Yusuf S, et al. for the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361: 1139–1151 Erratum in: N Engl J Med. 2010; 363: 1877 [DOI] [PubMed] [Google Scholar]
- 14.Patel MR, Mahaffey KW, Garg J, et al. for the ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365: 883–891 [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJ, et al. for the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365: 981–992 [DOI] [PubMed] [Google Scholar]
- 16.Mercaldi CJ, Siu K, Sander SD, et al. Long-term costs of ischemic stroke and major bleeding events among Medicare patients with nonvalvular atrial fibrillation. Cardiol Res Pract. 2012; 2012: 645469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA. 2005; 293: 699–706 [DOI] [PubMed] [Google Scholar]
- 18.Rose AJ, Berlowitz DR, Ash AS, et al. The business case for quality improvement: oral anticoagulation for atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011; 4: 416–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boccuzzi SJ, Martin J, Stephenson J, et al. Retrospective study of total healthcare costs associated with chronic nonvalvular atrial fibrillation and the occurrence of a first transient ischemic attack, stroke or major bleed. Curr Med Res Opin. 2009; 25: 2853–2864 [DOI] [PubMed] [Google Scholar]
- 20.Singh SN. Costs and clinical consequences of suboptimal atrial fibrillation management. Clinicoecon Outcomes Res. 2012; 4: 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotowycz MA, Filion KB, Joza J, et al. In-hospital management of atrial fibrillation: the CHADS2 score predicts increased cost. Can J Cardiol. 2011; 27: 506–513 [DOI] [PubMed] [Google Scholar]
- 22.Ghate SR, Biskupiak J, Ye X, et al. All-cause and bleeding-related health care costs in warfarin-treated patients with atrial fibrillation. J Manag Care Pharm. 2011; 17: 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WC, Lamas GA, Balu S, et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ. 2008; 11: 281–298 [DOI] [PubMed] [Google Scholar]
- 24.Suh DC, Nelson WW, Choi JC, Choi I. Risk of hemorrhage and treatment costs associated with warfarin drug interactions in patients with atrial fibrillation. Clin Ther. 2012; 34: 1569–1582 [DOI] [PubMed] [Google Scholar]
- 25.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004; 25: 119–141 [PMC free article] [PubMed] [Google Scholar]
- 26.Fang MC, Stafford RS, Ruskin JN, Singer DE. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004; 164: 55–60 [DOI] [PubMed] [Google Scholar]
- 27.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010; 138: 1093–1100 [DOI] [PubMed] [Google Scholar]
- 28.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006; 151: 713–719 [DOI] [PubMed] [Google Scholar]
- 29.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010; 123: 638–645 [DOI] [PubMed] [Google Scholar]
- 30.Reynolds MR, Shah J, Essebag V, et al. Patterns and predictors of warfarin use in patients with new-onset atrial fibrillation from the FRACTAL Registry. Am J Cardiol. 2006; 97: 538–543 [DOI] [PubMed] [Google Scholar]
- 31.Fitch K, Broulette J, Pyenson BS, et al. Utilization of anticoagulation therapy in Medicare patients with nonvalvular atrial fibrillation. Am Health Drug Benefits. 2012; 5 (3): 157–168 [PMC free article] [PubMed] [Google Scholar]