Abstract
The stability of cnidarian-dinoflagellate (genus Symbiodinium spp.) endosymbioses depends on the regulation of nutrient transport between Symbiodinium populations and their hosts. Previously, we successfully induced the production of lipid droplets in the free-living cultured Symbiodinium (clade B) under the nitrogen-deprivation condition for 5 days. Therefore, the present study aimed at understanding the disruption of the endosymbiotic relationship between the cnidarians and dinoflagellates by nitrogen deprivation using Aiptasia pulchella as an example. Transmission electron micrographs revealed the formation of lipid droplets induced by nitrogen deprivation, and the lipid analyses further showed that polyunsaturated fatty acids were drastically enriched in Symbiodinium after 30 days of nitrogen deprivation, although these were unaffected after 5 days of nitrogen starvation. The present study also suggested that the host provided nitrogen to the symbiotic cells during short-term environmental stress. However, the relationship started to deteriorate after 30 days. These findings provide a more detailed understanding of the mechanisms of the symbiotic relationship between the symbiotic dinoflagellates in terms of the nitrogen source, which might provide more information for the explanation of the regulatory mechanism underlying endosymbiotic associations.
Nutrition plays an important role in supporting endosymbiosis between cnidarians and unicellular dinoflagellates, particularly Symbiodinium spp.1,2. Numerous cnidarian-dinoflagellate mutualism studies have highlighted the crucial role of nutritional status and nutrient transfer in endosymbiosis2. Symbiodinium supplies organic carbon to its host and recycles essential nutrients. This nutrient transfer is largely responsible for the success of coral reefs in nutrient-deprivation tropical seas3. Symbiotic Symbiodinium derives nutrients from various sources, including exogenous seawater, as well as engages in catabolism and heterotrophy, with its host4,5. Nitrogen, which is one of the essential nutrients, of most organism, is excreted as ammonium by the host6,7.
Numerous investigations have been conducted on the mechanisms of nutrient metabolism in Symbiodinium in the last decade. Previous studies have shown that those endosymbionts could survive in nutrient-limited environments such as those with limited nitrogen sources8,9. Limited nutrients could induce stress in the host and affect the lipid droplet formation in Symbiodinium10,11.
Nitrogen deprivation is a routinely used method for inducing environmental stress in organisms and has been shown to severely affect the growth, development and metabolism of algae12. Upon exposure to nutritional stress induced by nitrogen or phosphate deprivation, algae synthesize triacylglycerols (TAGs), which are stored in lipid droplets13,14,9. Furthermore, the accumulation of lipid droplets in algal cells grown with limited nitrogen is specifically induced in response to the environmental changes15,16. Metabolite translocation from the host to endosymbionts and the cellular and molecular mechanisms leading to the biogenesis of lipid droplets in Symbiodinium are poorly understood.
Our previous study showed a significantly increased accumulation of lipid droplets in free-living Symbiodinium cultivated in nitrogen-deprived media for 5 days9. Therefore, the aim of this study was to investigate the effects of low concentrations of nitrogen on intracellular lipid content, fatty acid composition and morphology of Symbiodinium in Aiptasia pulchella. A. pulchella was cultured in the nitrogen-depleted media for 5 and 30 days, and then the Symbiodinium cells isolated from A. pulchella harvested after culturing for different days were compared with the cells isolated from the normal A. pulchella. The effects of nitrogen deprivation on cell morphology, lipid accumulation and lipid metabolism were assessed. Lipid analyses data revealed that TAG was the major lipid class in Symbiodinium freshly harvested from nitrogen-deprived culture conditions, and polyunsaturated fatty acids, such as C18:2, C20:5 and C22:6, were newly synthesized.
Results
Characterization of symbiont Symbiodinium cells from A. pulchella
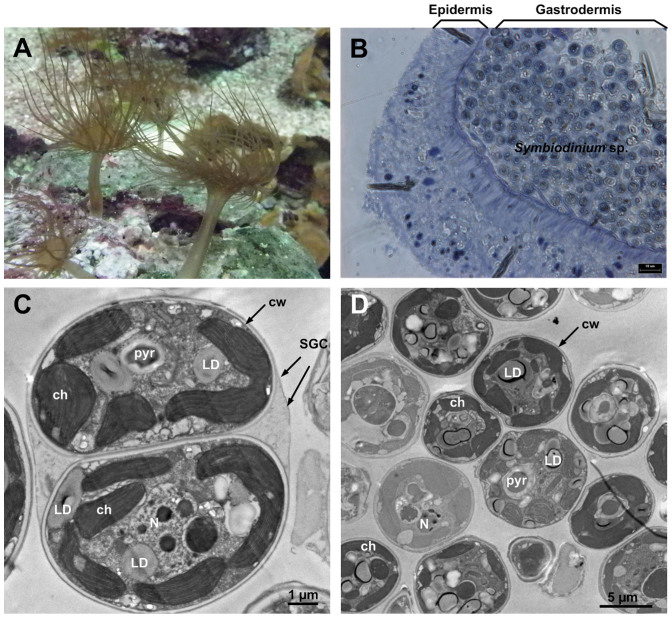
A. pulchella, sea anemone is described as a model system for cnidarian-dinoflagellate intracellular endosymbiosis mechanisms (Figure 1A)17. To determine the localization of Symbiodinium cells within its host, A. pulchella sections were stained with toluidine blue and observed under a light microscope. As shown in figure 1B, the Symbiodinium cells were mostly nested in the gastrodermal layer, which was surrounded by the epidermal layer. To characterize the ultrastructure of Symbiodinium cells in the host, we isolated the Symbiodinium cells from A. pulchella. The A. pulchella was blended to release the Symbiodinium cells from the host gastrodermal cells. The solution was loaded onto a Percoll gradient according to the method described previously18,19. The interface of the 60 to 80% Percoll layer obtained most symbiotic gastrodermal cells (SGCs) (Figure 1C), which are the symbionts containing host gastroderm cells and plays important roles in the initiation and maintenance of the endosymbiosis18, whereas the 80% to 100% Percoll layers only contained Symbiodinium cells without host cytoplasm contamination (Figure 1D). Phylogenetic analyses revealed that this symbiotic Symbiodinium cells belonged to clade B (data not shown). The chloroplast was the largest organelle in the Symbiodinium cell, occupying a considerable volume of the cytoplasm. The chloroplast was located adjacent to the cell wall with a parallel band pattern (Figure 1D). The pyrenoid was located at the center of the cell and surrounded by a capping vesicle (Figure 1C; 1D). Previous studies have reported that lipid droplet formation was discovered in the Symbiodinium cells in endosymbiotic associations with corals or anemones (Figure 1C)20. However, lipid droplet formation in free-living, cultured Symbiodinium cells was less frequently observed19.
Figure 1. The natural symbiotic association between cnidarian and Symbiodinium.
(A) The wild A. pulchella (B) The brown algae inside the host called Symbiodinium, which is always found in the gastrodermis layer. (C) Two Symbiodinium cells were surrounded by the symbiotic gastrodermal cell (SGC), (D) Symbiodinium cells isolated from A. pulchella containing several common organelles, such as nucleus (N), pyrenoid (pyr), chloroplasts (ch), cell wall (cw), lipid droplets (LDs).
Effect of nitrogen deprivation on the morphology of symbiotic dinoflagellates
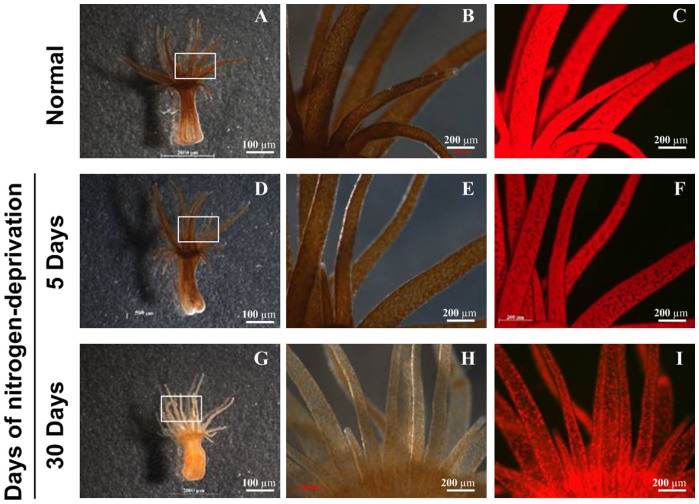
To assess the effect of nitrogen deprivation on symbiotic dinoflagellates obtained from sea anemones, A. pulchella cultured in the enriched media without any stress represented the normal strain, then transferred to a nitrogen-free medium and cultured for another 30 days. Light microscopy studies revealed the general distribution of symbiotic dinoflagellates in A. pulchella (Figure 2). Examination of the tentacles of A. pulchella showed a patchy distribution of brown Symbiodinium cells within the gastrodermis (Figures 2B, 2E, 2H). The Symbiodinium cells were densely clustered as indicated by the red fluorescence light in figure 2 (C, F, I). There was no difference in the cell density of the normal and 5-day-nitrogen-deprivation treatments, but the cell density decreased after 30 days of nitrogen deprivation (Table 1). This significant difference was confirmed by light and flourescene microscopy, meanwhile, the tentacle started to bleach as well (Figure 2). The 30-day nitrogen deprivation resulted in chloroplast degradation, and the reduction was 54.3% in chlorophyll a concentration compared to the normal cells (4.22 ± 0.01 to 1.93 ± 0.01 pg.cell−1, respectively) (Table 1). Fluorescence analysis performed by pulse amplitude modulation (PAM) fluorometry showed that the photosynthetic efficiency of the symbiotic cells was similar in the controls and nitrogen-deprived A. pulchella (Table 1).
Figure 2. The loss of color from the host A. pulchella in 30 days.
A. pulchella was cultured in enriched medium (A–C) and in medium depleted nitrogen source for the indicated times (D–I). The morphological changes of sea anemone growth under N sufficient and limited conditions (A)(D)(G). The tentacles of its host sea anemone went bleaching during 30 days; enlarged portions labeled by solid rectangular boxes (B)(E)(H). Fluorescence microscopy was applied to detect the auto-fluorescence of Symbiodinium chlorophyll (C)(F)(I). The scale bars indicate 200 μm.
Table 1. Influence of duration after nitrogen depletion on Symbiodinium cells.
| Normal | Starvation (Day 5) | Starvation (Day 30) | |
|---|---|---|---|
| Cell size (diameter, μm) | 6.49 ± 1.06a | 6.22 ± 0.88b | 6.49 ± 1.16a |
| (n = 688) | (n = 592) | (n = 446) | |
| Cell wall (thickness, μm) | 0.02 ± 0.005a | 0.03 ± 0.01b | 0.056 ± 0.02c |
| (n = 470) | (n = 994) | (n = 662) | |
| Chl a (pg cell−1) | 4.22 ± 0.01b | 4.8 ± 0.01a | 1.93 ± 0.01c |
| Lipid droplets size (area, μm2) | 0.38 ± 0.31b | 0.35 ± 0.27b | 1.17 ± 0.77a |
| (n = 166) | (n = 200) | (n = 1287) | |
| Lipid droplets number in one cells | 2.19 ± 1.54b | 1.63 ± 0.93b | 7.9 ± 2.87a |
| (n = 75) | (n = 126) | (n = 163) | |
| PAM | 0.44 ± 0.10a | 0.44 ± 0.06a | 0.45 ± 0.05a |
| (n = 6) | (n = 10) | (n = 4) | |
| Cell density (×106 cells per mg protein) | 15.44 ± 4.18a | 10.02 ± 2.09a | 2.12 ± 0.82b |
| (n = 4) | (n = 4) | (n = 4) |
Impact of the nutrient regime on the cell patterns (i.e., Cell size, cell wall, Chl a, Lipid droplets size, Lipid droplets number, PAM, cell density) between the Symbiodinium cells grown in normal and nitrogen-depleted media, and the cell patterns were analyzed by ANOVA. Upper level a–c denote statistical significance different between normal, starvation(day 5) and starvation(day 30), respectively (P<0.001). Data are presented as mean ± SD. Cell wall, Lipid droplets number and cell size: n = number of cell analyzed, Lipid droplets size: n = number of lipid droplets analyzed, PAM and Cell density: n = number of repetition.
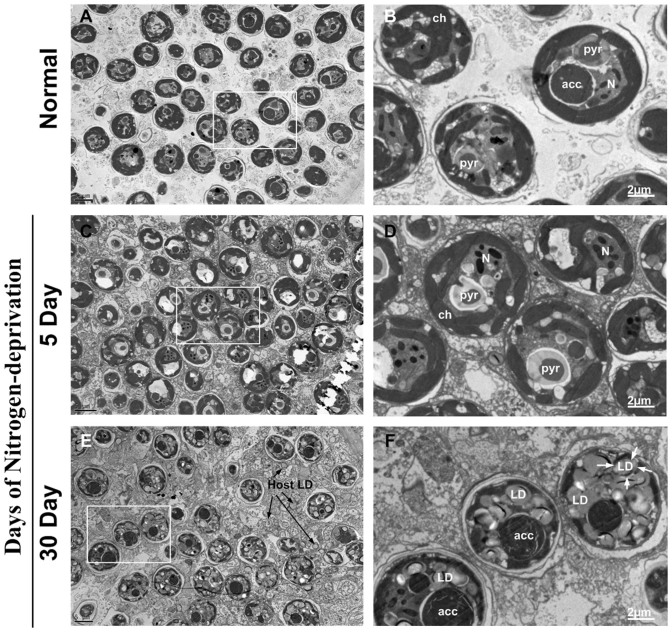
The ultrastructural changes in symbiotic Symbiodinium cells, A. pulchella was examined by transmission electron microscopy (TEM) (Figure 3). Nitrogen deprivation induced significant morphological changes in the Symbiodinium cells obtained from A. pulchella. The symbionts cultured in either enriched nutrient or nitrogen-depleted seawater for 5 days did not exhibit any obvious changes in cell morphology (Figures 3A, B, C, D). The chloroplasts, pyrenoids and starch granules appeared as major subcellular components in the cells. However, several morphological changes were found in the A. pulchella cultured in nitrogen-depleted seawater for 30 days. In addition, nitrogen deprivation induced the accumulation of lipid droplets that occupied most of the cytoplasm of the Symbiodinium cells. The lipid droplet number significantly increased from 2 compartements per cell in the A. pulchella cells of the normal treatment to 7 in the cells treated with nitrogen deprivation for 30 days (Table 1). Furthermore, the lipid droplet size significantly increased from 0.38 µm to 1.17 µm in the nitrogen-deprived A. pulchella. Although most nitrogen-deprived symbiotic Symbiodinium cells accumulated lipid droplets, there was no significant increase in cell size. However, the cell wall thickness of the nitrogen-deprived A. pulchella showed an approximate two-fold increase compared to that of the controls, from 0.02 µm to 0.05 µm.
Figure 3. The ultrastructural examination of morphological changes and lipid droplet formation in Symbiodinium after A. pulchella under nitrogen deprivation.
Transmission electron micrographs of A. pulchella in normal (A–B) and nitrogen-deprivation media (5 days: C–D; 30 days: E–F). Insets in A, C and E were magnified as B, D and F, respectively. Scale bars represent 5 μm and 2 μm. Abbreviations: LD, lipid droplet; Ch, chloroplast; Pyr, pyrenoids; N, nucleolus; acc: accumulation body, Host LD: host lipid droplet.
Nitrogen deprivation-induced lipid droplet formation and an increase in lipid content in symbiotic Symbiodinium
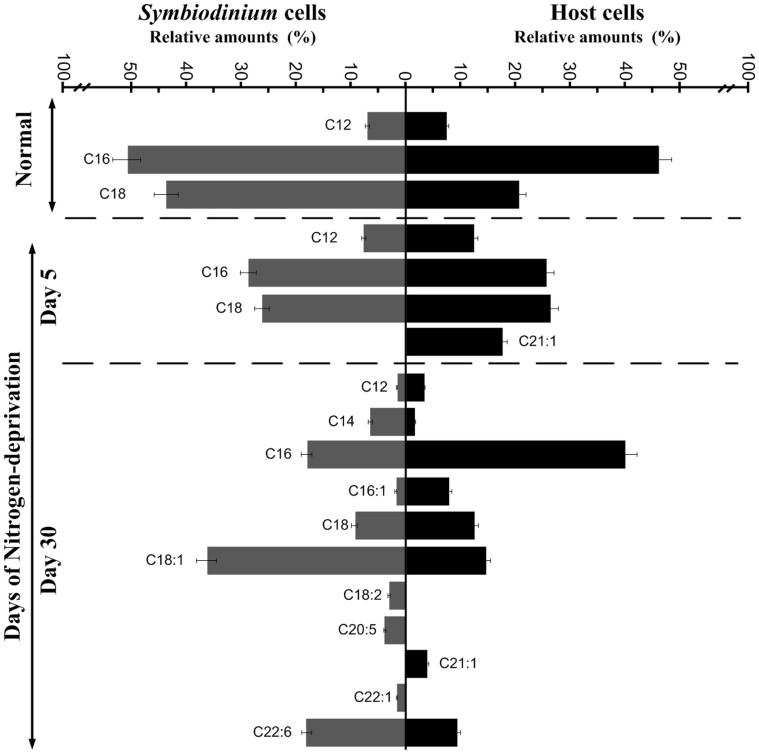
Nitrogen deprivation in the symbiotic Symbiodinium cells resulted in the accumulation of lipid droplets, which were isolated and the lipid content was analyzed using thin layer chromatography (TLC) (Table 2). The levels of neutral lipids, such as TAGs and CEs, increased after 30 days of nitrogen-deprivation treatment. Both TAGs and CEs were less detectable in the symbiotic Symbiodinium cells cultured in the normal conditions (Table 2). The concentration of TAGs and CEs were highest (21.77 ± 0.08 and 5.36 ± 0.15 pg cell−1, respectively) after 30 days of nitrogen-deprivation. Fatty acid analysis (Figure 4) of normal and 5-day nitrogen deprived symbiotic Symbiodinium cells revealed the predominance of saturated fatty acid (SFA), in which C16:0 and C18:0 were the most abundant fatty acids. Moreover, after 30 days of nitrogen deprived treatment the purified symbiotic Symbiodinium cells demonstrated that monounsaturated fatty acid (MUFA) (C16:1, C18:1, C22:1) and polyunsaturated fatty acid (PUFA) (C18:2, C22:6) were synthesized de novo. Furthermore, the fatty acid compositions of host A. pulchella cells showed that C12:0, C16:0 and C18:0 were the abundant fatty acids in normal condition. Interestingly, after nitrogen deprived treatment, the host started accumulating PUFA, however the C16:0 was still the majority fatty acid composition of the A. pulchella cells (Figure 4).
Table 2. TAGs and CEs accumulation in Symbiodinium cells.
| Lipid concentrations | Purified symbiotic Symbiodinium cells | ||
|---|---|---|---|
| (pg/cell) | Days of nitrogen-deprivation | ||
| Normal | Day 5 | Day 30 | |
| TAGs | 4.4 ± 0.09c | 7.87 ± 0.13b | 21.77 ± 0.08a |
| CEs | 1.16 ± 0.10c | 3.32 ± 0.11b | 5.36 ± 0.15a |
Lipid contents of purified symbiotic Symbiodinium in the normal and nitrogen-deprivation treatments were analyzed by TLC. Data are presented as mean ± SD (N = 3). Superscript a–c denote statistical significance within control and nitrogen-deprivation treatments (P<0.001).
Figure 4. Analyses of fatty acid compositions in TAGs of Symbiodinium cells and host cells (relative % w/w of total fatty acid groups) under normal and nitrogen-deprived conditions.
Symbiodinium cells and host cells were harvested from normal and after 5 and 30 days of growth from the starved Aiptasia pulchella. The lipid was extracted and analyzed by GC-MS/MS.
The effect of nitrogen-deprivation on the cellular morphology and protein expression of A. pulchella
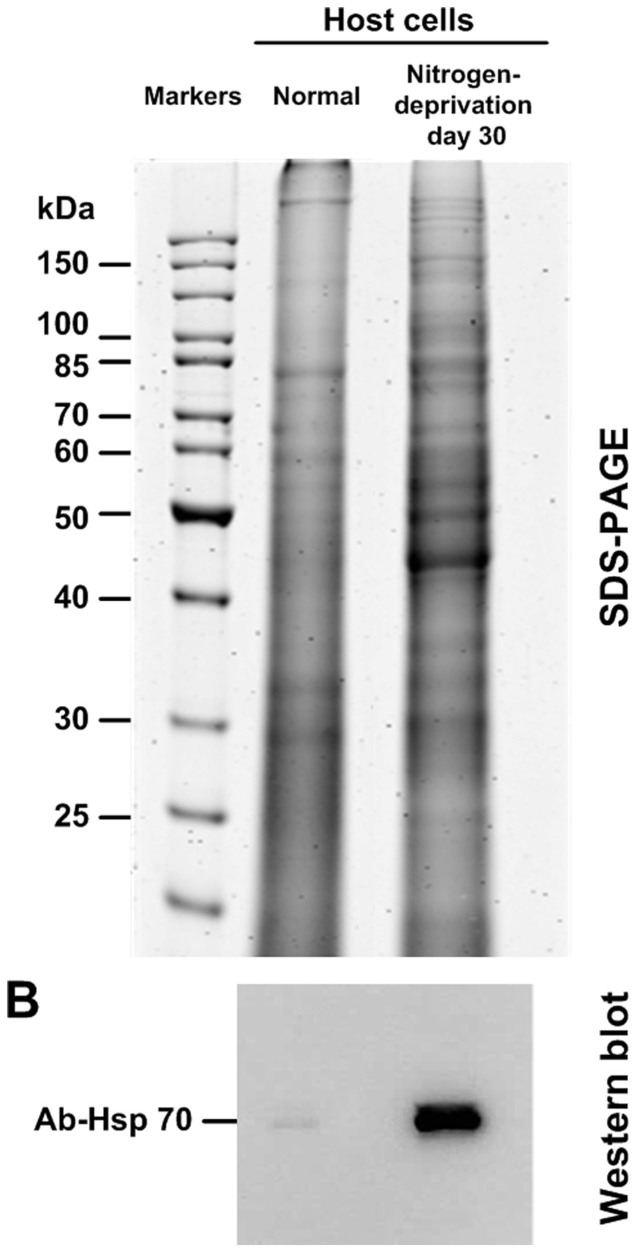
Nitrogen deprivation in the host not only affected the ultrastructure and lipid content of its symbiotic dinoflagellates, but also changed the cellular morphology and protein expression in the host. Furthermore, our data showed that A. pulchella with nitrogen deprivation for 30 days accumulated the lipid droplets in the host gastrodermis cell as observed by transmission electron microscopy (Figure 3E). To examine the effect of nitrogen deprivation on the A. pulchella protein, proteins from the host of the normal and the nitrogen deprivation for 30 days were extracted and subjected to immunological cross-recognition using anti-bodies against Hsp70. It was indicated that the expression level of heat shock protein 70 (Hsp 70) in A. pulchella cells significantly increased after 30 days of nitrogen deprivation (Figure 5).
Figure 5. SDS-PAGE and Western blot of the A. pulchella total protein under nitrogen-deprivation conditions.
(A) Total proteins were extracted from A. pulchella cells from the normal and the 30-day nitrogen-deprivation treatment. (B) Western blot analysis was carried out using an antibody against the 70 kDa Hsp70 protein.
Discussion
Nitrogen deprivation described as one of the nutrient limitations that disrupted the cells metabolism in microalgae. Our previous study demonstrated that the increased lipid content and lipid droplet accumulation in the free living Symbidinium spp. cells were caused by nitrogen deprivation9. These findings were similar to that of the studies involving nutrient-starved green algae, Scenedesmus obtusiusculus and Chlorella spp.21,22,14. Symbiotic dinoflagellates known as Symbiodinium spp. absorb carbon dioxide, phosphates and nitrates produced by its invertebrate host and transform these into nutrients that are essential to the holobiont (symbiotic dinoflagellates and invertebrate)23,24,25. Therefore, the metabolic proficiency of the host plays an important role in the nutrient status of photosynthetic dinoflagellates. In this study, we have characterized the endosymbiotic relationship between the cnidarians and dinoflagellates by using A. pulchella as a study model and observed the changes in cellular morphology and lipid content in the condition of nitrogen deprivation. A. pulchella treated with the nitrogen deprived media was observed on day 5 and day 30. On day 5, the Symbiodinium cells were isolated and purified, it was shown that no change in the endosymbiotic relationship of short- term environmental stress; however, this relationship deteriorated with the application of a long-term (30 days) environmental stress.
Nitrogen deprivation led to the synthesis of lipids and formation of lipid droplets in the symbionts26. It was shown that the symbiotic cells in the nitrogen-enriched corals contained less carbon and more nitrogen per cell than those in the nitrogen-deprived corals. A high carbon:nitrogen ratio induces an excess of photosynthetic products compared to that of protein synthesis, which results in the accumulation of starch and lipids. Our result showed that lipid droplets did not accumulate in Symbiodinium cells during endosymbiosis with a nitrogen starvation for less than 5 days. However, lipid droplet production occurred in the free-living cultured Symbiodinium cells treated with the nitrogen deprivation for 5 days9. Perhaps the host cells provided nitrogen to the symbiotic cells during this short-term nitrogen-deprivation stress.
Endosymbiotic Symbiodinium thriving in cnidarians utilizes its host's metabolites as nutrient sources23,24. Nitrogen-starved hosts showed a decrease in metabolism. In contrast, nitrogen-starved symbiotic cells remained metabolically active due to their photosynthetic efficiency, which caused lipid droplet accumulation in the symbiotic Symbiodinium cells. Hsp70 is routinely used as a biochemical stress indicator, as the elevated expression of Hsp70 is usually in response to environmental stress27. As expected, Hsp70 showed a higher expression in the host cells cultured for 30 days in the nitrogen-deprivated seawater compared to the cells cultured without nitrogen deprivation (Figure 5). A. pulchella might have up regulated its synthesis of Hsp70 in response to the nitrogen deprivation. The function of Hsp70 under stress conditions in A. pulchella remains to be investigated.
The fatty acid profiles of the normal, the nitrogen-deprived symbiotic cells and the host were analyzed by lipid extraction, transesterification and GC-MS analysis. Variations in fatty acid concentrations were observed in the host and the Symbiodinium isolated from A. pulchella under normal and nitrogen-deprived conditions. Palmitic acid (C16:0) and stearic acid (C18:0) were most abundant fatty acid in the host and the symbiotic Symbiodinium isolated from A. pulchella under normal condition and 5-day nitrogen deprivation. In contrast, the main fatty acid component of TAG in Symbiodinium isolated from A. pulchella cells treated with 30-day nitrogen deprivation was oleic acid (18:1). Furthermore, the host fatty acid isolated from A. pulchella treated with 30-day nitrogen deprivation was dominated by palmitic acid (C16:0) and oleic acid (18:1). It has been reported that palmitic acid (C16:0), stearic acid (C18:0) and oleic acid (18:1) were most identified fatty acid classes in the composite of coral and Symbiodinium spp.28. Similar composition of fatty acids was identified in both host sea anemone and symbiotic Symbidiniumin cells related to energy translocation and saturated lipid derived from Symbidinium during the endosymbiosis28,29,30. It has been hypothesized that the Symbiodinium cells residing in the host cell translocated the photosynthetically-derived lipid such as triacyglyceride since its nutrient requirements for photosynthesis were provided by the host9,31,32. In the present study, polyunsaturated fatty acid (PUFA) level increased in the Symbiodinium isolated from A.pulchella treated with nitrogen deprivation. This is similar with the previous reported, in which the accumulation of lipid droplets in the free-living Symbiodinium treated with nitrogen deprivation resulted in the induction of polyunsaturated fatty acid production due to further elongation and desaturation occurred during the TAG synthesis9,33.
Methods
Collection of sea anemone and the nitrogen-deprivation treatment
The sea anemone Aiptasia pulchella was collected from National Museum of Marine Biology Aquarium. They were maintained in filtered seawater (FSW) as the normal strain or in nitrogen-deficient artificial seawater at room temperature under a photosynthetically active radiation (PAR) of 40 µmol m−2s−1 in a 12-h light/12-h dark (12L/12D) cycle.
Isolation of Symbiodinium cells from A. pulchella Tissues
Symbiodinium isolated from its host tissue were subjected to further isolation method developed by Peng et al18. A. pulchella was collected from each treatment (normal, day 5 and day 30). They were then blended for releasing the Symbiodinium cells in FSW (filtered seawater) and homogenized with a glass grinder. The homogenate solution was then filtered through the 20-μm mesh and centrifuged at 4,000 × g for 5 min. The pellet collected after the centrifugation contained mostly Symbiodinium cells, which were resuspended in FSW and vortexed for 2 min. The mixture was centrifuged at 2,000 × g for 5 min. The pellet was retained and then resuspended in fresh FSW containing 1% (v/v) of triton X-100. The mixture was vortexed again for 2 min.
Symbiodinium clade identification
The genetic identity (18S rDNA) of the cultured Symbiodinium was examined by PCR-RFLP (Polymerase chain reaction-Restriction fragment length polymorphism) analysis34, and shown to be clade B. Symbiodinium DNA was extracted using a plant genomic DNA extraction miniprep system (VIOGENE, Taipei). Basically, Symbiodinium nuclear small subunit (n18S-rDNA) was amplified by PCR from 3 replicate extracts of each of the two cultures using the primers, ss5z (an equimolar mixture of the oligonucleotides 5′-GCAGTTATAATTTATTTGATGGTCACTGCTAC-3′ and 5′-GCAGTTATAGTTTATTTGATGGTTGCTGCTAC-3′) and ss3z (5′-AGCACTGCGTCAGTCCGAATAATTCACCGG-3′) and digested by the restriction enzymes, Taq I and Sau3A I (Promega, USA). Digestion products were separated by electrophoresis on 1.5% 0.5x TAE (Amresco, USA) agarose gels, to generate the RFLP pattern. RFLP pattern analysis was compared to the literature34 to assign each culture to one of the established Symbiodinium n18S-rDNA RFLP clades.
Cell density and Chlorophyll a determinations
The Symbiodinium cell density was examined with haemocytometer based cell counting and normalized to total protein of each homogenate of an individual anemone35. The protein content was determined using the BCA protein assay kit (Invitrogen, USA) on the same samples used for host homogenates. For chlorophyll a determination, the Symbiodinium were isolated and harvested by centrifugation at 12000 g for 10 min from A. pulchella. For each replicate during the analysis, a volume of culture containing 5 × 105 cells was used. Chlorophyll a was extracted in 90% acetone (v/v), and the amount of chlorophyll a was estimated according to the spectrophotometric quantification36.
Determination of photochemical efficiency
The normal and nitrogen deprived sea anemone were expressed as the ratio of variable (Fv) to maximal (Fm) fluorescence (Fv/Fm). In the present study, all the sea anemones were dark-adapted for 60 min at room temperature (25°C) using a Diving PAM (Walz, Germany).
Lipid analyses
All solvents for lipid analyses were analytical grade. Lipid contents of the 3 replicates of the symbiotic Symbiodinium from anemones cultured in the nature seawater and nitrogen-deprivation seawater were extracted by the Bligh and Dyer procedure37. Cells were extracted with 150 µl of chloroform/methanol (2:1, v/v). After centrifugation, the lower chloroform fraction was collected for the analysis by thin layer chromatography (TLC) (Analtech, USA) with the solvent system modified from previous reports38,39. Briefly, TLC was first developed to the Rf = 1 position in hexane. The plate was air-dried and then developed to the top (Rf = 1) in benzene. The plate was air-dried and then developed to the top (Rf = 0.5) in hexane: diethyl ether: acetic acid (70:30:1 v/v/v). The lipid visualization on TLC plates was performed by staining with 0.03% Coomassie blue R 250 (Sigma, USA) dissolved in 20% methanol containing 0.5% acetic acid40. Concentrations of individual lipid species were then quantified using the Metamorph Image Processing system (Molecular Devices Inc., Toronto, Canada) based on calibration curves of individual lipid standards (Wax ester: Sigma-Aldrich, USA; TAG: Sigma-Aldrich, USA; Cholesterol: Avanti Polar Lipids, USA; CE: Sigma-Aldrich, USA) co-run on the same TLC plate.
Analyses of fatty acid composition in cellular TAGs by gas chromatography-mass spectrometry (GC-MS)
Symbiotic Symbiodinium cells collected from A. pulchella were dried by lyophilization. Total lipids in the dried cells were extracted by the Bligh and Dyer procedure37, in which at the end the crude lipid was dissolved in hexane. Total lipids in dried cells were extracted and separated by TLC as previously described. TAGs on TLC plates was first identified by 0.001% primuline spraying (in 80% acetone), and then extracted by the Bligh and Dyer procedure37. Isolated TAGs were first saponified in 1N sodium hydroxide-methanol solution for 15 min at 80°C. The fatty acids were esterified in 14% boron trifluoride-methanol solution for 15 min at 100°C. After hexane extraction, the fatty acid methyl esters were analyzed on a gas chromatograph (GC, Varian CP-3800) and a mass spectrometer (Varian 320 MS) operated in full scan mode; scan range from 100 to 450 m/z. The column was a CP-Sil88 capillary column of length 20 m, 0.25 mm i.d., and the film thickness of the stationary phase was 0.2 µm. Helium was used as the carrier gas at a flow rate of 0.8 ml min−1. The temperature program was set as follows: holding at 50°C for 1 min, increasing from 50 to 200°C at a rate of 8°C min−1, holding at 200°C for 5 min, increasing from 200 to 230°C at a rate of 20°C min−1. Retention times and mass spectra were compared against the NIST02 library (National Institute of Standards and Technology, Gaithersburg, MD, USA) to identify fatty acids. Saturn GC/MS Workstation v6.9.3 software (Varian) was used to visualize spectra, integrate areas under peaks and search the library. Peaks of fatty acids were identified, and the relative amount of individual fatty acid was calculated by the integrated area percentage among the total fatty acids.
The transmission electron microscopy and imaging analysis
To investigate the intracellular accumulation of lipid droplets, A. pulchella under normal or nitrogen-limited seawater were collected and fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 100 mM sodium phosphate containing 5% sucrose (pH 7.3) for 2.5 h at 4°C. They were then rinsed with 100 mM sodium phosphate buffer at 4°C. Cells were then post-fixed in 1% OsO4 in 50 mM sodium phosphate (pH 7.3) for 1 h at 4°C. The cell aliquots were then washed 3 times for 15 min each with the same buffer and dehydrated by a graded ethanol series (50, 70, 80, 90, 95 and 100%) before embedding in LR white Resin. Thin sections (70 nm) cut by a Leica Reichert Ultracut R were collected on nickel grids, post-stained with 2.5% uranyl acetate and 0.4% lead citrate, rinsed 3 times with water, and the samples were viewed on a JEM-1400 transmission electron microscope (JEOL, Japan). In order to determine the lipid dropletsarea from the acquired images, the ratio of the actual length to pixel was first determined by distance calibration using the scale bar of the acquired TEM image. Individual lipid droplet was selected by threshold adjustment, and the area (μm2) of each lipid droplet was calculated with Metamorph's region measurement function.
SDS-PAGE and Western blotting
Proteins extracted from the A. pulchella cells (normal and day 30) were suspended in an equal volume of 2× sample buffer according to the suggestion in the Bio-Rad instruction manual and resolved by SDS-PAGE using 15% (w/v) polyacrylamide in the separating gel and 4.75% polyacrylamide in the stacking gel41. After electrophoresis, the gel was stained with Coomassie Blue R-250 and then destained. For immunoassay, proteins were transferred from SDS-PAGE onto a nitrocellulose membrane in a Trans-Blot system (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. The membrane was subjected to immunodetection using primary antibodies against Hsp 70. After washing, the membrane was supplemented with the secondary antibodies conjugated with goat anti-chicken horseradish peroxidase (HRP). The membrane was subsequently washed and visualized by SuperSignal West Pico Chemiluminescent substrate kits (Cat. 34080, Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's recommendations.
Author Contributions
J.P.L. and C.C.S. conceived the experiments. W.L.C., P.B., I.P.L. and C.H.T. performed the research. All authors contributed in analyzing the results and writing the manuscript.
Acknowledgments
The work was supported by a grant from the National Science Council, Taiwan, ROC (NSC 102-2313-B-291-001 to PL Jiang).
References
- Hastie L. C., Waston T. C. & Isamu T. Effect of nutrient enrichment on Tridacna derasa seed: dissolved in organic nitrogen increases growth rate. Aquaculture 106, 41–49 (1992). [Google Scholar]
- Belda C. A., Lucas J. S. & Yellowlees D. Nutrient limitation in the giant clam-zooxanthellae symbiosis: effect of nutrient supplements on growth of the symbiotic partners. Mar. Biol. 117, 655–664 (1993). [Google Scholar]
- Wang J. T. & Douglas A. E. Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis? J. Exp. Biol. 201, 2445–2453 (1998). [DOI] [PubMed] [Google Scholar]
- Szmant-Froelich A. & Pilson M. E. Q. Effects of feeding frequency and symbiosis with zooxanthellae on nitrogen metabolism and respiration of the coral Astrangia danae. Mar. Biol. 81,153–162 (1984). [Google Scholar]
- Steen R. G. Evidence of heterotrophy by zooxanthellae in symbiosis with Aiptasia pulchella. Biol. Bull. 170, 267–278 (1986). [Google Scholar]
- Rahav O., Dubinsky Z., Achituv Y. & Falkowski P. G. Ammonium metabolism in the zooxanthellate coral Stylophora pistillata. Proc. R. Soc. Lond. B. Biol. Sci. 236, 325–337 (1989). [Google Scholar]
- Falkowski P. G., Dubinsky Z., Muscatine L. & McCloskey L. Population control in symbiotic corals. BioScience 43, 606–611 (1993). [Google Scholar]
- Peng S. E. et al. Assessment of metabolic modulation in free-living versus endosymbiotic Symbiodinium using synchrotron radiation-based infrared microspectroscopy. Bio. Let. 23, 434–437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P. L., Pasaribu B. & Chen C. S. Nitrogen-deprivation elevates lipid levels in Symbiodinium spp. by lipid droplet accumulation: Morphological anc compositional analyses. PLOS ONE 9,e87416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Parker G., Lee K. W. & Cook C. B. Changes in the ultrastructure of symbiotic zooxanthellae (Symbiodinium sp., Dinophyceae) in fed and starved sea anemones maintained under high and low light. J. Phyco. 32, 987–994 (1996). [Google Scholar]
- Zhu B., Pan K. & Wang G. Effects of host starvation on the symbiotic dinoflagellates from sea anemone Stichodactyla mertensii. Mar. Eco. 32, 15–23 (2010). [Google Scholar]
- Hockin N. L., Mock T., Mulholland F., Kopriva S. & Malin G. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant Physiol. 158, 299–312 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin N. S. & Chisholm S. W. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J Phyco. 17, 374–384 (1981). [Google Scholar]
- Lin I. P., Jiang P. L., Chen C. S. & Tzen J. T. A unique caleosin serving as the major integral protein in oil bodies isolated from Chlorella sp. cells cultured with limited nitrogen. Plant Physiol. Biochem. 61, 80–87 (2012). [DOI] [PubMed] [Google Scholar]
- Gushina I. A. M. & Harwood J. L. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45, 160–186 (2006). [DOI] [PubMed] [Google Scholar]
- Pasaribu B., Lin I. P., Chen C. S., Lu C. Y. & Jiang P. L. Nutrient limitation in Auxenochlorella protothecoides induces qualitative changes of fatty acid and expression of caleosin as a membrane protein associated with oil bodies. Biotechnol. Lett. 36, 175–180 (2014). [DOI] [PubMed] [Google Scholar]
- Weis V. M., Davy S. K., Hoegh-Guldberg O., Rodriguez-Lanetty M. & Pringle J. R. Cell biology in model systems as the key to understanding corals. Trends in Ecology & Evolution 23, 369–376 (2008). [DOI] [PubMed] [Google Scholar]
- Peng S. E. et al. Proteomic analysis of symbiosome membranes in Cnidaria-dinoflagellate endosymbiosis. Proteomics 10, 1002–1016 (2010). [DOI] [PubMed] [Google Scholar]
- Pasaribu B. et al. SLDP: A Novel Protein Related to Caleosin is associated with the Endosymbiotic Symbiodinium Lipid Droplets from Euphyllia glabrescens. Mar biotech. 10.1007/s10126-014-9574-z (2014). [DOI] [PubMed] [Google Scholar]
- Chen W. N. U., Kang H. J., Weis V., Mayfield A. B. & Jiang P. L. et al. Diel rhythmicity of lipid-body formation in a coral-Symbiodinium endosymbiosis. Coral reef 31, 521–534 (2011). [Google Scholar]
- Van Donk E. & Hessen D. O. Grazing resistance in nutrient-stressed phyto- plankton. Oecologia 93, 508–511 (1993). [DOI] [PubMed] [Google Scholar]
- Tillberg J. E. & Rowley J. R. Physiological and structural effects of phosphorus starvation on the unicellular green algae. Scenedesmus. Physio. Plant. 75, 315–324 (1989). [Google Scholar]
- Weis V. M. Effect of dissolved inorganic carbon concentration on the photosynthesis of the symbiotic sea anemone Aiptasia pulchella Carlgren: role of carbonic anhydrase. J. Exp. Mar. Bio. Eco. 174, 209–225 (1993). [Google Scholar]
- Goiran C., Al-Moghrabi S. & Allemand D. Inorganic carbon uptake for photosynthesis by the symbiotic coral/dinoflagellate association. I. Photosynthetic performances of symbionts and dependence on sea water bicarbonate. J. Exp. Mar. Bio. Eco. 199, 207–225 (1996). [Google Scholar]
- Furla P., Benazet-Tambutte S. & Allemand D. Functional polarity of the tentacle of the sea anemone Anemonia viridis: role in inorganic carbon acquisition. American Journal of Physiol. 274, 303–310 (1998). [DOI] [PubMed] [Google Scholar]
- Muller-Parker G., McCloskey L. R., Hoegh-Guldberg O. & Mcauley P. J. Effect of ammonium enrichment on animal biomass of the coral Pocillopora damicornis. Pac. Sci. 48, 273–283 (1994). [Google Scholar]
- Tomanek L., & Sanford E. Heat-shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (genus Tegula). Bio. Bull. 205, 276–284 (2003). [DOI] [PubMed] [Google Scholar]
- Patton J. S., Abraham S. & Benson A. A. Lipogenesis in the intact coral Pocillopora eapitata and its isolated zooxanthellae: evidence for a iight-driven carbon cycle between symbiont and host. Mar. Biol. 44, 235–247 (1977). [Google Scholar]
- Meyers P. A. Fatty acids and hydrocarbons of Caribbean corals. [Taylor, D. L. (ed.) Rosenstiel School of Marine and Atmospheric Science, University of Miami, Florida]. Proc. 3rd int. Symp. coral Reefs 1, 529–535 (1997). [Google Scholar]
- Harland A. D., Fixter L. M., Spencer Davies P. & Anderson R. A. Distribution of lipids between the zooxanthellae and animal compartment in the symbiotic sea anemone Anemonia viridis: wax esters, triglycerides and fatty acids. Mar Biol 110, 13–19 (1991). [Google Scholar]
- Muscatine L., McCloskey L. R. & Marian R. E. Estimating the daily contribution of carbon from Zooxanthellae to coral animal respiration. Limnology and Oceanography. 26, 601–611(1981). [Google Scholar]
- Kellog R. B. & Patton J. S. Lipid droplets, medium of energy exchange in the symbiotic anemone Condylaetis gigantea: a model coral polyp. Mar. Biol. 75,137–149 (1983). [Google Scholar]
- Deng X., Fei X. & Li Y. The effects of nutritional restriction on neutral lipid accumulation in Chlamydomonas and Chlorella. Afri J Microbiol Res. 5, 260–270 (2011). [Google Scholar]
- Rowan R. & Powers D. A. Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar Ecol Prog Ser 71, 65–73 (1991). [Google Scholar]
- Tolleter D. et al. Coral bleaching independent of photosynthetic activity. Curr. Biol. 23, 1782–1786 (2013). [DOI] [PubMed] [Google Scholar]
- Jeffery S. W. & Humphrey G. F. New spectrophotometric equations for determining chlorophylls a, b, c1, and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 167, 191–194 (1975). [Google Scholar]
- Bligh E. G. & Dyer W. J. A rapid method for total lipid extraction and purification. Can J Biochem. Physiol. 37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- Oku H., Yamashiro H. & Onaga K. Lipid biosynthesis from [14C]-glucose in the coral Montipora digitata. Fish Sci. 69, 625–631 (2003). [Google Scholar]
- Fuchs B., Schiller J., Sub R. & Schurenberg M. A direct and simple method of coupling matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS) to thin layer chromatography (TLC) for the analysis of phospholipids from egg yolk. Anal Bioanal. Chem. 389, 827–834 (2007). [DOI] [PubMed] [Google Scholar]
- Abe A. Modification of the Coomassie brilliant blue staining method for sphingolipid synthesis inhibitors on silica gel thin-layer plate. Anal Biochem. 258, 149–150 (1998). [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970). [DOI] [PubMed] [Google Scholar]