This article analyzes the mechanism by which Pumilio represses the translation of its targets. The results show, rather surprisingly, that promotion of deadenylation is not required for expression. Instead, Pumilio interacts with poly(A) binding protein and somehow interferes with its activity.
Keywords: Pumilio, PUF, pAbp, poly(A) tail, deadenylation
Abstract
PUF proteins are potent repressors that serve important roles in stem cell maintenance, neurological processes, and embryonic development. These functions are driven by PUF protein recognition of specific binding sites within the 3′ untranslated regions of target mRNAs. In this study, we investigated mechanisms of repression by the founding PUF, Drosophila Pumilio, and its human orthologs. Here, we evaluated a previously proposed model wherein the Pumilio RNA binding domain (RBD) binds Argonaute, which in turn blocks the translational activity of the eukaryotic elongation factor 1A. Surprisingly, we found that Argonautes are not necessary for repression elicited by Drosophila and human PUFs in vivo. A second model proposed that the RBD of Pumilio represses by recruiting deadenylases to shorten the mRNA's polyadenosine tail. Indeed, the RBD binds to the Pop2 deadenylase and accelerates deadenylation; however, this activity is not crucial for regulation. Rather, we determined that the poly(A) is necessary for repression by the RBD. Our results reveal that poly(A)-dependent repression by the RBD requires the poly(A) binding protein, pAbp. Furthermore, we show that repression by the human PUM2 RBD requires the pAbp ortholog, PABPC1. Pumilio associates with pAbp but does not disrupt binding of pAbp to the mRNA. Taken together, our data support a model wherein the Pumilio RBD antagonizes the ability of pAbp to promote translation. Thus, the conserved function of the PUF RBD is to bind specific mRNAs, antagonize pAbp function, and promote deadenylation.
INTRODUCTION
Protein expression is controlled at multiple levels, including translation and mRNA stability (Garneau et al. 2007; Jackson et al. 2010; Schwanhausser et al. 2011). For example, the efficiency of mRNA translation is promoted by the 5′ 7-methyl guanosine cap and the 3′ poly(A) tail, which are, respectively, recognized by the eukaryotic initiation factor complex, eIF4F, and the poly(A) binding protein, pAbp (Jackson et al. 2010). Enzymatic removal of the poly(A) tail (i.e., deadenylation) and 5′ cap (i.e., de-capping) can antagonize translation and initiate mRNA degradation (Goldstrohm and Wickens 2008; Li and Kiledjian 2010).
Translation and mRNA stability are controlled by interaction of trans-acting regulators with cis-acting RNA elements. A prototypical example of these regulators is the PUF family of proteins, named after founding members Drosophila melanogaster Pumilio and Caenorhabditis elegans FBF (Fem-3 Binding Factor) (Wickens et al. 2002). PUFs are present in all eukaryotes and share a conserved RNA binding domain (RBD) composed of eight repeated motifs. The RBD binds with high affinity and specificity to 8–10 nt regulatory sequences that are predominantly found in 3′ untranslated regions (UTRs) of mRNAs (Zamore et al. 1997, 1999; Wang et al. 2002; Lu et al. 2009). PUF binding sites are prevalent in the transcriptome and hundreds of mRNAs copurify with individual PUFs (Gerber et al. 2004, 2006; Galgano et al. 2008; Morris et al. 2008; Hafner et al. 2010). As a consequence, the impact of PUFs on gene expression is likely substantial. Analysis of the biological functions of PUFs supports this idea: they control diverse functions, including development, fertility, cell proliferation, and neurological processes (Lehmann and Nusslein-Volhard 1987; Lin and Spradling 1997; Zhang et al. 1997; Forbes and Lehmann 1998; Asaoka-Taguchi et al. 1999; Crittenden et al. 2002; Dubnau et al. 2003; Mee et al. 2004; Ye et al. 2004).
PUF proteins repress target mRNA expression by inhibiting translation and/or inducing mRNA degradation (Miller and Olivas 2011), but the mechanisms and cofactors involved remain to be fully elucidated. Our recent results revealed that human and Drosophila PUFs possess multiple domains that contribute to repression (Weidmann and Goldstrohm 2012). For all PUFs studied to date, the conserved RBD contributes to repression; therefore, we focused on dissecting the mechanism of repression by the RBDs of Drosophila Pumilio and human PUFs, PUM1 and PUM2. To do so, we used recently developed assays that specifically measure their ability to repress target mRNAs (Van Etten et al. 2012; Weidmann and Goldstrohm 2012).
Multiple mechanisms have been proposed to account for repression by the RBD. Initially, the repressive activity of the Pumilio RBD was thought to depend on two partners, Nanos and Brain Tumor; however, later results revealed that they are not essential for Pumilio-mediated repression (Weidmann and Goldstrohm 2012). Early research in multiple organisms found that PUF repression correlated with shortening of the poly(A) tail of target mRNAs (Ahringer et al. 1992; Wreden et al. 1997; Olivas and Parker 2000; Chagnovich and Lehmann 2001). Subsequently, the RBD of PUFs from Saccharomyces cerevisiae, Drosophila, C. elegans, and human were shown to bind orthologs of Pop2, a deadenylase enzyme that shortens the poly(A) tail of mRNAs in a 3′ to 5′ direction, indicating a conserved role for the RBD in deadenylase recruitment to target mRNAs (Goldstrohm et al. 2006; Kadyrova et al. 2007; Suh et al. 2009; Van Etten et al. 2012). In addition, Pop2 forms a heterodimer with another deadenylase, Ccr4, as a part of the Ccr4-Not deadenylase complex (Goldstrohm and Wickens 2008). These facts support a model in which the PUF RBD represses mRNAs by recruiting deadenylases to enhance the rate of poly(A) tail shortening. Consistent with this model, functional data from yeast and humans demonstrate that deadenylases contribute to the efficiency of PUF repression (Goldstrohm et al. 2006, 2007; Hook et al. 2007; Van Etten et al. 2012). Because the poly(A) tail plays a pivotal role in translation through the action of pAbp (Kuhn and Wahle 2004; Jackson et al. 2010), PUF-enhanced shortening of the poly(A) tail could reduce synthesis of the encoded protein and/or promote mRNA decay. In the research presented here, we measured the impact of deadenylases, the poly(A) tail, and poly(A) binding protein in the mechanism of repression by Pumilio.
A recent study proposed, based on biochemical data, that PUF RBDs can inhibit translation by blocking polypeptide elongation (Friend et al. 2012). The RBD of the C. elegans PUF, FBF, was found to bind the CSR-1 protein, one of 27 nematode Argonaute orthologs (Wedeles et al. 2013). Together, FBF and CSR-1 were reported to interact with the translation elongation factor, eEF1A, and inhibit its GTPase activity, which is essential for translation. This mechanism may apply to the RBD of human PUMs as well (Friend et al. 2012). Like FBF, PUM2 bound to Argonaute orthologs and eEF1A, and specific mutations of conserved phenylalanine and threonine residues were reported to disrupt PUM2 binding to eEF1A and Argonautes, respectively. In vitro translation assays using a rabbit reticulocyte extract provided functional evidence that the PUM2 RBD inhibits translation. Wild-type PUM2 RBD impeded translation, whereas PUM2 RBD mutants, defective for binding to eEF1A or Argonautes, had no repressive effect. Given that the amino acids that mediate interaction with eEF1A and Argonautes are conserved in the RBDs of PUFs (Fig. 1A), this could be a conserved mechanism (Friend et al. 2012). In this report, we examine the role of Argonaute proteins in PUF repression in vivo.
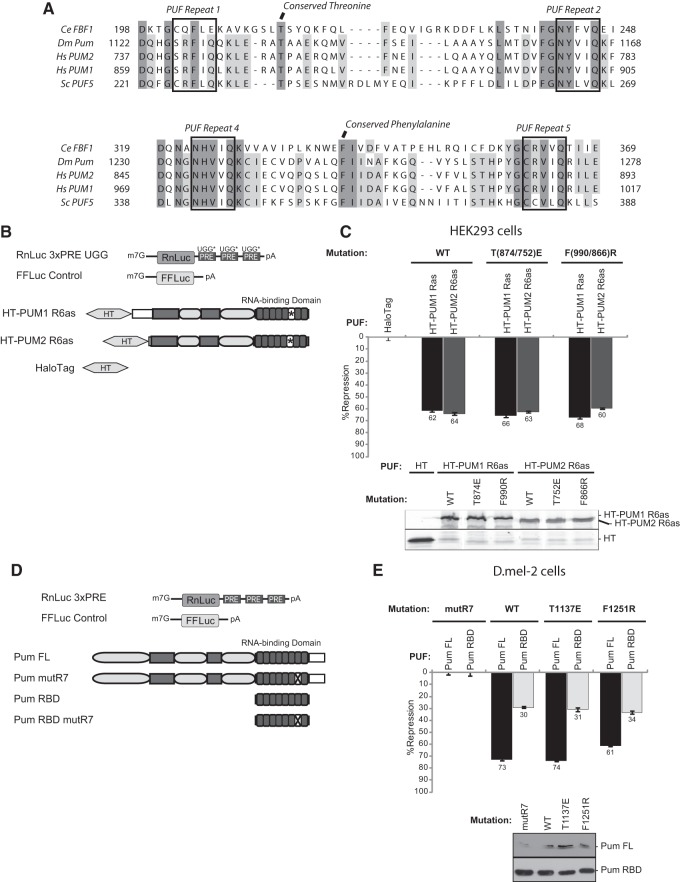
FIGURE 1.
Mutations in the Argonaute and eEF1A binding motifs do not alter PUF repression. (A) Alignment of Argonaute and eEF1A binding regions of PUFs from Caenorhabditis elegans (Ce FBF1), Drosophila melanogaster (Dm Pum), Homo sapiens (Hs PUM1 and PUM2), and Saccharomyces cerevisiae (Sc PUF5), reported by Friend et al. (2012). The conserved Argonaute and eEF1A binding amino acids are indicated at the top, and regions of complete and near complete conservation are highlighted in dark and light gray, respectively. RNA recognition motifs of each PUF repeat are also labeled. (B) Schematic of reporters and PUM constructs used in HEK293 cell experiments. Target Renilla luciferase reporter is labeled with UGG* to denote altered Pum response elements (PREs) in the 3′ untranslated region. A Firefly luciferase gene (FFLuc) served as an internal control for normalization. Human PUM1 and PUM2 were expressed as Halotag (HT) fusions and are marked with * in their RBDs to signify their altered specificity in PUF repeat 6 (R6as) which allows binding to UGG PREs, as previously described (Van Etten et al. 2012). Dark gray rectangles indicate regions of high conservation with Drosophila Pumilio, while light gray ovals represent the N-terminal repression domains (Weidmann and Goldstrohm 2012). Halotag alone served as the negative control. (C) Percent repression in human HEK293 cells of the RnLuc 3xPRE UGG reporter by human PUM1 R6as and PUM2 R6as test proteins. Note that the RnLuc 3xPRE UGG reporter is not regulated by endogenous PUM1 or PUM2 (Van Etten et al. 2012). The amino acid position of Argonaute-binding defective mutant (PUM1 T874E or PUM2 T752E) and eEF1A-binding defective mutant (PUM1 F990R and PUM2 F866R) is indicated at the top. Expression of each test protein was verified by fluorescent detection of TMR-labeled Halotag fusions. (D) Representation of constructs used in D.mel-2 cell experiments. The Renilla reporter 3′ UTR contains three wild-type PREs. Pumilio (Pum) constructs include full length (FL) and the RNA binding domain (RBD). Dark gray boxes mark regions of increased conservation, and light gray ovals denote N-terminal repression domains. The X in negative control Pum mutR7 constructs represents mutations in the RNA recognition amino acids of the seventh PUF repeat which block RNA binding and repression (Weidmann and Goldstrohm 2012). (E) Percent repression of RnLuc 3xPRE reporter by wild-type (WT), Argonaute-binding defective (T1137E), and eEF1A binding-defective (F1251R) Pum FL and Pum RBD test constructs in D.mel-2 cells. Western blot detection of test proteins using anti-V5 antibody is shown at the bottom. In all graphs, mean values derived from four replicates are plotted along with the standard error.
In the present study, we scrutinized the mechanisms that underlie repression mediated by the RBDs of Drosophila and human PUFs. Our results indicate that the PUF-Argonaute interactions are not required for PUF-mediated repression of protein expression. Instead, we find repression by the Pumilio RBD is completely dependent on the poly(A) tail. The RBD promotes deadenylation, dependent on the Pop2 and Ccr4 deadenylase enzymes, and interacts with Pop2. However, while blocking deadenylation stabilizes the mRNA, it does not prevent repression of protein synthesis. We find that the crucial mechanism of RBD-mediated repression depends on the poly(A) binding protein, pAbp. Consistent with these observations, the Pumilio RBD associates with pAbp. Together, our data support a mechanism wherein the Pumilio RBD targets pAbp to interfere with its ability to promote translation. The RBD does not displace pAbp from the mRNA but instead antagonizes its ability to promote translation. Upon triggering repression of protein synthesis, deadenylation occurs as a subsequent effect. Finally, our data reveal that the additional Pumilio repression domains inhibit protein expression by a pAbp- and poly(A)-independent mechanism.
RESULTS
Mutations in the Argonaute and eEF1A binding motifs do not alter PUF repression
We first tested the requirement of a PUF-Argonaute-eEF1A interaction using a mutational approach and cell-based reporter assays that we previously developed to measure PUF repression by Drosophila and human PUFs (Van Etten et al. 2012; Weidmann and Goldstrohm 2012). A PUF-responsive reporter gene was created by inserting three copies of the Pumilio Response Element (PRE) into the 3′ UTR of a Renilla luciferase gene to create RnLuc 3xPRE (Fig. 1B). Mutations within the RBD of C. elegans FBF and human PUM2 have been identified that abolish binding to Argonaute and eEF1A but do not affect RNA binding, as measured by in vitro binding assays (Friend et al. 2012). Alignment of C. elegans, human, Drosophila, and S. cerevisiae PUFs revealed that a threonine residue required for Argonaute-binding and the phenylalanine residue required for eEF1A-binding are conserved throughout PUFs (Fig. 1A; Friend et al. 2012). Interestingly, the Argonaute-binding threonine is conserved in S. cerevisiae PUFs, though Argonautes are not present in this species (Meister 2013). Conservation of these residues in Drosophila and human PUFs indicated that our functional assay could be used to assess their roles in repression. We created mutant versions of human PUM1 and PUM2 that correspond to the previously identified mutations that disrupt binding to Argonautes (T874E of PUM1; T752E of PUM2) or eEF1A (F990R of PUM1; F866R of PUM2) and tested their ability to repress the Renilla luciferase reporter in human cells (Friend et al. 2012). Because endogenous PUM1 and PUM2 in HEK293 cells repress the RnLuc 3xPRE reporter, we modified the PRE sequences (Fig. 1B, RnLuc 3xPRE UGG) so that only exogenously introduced PUF proteins with altered RNA binding specificity (Fig. 1B, PUM1 R6as or PUM2 R6as) can regulate the reporter, as previously described (Van Etten et al. 2012). When expressed in human cells, the altered specificity PUM1 and PUM2 repressed the RnLuc 3xPRE UGG reporter by 62% and 64%, respectively, relative to the negative control, Halotag (Fig. 1C). When mutations in the Argonaute or eEF1A binding sites were introduced into the altered specificity PUFs (PUM1 T874E or F990R; PUM2 T752E or F866R), their capacity for repression was not compromised (Fig. 1C), indicating that these binding interfaces are not required for repression by human PUMs in vivo.
We next assessed the role of Argonaute and eEF1A binding residues in repression by Drosophila Pumilio. The level of endogenous Pumilio in Drosophila D.mel-2 cells is insufficient to efficiently repress RnLuc 3xPRE (Fig. 1D), yet, moderate overexpression of Pumilio causes repression (Van Etten et al. 2012; Weidmann and Goldstrohm 2012). This system provides an excellent means of studying Pumilio structure and function. Full-length Drosophila Pumilio (Fig. 1D, Pum FL) potently repressed the PRE-bearing reporter, whereas the Pum RBD repressed to a lesser degree (Weidmann and Goldstrohm 2012). Mutations that inactivate Pumilio RNA binding activity (Fig. 1D, Pum mutR7) or change the PRE completely blocked repression (Weidmann and Goldstrohm 2012). Using this approach, we tested the activity of Pumilio with mutations in the predicted Argonaute (T1137E) and eEF1A (F1251R) binding residues by measuring repression of RnLuc 3xPRE (Fig. 1D). Full-length Pum T1137E retained the repression activity of wild-type Pum (Fig. 1E, Pum FL WT at 73% repression vs. Pum FL T1137E at 74% repression), and Pum F1251R repressed at slightly below wild-type level (Fig. 1E, Pum FL F1251R, 61% repression). We considered that the repressive activity of Pumilio's RBD might be obscured by additional repression domains that we previously identified in the amino terminus of the protein (Weidmann and Goldstrohm 2012). Therefore, we tested the effect of these mutations on the activity of the RBD (Fig. 1D). Wild-type Pum RBD, RBD T1137E, and RBD F1251R all repressed between 30% and 34% (Fig. 1E). We conclude that these conserved residues are not necessary for repression by full-length Pumilio or the conserved RBD.
Argonaute associates with human and Drosophila PUFs
The lack of detectable functional impact of mutation of the Argonaute binding residues of Drosophila and human PUFs compelled us to assess whether the mutations did, indeed, prevent this association. HEK293 cells were transfected with FLAG-tagged AGO1 and Halotag fusions of either wild- type or the T752E mutant PUM2. AGO1 was selected because it was reported to bind strongly (Friend et al. 2012). Halotag alone was used as a negative control, and a Halotag fusion of CNOT6L, a deadenylase known to associate with Argonaute served as a positive control (Fabian et al. 2009). Complexes were purified using a FLAG antibody from RNase-treated cell extracts. PUM2 and CNOT6L coimmunoprecipitated with FLAG-AGO1 but were not detected in the mock eluates (Fig. 2A, cf. lanes 14 and 16 to lanes 10 and 12). These data are consistent with biochemical data of Friend et al. (2012), but, surprisingly, we detect robust association between the PUM2 T752E mutant and AGO1 (Fig. 2A, lane 15).
FIGURE 2.
Argonaute associates with human and Drosophila PUFs. (A) Human PUM2 coimmunoprecipitates with Argonaute 1 (AGO1) from RNase-treated HEK293 cell extracts. Western blotting detected FLAG-tagged AGO1 in the inputs and eluates from anti-FLAG immunoprecipitations. Halotag (HT) or Halotag fused to wild-type or mutant (T752E) PUM2 or CNOT6L were detected by covalent labeling of Halotag with fluorescent TMR ligand. As a negative control, FLAG immunoprecipitations were performed from extracts that expressed Halotag prey proteins but not FLAG-AGO1. (B) Drosophila Argonaute 2 (Ago2) associates with the RNA binding domain (RBD) of Pumilio. Halotag alone or Halotag-Pum RBD fusions with V5 epitope tags were coexpressed in D.mel-2 cells with FLAG-tagged Ago2 or, as a negative control, myc-tagged Lsm11. Halotag pulldowns were performed from cell extracts, and bound proteins were eluted via TEV protease cleavage. Western blots of input cell extracts and Halotag eluates were probed with V5 antibody to detect wild-type or mutant (T1137E) Pum RBD. FLAG antibody Western blots detected Ago2, and a myc antibody Western blot detected Lsm11.
We also assessed binding of Drosophila Pumilio to Argonaute and the effect of the equivalent T1137E mutation. V5 epitope-tagged Pum RBD, fused to Halotag, was purified from RNase-treated D.mel-2 cell extracts that coexpressed FLAG-tagged Ago2 and the negative control protein, myc-tagged Lsm11. After washing, bound complexes were eluted by cleavage of the Halotag-Pum RBD fusion with TEV protease. We found that Ago2 coeluted with Pum RBD, whereas Lsm11 did not (Fig. 2B, lane 5). Interestingly, this was true for Drosophila Ago2, but no interaction between Pum and Ago1 was detected (data not shown). This result provides evidence that Drosophila Pum, like C. elegans FBF and human PUM2, associates with Argonaute. However, the Pum T1137E mutation did not disrupt binding of Pum to Ago2 (Fig. 2B, lane 6). Further, an alanine substitution, T1137A, also possessed wild-type repression activity and retained the ability to bind Argonaute (data not shown). In summary, our data show that the mutations reported to abrogate PUF interaction with Argonaute had no effect on repression or association with Argonaute.
The repression and Argonaute binding activities of the Pumilio RNA binding domain can be separated
Because mutation of the residues purported to mediate PUF binding to Argonaute did not, in fact, prevent the interaction, we sought an alternative way to assess the functional relevance to Pumilio repression. The tethered function assay provided an ideal means to dissect the regions of the RBD necessary for Argonaute binding and repression (Coller and Wickens 2002). In this assay, regions of Pumilio were fused to the phage protein MS2, which binds specific RNA stem–loop structures in the 3′ UTR of a Renilla reporter gene (Fig. 3A, RnLuc MS2). The regions of the RBD necessary for association with Ago2 and for repression of RnLuc MS2 were mapped via a series of truncations with C-terminal V5 epitope tags (Fig. 3A). These proteins were expressed in D.mel-2 cells with FLAG-tagged Ago2. Anti-FLAG immunoprecipitations were then performed from RNase-treated cell extracts. The RBD and a C-terminal truncation (ΔC) both copurified with FLAG-Ago2 (Fig. 3B, lanes 6 and 7), but the ΔR1-2, ΔR4-5, and ΔR7-8 deletions of the PUF repeats prevented the RBD-Ago2 interaction (Fig. 3B, lanes 8–10), indicating that multiple PUF repeats are necessary to contact Ago2.
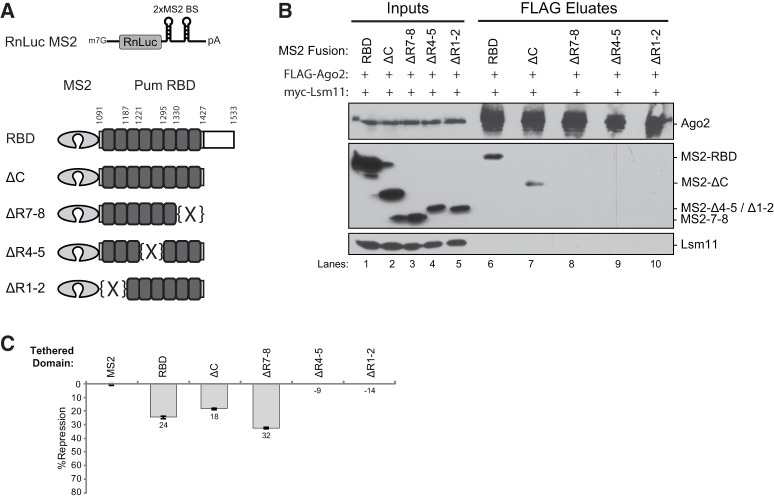
FIGURE 3.
The repression and Argonaute binding activities of the Pumilio RNA binding domain can be separated. (A) Diagrams of the tethered function reporter and Pum RBD constructs are shown. The RnLuc MS2 reporter contains two binding sites recognized by the MS2 RNA binding protein (2xMS2 BS) in its 3′ untranslated region. The RBD and depicted truncations were fused to the MS2 RNA binding protein. Amino acid numbers where truncations and PUF repeat deletions were made are indicated. (B) Western blots of anti-FLAG immunoprecipitations (eluates) of FLAG-tagged Ago2 from RNase-treated D.mel-2 cell extracts (Inputs). Pum RBD constructs were detected by anti-V5 Western blot. The negative control Lsm11 was coexpressed and detected by anti-myc Western blotting. (C) Tethered function reporter assays measured repression by Pum RBD constructs in D.mel-2 cells. Percent repression, calculated relative to MS2 negative control, is graphed. Mean values derived from four replicates are plotted along with the standard error.
Having identified Pum truncations that no longer bind Ago2, we next tested their repressive activity in the tethered function assay (Fig. 3C). We found that the RBD, ΔC, and ΔR7-8 each repressed the RnLuc MS2 reporter; however, neither ΔR4-5 nor the ΔR1-2 were active (Fig. 3C). Expression of each protein was verified by Western blotting (Fig. 3B, lanes 1–5). Most significantly, the tethered region lacking PUF repeats 7 and 8 (ΔR7-8) was active for repression though it did not bind Ago2 (Fig. 3B,C). Based on this analysis, we can conclude that the interaction between Pum and Ago2 is dispensable for repression by the RBD.
Depletion of Argonaute proteins does not hinder PUF repression in vivo
We further tested the requirement for Argonautes in repression by Pumilio by depleting Argonautes using RNA interference (RNAi). Efficient RNAi was demonstrated by the depletion of overexpressed Ago1 and Ago2 (Fig. 4A). We also confirmed RNAi depletion of endogenous Argonautes by quantitative reverse transcriptase coupled with polymerase chain reaction (qRT-PCR); Ago1 and Ago2 mRNA levels were depleted by up to 70% and 86%, respectively (Fig. 4B). In cells treated with nontargeting control dsRNA (NTC), Pum FL repressed RnLuc 3xPRE mRNA by 70%, and the Pum RBD repressed by 24% (Fig. 4B, NTC), consistent with our previous findings (Weidmann and Goldstrohm 2012). Depletion of either Argonaute, Ago1 or Ago2, did not affect repression by Pum FL or the Pum RBD (Fig. 4B, Ago1, Ago2) nor did simultaneous knockdown of both Ago1 and Ago2 (Fig. 4B, Ago1/Ago2). Therefore, depletion of Argonautes does not affect Pumilio repression in vivo.
FIGURE 4.
Depletion of Argonaute proteins does not hinder PUF repression in vivo. (A) Efficient depletion of Argonautes using dsRNAs to Ago1 or Ago2 was demonstrated via knockdown of FLAG-tagged proteins in D.mel-2 cells. Western blots were performed using anti-FLAG antibody and, as a loading control, anti-Actin. Nontargeting control (NTC)-treated extracts served as a control. Four times the amount of Ago-depleted extracts were loaded to highlight knockdown efficiency. (B) Percent repression of RnLuc 3xPRE by Dm Pum FL or Dm Pum RBD was measured in D.mel-2 cells. RNAi was performed using dsRNAs to Ago1, Ago2, or both Ago1 and Ago2. Negative control RNAi was performed using the NTC dsRNA. In each condition, percent repression by Pum FL or RBD was calculated relative to the respective RNA binding-defective mutant controls, Pum FL mutR7 or RBD mutR7. RNAi depletion of endogenous Argonaute mRNAs was verified via qRT-PCR, and the percent depletions relative to the NTC are indicated at the bottom of the panel. (C) Percent repression of RnLuc 3xPRE by human PUM1 was measured in D.mel-2 cells depleted of Ago1, Ago2, or both by RNAi. Repression by PUM1 was calculated relative to the negative control, empty expression vector. As in B, RNAi depletion of endogenous Argonaute mRNAs was verified by qRT-PCR. (D) Depletion of Pum, but not Ago1 and Ago2, alleviates repression of an mRNA with the Hunchback (Hb) 3′ untranslated region. Fold change in expression of the RnLuc reporter with the Hb 3′ UTR reporter was measured in D.mel-2 cells treated with NTC dsRNA, dsRNA targeting endogenous Pum, or dsRNAs targeting Ago1 and Ago2. Pum Response Elements 1 and 2 (PRE1 and PRE2) are binding sites for Pumilio and are also known as Nanos Response Elements. Reporter expression was normalized to a Firefly luciferase control and fold change was calculated relative to the NTC sample. In all experiments, mean values derived from four replicates are plotted with the standard error.
We also measured the effect of Argonaute depletion on the repressive activity of the human PUM1 in D.mel-2 cells. PUM1 achieved 53% repression in cells treated with the nontargeting control dsRNA (Fig. 4C), consistent with our previous observation (Van Etten et al. 2012). Efficient depletion of Ago1, Ago2, or both did not alleviate PUM1-mediated repression (Fig. 4C). Thus, like Drosophila Pumilio, human PUM1 is able to efficiently repress a PRE-containing mRNA when Argonautes are depleted. It is notable that, because eEF1A is an essential translation factor, it was not feasible to test its role in PUF repression using RNAi; however, because the eEF1A interaction with the RBD was Argonuate-dependent (Friend et al. 2012) and Argonaute is not necessary, this point is likely inconsequential.
Finally, we measured the effect of Argonaute depletion on the regulation of an mRNA bearing the 3′ UTR from a natural Pumilio target. Well-characterized targets of Pumilio, such as the mRNA encoding the morphogen Hunchback, are not expressed in D.mel-2 cells; therefore, we appended the 3′ UTR of the Hunchback mRNA to a Renilla luciferase reporter (Fig. 4D, RnLuc Hb 3′ UTR). To assess the effect of endogenous Pumilio and Argonaute on RnLuc Hb 3′ UTR expression, we performed RNAi with nontargeting control dsRNA, or dsRNA targeting Pum, or dsRNA targeting both Ago1 and Ago2. When Pum was depleted, the RnLuc Hb 3′ UTR reporter expression increased by about twofold (Fig. 4D). In contrast, no effect on RnLuc Hb 3′ UTR expression was detected when Argonautes were depleted (Fig. 4D). We conclude that endogenous Pumilio represses the Hb 3′ UTR reporter, but Argonautes are not required for this effect.
The RBD of Pumilio enhances deadenylation dependent on the Pop2 and Ccr4 deadenylases
The data reported above demonstrate that interaction with Argonautes is not necessary for RBD-mediated repression. Consequently, alternative mechanisms(s) must account for the observed PUF-mediated repression in vivo. In several model systems PUF repression correlates with shortening of the poly(A) tail of target mRNAs; therefore, we next measured the effect of the Pum RBD on deadenylation (Ahringer et al. 1992; Wreden et al. 1997; Olivas and Parker 2000; Chagnovich and Lehmann 2001; Gamberi et al. 2002; Goldstrohm et al. 2006). We investigated the ability of the wild-type and mutR7 Pum RBD to affect poly(A) tail length of the RnLuc 3xPRE reporter using a transcriptional shutoff strategy (Fig. 5A). RNA samples were collected at specific time points following inhibition of synthesis with actinomycin D. To measure poly(A) tail length, the 3′ end of the RnLuc 3xPRE mRNA was liberated by RNase H cleavage with a specific antisense DNA oligonucleotide and detected by Northern blotting. Inclusion of a polythymidine oligonucleotide in this reaction removed the poly(A) tail, serving as a marker (Fig. 5A, dT).
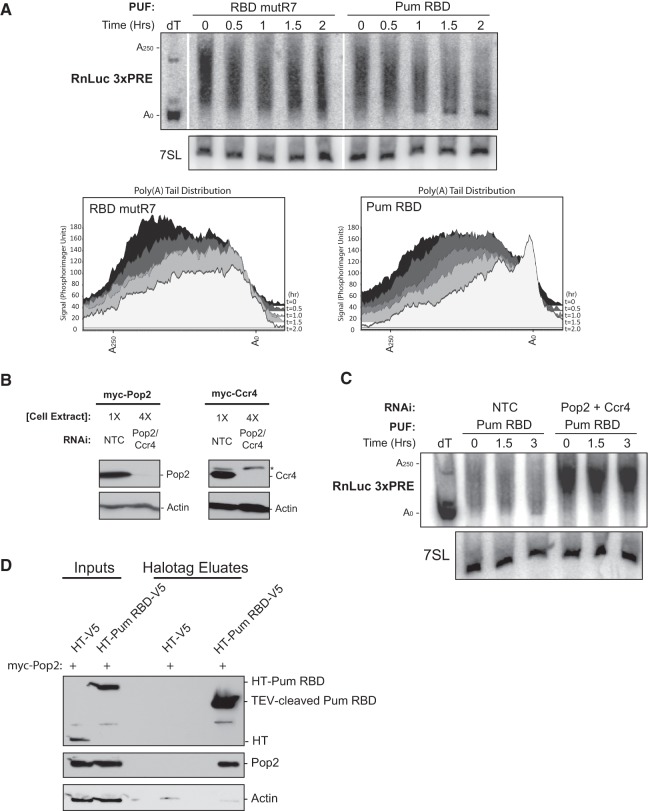
FIGURE 5.
The RBD of Pumilio enhances deadenylation dependent on the Pop2 and Ccr4 deadenylases. (A) (Top) Denaturing polyacrylamide Northern blotting of RNA prepared from D.mel-2 cells over a time course following addition of actinomycin D. Poly(A) shortening of the RnLuc 3xPRE mRNA was induced by wild-type Pum RBD or, as a negative control, the RNA binding-defective RBD mutR7. To resolve the poly(A) tail, the RnLuc 3xPRE mRNA was cleaved using a DNA oligo that is complementary to RnLuc and RNase H. A deoxy-thymidine oligo (dT) was also added to one reaction to remove the poly(A) tail (A0). Northern blot was probed to detect the RnLuc 3xPRE 3′ cleavage product and the internal control 7SL, a stable noncoding RNA. Length of poly(A) tails are marked on the left. (Bottom) Phosphorimager quantification of poly(A) length distribution for each lane from the Northern blot. (B) Western blotting of myc-tagged Pop2 and Ccr4 depletion by RNAi in D.mel-2 cells. Four times the amount of Pop2- and Ccr4-depleted extract was loaded relative to the NTC extracts to highlight knockdown efficiency. Myc antibody was used for Pop2 and Ccr4 detection. Western blot of actin served as a loading standard. A nonspecific band was also detected by the myc antibody in all cell extracts (*). (C) Denaturing polyacrylamide Northern blotting of RnLuc 3xPRE from D.mel-2 cells expressing Pum RBD and treated with either nontargeting control (NTC) dsRNA or both Pop2 and Ccr4 dsRNAs. (D) Western blotting of Halotag pulldown assay demonstrating association of Pop2 with Pumilio RBD. V5-tagged Halotag (HT-V5) alone or Halotag-Pum RBD (HT-Pum RBD-V5) fusions were coexpressed with myc-tagged Pop2. Cell extracts (Inputs) were treated with RNases. HaloLink-bound complexes were eluted with TEV protease (Eluates). Blots were probed with V5 antibody to detect Pum RBD or with myc antibody to detect Pop2. An actin Western blot served as a negative control.
At the initial time point, a heterogeneous population of poly(A) tails were present, including new mRNAs with long poly(A) tails of up to 250 nt, in addition to intermediates at various stages of deadenylation (Fig. 5A, time = 0). Over the 2-h time course, we observed that deadenylation of RnLuc 3xPRE progressed relatively slowly in the presence of RBD mutR7 (Fig. 5A). In contrast, when the wild-type Pum RBD was present, the poly(A) tail of RnLuc 3xPRE was more rapidly shortened, with the majority of RnLuc 3xPRE mRNA having almost no poly(A) tail after 2 h (Fig. 5A). In the last three time points (1, 1.5, 2 h), the deadenylated RnLuc 3xPRE intermediate accumulated (Fig. 5A, Pum RBD). As an internal control, the levels of nonadenylated 7SL RNA were unchanged over the time course that samples were collected (Fig. 5A, 7SL). These results demonstrate that the RBD of Pum is sufficient to accelerate deadenylation of the reporter mRNA.
In yeast, PUF-mediated deadenylation depends on the Pop2-Ccr4 deadenylase heterodimer, wherein the PUF directly interacts with the Pop2 subunit (Goldstrohm et al. 2006, 2007; Hook et al. 2007). This interaction is thought to be a conserved feature of PUF repression, because orthologs of PUFs and Pop2 from C. elegans and humans have been reported to bind each other (Goldstrohm et al. 2006; Suh et al. 2009; Van Etten et al. 2012). Furthermore, in vitro evidence indicates that Drosophila Pum binds to Pop2 (Kadyrova et al. 2007). We first asked whether RNAi depletion of Pop2 and Ccr4 (Drosophila Twin) would affect Pum RBD-promoted deadenylation of the RnLuc 3xPRE mRNA. We confirmed depletion of epitope-tagged Pop2 and Ccr4 by Western blotting (Fig. 5B). Next, Northern blotting was performed to measure the effect of deadenylase depletion on the poly(A) tail of RnLuc 3xPRE mRNA. In cells treated with nontargeting control RNAi, the reporter was deadenylated (Fig. 5C, NTC); however, depletion of Pop2 and Ccr4 prevented the ability of Pum RBD to accelerate deadenylation (Fig. 5C, Pop2 + Ccr4). In fact, deadenylation was completely blocked: the RnLuc 3xPRE mRNA was stabilized with a long poly(A) tail throughout the 3-h time course. These data indicate that the Pop2-Ccr4 deadenylase complex is necessary for Pum RBD-mediated deadenylation of RnLuc 3xPRE.
Given the observations that deadenylation of the Pum reporter mRNA depends on Pop2-Ccr4 deadenylase, we wished to confirm that Pum binds to the Pop2 subunit of the deadenylase complex. To do so, the Pum RBD was fused to Halotag and the V5 epitope (HT-RBD-V5) and coexpressed in D.mel-2 cells with myc-tagged Pop2. As a negative control, Pop2 was also coexpressed with Halotag-V5 alone (HT-V5). Expression of HT-V5, HT- RBD-V5, and the myc-Pop2 protein was confirmed in the cell extracts (Fig. 5D, Inputs). Halotag proteins were affinity-purified from RNase-treated extracts, and the bound proteins were eluted with TEV protease and detected by Western blotting. Pop2 substantially coeluted with the Pum RBD (Fig. 5D, HT-Pum RBD-V5) but was not detected in the Halotag control eluate (Fig. 5D, HT-V5). As a negative control, we probed for actin protein and found no enrichment by HT-RBD (Fig. 5D). These results demonstrate that the Pum RBD associates with Pop2. The fact that the association was maintained in the presence of RNases indicates that RNA does not mediate the interaction. Together with the data demonstrating that the RBD promotes deadenylation, the interaction with Pop2 suggests that the Pum RBD may recruit the deadenylase to the target mRNA to enhance deadenylation.
A poly(A) tail is necessary for repression by the Pumilio RNA binding domain
The observation that the RBD of Pum accelerated deadenylation indicated that poly(A)-dependent regulation may be the mechanism by which it represses protein expression. If so, then repression should depend on the presence of a poly(A) tail. To test this idea, we compared Pum-mediated repression of a reporter bearing a poly(A) tail to one that lacks poly(A). To create a nonadenylated 3′ end, the cleavage/polyadenylation elements of the RnLuc 3xPRE reporter were replaced by a histone stem–loop (HSL), which is processed by a unique 3′ end formation pathway (Fig. 6A; Marzluff et al. 2008). Consistent with earlier observations, full-length Pum repressed the RnLuc 3xPRE pA reporter by 79%, and the Pum RBD repressed by 19% (Fig. 6B, 3xPRE pA: Pum FL and Pum RBD). In contrast, repression of the nonadenylated RnLuc 3xPRE HSL reporter by Pum FL was diminished to 36% (Fig. 6B, 3xPRE HSL). Strikingly, the RBD was unable to repress an HSL reporter (Fig. 6B, 3xPRE HSL:Pum RBD) and instead promoted expression—the basis of this effect is currently unknown. We conclude that the 3′ poly(A) tail is necessary for repression by the Pum RBD. Consistent with this result, repression by full-length Pum is reduced in the absence of a poly(A) tail, reflecting the loss of repression by its RBD. The remaining poly(A) independent repressive activity of full-length Pum likely emanates from the N-terminal repression domains (Weidmann and Goldstrohm 2012).
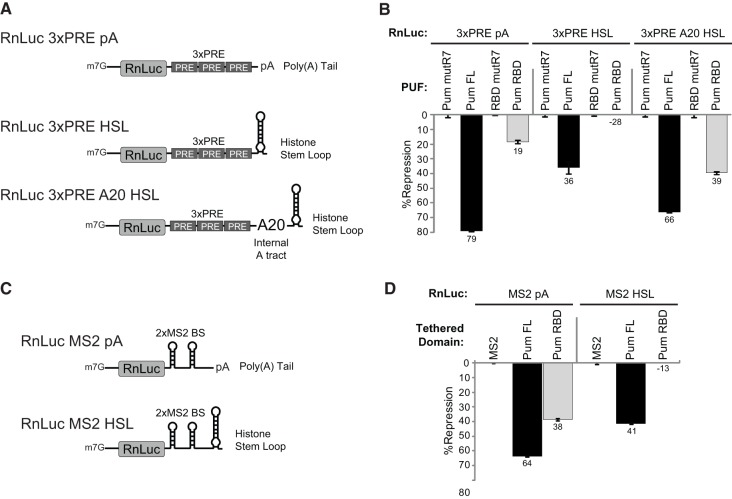
FIGURE 6.
A poly(A) tail is necessary for Pum RBD-mediated repression. (A) Diagrams represent Renilla luciferase (RnLuc) reporters with polyadenylated or nonadenylated 3′ ends. The RnLuc 3xPRE pA reporter contains three PUF response elements (PREs) and a poly(A) tail generated by an efficient cleavage/polyadenylation element. The RnLuc 3xPRE HSL reporter has a nonadenylated 3′ end generated by histone stem–loop (HSL) processing elements. RnLuc 3xPRE A20 HSL has an internal polyadenosine tract of 20 nt (A20) between the PREs and HSL. (B) Percent repression of the RnLuc 3xPRE reporters by full-length Pum FL and Pum RBD in D.mel-2 cells. Percent repression by Pum FL and RBD was calculated relative to Pum FL mutR7 and RBD mutR7, respectively. (C) RnLuc reporters with two MS2 protein binding sites were used for tethered function assays, including polyadenylated RnLuc MS2 pA and nonadenylated RnLuc MS2 HSL. (D) Percent repression of each MS2 reporter by MS2 protein fusions of Pum FL or Pum RBD. Percent repression was calculated relative to the negative control MS2 protein. All graphs show mean values with the standard error from four replicate samples.
The requirement of the poly(A) tail for repression can be interpreted in several ways. Pum RBD could repress by promoting shortening of the poly(A) tail, thereby reducing translation and/or mRNA stability. Alternatively, Pum RBD could interfere with the function of poly(A) binding protein, which coats the poly(A) tail and promotes translation initiation. To discriminate between these models, we created a new reporter mRNA with a nonadenylated HSL 3′ end and an internal poly(A) tract (Fig. 6A, RnLuc 3xPRE A20 HSL). The internal poly(A) tract is 20 nt long, which is sufficient to bind at least one molecule of pAbp (Kuhn and Wahle 2004). Importantly, because deadenylases are 3′ exoribonucleases that do not degrade internal poly(A) tracts (Goldstrohm and Wickens 2008), the RnLuc 3xPRE A20 HSL mRNA is not subject to deadenylation. Pum FL repressed the RnLuc 3xPRE A20 HSL mRNA by 66%, which is similar in magnitude to RnLuc 3xPRE pA with a normal 3′ poly(A) tail (Fig. 6B). Pum RBD repressed RnLuc 3xPRE A20 HSL by 39%, demonstrating that the internal poly(A) tract restored and strengthened repression (Fig. 6B). We conclude that Pum RBD repression depends on the presence of poly(A). These results suggest that RBD-mediated repression may require co-occupancy of the mRNA by Pum RBD and pAbp.
We also considered the possibility that poly(A) could promote repression by facilitating RNA-binding by Pum, perhaps mediated by pAbp. If so, then strengthening binding of Pum to the RNA should overcome the poly(A) requirement. Pum binds to the PRE with a dissociation constant in the low nano-molar range (Zamore et al. 1997, 1999). To strengthen this interaction, we utilized a modified MS2 coat protein and binding site interaction with an order of magnitude stronger binding (Lim et al. 1994; Johansson et al. 1998). Two reporters were used in this analysis: RnLuc MS2 pA—the 3′ poly(A) tail was generated using efficient cleavage/polyadenylation elements, whereas in the RnLuc MS2 HSL reporter, a nonadenylated 3′ end was generated by the HSL (Fig. 6C). The results corroborate those described for the 3xPRE reporter. When tethered, Pum FL repressed the RnLuc MS2 HSL reporter less efficiently (41%) than the RnLuc MS2 pA reporter (64%) (Fig. 6C). Importantly, tethered Pum RBD repressed the polyadenylated reporter by 38%, whereas repression of the HSL reporter was completely alleviated (Fig. 6D) and, as observed for the 3xPRE HSL reporter, Pum RBD slightly enhanced expression of RnLuc MS2 HSL. We conclude that poly(A), in the form of a 3′ tail or an internal poly(A) tract, is necessary for repression by the Pum RBD and contributes to the full magnitude of repression by the full-length Pumilio protein. Moreover, strengthening the association of Pum RBD with the mRNA did not lessen the poly(A) dependence, suggesting that poly(A) functions beyond facilitating the RBD-mRNA interaction.
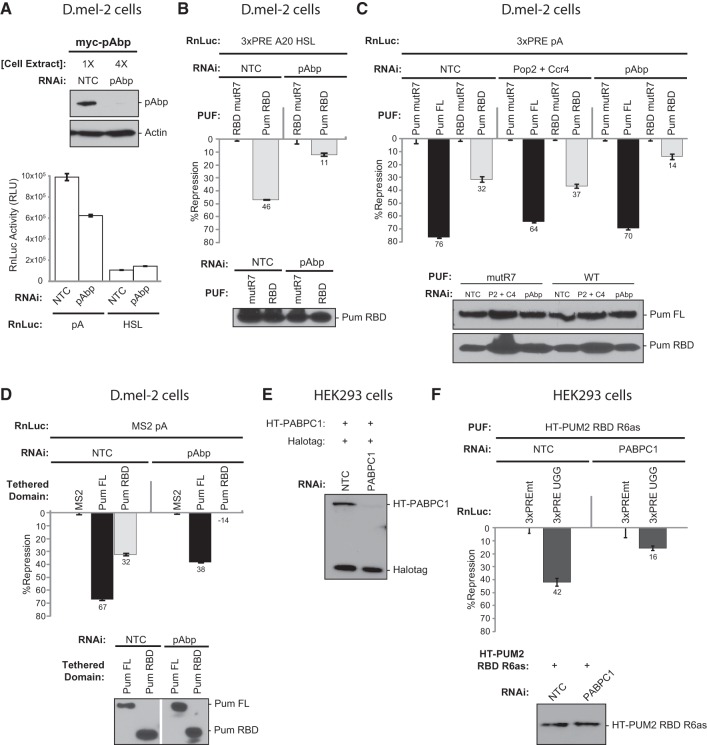
Poly(A) binding protein is necessary for repression by Drosophila and human PUF RNA binding domains
Having found that poly(A) is necessary for repression by the Pum RBD, we wished to determine if this property is mediated by pAbp. To do so, we depleted pAbp using RNAi. Depletion of epitope-tagged pAbp protein was confirmed by Western blotting (Fig. 7A). In addition, using qRT-PCR, we measured an 84% decrease of endogenous pAbp mRNA relative to the negative control RNAi. Next, we confirmed that depletion of pAbp reduced poly(A)-stimulated translation. Indeed, polyadenylated Renilla luciferase reporter expression was reduced by 40% relative to nontargeting control RNAi (Fig. 7A, RnLuc pA), consistent with pAbp's general role in promoting translation. Importantly, reporter luciferase activity remained more than three orders of magnitude above background, permitting measurement of Pumilio activity in subsequent experiments. As an additional control, we measured the effect of pAbp depletion on a nonadenylated RnLuc HSL reporter. Knockdown of pAbp did not reduce luciferase activity (Fig. 7A, RnLuc HSL), consistent with the fact that HSL translation does not utilize pAbp (Marzluff et al. 2008).
FIGURE 7.
Poly(A) binding protein is necessary for repression activity of the PUF RBD. (A) In the top panel, Western blotting demonstrated efficient RNAi depletion of myc-tagged pAbp from D.mel-2 cells. Four times the amount of pAbp-depleted extract was loaded relative to the nontargeting control (NTC)-treated extracts. Myc antibody was used to detect myc-pAbp, while an actin antibody was used to illustrate differences in loading. At the bottom, a graph of Renilla luciferase reporter activity, in relative light units (RLU), is plotted as measured in nontargeting control- or pAbp dsRNA-treated cells. Reporters included polyadenylated RnLuc (pA) and RnLuc HSL, with a nonadenylated histone stem–loop. (B) Percent repression of RnLuc 3xPRE A20 HSL reporter by the Pum RBD in D.mel-2 cells treated with dsRNA to pAbp or NTC dsRNA. Percent repression was calculated relative to the RBD mutR7 negative control. (C) Percent repression of RnLuc 3xPRE pA reporter by Pum FL and Pum RBD in D.mel-2 cells treated with NTC, Pop2 and Ccr4, or pAbp dsRNAs. Percent repression was calculated relative to the negative control Pum FL mutR7 and RBD mutR7. (D) Percent repression of RnLuc MS2 pA reporter by MS2 alone, a MS2-Pum FL fusion, and a MS2-Pum RBD fusion in D.mel-2 cells treated with NTC or pAbp dsRNAs. Percent repression by tethered constructs was calculated relative to MS2 alone. (E) Western blotting, using anti-Halotag antibody, demonstrating efficient RNAi depletion of human PABPC1, fused to Halotag (HT-PABPC1), in HEK293 cells that coexpressed an internal control Halotag protein. (F) Percent repression of RnLuc 3xPRE UGG reporter by human PUM2 RBD R6as, with altered RNA binding specificity, in HEK293 cells treated with either nontargeting control or PABPC1 siRNAs. Percent repression of the RnLuc 3xPRE UGG reporter was calculated relative to the negative control reporter, RnLuc 3xPREmt, wherein the PREs are inactivated by mutations. For each experiment, Western blotting confirmed expression of the PUF test constructs under each RNAi condition. All graphs represent mean values with standard errors calculated from four replicate samples, with the exception of panel F, which used three replicate samples.
To evaluate the role of pAbp in Pum repression, we first took advantage of the ability of the Pum RBD to repress the RnLuc 3xPRE A20 HSL mRNA, which bears an internal A20 tract and a nonadenylated 3′ end (Fig. 6A). This reporter permits analysis of the effect of pAbp independent of potential interplay with deadenylation. As shown in Figure 7B, the Pum RBD repressed the RnLuc 3xPRE A20 HSL by 46% in the control sample. When pAbp was depleted, repression was substantially diminished to 11% (Fig. 7B). This residual repression may result from incomplete pAbp depletion. We conclude that poly(A) tract-dependent repression by Pum RBD requires pAbp.
We next evaluated the roles of Pop2 and Ccr4 deadenylases and pAbp by depleting each protein using RNAi and measuring the effect on repression of the RnLuc 3xPRE pA reporter. Simultaneous knockdown of Pop2 and Ccr4 did not prevent repression by full-length Pum, but the activity was reduced from 74% to 64% (Fig. 7C). In contrast, depletion of Pop2 and Ccr4 did not alleviate repression by the RBD; indeed, it was slightly enhanced (Fig. 7C). Note that we confirmed efficient depletion of Pop2 and Ccr4 proteins by these dsRNAs, which blocked deadenylation (Fig. 5B,C). We conclude that deadenylation is not required for Pum RBD-mediated repression, despite the fact the RBD accelerates deadenylation dependent on Pop2 and Ccr4 deadenylases (Fig. 5).
When pAbp was knocked down, Pum RBD repression of RnLuc 3xPRE pA decreased from 32% to 14% (Fig. 7C); therefore, pAbp plays an important role in RBD-mediated repression of polyadenylated mRNA, in agreement with the internal poly(A) tract data (Fig. 7B). In contrast, Pum FL repression was largely unaffected (Fig. 7C), highlighting a pAbp-independent function of amino-terminal repression domains of Pumilio that we previously characterized (Weidmann and Goldstrohm 2012). It is important to note that the general effect of pAbp depletion (Fig. 7A) did not prevent measurement of Pumilio-mediated repression because the experimental design measures repression within each RNAi condition (e.g., repression by wild-type Pum RBD measured relative to the mutR7 negative control within pAbp-depleted cells).
To further analyze the role of pAbp in Pumilio repression, we used the tethered function approach. When fused to MS2, Pum FL and Pum RBD repress the RnLuc MS2 pA reporter to a degree comparable to their effect on the RnLuc 3xPRE, 67% and 32%, respectively (Fig. 7D), consistent with our previous analysis (Weidmann and Goldstrohm 2012). Knockdown of pAbp reduced Pum FL repression from 67% to 38%, whereas repression by the Pum RBD was entirely lost (Fig. 7D). In fact, without pAbp, the RBD slightly stimulated reporter protein expression (Fig. 7D) to a degree similar to the RBD's effect on HSL reporters (Fig. 6B,D). Western blotting confirmed that expression of tethered constructs was unaffected by pAbp knockdown (Fig. 7D). Interestingly, repression by tethered Pum FL and RBD is more sensitive to pAbp depletion. The results confirm that repression by the Pum RBD depends on pAbp, whereas the N-terminal repression domains in full-length Pumilio can repress independent of this cofactor.
The finding that pAbp was necessary for repression by the RBD of Drosophila Pumilio suggested that pAbp may also be involved in repression by the conserved RBD of human PUFs. To test this idea, we performed RNAi to deplete the human pAbp ortholog, PABPC1, from HEK293 cells. Efficient knockdown of epitope-tagged PABPC1 was confirmed by Western blotting (Fig. 7E). A coexpressed Halotag protein, included as an internal control, was not affected by RNAi depletion of PABPC1 (Fig. 7E). To specifically detect RBD-mediated repression, we utilized the 3xPRE UGG reporter (Fig. 1B) to measure repression activity of PUM2 RBD with altered RNA binding specificity, fused to Halotag (Fig. 7F, HT-PUM2 RBD-R6as). A mutant version of the reporter, RnLuc 3xPREmt, wherein the PREs were mutated to eliminate PUM2 binding and repression (Van Etten et al. 2012), was included as a negative control. We observed that PUM2 RBD R6as repressed RnLuc 3xPRE UGG by 42% in cells transfected with nontargeting control siRNA (Fig. 7F, NTC). In contrast, PABPC1 depletion substantially reduced PUM2 RBD R6as repression to 16% (Fig. 7F). Importantly, PABPC1 depletion did not affect expression of HT-PUM2 RBD R6as (Fig. 7F). We conclude that PABPC1 is required for repression by human PUM2 RBD. Taken together, our data demonstrate that poly(A) binding protein plays a conserved role in repression by the RBD of Drosophila and human PUFs.
The Pumilio RNA binding domain associates with pAbp
The functional connection between the RBD and pAbp prompted us to search for a physical association between these two proteins. To test this idea, we fused the Pum RBD to Halotag and a V5 epitope (HT-RBD-V5) and coexpressed this protein in D.mel-2 cells with either myc-tagged pAbp or, as a negative control, myc-tagged Lsm11. As a negative control, Lsm11 and pAbp were also coexpressed in cells with V5-tagged Halotag. Cell extracts were treated with RNases to degrade RNA that might bridge the two proteins. Halotag proteins were captured, and bound complexes were eluted using TEV protease. Western blotting with anti-V5 antibody shows that HT-V5 and HT-RBD-V5 expressed efficiently in D.mel-2 cells, and an equivalent amount of RBD was cleaved off of the resin in both Lsm11 and pAbp samples (Fig. 8, lanes 6 and 8). pAbp copurified with HT-RBD-V5, but not the HT control (Fig. 8, cf. lane 8 to lane 7). The Lsm11 control protein did not associate with HT or HT-RBD-V5, demonstrating specificity (Fig. 8, lanes 5 and 6). These results reveal that the Pum RBD associates with pAbp independent of RNA, providing a physical link between the Pum RBD and its cofactor.
FIGURE 8.

The Pumilio RNA binding domain associates with pAbp. V5-tagged Halotag (HT) alone or Halotag-Pum RBD fusions (HT-Pum RBD-V5) were coexpressed in D.mel-2 cells with myc-tagged pAbp or, as a negative control, myc-tagged Lsm11. Cell extracts were treated with RNases, and Halotag fusions were purified over a HaloLink resin. Bound proteins were eluted with TEV protease. Western blots were probed with V5 antibody for detection of Pum RBD and myc antibody for detection of prey proteins, Lsm11 or pAbp.
The Pumilio RNA binding domain does not displace pAbp from a target mRNA
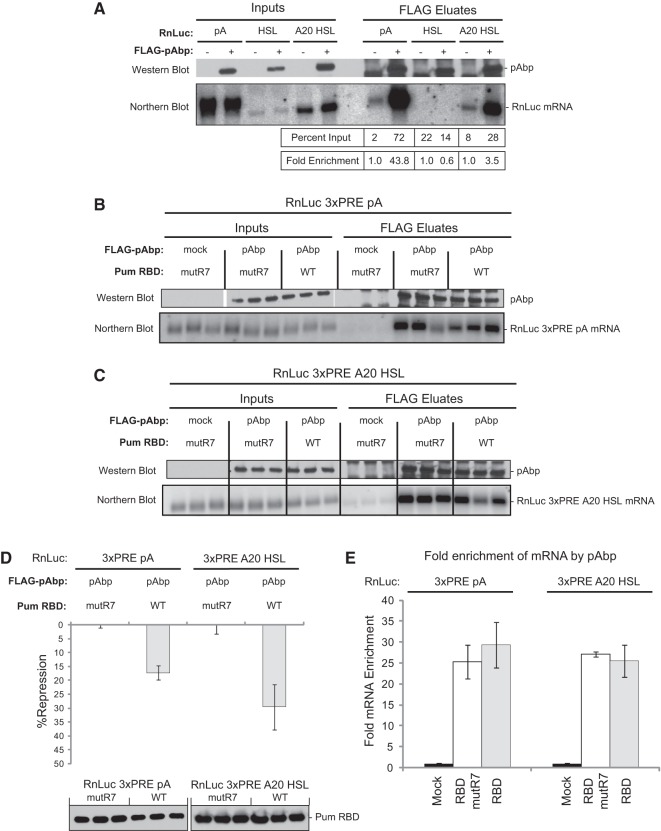
Based on our results showing that pAbp is necessary for poly(A)-dependent repression by the Pum RBD and that Pum interacts with pAbp, we hypothesized that Pum may promote repression by interfering with pAbp's ability to promote translation. One potential mechanism could be that Pum displaces pAbp from the poly(A) tail of a target mRNA. To test the hypothesis, we measured the effect of wild-type and mutant Pum RBD on the association of pAbp with the RnLuc 3xPRE mRNA. To do so, FLAG-tagged pAbp was immunoprecipitated, RNA was purified from the eluate, and pAbp-associated mRNA was then detected by Northern blotting. We first validated this RNA coimmunoprecipitation assay by coexpressing FLAG-tagged pAbp with RnLuc reporters bearing different 3′ ends, including a normal poly(A) tail, a histone stem–loop (HSL), or an internal 20-adenosine tract terminating in a HSL (A20 HSL). As negative controls, mock anti-FLAG immunoprecipitations were also performed from cells that expressed each reporter but not FLAG-pAbp (Fig. 9A). Immunoprecipitation of FLAG-pAbp was confirmed by Western blot (Fig. 9A). As expected, polyadenylated RnLuc mRNA was substantially enriched by pAbp, with 44-fold enrichment relative to the mock eluate (Fig. 9A). Importantly, the HSL reporter was not enriched; however, the introduction of the internal adenosine tract (A20 HSL) conferred pAbp enrichment (Fig. 9A). This control validates our ability to specifically enrich for pAbp-associated mRNAs.
FIGURE 9.
The Pumilio RNA binding domain does not displace pAbp from a target mRNA. (A) Control experiments demonstrating selective coimmunoprecipitation of polyadenylated mRNA with pAbp from D.mel-2 cells. Western blotting confirmed FLAG-tagged pAbp expression in inputs and in purified eluates. Northern blotting detected Renilla luciferase (RnLuc) reporter mRNAs in input and eluates, including RnLuc with a poly(A) tail (pA), a histone stem–loop (HSL), or an internal 20-adenosine tract preceding HSL (A20 HSL). Cell extracts were prepared from cells transfected with an FLAG-pAbp expression vector or from mock transfected cells. Renilla mRNA measured in each eluate was quantified as a percentage of the total RNA present in the input cell extracts. From these values, fold enrichment of each RNA in pAbp eluates was calculated relative to the corresponding mock transfection. In this way, values were normalized to the amount of reporter RNA present in the input samples. (B) FLAG-pAbp immunoprecipitations were performed from cells expressing either wild-type (WT) or mutR7 Pum RBD with the RnLuc 3xPRE pA reporter, as diagrammed in Figure 6A. Three replicates were performed for each sample. Expression and enrichment of pAbp in eluates was verified with Western blotting using a FLAG antibody. Northern blotting was performed to detect RnLuc 3xPRE pA RNAs in the input cell extracts and eluates. (C) The same experiment as in panel B except that a RnLuc 3xPRE A20 HSL reporter was used. (D) Verification of RBD repression using triplicate samples from panels B and C. In each case, Western blotting confirmed expression of Pum RBD test constructs. The graph represents mean values with standard errors calculated from three separate transfections with four replicates each. (E) Fold enrichment of RnLuc 3xPRE pA or RnLuc 3xPRE A20 HSL by pAbp with coexpressed wild-type or mutR7 Pum RBD as quantified from the experiments in panels B and C. The graph represents mean values with standard errors calculated from three replicate samples.
Using this RNA immunoprecipitation assay, we then tested whether the Pum RBD affects the association of pAbp with the PRE-containing reporter mRNA. Three replicate FLAG-pAbp immunoprecipitations were performed from cells that coexpressed either the RnLuc 3xPRE pA (Fig. 9B) or the RnLuc 3xPRE A20 HSL (Fig. 9C) mRNAs, along with either wild-type or mutant Pum RBD. Inputs and FLAG eluates were assayed by Northern blotting, and FLAG-pAbp enrichment was confirmed via Western blotting (Fig. 9B,C). RnLuc 3xPRE pA and RnLuc 3xPRE A20 HSL mRNAs were enriched by 25- to 30-fold in the FLAG-pAbp eluates but not in mock eluates. We also performed luciferase assays on these samples to verify that RBD-mediated repression is effective under these conditions (i.e., that expression of FLAG-pAbp does not alter repression) (Fig. 9D). Consistent with the results in Figure 6B, the RBD repressed the 3xPRE pA reporter by 17%, and repression of the 3xPRE A20 HSL reporter was slightly enhanced at 29% (Fig. 9D). Importantly, wild-type Pum RBD did not significantly change the level of enrichment of the RnLuc 3xPRE pA and RnLuc 3xPRE A20 HSL mRNAs in FLAG-pAbp eluates (Fig. 9E). We conclude that the RBD does not displace pAbp from an mRNA to elicit repression. Instead, the data suggests that the RBD-pAbp interaction antagonizes the ability of the poly(A)-bound pAbp to enhance translation.
DISCUSSION
Drosophila and human PUF proteins possess multiple domains that contribute to repression of protein expression from target mRNAs (Weidmann and Goldstrohm 2012). For example, Drosophila Pumilio has four repression domains that can function autonomously, including the RBD and three repression domains located in the amino terminus (Weidmann and Goldstrohm 2012). The current challenge is to dissect how each repression domain acts to inhibit translation and/or promote mRNA degradation. In this study, we focused on the mechanism of repression elicited by the evolutionarily conserved RBD. First, we evaluated two mechanisms proposed to account for RBD-mediated repression, including inhibition of translation elongation by a PUF-Argonaute-eEF1A ternary complex and acceleration of deadenylation achieved via recruitment of Pop2-Ccr4 deadenylases.
Biochemical data led to a model wherein the PUF RBD forms a complex with Argonaute that, in turn, binds eEF1A (Friend et al. 2012). Our analysis represents the first evaluation of this model in Drosophila and human cells. Our results confirm that human PUMs associate with Argonaute and reveal that the PUF-Argonaute interaction is conserved by Drosophila Pumilio. However, we found that mutations in conserved sites reported to inactivate PUF-Argonaute binding in vitro did not eliminate the PUF-Argonaute association of Drosophila and human PUFs. Moreover, these mutations had no effect on PUF repression in cells. In these contexts, multiple PUF-Argonaute contacts, or other protein partners, may complement the binding sites that were identified in vitro.
Our functional data indicate that the interaction of Drosophila and human PUFs with Argonautes is not essential for repression in vivo. First, we identified truncations of the RBD that eliminated the association with Argonaute but retained repressive activity. Multiple regions outside of the reported Argonaute binding site were found to be necessary for the PUF-Argonaute interaction. However, when tethered to mRNA, an RBD truncation (deletion of PUF repeats 7 and 8) that did not associate with Argonaute was still fully active for repression. Second, RNAi depletion of Argonautes did not alleviate repression by Drosophila Pumilio or human PUMs nor did mutations reported to prevent binding to eEF1A. These observations held true for target mRNAs with a minimal 3′ UTR with PRE elements and for a target mRNA that contains the 3′ UTR of the natural Pum target mRNA, Hunchback. Taken together, this evidence indicates that the PUF-Argonaute-eEF1A complex does not play an essential role in the mechanism of repression by Drosophila Pumilio or human PUFs. Notwithstanding, it remains possible that the PUF-Argonaute-eEF1A complex could contribute to regulation of particular target mRNAs in specific contexts. An intriguing possibility is that a PUF-Argonaute interaction could participate in combinatorial control of target mRNAs regulated by both PUFs and microRNAs. Interestingly, human and Drosophila PUMs were recently reported to collaborate with microRNA-mediated repression (Kedde et al. 2010; Miles et al. 2012). If true, we would anticipate that collaborative repression would be dependent on the major effector protein of microRNA-mediated repression, GW182 (Tritschler et al. 2010). Notably, we depleted GW182 from Drosophila cells and saw no effect on repression by the Pumilio RBD (data not shown). Our interpretation of this result is qualified by the fact that the reporters used in the present study are not predicted to be regulated by microRNAs, and the observations by Friend et al. were reported to be microRNA-independent (Friend et al. 2012). Thus, future studies are necessary to address whether the PUF-Argonaute interaction might participate in combinatorial control.
Multiple studies indicate that deadenylation plays a role in PUF RBD-mediated repression. For example, PUF repression correlates with deadenylation of target mRNAs (Ahringer et al. 1992; Wreden et al. 1997; Olivas and Parker 2000; Goldstrohm et al. 2006). Our data and previous work demonstrate that the conserved RBDs of yeast, C. elegans, Drosophila, and human PUFs interact with the Pop2 subunit of the Pop2-Ccr4 deadenylase complex (Goldstrohm et al. 2006; Hook et al. 2007; Kadyrova et al. 2007; Suh et al. 2009; Van Etten et al. 2012). In this study, we showed that Pop2 copurifies with the RBD of Pumilio. Genetic analysis in yeast demonstrated that PUF-mediated deadenylation depends on Pop2 and Ccr4, and the yeast Puf4 protein requires Pop2 and Ccr4 to repress protein expression (Goldstrohm et al. 2006, 2007; Hook et al. 2007). PUF-regulated deadenylation could be reconstituted with purified PUF RBD and Pop2-Ccr4 deadenylase complex (Goldstrohm et al. 2006, 2007; Hook et al. 2007). Furthermore, human PUFs interact with multiple isoforms of Pop2 and Ccr4 deadenylases, and their ability to repress is diminished when deadenylation is blocked (Van Etten et al. 2012). These findings all supported a model in which the RBD of PUF proteins recruits the deadenylases to target mRNAs, thereby promoting poly(A) tail shortening. This effect results in diminished translational output and, subsequently, can lead to mRNA decay. Indeed, we show here that the Pum RBD promotes deadenylation and requires the poly(A) tail to repress. Consistent with this model, we showed that deadenylation of the Pum target mRNA was fully dependent on Pop2 and Ccr4 deadenylases.
Surprisingly, we found that repression by Drosophila Pum RBD persists when Pop2 and Ccr4 are depleted by RNAi. Depletion of the deadenylases caused only a minor reduction in repression by full-length Pumilio. Further, while poly(A) is necessary for Pumilio repression, this requirement can be fulfilled by an internal poly(A) tract that is not susceptible to deadenylation. Therefore, deadenylation is not a primary mechanism of repression by Drosophila Pum RBD. We hypothesize that deadenylation may be a secondary effect and may serve to increase efficiency or reinforce the regulatory switch by diverting the repressed mRNA to the decay pathway.
If deadenylation is not the primary mechanism, then what is the trigger of Pum RBD-mediated repression? Our analysis revealed that repressive activity of the RBD is fully dependent on the presence of poly(A), whether at the 3′ end or as an internal poly(A) tract, in the PRE containing target mRNA. This led us to test the role of the poly(A) binding protein, pAbp. Indeed, we found that pAbp was necessary for RBD-mediated repression in vivo. We also identified a physical link between Pumilio and pAbp. Biochemical evidence further supports the importance of pAbp in PUF repression. Using a translationally active yeast extract, the pAbp ortholog, Pab1p, was shown to participate in translational inhibition by the RBD of yeast Puf5 (Chritton and Wickens 2011). In this same yeast extract, the RBD of C. elegans FBF also inhibited translation in a Pab1p-dependent manner (Chritton and Wickens 2011). In addition, pAbp colocalizes with PUFs in ribonucleoprotein granules in rat neurons (Vessey et al. 2010). Together with our in vivo evidence, these findings suggest that the involvement of pAbp in repression by PUFs may be an evolutionarily conserved feature. In support of this, we found that RNAi depletion of PABPC1 reduced repression by human PUM2 RBD in HEK293 cells. Future in vivo analysis of the importance of pAbp orthologs in repression by other PUF proteins will be necessary to confirm this prediction.
Poly(A) binding protein enhances translation, making it an opportune target for negative regulators of protein expression (Tritschler et al. 2010; Brook et al. 2012). For instance, the translational inhibitor PAIP2 (polyadenosine binding protein interaction protein 2), the sequence-specific repressor Musashi, and the microRNA induced silencing complex (miRISC) component GW182 have all been shown to bind pAbp orthologs (Khaleghpour et al. 2001; Karim et al. 2006; Kawahara et al. 2008; Fabian et al. 2009). Binding of PAIP2, Musashi, or GW182 to pAbp disrupts its interaction with the 5′ cap-bound eIF4F complex, resulting in reduced translation efficiency (Khaleghpour et al. 2001; Karim et al. 2006; Duncan et al. 2009; Moretti et al. 2012; Zekri et al. 2013). Together with SXL, the RNA binding protein UNR also targets pAbp but reduces translation by an alternate mechanism; UNR interferes with ribosome recruitment by the assembled initiation factors (Duncan et al. 2009). PAIP2 and miRISC also repress by displacing PABPC1 from the poly(A) tail (Karim et al. 2006; Duncan et al. 2009; Moretti et al. 2012; Zekri et al. 2013). In the case of miRISC, displacement of PABPC1 is thought to lead to subsequent deadenylation, mediated by miRISC recruitment of the Ccr4-Pop2 deadenylase complex (Moretti et al. 2012; Zekri et al. 2013). Drawing on these examples, we consider potential mechanisms for pAbp-dependent repression by Pumilio. Pumilio did not displace pAbp from the target mRNA, distinguishing the mechanism of repression from that of miRISC or PAIP2. Alternatively, Pumilio interaction with pAbp may interfere with pAbp binding to eIF4G. We note that our attempts to detect association of eIF4G with Pum RBD have been unsuccessful. Supporting this model, Chritton and Wickens showed that the Pab1-eIF4G interaction is required for repression by yeast Puf5 in vitro (Chritton and Wickens 2011). As a result, Pumilio would disrupt the “closed loop” contacts between 5′ cap-bound eIF4F and poly(A)-bound pAbp, resulting in diminished translation initiation. It is also possible that the pAbp-eIF4G interaction remains unaffected by the PUF-pAbp complex. In this scenario, Pum RBD would act like SXL-UNR, interacting with pAbp to block ribosome recruitment. Future detailed mechanistic analysis of Pumilio regulated translation will be necessary to distinguish these models.
While we focused on poly(A)-dependent repression by the RBD in the present study, our data emphasize that additional mechanisms of PUF repression exist. Full-length Drosophila and human PUFs retain repressive activity, albeit reduced in magnitude, which is independent of poly(A), pAbp, and Pop2-Ccr4 (Chagnovich and Lehmann 2001; Van Etten et al. 2012; this study). Our previous work showed that the Pum RBD is one of four repression domains in Pumilio and that the amino terminus of fruit fly and human PUFs exhibit robust repressive activity (Weidmann and Goldstrohm 2012). These observations argue that additional mechanisms of repression, elicited by the amino-terminal repression domains of Pumilio, PUM1, and PUM2, remain to be identified.
Originally, it was suspected, based on conservation of the RBD throughout eukaryotes, that members of the PUF family may repress by the same means (Wickens et al. 2002; Spassov and Jurecic 2003). Indeed, enhancement of mRNA decay by the RBD via recruitment of deadenylases is a conserved feature, though the contribution to the magnitude of repression appears to differ between PUFs in different organisms (Goldstrohm et al. 2006; Hook et al. 2007; Blewett and Goldstrohm 2012; Van Etten et al. 2012). While repression by Drosophila Pumilio is largely unaffected by depletion of deadenylases, repression by human PUM1 was substantially reduced by deadenylase depletion and by overexpression of a dominant negative deadenylase (Van Etten et al. 2012). Additional evidence comes from analysis of yeast PUFs. Both Puf4 and Puf5 accelerate deadenylation, and repression by Puf4 depends on Pop2 and Ccr4 (Goldstrohm et al. 2006; Hook et al. 2007; Blewett and Goldstrohm 2012). In contrast, while Puf5 does promote poly(A) shortening, it can circumvent deadenylation by recruiting the Eap1 protein to enhance de-capping of the target mRNA (Blewett and Goldstrohm 2012). Thus, an emerging principle is that individual PUFs can have different corepressor requirements and dominant repressive mechanisms. Analysis of yeast Puf6 lends additional support, as Puf6 was shown to uniquely target eIF5B to inhibit translation (Deng et al. 2008).
Beyond the RBD, PUF proteins from organisms such as yeast and C. elegans differ substantially at the amino acid level from those found in insects and vertebrates (Spassov and Jurecic 2003; Weidmann and Goldstrohm 2012). The divergent polypeptide sequences of different PUF proteins may confer unique regulatory functions. Further adding to the regulatory potential, PUFs from insects and vertebrates have evolved multiple repressive domains, each of which can act independently (Weidmann and Goldstrohm 2012). Thus, individual PUFs may assemble distinct regulatory complexes, depending on the context in vivo. We anticipate that members of this ancient protein family have evolved strategies of regulation that remain to be revealed, with the pAbp- and poly(A)-dependent mechanism of the RBD contributing to the maximal efficiency of repression.
MATERIALS AND METHODS
Plasmids
Plasmids used in this study included pAc5.1 FFluc, pAc5.1 RnLuc, pAc5.1 RnLuc 3xPRE, pIZ Pum FL, pIZ Pum FL mutR7, pIZ Pum RBD, and pIZ Pum RBD mutR7, all of which were previously described (Weidmann and Goldstrohm 2012). The PRE sequences were derived from the natural Pum target mRNA, Hunchback (Murata and Wharton 1995; Zamore et al. 1997, 1999). The pAc5.1 RnLuc Hb 3′ UTR reporter was created by inserting the 3′ untranslated region of the Drosophila Hunchback mRNA (NM_169233.2) into the XhoI and NotI restriction sites downstream from Renilla luciferase coding sequence in the pAc5.1 RnLuc vector. The Hb 3′ UTR was amplified from Drosophila genomic DNA using the following primers (Hb sequence underlined, restriction sites in bold):
Hb 3′ UTR Forward: 5′-GCAGCTCGAGGTTCCCCATCACCATCACCTTG
Hb 3′ UTR Reverse: 5′-CACCGCGGCCGCAATTTGACTTTGGACTGTTGGTATTGTTTG.
The pIZ PUM1 plasmid, expressing human PUM1, was previously described (Van Etten et al. 2012). Mutant versions of Drosophila Pumilio (NP_001262403.1), reported to inhibit binding to Argonaute or eEF1A (Friend et al. 2012), were created by site-directed mutagenesis with the following primers (mutations in bold):
Pum T1137E Forward: 5′-CCAACAGAAGTTGGAGCGGGCCGAGGCCGCCGAGAAGCAAATGG
Pum T1137E Reverse: 5′-CCATTTGCTTCTCGGCGGCCTCGGCCCGCTCCAACTTCTGTTGG
Pum F1251R Forward: 5′-GGACCCCGTGGCGCTGCAGCGCATCATCAATGCTTTCAAGGGTCAGG
Pum F1251R Reverse: 5′-CCTGACCCTTGAAAGCATTGATGATGCGCTGCAGCGCCACGGGGTCC.
The psiCheck1-based RnLuc 3xPRE, RnLuc 3xPRE UGG, and RnLuc 3xPREmt reporter plasmids, the pGL4.13 FFLuc internal control, and the pFN21A-based expression vectors for Halotag, Halotag human CNOT6L, or Halotag versions of human PUM1 and PUM2 R6SYE derivatives were previously described (Van Etten et al. 2012). For pFN21A PUM2 RBD R6as, aa705–1050 of human PUM2 (NP_056132.1) was cloned into the flexi sites of pFN21A (Promega), and R6as (N921S,Q925E) was generated via site-directed mutagenesis as in Van Etten et al. (2012). Human PUF mutants were created based on the mutations reported to abrogate binding to Argonautes or eEF1A (Friend et al. 2012), by site-directed mutagenesis of PUM1 (NP_001018494.1) and PUM2 (NP_056132.1) using the following primers:
PUM1 T874E Forward: 5′-GCTCAAACTGGAGCGTGCCGAACCAGCTGAGCGCCAGC
PUM1 T874E Reverse: 5′-GCTGGCGCTCAGCTGGTTCGGCACGCTCCAGTTTCAGC
PUM1 F990R Forward: 5′-GTGTACAGCCCCAGTCTTTGCAACGTATCATCGATGCGTTTAAGGGACAGG
PUM1 F990R Reverse: 5′-CCTGTCCCTTAAACGC ATCGATGATACGTTGCAAAGACTGGGGCTGTACAC
PUM2 T752E Forward: 5′-CATACAGCAAAAACTAGAGAGAGCTGAACCAGCTGAGCGACAGATGG
PUM2 T752E Reverse: 5′-CCATCTGTCGC TCAGCTGGTTCAGCTCTCTCTAGTTTTTGCTGTATG
PUM2 F866R Forward: 5′-GTGTTCAGCCACAGTCACTACAGCGCATCATTGATGCTTTCAAGGGACAAG
PUM2 F866R Reverse: 5′-CTTGTCCCTTGAAAGCATCAATGATGCGCTGTAGTGACTGTGGCTGAACAC.
For FLAG immunoprecipitation in HEK293 cells, the coding sequence for human AGO1 (NP_036331.1) was inserted with an N-terminal 3xFLAG tag into the pF5A vector (Promega) to create pF5A N3xFLAG AGO1. pFN21A PABPC1 was generated by inserting the coding sequence of human pAbp, PABPC1 (NP_002559.2) into the flexi sites of pFN21A (Promega). For Halotag pulldown assays, the Halotag coding sequence from pFN18A (Promega), a C-terminal TEV cleavage site, and the Sgf1 restriction site were inserted into the pIZ V5-His A vector (Invitrogen) to create the HT control. Drosophila Pum was then inserted in frame and C-terminal to Halotag and a TEV protease cleavage site. For HT-RBD, containing amino acids 1091–1533, the N terminus of Pum (aa1–1090) was deleted via inverse PCR. The pIZ myc-Lsm11 (NP_610522.1) vector and the empty pUB myc and pUB FLAG vectors (Ubiquitin 63E promoter, SV40 poly(A) site, and pUC19 backbone) were provided by Dr. Eric Wagner. pUB myc-pAbp was generated by inserting the coding sequence of Drosophila pAbp (NP_725750.1) into the pUB vector downstream from the myc tag, and pIZ myc-Pop2 and pIZ myc-Ccr4 were generated by inserting the coding sequences of Drosophila Pop2 (NP_648538.1) or Drosophila twin (NP_732966.1) with an N-terminal myc tag into pIZ. pUB FLAG-Ago1 and pUB FLAG-Ago2 were created by inserting the coding sequence of Drosophila Ago1 (NP_001246314.1) and Drosophila Ago2 (NP_730054.1) into the pUB FLAG vector downstream and in frame with the FLAG tag. For the tethered function assays, the pAc5.1 RnLuc 2xMS2 reporter, pIZ MS2-Pum FL, and pIZ MS2-RBD (aa1091–1533) were previously described (Weidmann and Goldstrohm 2012). To create truncations of the Pum RBD, fused to MS2, inverse PCR was used with pIZ MS2-RBD to delete aa1427–1533 (pIZ MS2-RBDΔC) and subsequently aa1330–1426 (pIZ MS2-RBDΔR7-8), aa1222–1294 (pIZ MS2-RBDΔR4-5), or aa1091–1186 (pIZ MS2-RBDΔR1-2). For the HSL reporters, a histone stem–loop (HSL) and a histone downstream element (HDE) were inserted in place of the SV40 cleavage/poly adenylation element to create pAc5.1 RnLuc HSL, pAc5.1 RnLuc 3xPRE HSL, and pAc5.1 RnLuc MS2 HSL (Weidmann and Goldstrohm 2012). The sequence added is as follows, with the HSL and histone downstream element underlined: 5′-GGTCCTTTTCAGGACCACAAACCAGATTCAATGAGATAAATTTTCTGTT. Inverse PCR was performed to insert 20 adenosines upstream of the HSL to create the pAc5.1 RnLuc A20 HSL and pAc5.1 RnLuc 3xPRE A20 HSL reporters.
Cell culture
D.mel-2 cells (Invitrogen) were cultured in Sf-900 III serum-free medium (Invitrogen) with 50 units/mL penicillin and 50 µg/mL streptomycin using standard cell culture techniques. Cells were grown at 28°C. HEK293 cells were cultured as previously described (Van Etten et al. 2012).
Transfections
Transfections were performed as previously described for D.mel-2 cells (Weidmann and Goldstrohm 2012) and HEK293 cells (Van Etten et al. 2012). When transfections were performed for transcription shutoff experiments, Effectene (Qiagen) reagents were scaled up to 20 mL total volume in a T-150 flask: 4.55 ng FFLuc, 9.1 ng RnLuc, 3636 ng pIZ vector, 818 µL EC buffer, 29.1 µL Enhancer, 36.36 µL Effectene, 14.6 mL D.mel-2 cells (1.5 × 106 cells/mL), and 5.4 mL Sf900III media. To inhibit transcription, actinomycin D (Sigma) was added at 5 µg/mL final concentration, 48 h post-transfection. Aliquots of cells at each indicated time point were removed, pelleted at 1000g for 3 min, and frozen at −80°C until RNA isolation. For experiments with RnLuc 3xPRE HSL and RnLuc 3xPRE A20 HSL reporters, 50 ng and 20 ng of the indicated reporter, respectively, was transfected per well of a six-well plate, to ensure comparable levels of expression to pA reporters. To compare the effect of pAbp depletion on RnLuc pA and RnLuc HSL, 50 ng of the indicated RnLuc plasmid and 400 ng of empty pIZ vector were transfected into one well of a six-well plate treated with either control or pAbp dsRNA.
In the siRNA experiment assessing PABPC1 knockdown efficiency, FuGENE HD (Promega) was used to transfect 80 ng pFN21A PABPC1 and 20 ng pFN21A Halotag control into 96-well plates treated with siRNAs. In experiments testing the effect of siRNA-mediated depletion of PABPC1 on repression activity of HT-PUM2 RBD R6as, FuGENE HD was used to transfect 5 ng pGL4.13 FFLuc, 10 ng psiCheck1 RnLuc 3xPRE UGG or RnLuc 3xPRE ACA, and 85 ng pFN21A PUM2 RBD R6as into 20,000 cells in each well of a 96-well plate.
RNA interference
As previously described, double-stranded RNAs (dsRNAs) were in vitro transcribed for RNAi, including nontargeting control (NTC) LacZ, PumN, Pop2, and Ccr4 (Van Etten et al. 2012; Weidmann and Goldstrohm 2012). The following primers were used to generate templates for production of Argonaute and pAbp dsRNAs, with T7 promoter sequence underlined and gene-specific regions bolded:
AGO1 Forward: 5′-GGATCCTAATACGACTCACTATAGGCCAATCACTTCCAGGTGACAATGC
AGO1 Reverse: 5′-GGATCCTAATACGACTCACTATAGGCCACTGCGAGGGCCTTACG
AGO2 Forward: 5′-GGATCCTAATACGACTCACTATAGGGGATGGAGCAACTCAGGTGGC
AGO2 Reverse: 5′-GGATCCTAATACGACTCACTATAGGGAGGAATAATCACAATTGCCAGATCG
pAbp Forward: 5′-GGATCCTAATACGACTC ACTATAGGGCGTATGCAGCAGCTGGGACAG
pAbp Reverse: 5′-GGATCCTAATACGACTCA CTATAGGCCTTGCAATTGCTGTGGAATTGGC.
Corresponding regions were amplified via PCR from D.mel-2 cDNA, and dsRNA was transcribed in vitro and purified as previously described (Van Etten et al. 2012; Weidmann and Goldstrohm 2012). For knockdown, cells in one well of a six-well plate, with total volume 1.6 mL, were treated with 6 µg of each dsRNA for 5 min before transfection. For knockdown during transcription shutoff assays, 20 mL total volume was treated with 60 µg of each dsRNA for 5 min before transfection.
For RNAi in HEK293 cells, depletion of PABPC1 was performed through the use of On-target Plus Smartpool siRNA (L-019598-00) or a Nontargeting Control siRNA (Dharmacon). Twenty thousand HEK293 cells were plated per well of a 96-well plate in antibiotic-free medium. Twenty four hours later, the cells were transfected with 10 fmol of siRNA using Dharmafect 1 (Dharmacon) according to the manufacturer's protocol. Forty-eight hours after siRNA treatment, cells were transfected with reporters and expression vectors. Forty-eight hours post-transfection, luciferase assays to measure RnLuc and FfLuc activities were performed and cell lysates were prepared for Western blot analysis.
Alignments
Alignments of the PUF RNA binding domain were performed using the open source bioinformatics software Jalview 2.8 (www.jalview.org) using the MafftWS alignment (Waterhouse et al. 2009).
Luciferase assays
Luciferase assays were performed as previously described using the Dual-Glo Luciferase Assay System (Promega) (Van Etten et al. 2012; Weidmann and Goldstrohm 2012). A relative response ratio (RRR), from RnLuc signal/FFLuc signal, is calculated for each sample. Percent repression is derived from the equation 100 × [1 − (RRRWT/RRRNegative Control)], where RRRWT is from a sample transfected with an active regulator and RRRNegative Control comes from a sample transfected with an equivalent amount of an inactive negative control. Inactive controls for Pum FL and Pum RBD were created by mutating the RNA recognition amino acids in the seventh PUF repeat, which prevents RNA binding and repression, to create pIZ Pum FL mutR7 and pIZ Pum RBD mutR7 plasmids (Weidmann and Goldstrohm 2012). Empty pIZ vector was used as the inactive control for human PUM1 in Drosophila cells. The control for tethered function experiments was the MS2 expression vector, pIZ MS2 (Weidmann and Goldstrohm 2012). For the RnLuc Hb 3′ UTR reporter, fold change reporter expression was calculated as RRRRNAi/RRRNTC, where RRRRNAi is from sample treated with targeting dsRNAs and RRRNTC is from sample treated with nontargeting control dsRNAs.
To measure repression by altered specificity human PUMs in HEK293 cells, the pFN21A Halotag expression vector served as a negative control, and percent repression was calculated as previously described (Van Etten et al. 2012). For RNAi depletion of PABPC1 in HEK293, percent repression of the RnLuc 3xPRE UGG reporter was calculated relative to the negative control reporter, RnLuc 3xPREmt, as previously described (Van Etten et al. 2012).
Immunoprecipitation
For FLAG immunoprecipitations from HEK293 cell, 3 mLs of 200,000 cells/mL were transfected. Mock samples were transfected using Fugene HD with 3 µg of Halotag prey plasmids (HT, HT-PUM2, HT-PUM2 T752E, and HT-CNOT6L), while FLAG-AGO1 samples were transfected with 750 ng pF5A FLAG-HsAGO1 bait and 2.25 µg of Halotag prey plasmids. Forty-eight hours post-transfection, cell pellets were resuspended in 500 µL lysis buffer containing 0.5% Igepal CA-630 (USB), 50 mM Tris-HCl pH 8.0, 0.5 mM EDTA, 2 mM MgCl2, and 150 mM NaCl. Protease inhibitors were also added to final concentrations of 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg/mL aprotinin, 10 µg/mL pepstatin, and 10 µg/mL leupeptin. Lysates were passed through a 25-gauge needle five times and were then cleared at 16,000g for 10 min at 4°C. Cleared lysates were treated with final concentrations of 20 units/mL RNase ONE (Promega), 8 µg/mL RNase A (Fermentas), and 500 nM HaloTag TMR ligand (Promega). A portion was kept as the input. The extract was then bound to 10 µL bed volume of EZview Red Anti-FLAG M2 affinity resin (Sigma) (equilibrated with lysis buffer and blocked for 30 min at 4°C with 500 µg/mL BSA). Binding proceeded for 12 h at 4°C. Beads were washed one time in 1 mL lysis buffer and three times in lysis buffer lacking detergent with 500 mM NaCl. Beads were resuspended in 30 µL elution buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.5 mM EDTA, and 2 mM MgCl2. To elute bound complexes, 3xFLAG peptide (Sigma) was added to 150 ng/µL. Elution proceeded with end-over-end rotation for 30 min at 4°C. Eluates were separated from the resin using a Micro Bio-Spin column (Bio-Rad).
For FLAG immunoprecipitations from D.mel-2 cells, 2 mL of 1.5 million cells/mL were transfected (Effectene) with 300 ng of MS2-V5 prey plasmids (RBD, RBDΔC, RBDΔR7-8, RBDΔR4-5, RBDΔR1-2), 50 ng FLAG-Dm Ago2 bait, and 50 ng myc-Lsm11 negative control. Immunoprecipitation proceeded similar to the above protocol with minor differences. Each cell pellet was lysed in 300 µL lysis buffer containing 0.5% Igepal CA-630 (USB), 50 mM Tris-HCl pH 8.0, 2 mM EDTA, 150 mM NaCl. Protease inhibitors were also added to final concentrations of 2 mM phenylmethylsulfonyl fluoride, 20 µg/mL aprotinin, 20 µg/mL pepstatin, and 20 µg/mL leupeptin. Lysates were treated with 50 units/mL RNase ONE and 10 µg/mL RNase A. After binding, the resin was washed three times in lysis buffer and three times in lysis buffer lacking detergent. Beads were eluted for 24 h at 4°C in 50 µL elution buffer containing 50 mM Tris pH 8.0, 300 nM NaCL, 2 mM EDTA, and 300 ng/µL of 3xFLAG peptide.
FLAG-pAbp RNA immunoprecipitation
The FLAG-pAbp RNA immunoprecipitations (RNA-IP) in D.mel-2 cells followed the FLAG immunoprecipitation described above with the following key differences. For control in Figure 9A, 2 mLs of D.mel-2 cells at 1.5 million cells/mL were transfected with 100 ng of RnLuc reporter plasmids (pA, HSL, A20 HSL) and 50 ng of either empty pUB vector or FLAG-pAbp. In the RNA-IPs with the Pum RBD, reporter plasmids (20 ng RnLuc 3xPRE pA or 30 ng RnLuc 3xPRE A20 HSL) were cotransfected with 50 ng of either pUB vector or FLAG-pAbp and 350 ng of either RBD mutR7 or RBD plasmids. The RNA-IP lysis buffer consisted of 50 mM Tris pH 8.0, 150 mM NaCl, 2 mM EDTA, protease inhibitors, 0.2% Igepal CA-630 (USB), and 200 units/mL RNasin. In addition, beads were washed one time in lysis buffer and three times in lysis buffer with reduced detergent (0.01%). No elution with 3xFLAG peptide was performed; input lysates and IP pellets were directly subjected to Trizol RNA purification or SDS elution.
Halotag pulldown assays
For pulldown assays, 300 ng bait plasmid (pIZ Halotag alone or pIZ Halotag-Pum RBD aa1091–1533) were cotransfected with 100 ng prey plasmid (pIZ myc-Lsm11, pIZ myc-Pop2, or pUB myc-pAbp) into one well of a six-well plate for each bait-prey combination. For pulldown of Ago2 by HT-RBD, 300 ng of pIZ Halotag-PumRBD or pIZ Halotag-PumRBD T1137E bait, 50 ng of pUB FLAG-Ago2 prey, and 50 ng of pUB myc-Lsm11 control were cotransfected into one well of a six-well plate. Two milliliters of transfected cells were pelleted at 1000g for 3 min and washed twice in phosphate buffered saline (PBS). Pellets were resuspended in 300 µL lysis buffer containing 0.5% Igepal CA-630 (USB), 50 mM Tris-HCl pH 8.0, 2 mM EDTA, and 150 mM NaCl. Protease inhibitors were also added to final concentrations of 2 mM phenylmethylsulfonyl fluoride, 20 µg/mL aprotinin, 20 µg/mL pepstatin, and 20 µg/mL leupeptin. Lysates were passed through a 25-gauge needle five times and incubated at 4°C for 1 h. Lysates were then cleared at 16,000g for 15 min at 4°C. A portion of the resulting supernatant was kept as the input. The extract was then bound to 20 µL bed volume of Halolink beads (Promega) (equilibrated with lysis buffer and blocked for 30 min at 4°C with 500 µg/mL BSA). During the binding, 100 units/mL RNase ONE (Promega) and 10 µg/mL RNase A were added to degrade RNA. Binding proceeded for 24 h at 4°C. Beads were washed three times in 1 mL lysis buffer and three times in lysis buffer lacking detergent with increased NaCl (300 nM NaCl for Ago2, 750 nM NaCl for pAbp). Beads were resuspended in 30 µL elution buffer containing 50 mM Tris-HCl pH 8.0 and 300 mM NaCl. To cleave Halotag fusions, thereby eluting bound complexes, 5 units of AcTEV protease (Invitrogen) were added and incubated for 12 h at 4°C. Eluates were separated from the Halolink beads using a Micro Bio-Spin column (Bio-Rad).
Western blotting
Western blotting from luciferase assay samples was performed as previously described (Weidmann and Goldstrohm 2012). For Western blotting of Halotag pulldowns, input samples were diluted 10-fold in elution buffer and were separated along with TEV elutions via SDS-polyacrylamide (12%) gel electrophoresis, and proteins were transferred onto Immobilon-P membranes (Millipore). All membranes were probed with either V5 monoclonal antibody (Invitrogen), c-myc (9E10) antibody (provided by Dr. Eric Wagner), anti-Halotag monoclonal antibody (Promega), or monoclonal anti-FLAG M2 antibody (Sigma). Secondary detection was performed using horseradish peroxidase conjugated goat anti-mouse IgG (Thermo Scientific). Signals were detected using Immobilon Western chemiluminescent substrate (Millipore) and autoradiography film.
Fluorescent labeling and visualization of Halotag protein constructs
Protein extracts from HEK293 cells expressing Halotag fusions were harvested from each well of a 96-well plate in 20 µL of lysis buffer (0.5% Igepal CA-630 [USB], 50 mM Tris-HCl pH 8.0, 0.5 mM EDTA, 2 mM MgCl2, 150 mM NaCl) with 1× Protease Inhibitor cocktail (Promega) and mixed with 900 nM Halotag TMR Ligand (Promega) for 30 min on ice, protected from light. For labeling of FLAG IPs, refer to FLAG IP methods. After labeling, extracts were separated via SDS-polyacrylamide (12%) gel electrophoresis and detected by fluorescence imaging with a Typhoon Trio imager (GE Healthcare).
RNA isolation
For isolation of RNA, 4 mL of transfected D.mel-2 cells were centrifuged at 1000g for 3 min, washed twice in PBS, and RNA was purified from cell pellets using Maxwell 16 LEV simplyRNA Tissue Kit and the Maxwell 16 Instrument (Promega). Total RNA preparations were utilized for Northern blotting or cDNA preparation. For pAbp RNA-IP experiments, Trizol reagent (Ambion) was used for RNA purification according to the manufacturer's protocols.
Northern analysis
Northern blotting was performed as previously described (Blewett and Goldstrohm 2012). RNA was separated in a denaturing 0.85% agarose MOPS/formaldehyde gel. RNAs were transferred to Immobilon NY+ membrane (Millipore). Membranes were then UV-crosslinked and probed for the RNAs indicated. For the RnLuc reporter, a [32P]-body-labeled, antisense RNA probe was created by in vitro transcription. The following primers were used to amplify templates for creation of RnLuc RNA probes. The T7 promoter sequence is underlined and gene-specific regions are bolded:
RnLuc forward primer, 5′-GCCCGTGGCTAGATGCATCATCC
RnLuc reverse primer, 5′-GGATCCTAATACGACTCACTATAGGGCGGACAATCTGGACGACGTCGG.
For detection of the RnLuc reporter for poly(A) tail analysis, primers included the
RnLuc 3′ forward primer: 5′-GGGCGAGGTTAGACGGCCTACCCT
RnLuc 3′ reverse primer: 5′-GGATCCTAATACGACTCACTATAGGGCGGCCAGCGGCCTTGG.
The 7SL RNA was detected on Northern blots using a [32P]-5′ end-labeled DNA oligo with the following sequence:
7SL Probe: 5′-CACCCCTGGCCCGGTTCATCCCTCCTTAGCCAACCTGAATGCCACGG.
Northern blots of poly(A) tail length, including RNase H cleavage, were performed as previously described (Blewett and Goldstrohm 2012). Total RNA, 20 μg, was annealed to a cleavage oligo specific to the RnLuc reporter. The oligo used for RNase H cleavage bore the sequence 5′-CCTTGAATGGCTCCAGGTA. Oligo dT cleaved control reactions contained 5 μg of oligo dT15 (IDT). Two units of RNase H (NEB) were added to each reaction. RNAs were cleaved for 1 h at 37 °C, then precipitated with 0.3 M sodium acetate, and three volumes of 100% ethanol for 1 h at −20°C. RNAs were pelleted, washed with 70% ethanol, and resuspended in denaturing RNA loading buffer. RNAs were separated in 5% polyacrylamide, 7 M urea gels with 0.5 X Tris-borate-EDTA running buffer. Electrophoretic transfer to Immobilon NY+ was performed with TransBlot (Bio-Rad) for 1 h at 60 V. Membranes were then probed as described above. Blots were visualized using a Typhoon Trio (GE) phosphoimager and quantitated using ImageQuant software (GE).
cDNA preparation and qPCR
For measurement of endogenous mRNA knockdown, RNAs were primed with random hexamers (IDT) for synthesis of cDNAs using GoScript reverse transcriptase (Promega). The final concentration of RNA in these reactions was 500 µg/mL. To measure endogenous mRNA levels, quantitative PCR (qPCR) was performed on 5 µL of cDNA product in a 50-µL reaction using 100 nM of gene-specific primers and GoTaq qPCR master mix (Promega) as described previously (Weidmann and Goldstrohm 2012). Standard negative control reactions were performed without reverse transcriptase. Differences in mRNA levels were calculated using the ΔΔCT method. CT values were measured and normalized to the internal control Rpl32 mRNA to generate ΔCT. ΔΔCT was derived relative to the nontargeting control ΔCT (Livak and Schmittgen 2001; Schmittgen and Livak 2008). qPCR primers for Pop2 and Ccr4 were previously published (Van Etten et al. 2012). The qPCR primer sequences for additional Drosophila genes are as follows:
Rpl32 Forward: 5′-GCCCAAGGGTATCGACAACAG
Rpl32 Reverse: 5′-GCACGTTGTGCACCAGGAAC
Ago1 Forward: 5′-CCAGATGCGTCGCAAGTATCG
Ago1 Reverse: 5′-CGGGTAGCGCAATTTCATGC
Ago2 Forward: 5′-GTGAGCGACGGCCAGTTTCC
Ago2 Reverse: 5′-GAACTTGTTCGATGTCGTTACGTCG
pAbp Forward: 5′-CGTCGCTCGTTGGGCTATGC
pAbp Reverse: 5′-GCGACGAAGAGAAGGATCACGC.
ACKNOWLEDGMENTS
We thank Dr. Eric Wagner, Dr. Craig Smibert, and Dr. Nils Walter for comments on the manuscript. Dr. Wagner provided valuable technical advice, antibodies, and plasmids. Dr. Trista Schagat provided valuable technical advice. This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM105707. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. C.A.W. and N.A.R. were supported by the Michigan Predoctoral Training Program in Genetics through NIH National Research Service Award 5T32GM007544-33. A.C.G. was supported by a Research Scholar Grant, RSG-13-080-01-RMC, from the American Cancer Society, and a grant from the Edward Mallinckrodt Jr. Foundation.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.046029.114.
REFERENCES
- Ahringer J, Rosenquist TA, Lawson DN, Kimble J 1992. The Caenorhabditis elegans sex determining gene fem-3 is regulated post-transcriptionally. EMBO J 11: 2303–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, Kobayashi S 1999. Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat Cell Biol 1: 431–437 [DOI] [PubMed] [Google Scholar]
- Blewett NH, Goldstrohm AC 2012. A eukaryotic translation initiation factor 4E-binding protein promotes mRNA decapping and is required for PUF repression. Mol Cell Biol 32: 4181–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook M, McCracken L, Reddington JP, Lu ZL, Morrice NA, Gray NK 2012. The multifunctional poly(A)-binding protein (PABP) 1 is subject to extensive dynamic post-translational modification, which molecular modelling suggests plays an important role in co-ordinating its activities. Biochem J 441: 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnovich D, Lehmann R 2001. Poly(A)-independent regulation of maternal hunchback translation in the Drosophila embryo. Proc Natl Acad Sci 98: 11359–11364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chritton JJ, Wickens M 2011. A role for the poly(A)-binding protein Pab1p in PUF protein-mediated repression. J Biol Chem 286: 33268–33278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J, Wickens M 2002. Tethered function assays using 3′ untranslated regions. Methods 26: 142–150 [DOI] [PubMed] [Google Scholar]
- Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J 2002. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417: 660–663 [DOI] [PubMed] [Google Scholar]
- Deng Y, Singer RH, Gu W 2008. Translation of ASH1 mRNA is repressed by Puf6p-Fun12p/eIF5B interaction and released by CK2 phosphorylation. Genes Dev 22: 1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, et al. 2003. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol 13: 286–296 [DOI] [PubMed] [Google Scholar]
- Duncan KE, Strein C, Hentze MW 2009. The SXL-UNR corepressor complex uses a PABP-mediated mechanism to inhibit ribosome recruitment to msl-2 mRNA. Mol Cell 36: 571–582 [DOI] [PubMed] [Google Scholar]
- Fabian MR, Mathonnet G, Sundermeier T, Mathys H, Zipprich JT, Svitkin YV, Rivas F, Jinek M, Wohlschlegel J, Doudna JA, et al. 2009. Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol Cell 35: 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A, Lehmann R 1998. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125: 679–690 [DOI] [PubMed] [Google Scholar]
- Friend K, Campbell ZT, Cooke A, Kroll-Conner P, Wickens MP, Kimble J 2012. A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nat Struct Mol Biol 19: 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgano A, Forrer M, Jaskiewicz L, Kanitz A, Zavolan M, Gerber AP 2008. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS One 3: e3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberi C, Peterson DS, He L, Gottlieb E 2002. An anterior function for the Drosophila posterior determinant Pumilio. Development 129: 2699–2710 [DOI] [PubMed] [Google Scholar]
- Garneau NL, Wilusz J, Wilusz CJ 2007. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol 8: 113–126 [DOI] [PubMed] [Google Scholar]
- Gerber AP, Herschlag D, Brown PO 2004. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol 2: E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber AP, Luschnig S, Krasnow MA, Brown PO, Herschlag D 2006. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proc Natl Acad Sci 103: 4487–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm AC, Wickens M 2008. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol 9: 337–344 [DOI] [PubMed] [Google Scholar]
- Goldstrohm AC, Hook BA, Seay DJ, Wickens M 2006. PUF proteins bind Pop2p to regulate messenger RNAs. Nat Struct Mol Biol 13: 533–539 [DOI] [PubMed] [Google Scholar]
- Goldstrohm AC, Seay DJ, Hook BA, Wickens M 2007. PUF protein-mediated deadenylation is catalyzed by Ccr4p. J Biol Chem 282: 109–114 [DOI] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M Jr, Jungkamp AC, Munschauer M, et al. 2010. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook BA, Goldstrohm AC, Seay DJ, Wickens M 2007. Two yeast PUF proteins negatively regulate a single mRNA. J Biol Chem 282: 15430–15438 [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV 2010. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 11: 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson HE, Dertinger D, LeCuyer KA, Behlen LS, Greef CH, Uhlenbeck OC 1998. A thermodynamic analysis of the sequence-specific binding of RNA by bacteriophage MS2 coat protein. Proc Natl Acad Sci 95: 9244–9249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyrova LY, Habara Y, Lee TH, Wharton RP 2007. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development 134: 1519–1527 [DOI] [PubMed] [Google Scholar]
- Karim MM, Svitkin YV, Kahvejian A, De Crescenzo G, Costa-Mattioli M, Sonenberg N 2006. A mechanism of translational repression by competition of Paip2 with eIF4G for poly(A) binding protein (PABP) binding. Proc Natl Acad Sci 103: 9494–9499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Imai T, Imataka H, Tsujimoto M, Matsumoto K, Okano H 2008. Neural RNA-binding protein Musashi1 inhibits translation initiation by competing with eIF4G for PABP. J Cell Biol 181: 639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedde M, van Kouwenhove M, Zwart W, Oude Vrielink JA, Elkon R, Agami R 2010. A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol 12: 1014–1020 [DOI] [PubMed] [Google Scholar]
- Khaleghpour K, Svitkin YV, Craig AW, DeMaria CT, Deo RC, Burley SK, Sonenberg N 2001. Translational repression by a novel partner of human poly(A) binding protein, Paip2. Mol Cell 7: 205–216 [DOI] [PubMed] [Google Scholar]
- Kuhn U, Wahle E 2004. Structure and function of poly(A) binding proteins. Biochim Biophys Acta 1678: 67–84 [DOI] [PubMed] [Google Scholar]
- Lehmann R, Nusslein-Volhard C 1987. Involvement of the pumilio gene in the transport of an abdominal signal in the Drosophila embyro. Nature 329: 167–170 [Google Scholar]
- Li Y, Kiledjian M 2010. Regulation of mRNA decapping. Wiley Interdiscip Rev RNA 1: 253–265 [DOI] [PubMed] [Google Scholar]
- Lim F, Spingola M, Peabody DS 1994. Altering the RNA binding specificity of a translational repressor. J Biol Chem 269: 9006–9010 [PubMed] [Google Scholar]
- Lin H, Spradling AC 1997. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124: 2463–2476 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lu G, Dolgner SJ, Hall TM 2009. Understanding and engineering RNA sequence specificity of PUF proteins. Curr Opin Struct Biol 19: 110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff WF, Wagner EJ, Duronio RJ 2008. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat Rev Genet 9: 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee CJ, Pym EC, Moffat KG, Baines RA 2004. Regulation of neuronal excitability through pumilio-dependent control of a sodium channel gene. J Neurosci 24: 8695–8703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G 2013. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet 14: 447–459 [DOI] [PubMed] [Google Scholar]
- Miles WO, Tschop K, Herr A, Ji JY, Dyson NJ 2012. Pumilio facilitates miRNA regulation of the E2F3 oncogene. Genes Dev 26: 356–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Olivas WM 2011. Roles of Puf proteins in mRNA degradation and translation. Wiley Interdiscip Rev RNA 2: 471–492 [DOI] [PubMed] [Google Scholar]
- Moretti F, Kaiser C, Zdanowicz-Specht A, Hentze MW 2012. PABP and the poly(A) tail augment microRNA repression by facilitated miRISC binding. Nat Struct Mol Biol 19: 603–608 [DOI] [PubMed] [Google Scholar]
- Morris AR, Mukherjee N, Keene JD 2008. Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol Cell Biol 28: 4093–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Wharton RP 1995. Binding of Pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell 80: 747–756 [DOI] [PubMed] [Google Scholar]
- Olivas W, Parker R 2000. The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J 19: 6602–6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108 [DOI] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M 2011. Global quantification of mammalian gene expression control. Nature 473: 337–342 [DOI] [PubMed] [Google Scholar]
- Spassov DS, Jurecic R 2003. The PUF family of RNA-binding proteins: does evolutionarily conserved structure equal conserved function? IUBMB Life 55: 359–366 [DOI] [PubMed] [Google Scholar]
- Suh N, Crittenden SL, Goldstrohm A, Hook B, Thompson B, Wickens M, Kimble J 2009. FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics 181: 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritschler F, Huntzinger E, Izaurralde E 2010. Role of GW182 proteins and PABPC1 in the miRNA pathway: a sense of deja vu. Nat Rev Mol Cell Biol 11: 379–384 [DOI] [PubMed] [Google Scholar]
- Van Etten J, Schagat TL, Hrit J, Weidmann CA, Brumbaugh J, Coon JJ, Goldstrohm AC 2012. Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs. J Biol Chem 287: 36370–36383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Schoderboeck L, Gingl E, Luzi E, Riefler J, Di Leva F, Karra D, Thomas S, Kiebler MA, Macchi P 2010. Mammalian Pumilio 2 regulates dendrite morphogenesis and synaptic function. Proc Natl Acad Sci 107: 3222–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McLachlan J, Zamore PD, Hall TM 2002. Modular recognition of RNA by a human pumilio-homology domain. Cell 110: 501–512 [DOI] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ 2009. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeles CJ, Wu MZ, Claycomb JM 2013. A multitasking Argonaute: exploring the many facets of C. elegans CSR-1. Chromosome Res 21: 573–586 [DOI] [PubMed] [Google Scholar]
- Weidmann CA, Goldstrohm AC 2012. Drosophila Pumilio protein contains multiple autonomous repression domains that regulate mRNAs independently of Nanos and brain tumor. Mol Cell Biol 32: 527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M, Bernstein DS, Kimble J, Parker R 2002. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet 18: 150–157 [DOI] [PubMed] [Google Scholar]
- Wreden C, Verrotti AC, Schisa JA, Lieberfarb ME, Strickland S 1997. Nanos and pumilio establish embryonic polarity in Drosophila by promoting posterior deadenylation of hunchback mRNA. Development 124: 3015–3023 [DOI] [PubMed] [Google Scholar]
- Ye B, Petritsch C, Clark IE, Gavis ER, Jan LY, Jan YN 2004. Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr Biol 14: 314–321 [DOI] [PubMed] [Google Scholar]
- Zamore PD, Williamson JR, Lehmann R 1997. The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. RNA 3: 1421–1433 [PMC free article] [PubMed] [Google Scholar]
- Zamore PD, Bartel DP, Lehmann R, Williamson JR 1999. The PUMILIO–RNA interaction: a single RNA-binding domain monomer recognizes a bipartite target sequence. Biochemistry 38: 596–604 [DOI] [PubMed] [Google Scholar]
- Zekri L, Kuzuoglu-Ozturk D, Izaurralde E 2013. GW182 proteins cause PABP dissociation from silenced miRNA targets in the absence of deadenylation. EMBO J 32: 1052–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Gallegos M, Puoti A, Durkin E, Fields S, Kimble J, Wickens MP 1997. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature 390: 477–484 [DOI] [PubMed] [Google Scholar]