Abstract
Background and purpose
We previously evaluated a new uncemented femoral stem designed for elderly patients with a femoral neck fracture and found stable implant fixation and good clinical results up to 2 years postoperatively, despite substantial periprosthetic bone mineral loss. We now present the medium-term follow-up results from this study.
Patients and methods
In this observational prospective cohort study, we included 50 patients (mean age 81 (70–92) years) with a femoral neck fracture. All patients underwent surgery with a cemented cup and an uncemented stem specifically designed for fracture treatment. Outcome variables were migration of the stem measured with radiostereometry (RSA) and periprosthetic change in bone mineral density (BMD), measured with dual-energy X-ray absorptiometry (DXA). Hip function and health-related quality of life were assessed using the Harris hip score (HHS) and the EuroQol-5D (EQ-5D). DXA and RSA data were collected at regular intervals up to 4 years, and data concerning reoperations and hip-related complications were collected during a mean follow-up time of 5 (0.2–7.5) years.
Results
At 5 years, 19 patients had either passed away or were unavailable for further participation and 31 could be followed up. Of the original 50 patients, 6 patients had suffered a periprosthetic fracture, all of them sustained after the 2-year follow-up. In 19 patients, we obtained complete RSA and DXA data and no component had migrated after the 2-year follow-up. We also found a continuous total periprosthetic bone loss amounting to a median of –19% (–39 to 2). No changes in HHS or EQ-5D were observed during the follow-up period.
Interpretation
In this medium-term follow-up, the stem remained firmly fixed in bone despite considerable periprosthetic bone mineral loss. However, this bone loss might explain the high number of late-occurring periprosthetic fractures. Based on these results, we would not recommend uncemented femoral stems for the treatment of femoral neck fractures in the elderly.
In Sweden, cemented stems have been used primarily for patients with a femoral neck fracture (FNF), but with the introduction of modern hydroxyapatite- (HA-) coated implants, uncemented fixation has increased in popularity (Garellick et al. 2011). Excellent long-term results have been reported for patients with primary osteoarthritis (OA) of the hip (Boden et al. 2006a). The concept of inserting an uncemented femoral component is attractive to many surgeons, as the cementing procedure can induce cardiac arrhythmia and/or cardiorespiratory collapse (Parvizi et al. 1999). However, a recent report from the Swedish Hip Arthroplasty Register indicated that uncemented implants used for patients with an FNF are associated with a 20-fold higher risk of reoperation due to periprosthetic fracture than cemented matte stems (Leonardsson et al. 2012).
We have already published an evaluation of a new uncemented femoral stem designed for elderly patients with an FNF; this showed good clinical results and stable implant fixation up to 2 years after surgery despite substantial periprosthetic bone loss (Sköldenberg et al. 2011). We now present the medium-term follow-up from this study.
Patients and methods
This observational, prospective single-cohort study was carried out between October 2005 and August 2013 (inclusion period October 2005 to March 2008) at the Department of Orthopedic Surgery, Danderyd Hospital, Stockholm, Sweden. The study was approved by the Ethics Committee of Karolinska Institutet (Dnr 04-086/4) and the Radiation Safety Committee at Danderyd Hospital (Dnr 005-5).
Patients
We included 50 patients ≥ 70 years of age with a displaced femoral neck fracture (Garden III or IV) who had an intact cognitive function (at least 8 correct answers on a 10-item (SPMSQ) mental test) (Pfeiffer 1975), who could walk independently with or without the help of walking aids, and who were willing to participate in the study. All patients gave their written consent to participate. Exclusion criteria and details of the study protocol have been presented previously (Sköldenberg et al. 2011).
Stem design
The implant, the Biomet Fracture Stem (BFX; Biomet UK Ltd., Bridgend, UK), is a tapered, collared stem intended for uncemented fixation. It is made of titanium alloy (Ti-6Al-4V) with a grit-blasted surface roughness of 7.5–10 µm. It has a straight 3° proximal-to-distal taper in 2 planes and a taper from the lateral shoulder to the medial calcar area. It has plasma-sprayed hydroxyapatite (HA) on the entire surface (thickness 65–95 µm, crystallinity 50–70%, purity > 95%) to enable fast ingrowth into osteoporotic bone. The geometry of the stem, except for the collar, is identical to that of the Bi-Metric stem (Meding et al. 2004, Boden et al. 2006).
Surgery
The patients underwent THA surgery using the new stem articulating on a 32-mm cobalt-chrome head against a cemented cup (Müller; Biomet UK). A posterior approach, with repair of the posterior capsule and external rotator muscles, was used in all patients. The femur was reamed until cortical bone contact was obtained. Then the proximal femur was prepared with broaches of increasing size until rotational stability was achieved. Before the final implant was inserted, 5–9 tantalum marker beads (1.0 mm in diameter) were inserted in the cancellous bone of the proximal femur. The patients were mobilized using a standard physiotherapy program. They were encouraged to mobilize with full weight bearing using crutches for support.
Outcome measures
The outcome variables were migration of the stem measured with RSA, changes in bone mineral density (BMD) in 7 Gruen zones around the stem, the occurrence of periprosthetic fractures and other hip-related complications, and clinical outcome.
Radiostereometric analysis. The RSA method followed the published guidelines for RSA (Valstar et al. 2005). We took digital calibrated radiographs (Bucky Diagnostic; Philips, Eindhoven, the Netherlands) using a fixed and a mobile X-ray (Roentgen) source (120 kV, 4–6 mAs), and a uniplanar calibration cage (Uniplanar digital 43; RSA Biomedical, Umeå, Sweden). All data were analyzed using UmRSA computer software (RSA Biomedical). Based on double examinations at 12 months, we calculated the precision of RSA. For translation along the x- (transverse), y- (vertical), and z- (anteroposterior (AP)) axes, this was 0.27, 0.19, and 0.52 mm, respectively. For rotation about the x- (flexion/extension), y- (ante-/retroversion), and z- (varus/valgus) axes, the values were 0.52°, 0.76°, and 0.27°, respectively, and for the maximal total point motion (MTPM) it was 0.74 mm. Further details of the RSA method in this study have been presented in the report on 2-year follow-up (Sköldenberg et al. 2011).
Bone densitometric analysis. BMD of the periprosthetic femur in the frontal plane was measured in the 7 Gruen zones in 1 plane using DXA (DPX-L; Lunar Co., Madison, WI). The change in frontal periprosthetic BMD ratio in all the individual zones, as well as in the entire periprosthetic region (zones 1–7), was calculated by dividing the BMD value at each follow-up visit by the postoperative BMD and converting it to a percentage change. We had previously conducted double measurements and validated the method. Postoperatively, the BMDs of vertebrae L1–L4 (lumbar spine) of all patients were measured to assess the patient’s general bone mass. The BMDs of the L1–L4 vertebrae were also measured at the 4-year follow-up.
Clinical outcome. Hip function was evaluated with the Harris hip score (HHS) at all follow-ups. This score has been validated for patients with FNFs (Frihagen et al. 2008). Health-related quality of life was assessed with the EQ-5D (EuroQoL) questionnaire. After inclusion, but before surgery, we asked the patients to estimate their HHS and EQ-5D for the week prior to the occurrence of the fracture.
Hip-related complications and reoperations. We used the Swedish unique personal ID-number to identify all hip-related complications during the study period. We searched digital medical charts at Danderyd Hospital, the Swedish Hip Arthroplasty Register, and the Swedish Patient Registry. All hip-related complications of the study were managed and registered at our department, and no other reoperations or complications were found to have occurred at other hospitals in Sweden.
Sample size
In the previously published paper (Sköldenberg et al. 2011), we used a sample size that indicated that we would need to recruit 50 patients to allow for loss to follow-up and to allow for analysis of subgroups of patients with high and low BMD. Before the 4-year follow-up, a new sample size analysis was performed to indicate how many patients would be needed for investigation of the migration pattern of the stems in the patients who were still living. With 18 patients, the study would have a power (2-sided, p = 0.01) of more than 99%, and of 93% to detect a continuous migration in MTPM and y-translation. These estimates were based on a previous RSA study with the HA-coated version of the Bi-Metric stem (Søballe et al. 1993) where mean MTPM was 1.9 (SD 1.3) mm and mean y-translation was 0.2 (0.2) mm.
Statistics
We used a simple linear regression analysis to investigate factors predictive of stem migration (expressed as MTPM) or bone loss (percentage change in BMD in zones 1–7). Due to the low number of patients available for migration and BMD analysis at 4 years (n = 19), we did not consider it appropriate to use a multivariate analysis. Factors investigated separately in the analysis were covariates which, from the results of previous studies, are known to influence migration or bone remodeling around implants. These factors are (1) postoperative periprosthetic BMD (zones 1–7) (Sköldenberg et al. 2011), (2) postoperative BMD of the lumbar spine (Alm et al. 2009), (3) age (Brodner et al. 2004), (4) gender (Brodner et al. 2004), and (5) stem size (Sköldenberg et al. 2006). We used the Shapiro-Wilk normality test to confirm the normality of residuals. Comparisons between migration, BMD, and clinical scores at 2 and 4 years and also comparisons of bone mineral loss in patients with and without periprosthetic fractures were analyzed with non-parametric tests. All p-values ≤ 0.05 were considered significant. We used SPSS software version 20 for Mac.
Results
Patients
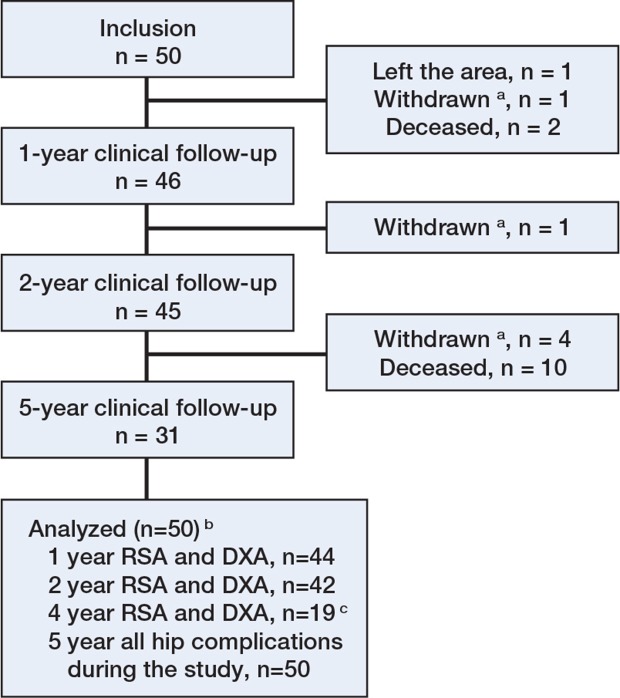
At the final follow-up in August 2013, 12 of the 50 patients had died, 7 were unable to participate further in the study (due to ill-health or dementia (n = 6) or from the fact that they had moved out of the Stockholm area (n = 1)) and 31 were available for clinical follow-up (Figure 1 and Table 1). For 19 of these 31 patients, we were able to obtain complete RSA and DXA data at 4 years. The mean follow-up time for the whole cohort was 5.3 (0.2–7.5) years.
Figure 1.
Diagram of patient flow through the study. The patient flow up to the 1-year follow-up is presented in more detail in a previous paper (Sköldenberg et al. 2011).
aWithdrawn from the study due to illness or dementia.
bAnalyzed for the occurrence of hip-related complications and reoperations.
c 12 patients had 4-year clinical follow-up (HHS and EQ-5D) but we were unable to obtain complete RSA and DXA measurements. This was for technical reasons in 3 patients (i.e. tantalum marker occlusion on radiographs) and 9 patients were too frail to attend follow-up at the hospital and were interviewed in a nursing home.
Table 1.
Baseline characteristics of subjects (n = 50)
| Age in years, mean (SD) | 81 (5) |
| Female/male | 36/14 |
| Body mass index, mean (SD) | 24 (4) |
| ASA classification (Owens et al. 1978) (1–2/3–4) | 29/21 |
| Charnley classification (Charnley 1972) (A/B/C) | 31/5/14 |
| Bone mineral density, total hip a | |
| WHO classification (2011) | |
| Normal bone density | 9 |
| Osteopenia | 14 |
| Osteoporosis | 16 |
| Density in g/cm2, mean (SD) | 0.78 (0.14) |
| Bone mineral density, L1-L4 vertebrae b | |
| WHO classification (2011) | |
| Normal bone density | 16 |
| Osteopenia | 13 |
| Osteoporosis | 16 |
| Density in g/cm2, mean (SD) | 1.05 (0.25) |
| Stem size in mm (9–11/12–14/15–17) | 13/22/15 |
measured in 39 patients with a healthy contralateral hip at inclusion.
measured in 45 patients in whom the lumbar spine BMD could be evaluated at inclusion.
Outcome variables
Radiostereometry. We found no migration of the stems between 2 and 4 years. At 4 years, the median migration for translations compared to the postoperative examinations was small: –0.07, –0.06, and 0.06 mm on the x-, y-, and z-axes. Likewise, the median rotation was small at –0.6, –0.5, and –0.08 degrees on the x-, y-, and z-axes. The total migration (MTPM) was 1.5 mm at 4 years (Table 2). We found no correlation between migration and the initial periprosthetic or lumbar spine BMD, age, gender, or stem size. None of the patients who had suffered a dislocation showed any migration in any direction.
Table 2.
Migration and percentage change in BMD at 2 and 4 years for 19 patients with complete data at 4 years. The p-values were derived from the Wilcoxon signed-rank test
| 2 years | 4 years | p-value | |||||
|---|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | ||
| Migration | |||||||
| Translation, mm | |||||||
| Transverse (x) | –0.08 | –0.2 | 0.5 | –0.07 | –0.3 | 0.3 | 0.3 |
| Vertical (y) | –0.07 | –0.3 | 0.3 | –0.06 | –1.0 | 0.6 | 0.5 |
| Anterioposterior (z) | 0.01 | –1.5 | 0.5 | 0.06 | –1.5 | 0.6 | 0.1 |
| Rotation (°) | |||||||
| Flexion/extension (x) | –0.5 | –3.3 | 0.5 | –0.6 | –3.1 | 0.5 | 1.0 |
| Ante-/retroversion (y) | –0.5 | –4.7 | 0.2 | –0.5 | –4.3 | 1.0 | 0.1 |
| Varus/valgus (z) | –0.03 | –1.9 | 0.5 | –0.08 | –1.8 | 0.5 | 0.2 |
| Total migration | |||||||
| MTPM | 1.6 | 0.8 | 5.5 | 1.5 | 0.6 | 5.2 | 0.4 |
| Percentage change in BMD versus postop | |||||||
| All zones (1 to 7) | –8.8 | –39.1 | 1.3 | –18.8 | –36.9 | 2.4 | 0.01 |
| Zone 1 | –33.5 | –59.6 | –13.7 | –36.2 | –59.6 | –13.7 | 0.01 |
| Zone 2 | –12.0 | –61.1 | 10.6 | –21.6 | –47.0 | 17.7 | 0.03 |
| Zone 3 | –4.4 | –60.5 | 13.6 | –9.6 | –39.0 | 14.2 | 0.4 |
| Zone 4 | –2.3 | –37.8 | 17.5 | –6.2 | –64.1 | 4.3 | 0.07 |
| Zone 5 | 0.0 | –31.6 | 21.7 | –8.5 | –29.8 | 13.4 | 0.05 |
| Zone 6 | –16.7 | –43.9 | 3.3 | –24.1 | –52.1 | 3.0 | 0.02 |
| Zone 7 | –26.4 | –43.8 | 11.0 | –25.9 | –56.9 | 8.0 | 0.2 |
| Lumbar spine | 0.03 | –5.1 | 7.0 | 0.05 | –4.06 | 6.01 | 0.7 |
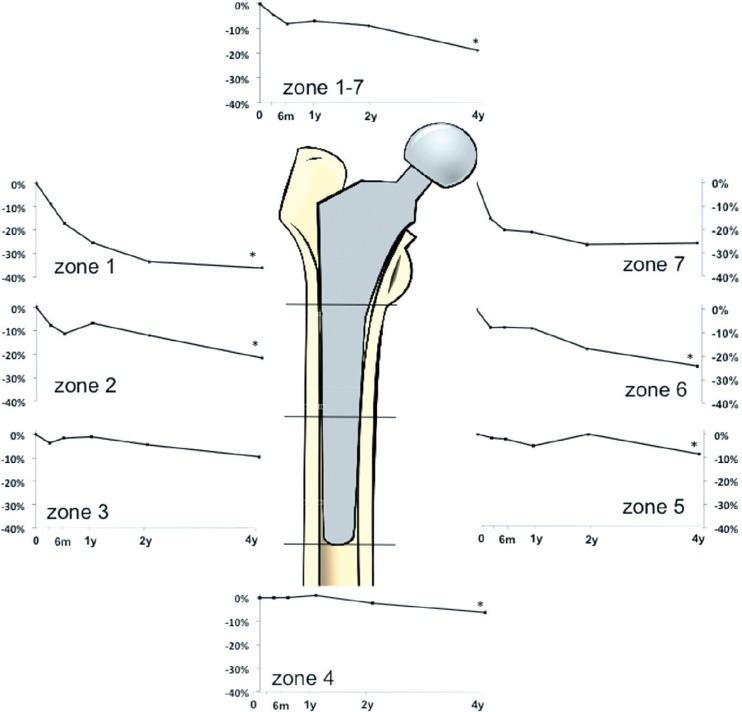
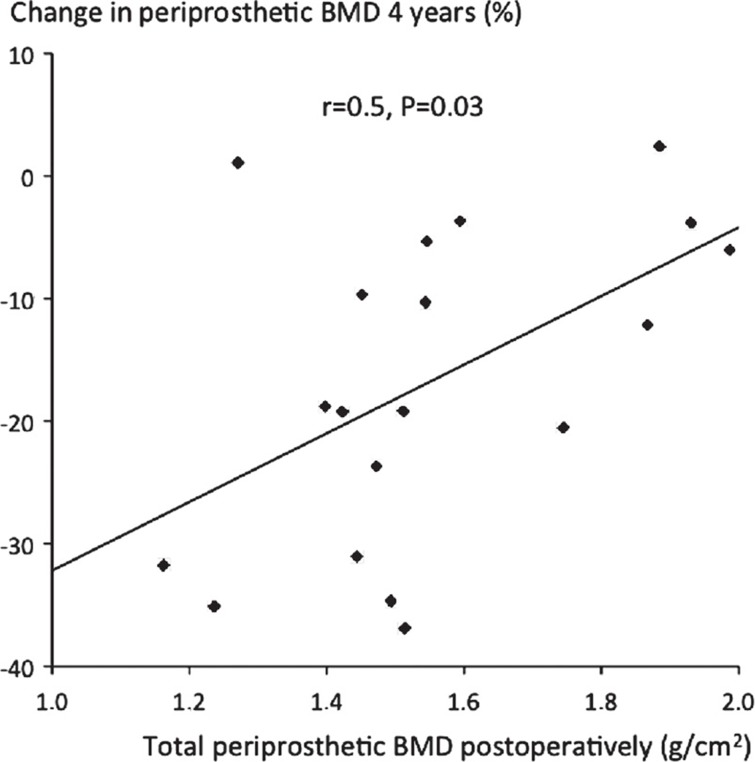
Bone densitometry. We found a decrease in periprosthetic BMD in all Gruen zones (1–7): median –19%, which was statistically significant compared to the 2-year value of –8.8% (p = 0.01, Wilcoxon signed-rank test). In individual zones, the decrease in periprosthetic BMD was continuous in all zones except zones 3 and 7 (Figure 2). The bone loss was greatest in zones 1 and 7, with a decrease of 36% and 26% (Table 2). The loss of BMD in zones 1–7 correlated with the initial BMD surrounding the implant (R = 0.5, p = 0.03): the lower the initial BMD, the greater the bone loss (Figure 3). We found no correlation between bone loss and the BMD of the lumbar spine, age, gender, or stem size.
Figure 2.
Graphs showing periprosthetic bone remodeling in zones 1–7 with median percentage change in bone mineral density (BMD) around the implant.* p ≤ 0.05 compared to the 2-year follow-up (Wilcoxon signed-rank test).
Figure 3.
Periprosthetic postoperative BMD (x-axis) plotted against percentage change in BMD around the implant (zones 1–7) (y-axis) at 4 years.
Clinical outcome. Mean HHS was 83 (95% CI: 78–88), with no statistically significant change compared to the 2-year value. Similarly, the health-related quality of life (EQ-5D) remained unchanged compared to the 2-year value (mean 0.7, 95% CI: 0.5–0.8). We found no correlation between clinical outcome data and migration or bone loss (data not shown).
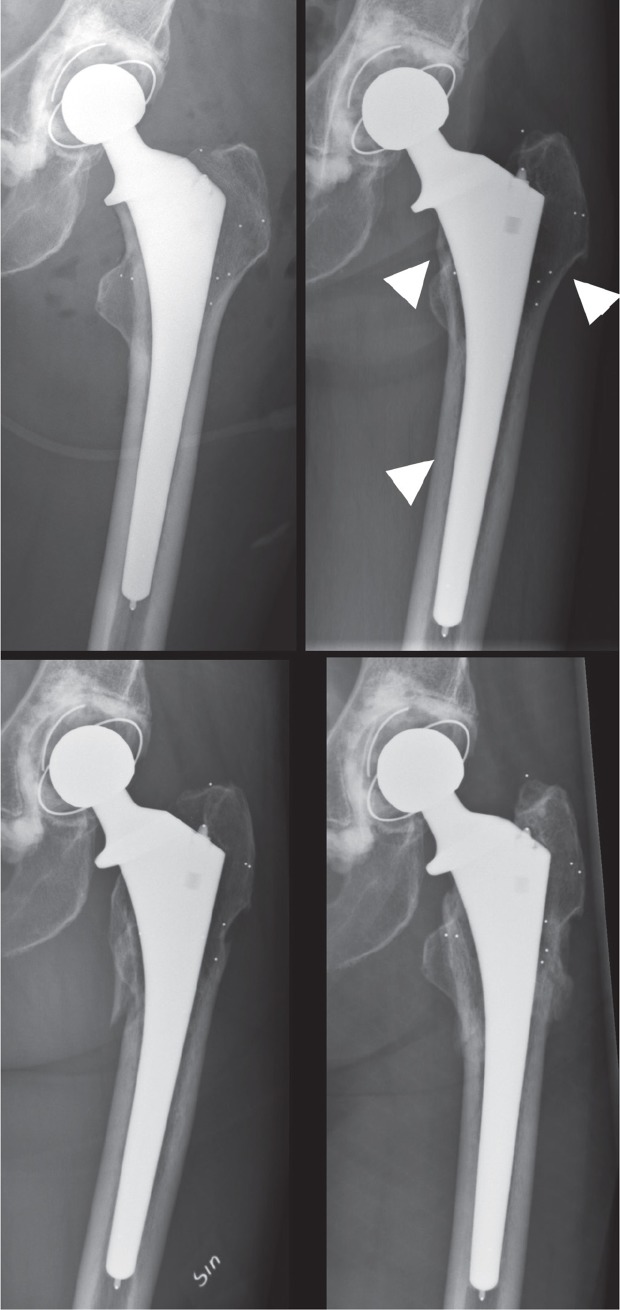
Hip-related complications and reoperations. There were 2 periprosthetic joint infections, 1 early and 1 hematogenous. Both patients underwent debridement, antibiotics and implant retention (DAIR) and recovered successfully. 7 patients sustained a dislocation of the hip at a median of 24 (2–92) days after surgery. All were treated with closed reduction under sedation. 3 patients experienced a subsequent dislocation, one of which required a revision of the acetabular component. 6 patients sustained a periprosthetic fracture after low-energy trauma. These fractures occurred 2.1, 2.1, 2.2, 2.9, 5.5, and 5.7 years after surgery. 3 were classified as Vancouver B1 (undisplaced with stable stem) and were treated nonoperatively with protected weight bearing (Figure 4). 1 was a B2 fracture (loose stem), which was treated with a long revision stem, and 2 were C type fractures (distal to the stem); these were treated with open reduction and internal fixation. All fractures healed.
Figure 4.
Example of a late-occurring periprosthetic fracture. a. Postoperatively. b. 2-year follow-up with radiographic signs of stress-shielding (arrows) including calcar atrophy and hypoattenuation of bone mass in the greater trochanter and diaphysis. At 2 years, the total decrease in BMD around the stem was –16% compared to the postoperative value. c. The periprosthetic fracture that was sustained after a low-energy trauma at 2.2 years. d. The healed fracture at 2.7 years, treated with protected weight bearing.
The patients who sustained a periprosthetic fracture after the 2-year follow-up but before the 4-year follow-up could not be evaluated with RSA or DXA at 4 years. However, we had BMD data at 2 years on 5 of the 6 patients. The median change in BMD in zones 1–7 at 2 years was –26% (–38% to –13%) compared to the initial postoperative value. For the remaining 44 patients, we had 2-year data on 38 patients with a median change in BMD of –14% (–49% to 1%).
Discussion
In this observational, prospective cohort study on a selected group of cognitively intact elderly patients, we found a 1-tenth incidence rate of periprosthetic fractures up to 5 years after surgery. The implant functioned well and was radiographically stable despite substantial progressive periprosthetic bone mineral loss. We believe that this bone mineral loss—in combination with fracture patients’ propensity for falls—would explain our findings.
Uncemented stems and femoral neck fractures
Our results are in keeping with the short-term (1-year) results of a randomized clinical trial (RCT) comparing an HA-coated stem (Corail) and a cemented stem (Spectron) in patients with FNF, where the authors noted an incidence of postoperative periprosthetic fractures of 4% (Figved et al. 2009) in the uncemented group as compared to 1% in the cemented group. The same research group have recently published their 5-year data and found an even higher incidence of late-occurring postoperative periprosthetic fractures in the uncemented group (9.4%) compared to the cemented group (1%) (Langslet et al. 2014). In general, there appears to be an increased risk of intraoperative fractures and postoperative fractures associated with the use of uncemented stems in FNF patients (Parker and Gurusamy 2006). In a recent report from the Swedish Hip Arthroplasty Register (Garellick et al. 2011), a comparison of survival curves between cemented and uncemented stems used for patients with osteoarthritis of the hip showed a biphasic progression. During the first 5 years after surgery, the risk of stem revision, regardless of cause, was reduced by about 50% when using cemented fixation. During the second period (8–16 years), the survival of the uncemented stems was better than that of cemented stems due to the increasing revision rate resulting from aseptic loosening of the latter stem type.
We recently published a report of elderly patients with a displaced FNF with similar demographics (Chammout et al. 2012) as in the current study, and we found mortality rates of 75% after 11 years and of 87% after 17 years. This has also been reported by others, and it could therefore be argued that, in this patient group, the superior short-term results found with the use of a cemented stem are of greater relevance than long-term stem survival. The risk of periprosthetic fracture should be considered when choosing the type of stem to be used in elderly patients. A cemented stem is associated with substantially lower risk of fracture (Leonardsson et al. 2012), and this should outweigh the potential risks to the patient associated with the use of cement (Parvizi et al. 1999).
Migration and bone remodeling
We used RSA to evaluate the fixation of the stem, and although RSA has been used to study the healing of FNFs after internal fixation (Ragnarsson and Kärrholm 1991), only 3 other studies using RSA have been published on the subject of hip arthroplasty in this patient group. The first involved the 2-year results of the current study and the other 2 studies dealt with the subject of cartilage wear and implant stability following hemiarthroplasty (Figved et al. 2012, Schewelov et al. 2012). Similarly, bone remodeling around femoral stems after THA in patients with degenerative joint disease has been extensively studied using DXA (Kilgus et al. 1993, Ang et al. 1997, Sköldenberg et al. 2006) but it has never been investigated in patients with FNF. In our study, the migration patterns of the stems at 3 months did not differ from the results of previously published papers on well-functioning uncemented stems for patients with osteoarthritis (Søballe et al. 1993). This indicates that early osseointegration can be achieved in osteoporotic bone and that immediate weight bearing is well tolerated postoperatively. Disuse-periprosthetic-proximal atrophy of the femur, also known as stress-shielding, is a well-documented phenomenon (Kilgus et al. 1993, Ang et al. 1997, Boden et al. 2006b) but to date there have been few reported clinical consequences (Hsieh et al. 2005). This periprosthetic bone loss may be one possible explanation for the increased risk of periprosthetic fractures found in patients who have undergone surgery for FNF with uncemented implants (Leonardsson et al. 2012), particularly as we found the BMD loss to be more pronounced in those who later sustained a periprosthetic fracture (Figure 4). This bone loss seems to be associated with larger stem size and a low initial BMD (Engh and Bobyn 1988, Rahmy et al. 2004, Sköldenberg et al. 2006) (Figure 3), though we were unable to verify the effect of stem size, possibly due to the low number of patients available for DXA at follow-up. The BMD gradually decreases even more over time, and generally more in the proximal areas than in those that are distal. 2 recent publications have further supported the hypothesis that there is indeed a lower reoperation rate for cemented stems than for uncemented stems (Gjertsen et al. 2012, Viberg et al. 2013).
Strengths and weaknesses of the study
To our knowledge, the has been the first study to measure periprosthetic BMD around a femoral stem in hip fracture patients. We could also correlate this to the incidence of periprosthetic fractures. With our extensive database search of all possible complications, we were able to identify periprosthetic fractures that would not normally have been reported to the Swedish Hip Arthroplasty Register. The greatest weakness was that we lacked a control group, and since there have been no previous publications on FNF patients and periprosthetic change in BMD, we can only compare our results with those for OA patients. In addition, our sample size calculation was performed for RSA data and not to estimate the incidence of complications. The study was also performed on a selected group of cognitively intact FNF patients, whose results may have differed from the general hip fracture population.
Conclusion
In conclusion, we found an important clinical effect of stress-shielding: a high incidence of periprosthetic fractures. Thus, even though the stem can easily achieve safe fixation even in osteoporotic bone and give good clinical results, we would not recommend use of this uncemented stem in the treatment of femoral neck fracture in elderly patients.
Acknowledgments
OS initiated the study, followed up patients, and wrote the manuscript. HS, PKP, and OM followed up patients and wrote the manuscript. TE and AS wrote the manuscript.
We express our sincere thanks and gratitude to all the staff of the Department of Nuclear Medicine and the Department of Radiology, Danderyd Hospital, who assisted us with this study, particularly Hans-Jerker Lundberg for his technical assistance with the DEXA analyses and Lise-Lotte Widmark for her invaluable help and assistance in conducting the RSA examinations. We also thank Dr Max Gordon for his help with the statistical analysis.
No competing interests declared.
References
- Alm JJ, Makinen TJ, Lankinen P, Moritz N, Vahlberg T, Aro HT. Female patients with low systemic BMD are prone to bone loss in Gruen zone 7 after cementless total hip arthroplasty . Acta Orthop. 2009;80(5):531–7. doi: 10.3109/17453670903316801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KC, Das De S, Goh JC, Low SL, Bose K. Periprosthetic bone remodelling after cementless total hip replacement. A prospective comparison of two different implant designs . J Bone Joint Surg (Br) 1997;79(4):675–9. doi: 10.1302/0301-620x.79b4.7410. [DOI] [PubMed] [Google Scholar]
- Boden H, Salemyr M, Skoldenberg O, Ahl T, Adolphson P. Total hip arthroplasty with an uncemented hydroxyapatite-coated tapered titanium stem: results at a minimum of 10 years’ follow-up in 104 hips . J Orthop Sci. 2006a;11(2):175–9. doi: 10.1007/s00776-005-0986-5. [DOI] [PubMed] [Google Scholar]
- Boden HS, Skoldenberg OG, Salemyr MO, Lundberg HJ, Adolphson PY. Continuous bone loss around a tapered uncemented femoral stem: a long-term evaluation with DEXA . Acta Orthop. 2006b;77(6):877–85. doi: 10.1080/17453670610013169. [DOI] [PubMed] [Google Scholar]
- Brodner W, Bitzan P, Lomoschitz F, Krepler P, Jankovsky R, Lehr S, et al. Changes in bone mineral density in the proximal femur after cementless total hip arthroplasty. A five-year longitudinal study . J Bone Joint Surg (Br) 2004;86(1):20–6. [PubMed] [Google Scholar]
- Chammout GK, Mukka SS, Carlsson T, Neander GF, Helge Stark AW, Skoldenberg OG. Total hip replacement versus open reduction and internal fixation of displaced femoral neck fractures: A randomized long-term follow-up study . J Bone Joint Surg (Am) 2012;94(21):1921–8. doi: 10.2106/JBJS.K.01615. [DOI] [PubMed] [Google Scholar]
- Engh CA, Bobyn JD. The influence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty . Clin Orthop. 1988;231:7–28. [PubMed] [Google Scholar]
- Figved W, Opland V, Frihagen F, Jervidalo T, Madsen JE, Nordsletten L. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures . Clin Orthop. 2009;467(9):2426–35. doi: 10.1007/s11999-008-0672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figved W, Dahl J, Snorrason F, Frihagen F, Rohrl S, Madsen JE, et al. Radiostereometric analysis of hemiarthroplasties of the hip—a highly precise method for measurements of cartilage wear . Osteoarthritis Cartilage. 2012;20(1):36–42. doi: 10.1016/j.joca.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Garellick G, Kärrholm J, Rogmark C, Rolfson O, Herberts P. Swedish Hip Arthroplasty Register Annual Report 2011. 2011.
- Gjertsen JE, Lie SA, Vinje T, Engesaeter LB, Hallan G, Matre K, et al. More re-operations after uncemented than cemented hemiarthroplasty used in the treatment of displaced fractures of the femoral neck: an observational study of 11,116 hemiarthroplasties from a national register . J Bone Joint Surg (Br) 2012;94(8):1113–9. doi: 10.1302/0301-620X.94B8.29155. [DOI] [PubMed] [Google Scholar]
- Hsieh PH, Chang YH, Lee PC, Shih CH. Periprosthetic fractures of the greater trochanter through osteolytic cysts with uncemented MicroStructured Omnifit prosthesis: retrospective analyses of 23 fractures in 887 hips after 5-14 years . Acta Orthop. 2005;76(4):538–43. doi: 10.1080/17453670510041538. [DOI] [PubMed] [Google Scholar]
- Kilgus DJ, Shimaoka EE, Tipton JS, Eberle RW. Dual-energy X-ray absorptiometry measurement of bone mineral density around porous-coated cementless femoral implants. Methods and preliminary results . J Bone Joint Surg (Br) 1993;75(2):279–87. doi: 10.1302/0301-620X.75B2.8444950. [DOI] [PubMed] [Google Scholar]
- Langslet E, Frihagen F, Opland V, Madsen JE, Nordsletten L, Figved W. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: 5-year followup of a randomized trial. Clin Orthop. 2014;472(4):1291–9. doi: 10.1007/s11999-013-3308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardsson O, Kärrholm J, Åkesson K, Garellick G, Rogmark C. Higher risk of reoperation for bipolar and uncemented hemiarthroplasty . Acta Orthop. 2012;83(5):459–66. doi: 10.3109/17453674.2012.727076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meding JB, Keating EM, Ritter MA, Faris PM, Berend ME. Minimum ten-year follow-up of a straight-stemmed, plasma-sprayed, titanium-alloy, uncemented femoral component in primary total hip arthroplasty . J Bone Joint Surg (Am) 2004;86(1):92–7. doi: 10.2106/00004623-200401000-00014. [DOI] [PubMed] [Google Scholar]
- Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings . Anesthesiology. 1978;49(4):239–43. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- Parker MJ, Gurusamy K. Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. Cochrane Database Syst Rev. 2006;3:CD001706. doi: 10.1002/14651858.CD001706.pub3. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty . Clin Orthop. 1999;369:39–48. doi: 10.1097/00003086-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients . J Am Geriatr Soc. 1975;23(10):433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Ragnarsson JI, Kärrholm J. Stability of femoral neck fracture. Roentgen stereophotogrammetry of 29 hook-pinned fractures . Acta Orthop Scand. 1991;62(3):201–7. doi: 10.3109/17453679108993593. [DOI] [PubMed] [Google Scholar]
- Rahmy AI, Gosens T, Blake GM, Tonino A, Fogelman I. Periprosthetic bone remodelling of two types of uncemented femoral implant with proximal hydroxyapatite coating: a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss . Osteoporos Int. 2004;15(4):281–9. doi: 10.1007/s00198-003-1546-5. [DOI] [PubMed] [Google Scholar]
- Schewelov T, Ahlborg H, Sanzen L, Besjakov J, Carlsson A. Fixation of the fully hydroxyapatite-coated Corail stem implanted due to femoral neck fracture: 38 patients followed for 2 years with RSA and DEXA . Acta Orthop. 2012;83(2):153–8. doi: 10.3109/17453674.2011.641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköldenberg OG, Boden HS, Salemyr MO, Ahl TE, Adolphson PY. Periprosthetic proximal bone loss after uncemented hip arthroplasty is related to stem size: DXA measurements in 138 patients followed for 2-7 years . Acta Orthop. 2006;77(3):386–92. doi: 10.1080/17453670610046307. [DOI] [PubMed] [Google Scholar]
- Sköldenberg OG, Salemyr MO, Boden HS, Lundberg A, Ahl TE, Adolphson PY. A new uncemented hydroxyapatite-coated femoral component for the treatment of femoral neck fractures: two-year radiostereometric and bone densitometric evaluation in 50 hips . J Bone Joint Surg (Br) 2011;93(5):665–77. doi: 10.1302/0301-620X.93B5.25374. [DOI] [PubMed] [Google Scholar]
- Søballe K, Toksvig-Larsen S, Gelineck J, Fruensgaard S, Hansen ES, Ryd L, et al. Migration of hydroxyapatite coated femoral prostheses. A Roentgen Stereophotogrammetric study . J Bone Joint Surg (Br) 1993;75(5):681–7. doi: 10.1302/0301-620X.75B5.8397213. [DOI] [PubMed] [Google Scholar]
- Viberg B, Overgaard S, Lauritsen J, Ovesen O. Lower reoperation rate for cemented hemiarthroplasty than for uncemented hemiarthroplasty and internal fixation following femoral neck fracture: 12- to 19-year follow-up of patients aged 75 years or more . Acta Orthop. 2013;84(3):254–9. doi: 10.3109/17453674.2013.792033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Scientific group on the assessment of osteoporosis at primary health care level. 2011.