Abstract
Background and purpose
Neurological deficits and pain are common after displaced sacral fractures. However, little is known about the association between the long-term clinical outcomes and radiological findings. We examined the long-term radiological findings and their correlations with lumbosacral pain and neurological deficits in the lower extremities after surgery for sacral fractures.
Methods
28 consecutive patients with operatively treated displaced sacral fractures were followed for mean 11 (8–13) years. Sensorimotor impairments of the lower extremities were classified according to the American Spinal Injury Association (ASIA). Pain was assessed using a visual analog scale (VAS). All patients underwent conventional radiographic examination and CT, and the images were scrutinized for nonunion, residual displacement, narrowing of the sacral foramina, and post-foraminal encroachment of the L5 and S1 nerves.
Results
There was residual displacement of ≥ 10 mm in 16 of the 28 patients. 26 patients had narrowing of 1 or more neural root foramina in L5-S4. 8 patients reported having no pain, 11 had pain only in the lumbosacral area, and 9 had pain in combination with radiating leg pain. Statistically significant correlations were found between narrowing of the sacral foramina and neurological deficits in the corresponding dermatomes. Significant correlations were also found between post-foraminal encroachment of L5 nerves and both sensory and motor deficits. No correlations were found between pain and radiological findings.
Interpretation
Pathological radiological findings are common 11 years after operatively treated displaced sacral fractures. Sacral foraminal and L5 post-foraminal bony encroachments were common findings and correlated with neurological deficits. However, lumbosacral pain did not correlate with radiological sequelae after fracture healing.
High-energy trauma with displaced sacral fracture is frequently associated with concomitant injuries to the intrapelvic soft tissue structures, including the lumbosacral plexus (Huittinen 1972, Denis et al. 1988, Majeed 1992). These injuries may cause considerable morbidity (Pohlemann et al. 1994, Tornetta and Matta 1996, Tötterman et al. 2006). However, little is known about which factors determine long-term clinical outcome in these patients, or what may explain the progression of neurological symptoms observed in a small proportion of patients (Adelved et al. 2012). Pelvic malunions and nonunions have been put forward as prognostic factors for impaired long-term outcome (Matta et al. 1996, Mears and Velyvis 2003, Oransky and Tortora 2007), but long-term structural changes of the sacrum after fracture healing have not been explored.
Our primary aim was to assess long-term radiological findings after surgically treated displaced sacral fractures. In addition, we wanted to assess whether pathological radiological findings, including bony structural changes of the sacrum, may contribute to neurological dysfunctions of the lower extremities or to the occurrence of pelvis-related pain.
Patients and methods
From July 1996 through October 2001, 39 consecutive patients with operatively treated displaced sacral fractures were prospectively registered at Oslo University Hospital, Ullevaal. Tötterman et al. (2006) conducted a 1-year follow-up study of these patients, where 32 patients were available for follow-up.
In the present long-term study, 28 of these 32 patients were available for clinical and radiological follow-up. Of the 4 patients who were lost to follow-up, 1 died, 2 declined to participate, and 1 patient was excluded due to a complete spinal cord injury with paraplegia. Of the patients included, 1 was excluded from the neurological examination in the study protocol due to hemiparesthesia after a head injury, but fulfilled the rest of the protocol. Mean follow-up time was 11 (8–13) years, female-to-male ratio was 7:21, and mean age was 43 (26–67) years. The mechanisms of injury included 20 motor vehicle accidents, 7 falls from heights, and 1 crush injury. Pelvic ring injuries were classified according to the AO/OTA fracture classification (Fracture and dislocation compendium. Orthopaedic Trauma Association Committee for Coding and Classification 1996). In 26 cases, the sacral fractures were part of a vertical shear pelvic ring disruption, classified as AO/OTA type-C injuries, and 2 patients had H-shaped sacral fractures. The sacral fractures were further classified according to Denis et al. (1988) as zone I (n = 1), zone II (n = 20), and zone III (n = 7). All fractures were treated operatively: 22 with open reduction and internal fixation, 12 with concomitant sacral laminectomy, and 6 with closed reduction and percutaneous SI-screw fixation (Adelved et al. 2012). Additional anterior plating was performed in 14 patients. The functional outcome of these patients after a mean of 11 years post injury has already been reported (Adelved et al. 2012).
Radiological examination
At follow-up, all patients underwent conventional radiographic examination of the pelvis and the lumbar spine. This included pelvic anteroposterior, inlet, and outlet views according to standard radiographic protocol (Bontrager and Lampignano 2005).
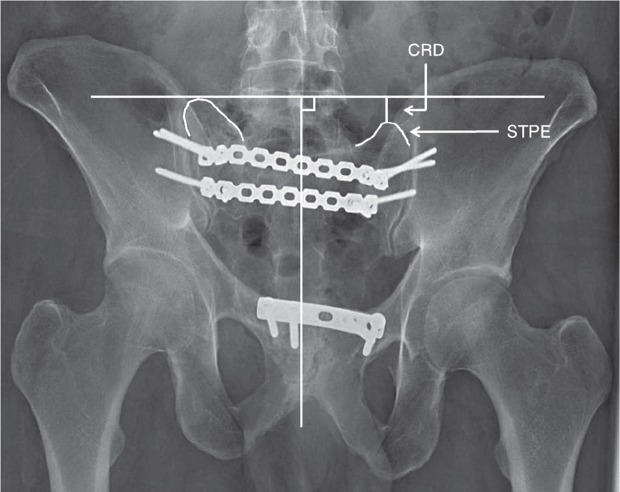
Residual displacement (RD) was defined as a cephalad or posterior displacement of the hemipelvis and sacrum, and was graded as < 10 mm or ≥ 10 mm. In the outlet views, cephalad residual displacement (CRD) was recorded by measuring the difference in height between the top lateral prominences of the 2 sacral transverse process elements (Standring et al. 2008) (Figure 1). In the inlet views, posterior residual displacement (PRD) was recorded by measuring the difference in height between the posterior superior iliac spines (Mears and Velyvis 2003) (Figure 2). In 4 cases, PRD was determined using the difference in height between the ischial spines in the inlet views, since in these images the posterior pelvic borders were not sufficiently visualized in the films.
Figure 1.
Measurement of cephalad residual displacement (CRD) of the right hemipelvis and sacrum illustrated on a pelvic outlet image. A midline vertical line is drawn along the axis of the central portion of the sacrum. A horizontal line, perpendicular to the vertical line, is drawn on the highest of the 2 measurement points—in this case, the lateral top points of the sacral transverse process elements (STPE). The difference in height between the horizontal line and the lowest of the 2 measurement points is the CRD.
Figure 2.
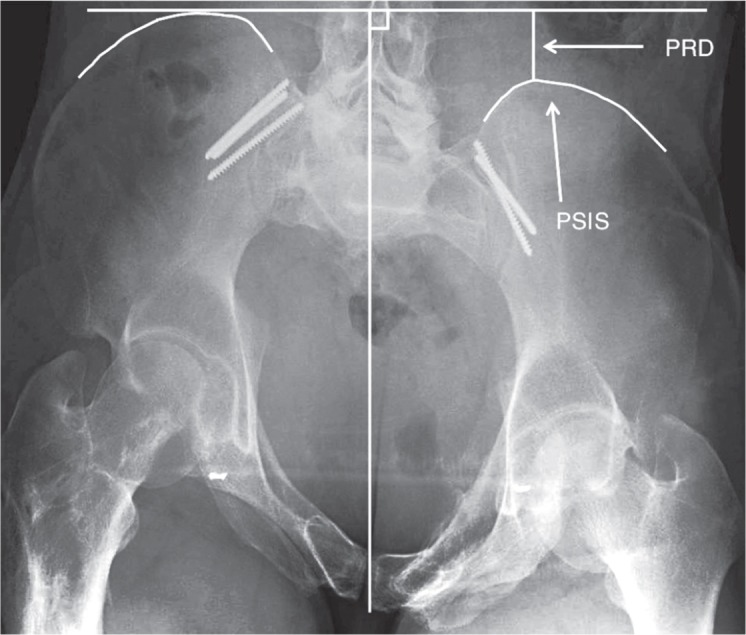
Measurement of posterior residual displacement (PRD) of the right hemipelvis and sacrum illustrated on a pelvic inlet image. A midline vertical line is drawn along the axis of the central portion of the sacrum. A horizontal line, perpendicular to the vertical line, is drawn on the highest of the 2 measurement points—in this case the posterior superior iliac spines (PSIS). The difference in height between the 2 PSIS is the PRD.
All patients were also examined with a 64-channel multi-detector computer tomography (MDCT). The CT images were scrutinized for nonunion, ankylosis, osteoarthritis (OA) in the L5-S1 facet joints and the SI-joints, and heterotopic ossification. Fracture healing was confirmed by the presence of bridging trabecular bone across the fracture lines on CT.
To identify any bony entrapment of the nerves, all 3 sets of 2D CT images were used, following each nerve from the spinal canal to the point where the nerve was peripheral to the sacrum. Narrowing of the neural foramina were recorded and then divided into 4 categories: 1: no narrowing; 2: less than 50% narrowing; 3: more than 50% narrowing; and 4: total occlusion of the foramen (Figure 3).
Figure 3.
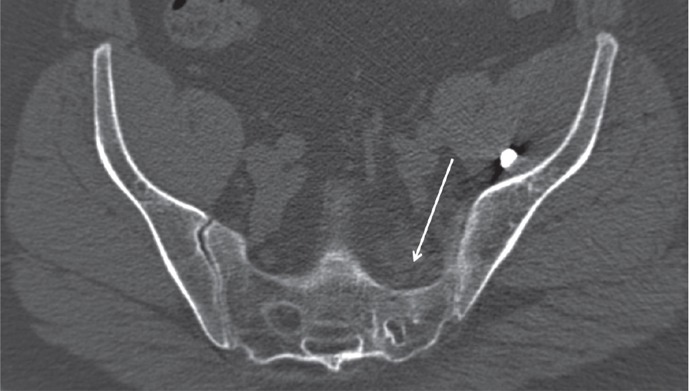
Considerable distortion and narrowing of the left S1 neural foramen, marked with an arrow. Note the unaffected contralateral foramen for comparison.
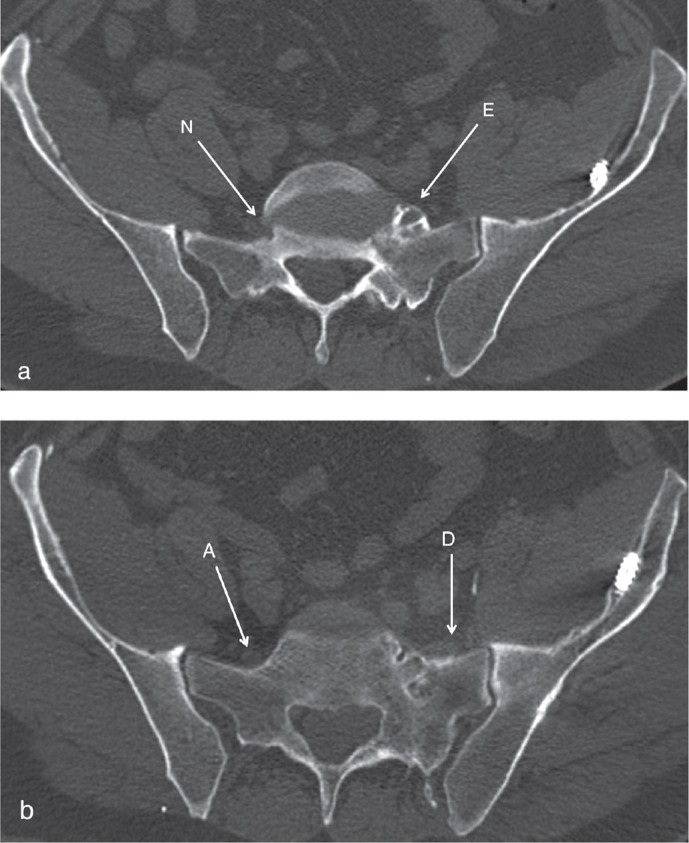
L5 and S1 nerves were then followed in their post-foraminal course and any changes in their path were recorded—i.e. displacement of the nerves by pathological bony structures and thus diversion from the assumed anatomical course or entrapment/overgrowth of the nerves by bony structures (Figure 4). The S2-S4 nerves were not as readily identifiable post-foraminally as L5 and S1, and they were therefore not included in the post-foraminal assessment. Finally, any narrowing of the spinal canal was recorded, using the midline sagittal images evaluating the inner tapering AP diameter.
Figure 4.
a. Bony encroachment of the left L5 nerve post-foraminally (E). The contralateral L5 is unaffected in its post-foraminal path (N). b. A few slices more distally. The left L5 is dislocated laterally from its anatomical path (D), running through the area with fracture sequelae, with topographical changes of the adjacent bony surface. (A) shows the unaffected contralateral L5 nerve running in its anatomic path.
A radiologist who was experienced in pelvic traumatology (JCH) reviewed all the radiographs and CT scans. He was blinded regarding the clinical information.
Clinical examination
Sensorimotor impairments were classified according to the American Spinal Injury Association (ASIA) score (Maynard et al. 1997, Adelved et al. 2012). The clinical assessment focused on neurological function in the lower extremities and the perineum.
Pain was assessed using a visual analog scale (VAS) ranging from 0 to 10, where zero represented no pain and 10 the most severe pain. The patients were asked to grade their average pain specifically in the lower back and posterior pelvic area. When present, radiating pain to the lower extremities was also recorded. Peripheral pain in the lower limbs was not considered when there were sequelae after lower extremity injuries.
Statistics
Due to small sample size and skewed distribution, non-parametric methods (namely, Spearman correlation coefficients) were used and p-values of ≤ 0.05 were considered to be statistically significant. We used PASW Statistics 18 software.
Ethics
The study was reviewed and approved by the Regional Committee for Medical and Health Research Ethics, Region South-East Norway. All patients signed an informed consent document at follow-up.
Results
Radiological findings
Residual displacement of more than 10 mm was observed in 16 patients: 6 with CRD, 3 with PRD, and 7 with a combination of CRD and PRD. In the remaining 12 patients with residual displacement of less than 10 mm, 10 had a combination of CRD and PRD, 1 had a pure CRD, and the other a pure PRD (Table 1). The average CRD of ≥ 10 mm was 15 (10–28) mm and the average PRD of ≥ 10 mm was 19 (11–35) mm. The average CRD of < 10 mm was 4 mm and the average PRD of < 10 mm was 6 mm (Table 2).
Table 1.
Residual displacements in the posterior pelvic ring assessed with inlet- and outlet radiographs at 11 years; n = 28
| n | |
|---|---|
| RD ≥ 10 mm | 16 |
| Combination of CRD and PRD | 7 |
| CRD | 6 |
| combined with PRD < 10 mm | 5 |
| pure CRD | 1 |
| PRD | 3 |
| combined with CRD < 10 mm | 2 |
| pure PRD | 1 |
| RD < 10 mm | 12 |
| Combination of CRD and PRD | 10 |
| Pure CRD | 1 |
| Pure PRD | 1 |
RD: residual displacement;
CRD: cephalad residual displacement;
PRD: posterior residual displacement.
Table 2.
Residual displacement in the posterior pelvic ring at 11 years, measured on the inlet- and outlet radiographs; n = 28
| Average (range) | |
|---|---|
| CRD ≥ 10 mm | 15 (10–28) mm |
| PRD ≥ 10 mm | 19 (11–35) mm |
| CRD < 10 mm | 4 mm |
| PRD < 10 mm | 6 mm |
CRD: cephalad residual displacement;
PRD: posterior residual displacement.
Nonunions were seen in only 2 patients, both of them occurring in the anterior pelvic ring. These patients were operated with both anterior and posterior fixation: in 1 of them all the implants were intact, while both anterior and posterior implant failure was noted in the other patient. All sacral fractures were healed.
26 of 28 patients had narrowing of 1 or more neural foramina from L5 to S4. Post-foraminal bony encroachment of the L5 nerve was observed in 22 patients (Table 3).
Table 3.
Radiological findings assessed with CT: changes in the lumbosacral area and the SI-joints, and bony structural changes in L5 and sacrum; n = 28
| n | |
|---|---|
| L5-S1 | |
| Ankylosis, facet joints | 9 |
| bilateral | 4 |
| with OA in contralateral joint | 3 |
| with normal contralateral joint | 2 |
| L5-TP fusion to sacrum | 3 |
| L5-S1 disc space narrowing | 14 |
| OA, facet joints | 18 |
| SI-joint(s) | |
| Ankylosis | 8 |
| unilateral | 6 |
| bilateral | 2 |
| OA | 12 |
| bilateral | 2 |
| with ankylosis in contralateral joint | 2 |
| with normal contralateral joint | 8 |
| Heterotopic ossification | 13 |
| Ilio-lumbar ligaments (ILL) | 4 |
| Pelvic floor (PF) | 5 |
| Both in PF and ILL | 4 |
| Sacral canal narrowing | 8 |
| L5 vertebral canal narrowing a | 1 |
| Neural root foramen narrowing | 26 |
| L5 | 17 |
| S1 | 20 |
| S2 | 15 |
| S3 | 9 |
| S4 | 9 |
| Post-foraminal bony encroachment, L5 nerve | 22 |
| Post-foraminal bony encroachment, S1 nerve | 16 |
sequelae after unstable L5 fracture.
SI: sacro-iliac; OA: osteoarthritis;
TP: transverse process.
In 9 patients, the implants had been removed due to posterior pelvic pain. In the remaining 19, breakage or loosening of implants was seen in 8 cases (involving anterior implant failure in 3 cases, posterior implant failure in 3, and both anterior and posterior implant failure in 2 cases).
Clinical findings
Neurological assessment in 1 patient was not possible due to sensory impairments in the right side of the body after a head injury. In the remaining 27 patients, 26 had neurological deficits in the lower extremities. 12 had minor to moderate sensory deficits affecting the L5-S4/S5 dermatomes. The L5-S1 dermatomes were mainly affected. Combined sensory deficits and muscle weakness were present in 14 patients. The deficits were unilateral in 9 and bilateral in 14. Uni- or bilaterality of neurological deficits could not be assessed in 3 patients who had undergone unilateral amputation below the knee: 2 initially due to crush injuries and 1 due to sequelae after severe foot and ankle fractures 5 years post injury.
8 patients reported having no pain at the long-term follow-up. Of the remaining 20 patients, 11 reported pain limited to the lumbosacral area while 9 reported both lumbosacral pain and radiating pain involving the L5-S2 dermatomes. However, 15 patients had scores of ≤ 2 on the VAS scale, indicating slight or no pain. Of the remaining 13 patients, 5 had VAS scores between 4 and 7, and 5 had scores of ≥ 8.
Correlation of clinical and radiological findings
Narrowing of the sacral foramina in S1-S3 had a statistically significant correlation with neurologic deficits in the corresponding dermatomes. Similarly, there was a correlation between narrowing of the sacral canal and neurological deficits at the S2 level. Post-foraminal involvement of the L5 nerves was significantly correlated with both sensory and motor deficits in the corresponding dermatomes (Table 4). No significant correlations were found between any of the radiological findings and posterior pelvic pain (Table 5).
Table 4.
Correlation between the radiologically verified narrowing of the neural foramina and neurological deficits in corresponding dermatomes; n = 27
| Spearman’s correlation coefficient | p-value | |
|---|---|---|
| Narrowing of the sacral central canal | 0.42 | 0.03 a |
| Neural foramen level | ||
| L5 | ||
| sensory | 0.22 | 0.1 |
| motor | 0.08 | 0.6 |
| S1 | ||
| sensory | 0.30 | 0.03 |
| motor | 0.15 | 0.3 |
| S2 | 0.50 | < 0.001 |
| S3 | 0.45 | 0.001 |
| S4 | 0.22 | 0.1 |
| L5-post-foraminal boney encroachment | ||
| Sensory | 0.31 | 0.03 |
| Motor | 0.35 | 0.01 |
significance only at S2-level.
Table 5.
Correlation between radiological findings and lumbosacral pain; n = 28
| Spearman’s correlation coefficient | p-value | |
|---|---|---|
| L5-S1 | ||
| Disc space narrowing | 0.09 | 0.7 |
| Facet joint osteoarthritis | 0.28 | 0.2 |
| Facet joint ankylosis | 0.27 | 0.2 |
| SI-joint | ||
| Ankylosis | 0.01 | 1.0 |
| Osteoarthritis | 0.05 | 0.8 |
| Presence of implants | 0.29 | 0.1 |
| Residual displacement ≥ 10 mm | ||
| Cephalad | 0.14 | 0.5 |
| Posterior | 0.005 | 1.0 |
Discussion
There are no established validated protocols for radiological quantification and measurement of pelvic deformities and other sequelae after pelvic ring disruptions (Lefaivre et al. 2012). For measurement of residual displacement, we used conventional radiographs, since they are readily available and are most commonly used by most orthopedic surgeons in outpatient clinical settings. We used the methods described by Mears and Velyvis (2003), but modified the landmarks for the CRD measurements on the outlet films; we were mainly interested in the sacral fracture and any displacements of the sacral bone. In addition, a few patients sustained sequelae after iliac wing fractures, resulting in inaccurate measurements when the tops of the iliac wings were used as reference landmarks. We therefore used the sacral transverse process elements of the lateral masses as CRD landmarks.
Malunion is imprecisely defined after pelvic ring injuries, but most authors have considered displacement in any dimension of less than 10 mm, with no gross rotational malalignment, to be a satisfactory result (Tornetta and Matta 1996, Lindahl et al. 1999). The consequences and treatment of sacral malunions after surgery are poorly documented. Most reports have been case reports or small case series, and late surgical decompression of the neural roots in these patients has seldom been described in the literature (Alexander et al. 2013).
There have been a few publications describing clinical manifestations and surgical treatment of pelvic malunions and nonunions (Pennal and Massiah 1980, Matta et al. 1996, Mears and Velyvis 2003, Oransky and Tortora 2007). In these series, the initial treatment of the fractures consisted of either nonoperative treatment or external fixation in the majority of cases, resulting in inadequate posterior pelvic stability (Lindahl et al. 1999, Kanakaris et al. 2009). A substantial number of the patients in these reports had major pelvic ring deformities with considerable morbidity related to the deformity.
Our series is therefore not directly comparable to these studies. All our patients were initially treated with internal fixation, and at the long-term follow-up, none of them had major deformities or nonunions in the posterior pelvic ring. As reported in our previous publication, 26 of our 28 patients were able to walk independently (Adelved et al. 2012).
Numerous authors have considered a residual cephalad displacement of more than 10 mm to be a poor prognostic factor (McLaren et al. 1990, Matta and Tornetta 1996, Matta et al. 1996, Tornetta and Matta 1996, Lindahl and Hirvensalo 2005) and have recommended accurate reduction of a displaced vertically unstable sacral fracture.
Other studies have shown conflicting results. Nepola et al. (1999) presented the results of 33 patients with type-C vertical shear injuries, treated with external fixation or nonoperatively, with residual displacement ranging from 2 mm to 52 mm. They found no correlation between residual displacement and functional outcome, including pain. Pohlemann et al. (1996) reported the results of 30 patients with type-C, vertically unstable pelvic ring fractures, treated with internal and/or external fixation. 18 had slight pain or no pain, 28 had residual displacement of < 10 mm, and only 8 had good or excellent outcome.
Displaced unstable sacral fractures are frequently associated with neurological lesions (Huittinen 1972, Gibbons et al. 1990, Majeed 1992). The configuration and size of the anterior sacral foramina may be altered, either due to insufficient reduction, or later due to callus formation during the bone-healing process. In the present study, narrowing of the sacral foramina was observed in several patients and correlated with neurological deficits. As opposed to sacral foramina, structural changes in the L5 neural foramina did not appear to have any effect on neurological deficits. However, post-foraminal encroachment of the L5 nerves correlated well with neurological deficits in the corresponding dermatomes. Post-foraminally, impingement of the L5 nerve may be caused by several structures, formed by the lumbosacral ligament (Nathan et al. 1982). Also, it runs distally along the anterior aspect of the lateral mass between the SI-joint and the sacral foramina. This path is usually the main fracture area in a vertically unstable sacral fracture, and the L5 nerve may thus be avulsed or stretched by bony fragments in cases of severe fracture displacement (Huittinen 1972, Denis et al. 1988). Later, during fracture healing, bony encroachment caused by callus or ectopic bone formation may contribute to further neurological dysfunction. However, the exact cause of L5 pathology observed in our patients cannot be determined from this study.
Several studies have shown limited long-term recovery of the neurological injuries after pelvic fractures (Matta and Saucedo 1989, Tornetta and Matta 1996, Rommens and Hessmann 2002). To our knowledge, changes in the sacral topography—including changes in the shape and diameter of the anterior sacral foramina after fracture healing—have not been studied in detail. In the present study, with no CT scans from previous follow-ups, comparison with earlier radiographs and recording of changes over time was not possible. Thus, we cannot determine whether the neurological deficits were caused or deteriorated by gradual changes in the topography of the bony structures, although we found a close relationship between boney impingement/encroachment of neural structures and neurological deficits.
We observed a substantial proportion of patients with osteoarthritis of the facet joints, the lumbosacral junction, and the SI-joints. Degenerative processes in the lumbosacral spine, including facet joint OA and L5-S1 disc space reduction, are often reported in epidemiological and cadaveric studies (Eubanks et al. 2007, Kalichman et al. 2010). These studies also suggest that the association between these findings and low back pain is variable. There are no epidemiological data on the prevalence of OA in SI-joints. Radiological changes in the SI-joints have been suggested to contribute to low back pain (Hodge and Bessette 1999). Yet, other authors have shown normal variations in the appearance of the SI-joints, depending on factors such as sex and age (Vogler et al. 1984, Faflia et al. 1998). In these studies, degenerative changes were frequent with increasing age in individuals without back pain. Our results back up these studies. 15 out of 28 of our patients had no pain or only slight pain in the posterior pelvic area (VAS ≤ 2). For the remaining 13 with moderate to severe pain, no correlations were found between pain and any of the radiologic findings, including residual displacement.
There were some methodological differences between our study and the studies mentioned: all our patients were treated with internal fixation and we used a VAS scale to quantify pain. Despite these differences, our results support the results of the studies indicating a lack of association between residual displacement and pain. Our results indicate that foraminal architecture and bony changes around the fracture area may play a greater role in the long-term outcome than the overall pelvic alignment.
The present study had some limitations. The number of patients was low, so the results of the statistical analyses should be interpreted with caution. In addition, due to limited access to postoperative and 1-year follow-up radiographs, comparison of the 11-year radiological results with the earlier images was not possible. The strength of the study lies in the long-term follow-up and high response rate, and also the thorough neurological and radiological assessments.
In summary, pathological radiological findings are common after operatively treated displaced sacral fractures. Sacral foraminal and L5 post-foraminal encroachments correlated with neurologic deficits. Lumbosacral pain did not correlate with radiological sequelae after fracture healing, including residual displacement.
Acknowledgments
AA: Design, data collection, examination of all patients, data analysis, and writing of the manuscript. AT: design, surgery, and critical review of the manuscript. JCH: analysis and interpretation of the radiographs and CT scans. TG: data collection, examination of all patients, data analysis, statistical assistance, and critical review of the manuscript. JEM and OR: design, surgery, and critical review of the manuscript.
This work was supported by research funds from the Sophies Minde Ortopedi AS Foundation, Oslo, Norway.
No competing interests declared.
References
- Adelved A, Tötterman A, Glott T, Madsen JE, Røise O. Functional outcome 10 years after surgical treatment of displaced sacral fractures . Spine. 2012;37(16):E1009–16. doi: 10.1097/BRS.0b013e31823a0d83. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Brennan M, Rahm MD. L5 nerve root decompression after malunion of surgically managed vertically unstable pelvic ring injuries . Am J Orthop. 2013;42(4):E23–5. [PubMed] [Google Scholar]
- Bontrager KL, Lampignano JP. Textbook of radiographic positioning and related anatomy. 6th ed. 2005. pp. 275–8. In. Elsevier Mosby, St. Louis.
- Denis F, Davis S, Comfort T. Sacral fractures: an important problem. Retrospective analysis of 236 cases . Clin Orthop. 1988;227:67–81. [PubMed] [Google Scholar]
- Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Prevalence of lumbar facet arthrosis and its relationship to age, sex, and race: an anatomic study of cadaveric specimens . Spine. 2007;32:19, 2058–62. doi: 10.1097/BRS.0b013e318145a3a9. [DOI] [PubMed] [Google Scholar]
- Faflia CP, Prassopoulos PK, Daskalogiannaki ME, Gourtsoyiannis NC. Variation in the appearance of the normal sacroiliac joint on pelvic CT . Clin Radiol. 1998;53:10, 742–6. doi: 10.1016/s0009-9260(98)80316-4. [DOI] [PubMed] [Google Scholar]
- Fracture and dislocation compendium Orthopaedic Trauma Association Committee for Coding and Classification . J Orthop Trauma. 1996;10(Suppl 1):66–70. [PubMed] [Google Scholar]
- Gibbons KJ, Soloniuk DS, Razack N. Neurological injury and patterns of sacral fractures . J Neurosurg. 1990;72(6):889–93. doi: 10.3171/jns.1990.72.6.0889. [DOI] [PubMed] [Google Scholar]
- Hodge JC, Bessette B. The incidence of sacroiliac joint disease in patients with low-back pain . Can Assoc Radiol J. 1999;50:5, 321–3. [PubMed] [Google Scholar]
- Huittinen VM. Lumbosacral nerve injury in fracture of the pelvis. A postmortem radiographic and patho-anatomical study . Acta Chir Scand. 1972. pp. 3–43. [PubMed]
- Kalichman L, Kim DH, Li L, Guermazi A, Hunter DJ. Computed tomography-evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain . Spine J. 2010;10:3, 200–8. doi: 10.1016/j.spinee.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakaris NK, Angoules AG, Nikolaou VS, Kontakis G, Giannoudis PV. Treatment and outcomes of pelvic malunions and nonunions: a systematic review . Clin Orthop. 2009;467(8):2112–24. doi: 10.1007/s11999-009-0712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaivre KA, Slobogean G, Starr AJ, Guy P, O’brien PJ, Macadam SA. Methodology and interpretation of radiographic outcomes in surgically treated pelvic fractures: a systematic review . J Orthop Trauma. 2012;26:8, 474–81. doi: 10.1097/BOT.0b013e3182323aa2. [DOI] [PubMed] [Google Scholar]
- Lindahl J, Hirvensalo E. Outcome of operatively treated type-C injuries of the pelvic ring . Acta Orthop. 2005;76:5, 667–78. doi: 10.1080/17453670510041754. [DOI] [PubMed] [Google Scholar]
- Lindahl J, Hirvensalo E, Böstman O, Santavirta S. Failure of reduction with an external fixator in the management of injuries of the pelvic ring. Long-term evaluation of 110 patients . J Bone Joint Surg (Br) 1999;81:6, 955–62. doi: 10.1302/0301-620x.81b6.8571. [DOI] [PubMed] [Google Scholar]
- Majeed SA. Neurologic deficits in major pelvic injuries . Clin Orthop. 1992;282:222–8. [PubMed] [Google Scholar]
- Matta JM, Saucedo T. Internal fixation of pelvic ring fractures . Clin Orthop. 1989;242:83–97. [PubMed] [Google Scholar]
- Matta JM, Tornetta PI. Internal fixation of unstable pelvic ring injuries . Clin Orthop. 1996;329:129–40. doi: 10.1097/00003086-199608000-00016. [DOI] [PubMed] [Google Scholar]
- Matta JM, Dickson KF, Markovich GD. Surgical treatment of pelvic nonunions and malunions . Clin Orthop. 1996;329:199–206. doi: 10.1097/00003086-199608000-00024. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr.,, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35:5, 266–74. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- McLaren AC, Rorabeck CH, Halpenny J. Long-term pain and disability in relation to residual deformity after displaced pelvic ring fractures . Can J Surg. 1990;33:6, 492–4. [PubMed] [Google Scholar]
- Mears DC, Velyvis J. Surgical reconstruction of late pelvic post-traumatic nonunion and malalignment . J Bone Joint Surg (Br) 2003;85:1, 21–30. doi: 10.1302/0301-620x.85b1.13349. [DOI] [PubMed] [Google Scholar]
- Nathan H, Weizenbluth M, Halperin N. The lumbosacral ligament (LSL), with special emphasis on the “lumbosacral tunnel” and the entrapment of the 5th lumbar nerve . Int Orthop. 1982;6:3, 197–202. doi: 10.1007/BF00267730. [DOI] [PubMed] [Google Scholar]
- Nepola JV, Trenhaile SW, Miranda MA, Butterfield SL, Fredericks DC, Riemer BL. Vertical shear injuries: is there a relationship between residual displacement and functional outcome? . J Trauma. 1999;46:6, 1024–9. doi: 10.1097/00005373-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Oransky M, Tortora M. Nonunions and malunions after pelvic fractures: why they occur and what can be done? . Injury. 2007;38:4, 489–96. doi: 10.1016/j.injury.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Pennal GF, Massiah KA. Nonunion and delayed union of fractures of the pelvis . Clin Orthop. 1980;151:124–9. [PubMed] [Google Scholar]
- Pohlemann T, Bosch U, Gänsslen A, Tscherne H. The Hannover experience in management of pelvic fractures . Clin Orthop. 1994;305:69–80. [PubMed] [Google Scholar]
- Pohlemann T, Gänsslen A, Schellwald O, Culemann U, Tscherne H. Outcome after pelvic ring injuries . Injury. 1996;27(Suppl 2):B31–B38. [PubMed] [Google Scholar]
- Rommens PM, Hessmann MH. Staged reconstruction of pelvic ring disruption: differences in morbidity, mortality, radiologic results, and functional outcomes between B1, B2/B3, and C-type lesions . J Orthop Trauma. 2002;16:2, 92–8. doi: 10.1097/00005131-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Standring S, Gray H, Borley NR. Gray’s anatomy: the anatomical basis of clinical practice. 40th ed. 2008. pp. 724–8. In. Churchill Livingstone Elsevier, Edinburgh.
- Tornetta PI, Matta JM. Outcome of operatively treated unstable posterior pelvic ring disruptions . Clin Orthop. 1996;329:186–93. doi: 10.1097/00003086-199608000-00022. [DOI] [PubMed] [Google Scholar]
- Tötterman A, Glott T, Madsen JE, Røise O. Unstable sacral fractures: associated injuries and morbidity at 1 year . Spine. 2006;31(18):E628–35. doi: 10.1097/01.brs.0000231961.03527.00. [DOI] [PubMed] [Google Scholar]
- Vogler JB, III, Brown WH, Helms CA, Genant HK. The normal sacroiliac joint: a CT study of asymptomatic patients . Radiology. 1984;151:2, 433–7. doi: 10.1148/radiology.151.2.6709915. [DOI] [PubMed] [Google Scholar]