Abstract
Objective
The purpose of this study was to compare the image quality of standard single-shot echo-planar imaging (ss-EPI) and that of readout-segmented EPI (rs-EPI) in patients with breast cancer.
Materials and Methods
Seventy-one patients with 74 breast cancers underwent both ss-EPI and rs-EPI. For qualitative comparison of image quality, three readers independently assessed the two sets of diffusion-weighted (DW) images. To evaluate geometric distortion, a comparison was made between lesion lengths derived from contrast enhanced MR (CE-MR) images and those obtained from the corresponding DW images. For assessment of image parameters, signal-to-noise ratio (SNR), lesion contrast, and contrast-to-noise ratio (CNR) were calculated.
Results
The rs-EPI was superior to ss-EPI in most criteria regarding the qualitative image quality. Anatomical structure distinction, delineation of the lesion, ghosting artifact, and overall image quality were significantly better in rs-EPI. Regarding the geometric distortion, lesion length on ss-EPI was significantly different from that of CE-MR, whereas there were no significant differences between CE-MR and rs-EPI. The rs-EPI was superior to ss-EPI in SNR and CNR.
Conclusion
Readout-segmented EPI is superior to ss-EPI in the aspect of image quality in DW MR imaging of the breast.
Keywords: Breast neoplasm, Magnetic resonance imaging, Diffusion-weighted MRI
INTRODUCTION
Magnetic resonance imaging of the breast is well-established as a valuable technique in the diagnosis of breast cancer. The contrast enhanced MR imaging (CE-MRI), particularly, have been widely performed for the evaluation of breast tumors (1, 2, 3). Breast MRI has a high sensitivity but only a moderate specificity for the characterization of breast lesions (4). In recent years, results of many studies have shown that diffusion-weighted (DW) imaging may improve lesion characterization in MR imaging (5, 6, 7). DW imaging provides physiological information about the functional environment and movement of water in normal versus abnormal tissues (6), and it also allows measurement of the apparent diffusion coefficient (ADC) in breast tissue. The previous studies suggest that ADCs could be an effective parameter in distinguishing between malignant and benign breast lesions (7), whereby low ADCs are associated with malignancy (5, 6). Single-shot echo-planar imaging (ss-EPI) is the routine sequence for clinical DW imaging. However, they suffer from susceptibility artifacts that manifest as geometric distortion, signal dropout, and image blurring (8). The distortions are mainly ascribed to the slow traversal through k-space along the phase-encoding direction (9), leading to a low bandwidth in this direction that causes blurring and geometric distortion. These artifacts can be diminished by accelerating the k-space traversal along the phase-encoding direction. This distortion can be reduced by readout-segmented EPI (rs-EPI). This multi-shot sequence, in which an EPI readout is used to sample a subset of k-space points in the readout direction at each shot, allows a substantial reduction in echo spacing and an associated reduction in the time taken to traverse k-space in the phase-encoding direction (10). This leads to reduction in distortion and blurring caused by T2* decay during the readout. The readout-segmented sampling scheme has been known for a number of years (11), but recent technical improvements (10, 12) have integrated the method with 2-dimensional navigator correction, to allow a robust correction for the shot-to-shot phase errors that occur in diffusion-weighted imaging (13). A number of studies have assessed the image quality of rs-EPI in the brain, but there are fewer reports concerning applications in the breast imaging (14).
The purpose of our study was to compare the image quality between the standard ss-EPI and the rs-EPI in patients with breast cancer.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the Institutional Review Board, with the requirement for informed patient consent waived.
Of 736 patients who underwent breast MRI from January 2012 to December 2012, 178 patients underwent both ss-EPI and rs-EPI. We retrospectively reviewed the radiology and pathology records of those potential patients. Breast MRI findings were reported according to the level of suspicion of malignancy, by using the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) lexicon. Along with already known biopsy-proven cancers (BI-RADS category 6), BI-RADS category 4 or 5 lesions which were later pathologically confirmed as malignancy were also included. Of the 178 patients, 84 were excluded for not having a suspicious abnormality on dynamic images, 16 were excluded for having non-mass enhancement type lesions, 6 were excluded for not having pathological verification, and one was excluded because the lesion was not discernible on CE-MRI due to prominent background parenchymal enhancement of the breast. Therefore, 71 patients with 74 lesions were enrolled. The mean age of the patients was 52.7 ± 11.4 years (range, 30-88 years). All lesions were pathologically verified either by surgery (n = 65) or by imaging-guided biopsy (n = 9).
Image Acquisition
Imaging was performed with a 3.0 T MAGNETOM Verio MR system (Siemens, Erlangen, Germany) by using a dedicated surface breast coil with the patient in a prone position. The MR images were acquired using the following sequences: 1) axial, turbo spin-echo T2-weighted imaging sequence; repetition time (TR)/echo time (TE), 3530/93 ms; flip angle, 80°; field-of-view (FOV), 320 × 320 mm; matrix size, 576 × 403; slice thickness, 4 mm; and acquisition time, 2 minutes 28 seconds. 2) two DW imaging sequences (i.e., ss-EPI and rs-EPI); b values, 0 and 750 s/mm2; TR/TE, 9800/87 ms and 5600/55 ms, respectively; FOV, 340 × 117 mm and 360 × 180 mm, respectively; matrix size, 192 × 82; slice thickness, 4 mm; acquisition time, 2 minutes 47 seconds and 2 minutes 31 seconds, respectively; and 5 readout segments for rs-EPI. 3) apparent diffusion coefficient (ADC) maps were calculated automatically by using MRI software from the DW images. 4) pre- and post-contrast, axial T1-weighted flash three-dimensional Volumetric Interpolated Breath-Hold Examination sequence; TR/TE, 4.4/1.7 ms; flip angle, 10°; FOV, 320 × 320 mm; matrix size, 512 × 292; slice thickness, 1.2 mm; acquisition time, 6 minutes 7 seconds; obtained before and at 7, 67, 127, 187, 247, and 307 seconds after an injection of 0.1 mmol/kg body weight of gadobutrol (Gadovist, Bayer Healthcare, Berlin, Germany). After the examination, the unenhanced images were subtracted from the post-contrast images. Maximum-intensity projection images were created from the early phase of the subtraction images.
Qualitative Comparison of Image Quality
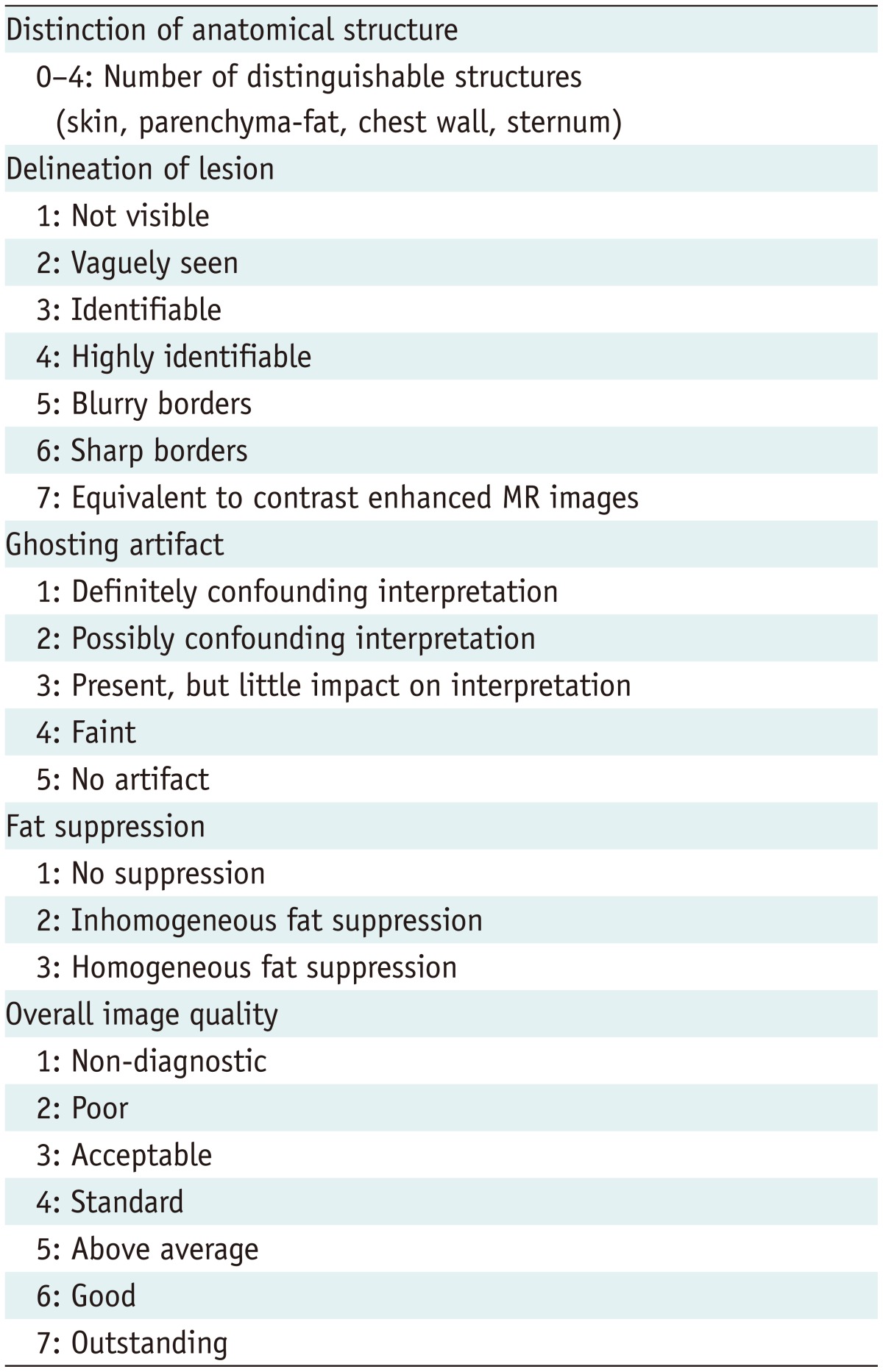
For the assessment of DW image quality, three fellowship-trained breast radiologists, each of whom had at least 8 years of experience in interpreting breast MR imaging, independently reviewed and visually assessed the two sets of DW images and ADC maps with CE-MR images as references. The readers scored the images based on five criteria: distinction of the anatomical structure, delineation of the lesion, degree of ghosting artifact, uniformity of fat suppression, and overall image quality (Table 1). Before the study, one of the readers reviewed all breast MR images, including T2-weighted images, dynamic contrast-enhanced images, DW images, and ADC maps, to identify pathologically proven lesions. The reader provided reference images corresponding to each score for each criterion. After training for the scoring system at the consensus meeting, each reader independently assessed the ss-EPI and rs-EPI images from the patients. The included cases were anonymized and randomized, and then they were provided to the readers.
Table 1.
Criteria for Qualitative Comparison of Image Quality in Diffusion-Weighted Imaging in Patients with Breast Cancer
Quantitative Comparison of Image Quality
Geometric distortion was evaluated by comparing lesion lengths between CE-MR images and the corresponding DW images. Anterior-posterior (AP) length and left-right (LR) width of the lesions were measured and the differences were calculated.
For the assessment of image parameters, regions-of-interest (ROIs) were manually drawn on the same area of both DW images in all 74 lesions and normal breast parenchyma. Lesion ROIs were drawn in the center of the mass with high signal intensity in DW images then copied to the ADC map (Fig. 1). ROIs in DW images were drawn with reference to CE-MR images, while avoiding fatty tissue or the necrotic portion. If the lesion was not visible in DW images, the case was excluded from the quantitative analysis. Normal tissue ROIs were drawn in homogeneous breast parenchyma without enhancement in the contralateral breast. For each ROI, mean signal intensity and standard deviation were determined.
Fig. 1.
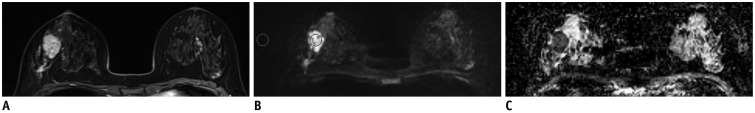
MR images in 39-year-old woman with invasive ductal carcinoma in her right breast.
Contrast-enhanced T1-weighted MR image (A), readout-segmented echo-planar image (B), and ADC map (C). Lesion ROI was drawn in center of mass with high signal intensity in DW image then it was copied to ADC map. Normal tissue ROI was drawn in homogeneous breast parenchyma in contralateral breast. Another ROI was drawn at periphery of DW image to measure background noise. SNR, contrast, CNR, and lesion ADC were 304.5, 2.6, 3.4, and 1.06 × 10-3 mm2/sec, respectively. ADC = apparent diffusion coefficient, CNR = contrast-to-noise ratio, DW = diffusion-weighted, ROI = regions-of-interest, SNR = signal-to-noise ratio
Signal-to-noise ratio (SNR) was determined by the ratio between the mean signal intensity inside the ROI (SROI) and the standard deviation of the background noise (σBG) (SNR = SROI / σBG). Contrast was determined by the ratio between SROI and that of normal tissue on DW images (ST) (contrast = SROI / ST). Contrast-to-noise ratio (CNR) was determined by the difference between SROI and ST divided by the standard deviation in the lesion ROI (σROI) and normal tissue ROI (σT), using the following equation:
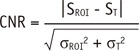 |
The ADC and size of the ROI were recorded. These quantitative measurements were performed by a breast radiology fellow.
Statistical Analysis
All statistical analyses were carried out using SAS (version 9.2, SAS Institute Inc., Cary, NC, USA). Regarding the image quality, inter-observer agreement was assessed with the kappa statistics. The average scores of the three readers were calculated and used for analysis. Differences in image quality, discrepancy in lesion lengths, and image parameters between ss-EPI and rs-EPI were compared using the paired t test. P < 0.05 was considered as statistically significant.
RESULTS
Patients
A total of 74 malignant lesions were identified in 71 patients. One patient had two lesions and another patient had three lesions. According to BI-RADS classification, there was one case of category 4 and one case of category 5 in MR imaging. The other 72 cases were category 6 lesions. Malignant lesions were identified as 59 (79.7%) invasive ductal carcinomas, 5 (6.8%) ductal carcinoma in situ, 5 (6.8%) invasive lobular carcinomas, 2 (2.7%) mucinous carcinomas, 2 (2.7%) invasive mammary carcinomas, and 1 (1.3%) invasive tubular carcinoma. The maximum diameter of the lesions ranged from 4.4 to 88.8 mm with a mean size of 20.0 mm on contrast-enhanced T1-weighted images.
Qualitative Comparison of Image Quality
The three readers were in fair inter-observer agreement regarding the overall image quality (κ = 0.428). The average numbers given by the three readers for distinguishable anatomical structures (total 4 structures) were 1.7 for ss-EPI and 3.5 for rs-EPI. For delineation of the lesion, the mean scores were 3.0 (identifiable) for ss-EPI and 4.6 (well identifiable to blurry borders) for rs-EPI. For ghosting artifact, they were 3.0 (present, but little impact on interpretation) for ss-EPI and 3.2 for rs-EPI. For fat suppression, they were 2.5 (inhomogeneous to homogeneous fat suppression) for ss-EPI and 2.3 for rs-EPI. For overall image quality, they were 2.7 (poor to acceptable) for ss-EPI and 4.7 (standard to above average) for rs-EPI (Table 2). The rs-EPI was superior to ss-EPI in most criteria regarding the image quality (Fig. 2). Distinction of anatomical structures (p < 0.001), delineation of the lesion (p < 0.001), ghosting artifact (p = 0.034), and overall image quality (p < 0.001) were significantly better in rs-EPI. Fat suppression did not differ statistically between the two groups (p = 0.055).
Table 2.
Qualitative Comparison of Image Quality between Single-Shot Echo-Planar Imaging (ss-EPI) and Readout-Segmented EPI (rs-EPI) in Patients with Breast Cancer
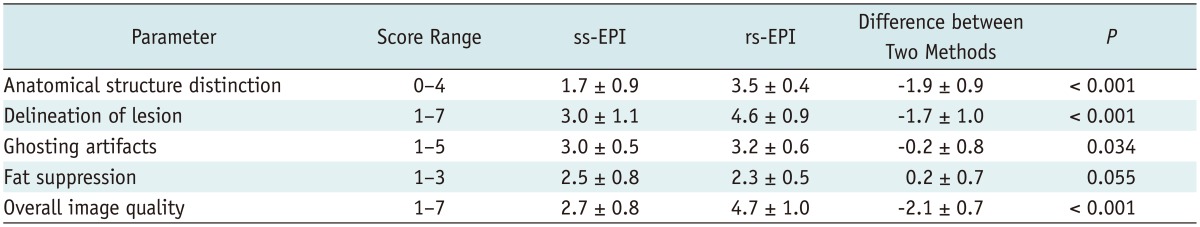
Note.-Numbers shows mean values ± standard deviation except for score range and p value.
Fig. 2.
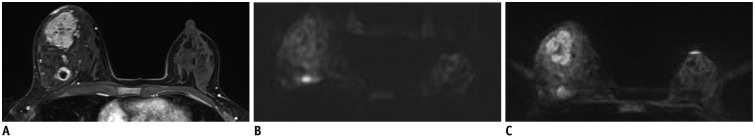
MR images in 45-year-old woman with two malignant masses in her right breast (invasive ductal carcinoma).
Contrast-enhanced T1-weighted MR image (A), single-shot echo-planar image (ss-EPI) (B), and readout-segmented echo-planar image (rs-EPI) (C). While posteriorly located rim-enhancing mass is relatively well identifiable on both sequences, other larger mass is poorly defined on ss-EPI. Average scores given by three readers for overall image quality were 2.3 (poor) for ss-EPI and 5.3 (above average) for rs-EPI. Other variables are scored as follows (ss-EPI vs. rs-EPI); anatomical structure, 2.0 vs. 4.0; delineation of lesion, 2.7 vs. 5.7; ghosting artifacts, 2.7 vs. 3.0; fat suppression, 3.0 vs. 3.0; SNR, 78.0 vs. 232.0; contrast, 1.0 vs. 2.2; CNR, 0.1 vs. 3.8; ADC, 1.35 × 10-3 mm2/sec vs. 1.33 × 10-3 mm2/sec. ADC = apparent diffusion coefficient, CNR = contrast-to-noise ratio, SNR = signal-to-noise ratio
Regarding the delineation of the lesion, all lesions were visible by all readers on rs-EPI. Meanwhile, 19 lesions were not identified by all or some readers in ss-EPI (Table 3). Of the 19 lesions, 10 lesions were not identified by any of the readers and 9 lesions were only identified by some reader(s). Among 17 lesions which is less than 1 cm in the maximum length, 9 lesions were visible by all readers and 8 lesions were not visible by some or all readers.
Table 3.
Detected Lesion on Single-Shot Echo-Planar Imaging According to Size
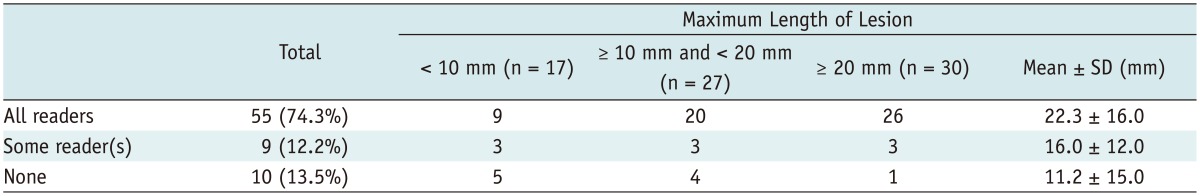
Note.-Numbers show number of lesions except for mean ± standard deviation (SD). All lesions were visible by all readers on readout-segmented echo-planar imaging.
Quantitative Comparison of Image Quality
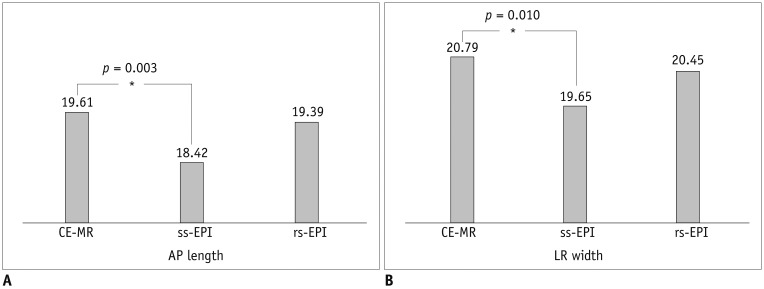
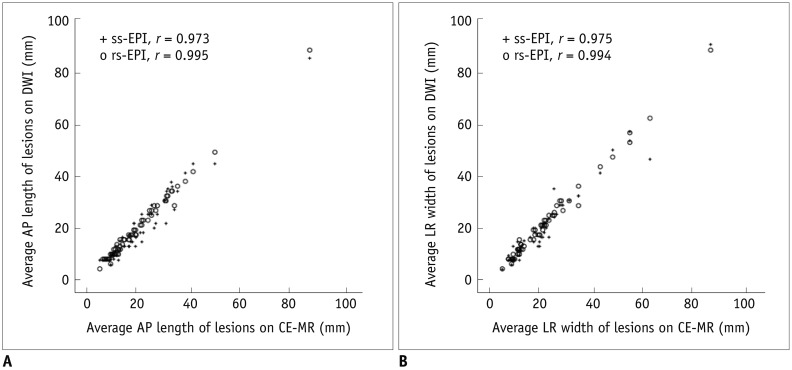
Twelve lesions were not visible or not measurable on ss-EPI, while all lesions were visible on rs-EPI on quantitative evaluation. The twelve cases were excluded from the quantitative analysis. The average AP length of the lesions was 19.6 ± 13.5 mm on CE-MRI, 18.4 ± 13.3 mm on ss-EPI, and 19.4 ± 13.3 mm on rs-EPI. The average LR width was 20.8 ± 15.3 mm on CE-MRI, 19.7 ± 15.0 mm on ss-EPI, and 20.4 ± 15.1 mm on rs-EPI (Fig. 3). They were measured to be smaller in both sets of DW images. Whereas there were no significant differences in AP length and LR width between CE-MR and rs-EPI (p = 0.212, p = 0.107), AP length and LR width were significantly smaller in ss-EPI (p = 0.003, p = 0.010) (Fig. 4). There were high correlations for the lesion lengths between CE-MR images and each DW image (Fig. 5). The Pearson correlation coefficient for the maximum size between the histopathology and the CE-MRI was 0.708.
Fig. 3.
Comparison of average lesion lengths in contrast-enhanced (CE)-MR and diffusion-weighted (DW) images.
Both average anterior-posterior (AP) length (A) and left-right (LR) width (B) of lesions were measured smaller in both sets of DW images. Whereas there were no significant differences in AP length and LR width between CE-MR and readout-segmented echo-planar image (rs-EPI), they were significantly smaller in single-shot echo-planar image (ss-EPI).
Fig. 4.
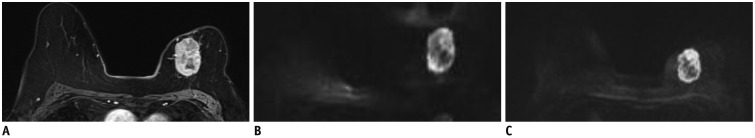
MR images in 88-year-old woman with invasive ductal carcinoma in her left breast.
Contrast-enhanced T1-weighted MR image (A), single-shot echo-planar image (ss-EPI) (B), and readout-segmented echo-planar image (rs-EPI) (C). rs-EPI provides superior anatomical detail and lesion delineation. Spatial distortion is more prominent on ss-EPI. Average scores given by three readers for overall image quality were 3 (acceptable) for ss-EPI and 5 (above average) for rs-EPI. Other variables are scored as follows (ss-EPI vs. rs-EPI); anatomical structure, 0 vs. 4.0; delineation of lesion, 4.3 vs. 6.0; ghosting artifacts, 2.7 vs. 4.0; fat suppression, 1.3 vs. 2.0; SNR, 126.5 vs. 556.9; contrast, 3.6 vs. 8.8; CNR, 6.3 vs. 15.4; ADC, 0.62 × 10-3 mm2/sec vs. 0.63 × 10-3 mm2/sec. ADC = apparent diffusion coefficient, CNR = contrast-to-noise ratio, SNR = signal-to-noise ratio
Fig. 5.
Correlation for lesion length between contrast-enhanced (CE) T1-weighted MRI and diffusion-weighted images (DWI).
Pearson correlation coefficients of anterior-posterior (AP) length (A) and left-right (LR) width (B) are slightly higher in readout-segmented echo-planar image (r = 0.995, r = 0.994) than in single-shot echo-planar image (r = 0.973, r = 0.975). rs-EPI = readout-segmented echo-planar imaging, ss-EPI = single-shot echo-planar imaging
The comparison of results for image parameters between ss-EPI and rs-EPI are listed in Table 4. SNR, contrast, and CNR were 104.6, 3.4, and 3.2 for ss-EPI, respectively, and 301.7, 3.0, and 4.9 for rs-EPI, respectively. The rs-EPI was superior to ss-EPI in SNR (p < 0.001) and CNR (p = 0.013). There were no significant differences in ROI size, contrast, and ADC between ss-EPI and rs-EPI (p = 0.578, p = 0.122, p = 0.631). The Pearson correlation coefficient of the ADC between ss-EPI and rs-EPI was 0.902.
Table 4.
Comparison of Image Parameters between Single-Shot Echo-Planar Imaging (ss-EPI) and Readout-Segmented EPI (rs-EPI) in Patients with Breast Cancer
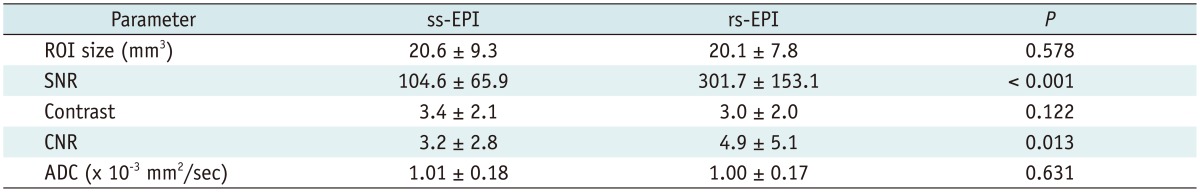
Note.-ADC = apparent diffusion coefficient, CNR = contrast-to-noise ratio, SNR = signal-to-noise ratio
DISCUSSION
The diagnostic utility of DWI has been limited in the assessment of breast lesions, because of its inferior image quality particularly compared to CE-MRI. However, the advancements in imaging technology are providing evidence for the potential use of this MR imaging technique, which does not require the use of intravenous contrast agent (5, 6, 7, 14). If the image quality is sufficiently good, DW images can be used not only for measurement of signal intensity and ADC, but also for morphological analysis of the lesions.
Our study demonstrates that the quality of DW imaging can be significantly improved by using the rs-EPI sequence. Regarding the qualitative assessment, rs-EPI was found to be superior to ss-EPI in overall image quality, anatomical structure distinction, and conspicuity of the lesions. It is consistent with previously published studies (8, 14, 15, 16). In a recent study investigating the diagnostic value of rs-EPI in 47 patients of breast lesions, rs-EPI provided higher image quality and lesion conspicuity compared to ss-EPI (14). The study also reported that rs-EPI reached higher diagnostic accuracy for the differentiation of benign and malignant breast lesions. The other study compared rs-EPI and ss-EPI in DW images of the pediatric brain and concluded that rs-EPI provided improved image quality (8).
Regarding the delineation of the lesion, 19 lesions were not identified by all or some readers in ss-EPI. In contrast, there was no lesion that was not visible by any readers in rs-EPI. The difference in visibility of lesions between the two sequences can be attributed to the improved point spread function (PSF) with rs-EPI, because of the reduction in T2* blurring. This effectively improves resolution.
The spatial distortion is a major disadvantage of ss-EPI in DW images. Because the distortions in ss-EPI are mainly due to slow traversal through the k-space along the phase-encoding direction, it can be improved by accelerating the k-space traversal along the direction (9, 10). The rs-EPI sequence provides a significantly higher overall quality by reducing spatial distortion and image blurring. Previous studies compared the distortions in the two DW images sequences either qualitatively or quantitatively and demonstrated the reduced distortion in rs-EPI (8, 14, 15, 16). In a study of DW in stroke patients, the distortion was evaluated by measuring the AP length of the pons (16). The rs-EPI images exhibited significantly less pontine distortion. We quantitatively assessed image distortion by comparing the discrepancy in lesion lengths between CE-MR images and each corresponding DW image. AP length and LR width were significantly smaller in ss-EPI when compared to CE-MRI. In particular, the discrepancy in AP length was greater. Some lesions were flatter while some lesions were longer in the AP direction. Sometimes, this spatial distortion caused marked change in the location of the lesion.
With regard to the image parameters, rs-EPI was superior to ss-EPI in SNR and CNR. Our results are consistent with those of Morelli et al. (16), who compared ss-EPI and rs-EPI in the brain MRI. However, SNR in rs-EPI was lower than that in ss-EPI in the other studies (8, 14). In a study of breast cancer patients, no difference was found in CNR between the two DW images (14).
Regarding the SNR, the reduction in the overall length of rs-EPI is disadvantageous, because noise scales inversely with the square root of the readout time. However, the shorter readout also means that the echo time is reduced, which results in the increased signal level and the SNR. The degree of signal increase will depend on the TE and on the T2 value for the tissue being measured. The effect of PSF is also important. The wider PSF with ss-EPI improves SNR at the cost of resolution. This is partly offset in rs-EPI by a shorter TE, particularly for tissue with short T2.
In addition to the ones mentioned above, there are many factors affecting SNR and CNR. It is hard to say in general whether they are higher for ss-EPI or rs-EPI, because it will depend on the exact protocol being used. The rs-EPI was developed with emphasis on reduced artifacts and sharper images, while maintain an acceptable SNR.
A longer scan time has been mentioned as a drawback to the use of rs-EPI in the brain MRI. The rs-EPI is slower than ss-EPI by an amount that is approximately proportional to the number of readout segments used (8). However, in most applications, it is necessary to acquire multiple averages when using ss-EPI to achieve sufficient SNR, so the difference in scan time using the two sequences is much less than this, in practice. Indeed, in the scanning protocols used in our study, a slightly shorter scan time was used for the rs-EPI sequence: 2 minutes 47 seconds for ss-EPI and 2 minutes 31 seconds for rs-EPI.
The first limitation of our study is that it was retrospective. Therefore, some acquisition parameters were not matched for the two DW images sequences. Secondly, our study subjects included only the malignant tumors with mass type lesion and most of them were invasive ductal carcinomas. Larger scale studies with non-mass type lesions and various histopathologic subtypes are required to prove the validity of high resolution DW images.
Compared to a relatively consistent image quality in CE-MRI, there is a considerable difference in the level of image quality, from patient to patient, using both DW images techniques. This point restricts the practical application of DW images, and the reason was not identified in this study. Although the overall image quality is much better in rs-EPI, other factors including patient-related issues and the choice of acquisition parameters affect the image quality and should be further investigated.
We demonstrated that the rs-EPI showed superior or equivalent image quality compared to ss-EPI in all categories. Anatomical structure distinction, delineation of the lesion, ghosting artifact, and overall image quality were significantly better in rs-EPI. Geometric distortion, SNR, and CNR were superior in rs-EPI as well. In this respect, rs-EPI is a useful alternative to ss-EPI in DW for evaluating breast pathology. We also expect the possibility of rs-EPI as screening sequence, which does not require the use of intravenous contrast agent.
In conclusion, rs-EPI is superior to ss-EPI in the aspect of image quality in breast DW MR imaging.
Footnotes
This work was based on a works-in-progress package provided by Siemens.
The statistical consultation was supported by Catholic Research Coordinating Center of the Korea Health 21 R&D Project (A070001), Ministry of Health & Welfare, Republic of Korea.
References
- 1.Huang W, Fisher PR, Dulaimy K, Tudorica LA, O'Hea B, Button TM. Detection of breast malignancy: diagnostic MR protocol for improved specificity. Radiology. 2004;232:585–591. doi: 10.1148/radiol.2322030547. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–378. doi: 10.1148/radiol.2442051620. [DOI] [PubMed] [Google Scholar]
- 3.Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007;244:672–691. doi: 10.1148/radiol.2443051661. [DOI] [PubMed] [Google Scholar]
- 4.Bluemke DA, Gatsonis CA, Chen MH, DeAngelis GA, DeBruhl N, Harms S, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004;292:2735–2742. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 5.Bogner W, Gruber S, Pinker K, Grabner G, Stadlbauer A, Weber M, et al. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology. 2009;253:341–335. doi: 10.1148/radiol.2532081718. [DOI] [PubMed] [Google Scholar]
- 6.Ei Khouli RH, Jacobs MA, Mezban SD, Huang P, Kamel IR, Macura KJ, et al. Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology. 2010;256:64–67. doi: 10.1148/radiol.10091367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–178. doi: 10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 8.Yeom KW, Holdsworth SJ, Van AT, Iv M, Skare S, Lober RM, et al. Comparison of readout-segmented echo-planar imaging (EPI) and single-shot EPI in clinical application of diffusion-weighted imaging of the pediatric brain. AJR Am J Roentgenol. 2013;200:W437–W443. doi: 10.2214/AJR.12.9854. [DOI] [PubMed] [Google Scholar]
- 9.Farzaneh F, Riederer SJ, Pelc NJ. Analysis of T2 limitations and off-resonance effects on spatial resolution and artifacts in echo-planar imaging. Magn Reson Med. 1990;14:123–139. doi: 10.1002/mrm.1910140112. [DOI] [PubMed] [Google Scholar]
- 10.Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009;62:468–475. doi: 10.1002/mrm.22024. [DOI] [PubMed] [Google Scholar]
- 11.Robson MD, Anderson AW, Gore JC. Diffusion-weighted multiple shot echo planar imaging of humans without navigation. Magn Reson Med. 1997;38:82–88. doi: 10.1002/mrm.1910380113. [DOI] [PubMed] [Google Scholar]
- 12.Porter DA, Mueller E. Multi-shot diffusion-weighted EPI with readout mosaic segmentation and 2D navigator correction. Proc Intl Soc Mag Reson Med. 2004;11:442. [Google Scholar]
- 13.Miller KL, Pauly JM. Nonlinear phase correction for navigated diffusion imaging. Magn Reson Med. 2003;50:343–353. doi: 10.1002/mrm.10531. [DOI] [PubMed] [Google Scholar]
- 14.Bogner W, Pinker-Domenig K, Bickel H, Chmelik M, Weber M, Helbich TH, et al. Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology. 2012;263:64–67. doi: 10.1148/radiol.12111494. [DOI] [PubMed] [Google Scholar]
- 15.Holdsworth SJ, Yeom K, Skare S, Gentles AJ, Barnes PD, Bammer R. Clinical application of readout-segmented- echo-planar imaging for diffusion-weighted imaging in pediatric brain. AJNR Am J Neuroradiol. 2011;32:1274–1279. doi: 10.3174/ajnr.A2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morelli J, Porter D, Ai F, Gerdes C, Saettele M, Feiweier T, et al. Clinical evaluation of single-shot and readout-segmented diffusion-weighted imaging in stroke patients at 3 T. Acta Radiol. 2013;54:299–306. doi: 10.1258/ar.2012.120541. [DOI] [PubMed] [Google Scholar]