Abstract
Adenomyosis is defined by the presence of endometrial glands and stroma within the myometrium. Despite its frequent occurrence, the precise aetiology and physiopathology of adenomyosis is still unknown. WNT/β-catenin signalling molecules are important and should be tightly regulated for uterine function. To investigate the role of β-catenin signalling in adenomyosis, the expression of β-catenin was examined. Nuclear and cytoplasmic β-catenin expression was significantly higher in epithelial cells of human adenomyosis compared to control endometrium. To determine whether constitutive activation of β-catenin in the murine uterus leads to development of adenomyosis, mice that expressed a dominant stabilized β-catenin in the uterus were used by crossing PR-Cre mice with Ctnnb1f(ex3)/+ mice. Uteri of PRcre/+ Ctnnb1f(ex3)/+ mice displayed an abnormal irregular structure and highly active proliferation in the myometrium, and subsequently developed adenomyosis. Interestingly, the expression of E-cadherin was repressed in epithelial cells of PRcre/+ Ctnnb1f(ex3)/+ mice compared to control mice. Repression of E-cadherin is one of the hallmarks of epithelial–mesenchymal transition (EMT). The expression of SNAIL and ZEB1 was observed in some epithelial cells of the uterus in PRcre/+ Ctnnb1f(ex3)/+ mice but not in control mice. Vimentin and COUP-TFII, mesenchymal cell markers, were expressed in some epithelial cells of PRcre/+ Ctnnb1f(ex3)/+ mice. In human adenomyosis, the expression of E-cadherin was decreased in epithelial cells compared to control endometrium, while CD10, an endometrial stromal marker, was expressed in some epithelial cells of human adenomyosis. These results suggest that abnormal activation of β-catenin contributes to adenomyosis development through the induction of EMT.
Keywords: β-catenin, adenomyosis, epithelial–mesenchymal transition, uterus
Introduction
Adenomyosis is a common, benign, gynaecological disorder and is defined by the presence of endometrial glands and stroma deep within the myometrium, associated with adjacent myometrial hypertrophy and hyperplasia [1–3]. Adenomyosis occurs when the normal boundary between the endometrial basal layer and the myometrium is disrupted [4–7]. Invasion of the basal endometrium leads to dysfunctional myometrial hyperperistalsis, increased intrauterine pressure and impairment of proper uterine function [8]. This condition can interfere with normal implantation and can cause subfertility [9–13]. Adenomyosis is frequently diagnosed in infertility patients [9,14], with a prevalence of 10–66% [5]. Symptoms include menorrhagia, dyspareunia, dyschezia, dysmenorrhoea and chronic pelvic pain [15]. The spread of adenomyosis also correlates significantly with pelvic pain [16]. However, early diagnosis of adenomyosis is difficult because of the absence of pathogenic symptoms and biomarkers. Therefore, most women are not diagnosed until later stages; severely symptomatic women who do not respond to pharmacological therapy require invasive surgical intervention, which involves a hysterectomy. Despite its prevalence, the precise aetiology and pathophysiology of adenomyosis is poorly understood.
Endometrial hyperplasia is observed more frequently among cases with adenomyosis [17]. Adenomyosis has been suggested to be an ovarian steroid hormone-dependent disorder. High concentrations of oestrogen without appropriate protection from progesterone have been linked to endometrial cancer, endometrial hyperplasia and adenomyosis [18]. When the relationship between adenomyosis and endometrial carcinoma was evaluated in hysterectomy specimens, this disease was positively associated with endometrial carcinomas in 17% of cases [19–25]. Interestingly, β-catenin signalling was activated in endometrial cancer and induced by high levels of oestrogen [18,26].
β-catenin (CTNNB1) functions in a dual manner in epithelial cells, depending on its intracellular localization. At the plasma membrane, β-catenin is an essential component of the E-cadherin–catenin unit and is important for cell differentiation and the maintenance of normal tissue architecture. β-catenin can also act as the main effector of canonical Wnt signalling in the nucleus, which is critically involved in tissue differentiation during embryonic development. In the absence of Wnt signals in normal cells, β-catenin forms a complex with glycogen synthase kinase 3β (GSK-3β) and adenomatous polyposis coli (APC). GSK-3β phosphorylates β-catenin, which marks ubiquitin-dependent degradation by the proteasome, thereby maintaining low levels of free cytoplasmic β-catenin [27]. However, mutations in exon 3 of β-catenin result in protein stabilization, cytoplasmic and nuclear accumulation and participation in signal transcriptional activation through DNA binding [28]. In adenocarcinomas, elevated β-catenin levels caused by mutations in CTNNB1 or APC result in the activation of the Wnt/β-catenin pathway. Recent evidence suggests that oestrogen-dependent proliferation depends on activated Wnt signalling, since it was shown to induce Wnt pathway components in the endometrium of oestrogen-treated women. Additionally, up to 30% of oestrogen-associated cancers exhibit nuclear β-catenin expression, the hallmark of canonical Wnt signalling [29,30]. Mutations of the Wnt/β-catenin pathway members result in aberrant activation of the target genes, including those encoding for activators of epithelial–mesenchymal transition (EMT) [31].
EMT is a programmed development of biological cells, characterized by loss of cell adhesion, repression of E-cadherin expression and an increase in cell mobility [32]. EMT endows cells with migratory and invasive properties and can be induced by oestrogen. One of the signals initiating an EMT is the canonical Wnt pathway, whose stimulation triggers the translocation of β-catenin to the nucleus [33]. A key initial step of EMT is the down-regulation of E-cadherin, which is repressed by several factors, namely ZEB1, ZEB2, SNAI1, SNAI2, Twist1, Twist2 and E12/E47 [32]. In inducing EMT, these factors not only transcriptionally repress epithelial markers such as E-cadherin but also activate mesenchymal genes [34]. Nonetheless, these phenomena are poorly understood in reproductive biology.
Previously we have generated a dominant stabilized β-catenin in the murine uterus (PRcre/+ Ctnnb1f(ex3)/+), which results in infertility, hormone insensitivity and endometrial glandular hyperplasia [35]. These analyses demonstrated that β-catenin has important roles in normal uterine function as well as in tumourigenesis. Here, we utilized the conditionally stabilized β-catenin mouse model and human adenomyosis samples to demonstrate that β-catenin is an important molecule in the aetiology and pathology of adenomyosis.
Materials and methods
Human adenomyosis samples
Tissue samples of adenomyosis with their corresponding eutopic endometrium were collected from surgical hysterectomy specimens from 19 women. Controls comprised 24 regularly cycling premenopausal women undergoing an endometrial hysterectomy for benign conditions with no history or evidence of adenomyosis, who were documented not to be pregnant and who had not been on hormonal therapies for at least 3 months before tissue sampling. These samples were obtained by the Michigan State University’s Center for Women’s Health Research Female Reproductive Tract Biorepository, with MSUIRB approval, and these samples were obtained with informed written consent from the patients. Full-thickness endometrium was collected at Greenville Hospital System from hysterectomy samples, using approved IRB protocols. Histological samples were examined by an independent pathologist, and phases were assigned according to the Noyes criteria [36].
Animals and tissue collection
Mice were cared for and used in the designated animal care facility according to Michigan State University institutional guidelines. All animal procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University (12/10-198-00). Control (PRcre/+ and Ctnnb1f(ex3)/+) and mutant (PRcre/+ Ctnnb1f(ex3)/+) mice were sacrificed at 2, 4 and 6 months of age to examine adenomyosis development. For the study of ovarian steroid hormone dependency of the adenomyosis phenotype, control and mutant mice were ovariectomized at 6 weeks. The mice were sacrificed to examine adenomyosis development at 6 months of age (n = 5).
Immunohistochemistry and immunofluorescence
Immunohistochemistry and immunofluorescence were performed as previously described [35,37]. The antibodies used are listed in Table S1 (see supplementary material). Images were captured with a confocal microscope (510 NLO confocal microscope with a META detector; Carl Zeiss, Thornwood, NY, USA) or fluorescent microscope (Nikon Instruments, Melville, NY, USA). Analysis of β-catenin expression was undertaken by light microscopy and scored according to the intensity of staining on a scale of zero (no staining) to five (strong staining).
Statistical methods
Statistical analyses were performed using one-way ANOVA analysis, followed by Tukey’s post hoc multiple range test or Student’s t-test, using the Instat package from GraphPad (San Diego, CA, USA). p < 0.05 was considered statistically significant.
Results
Activation of β-catenin signalling in human adenomyosis
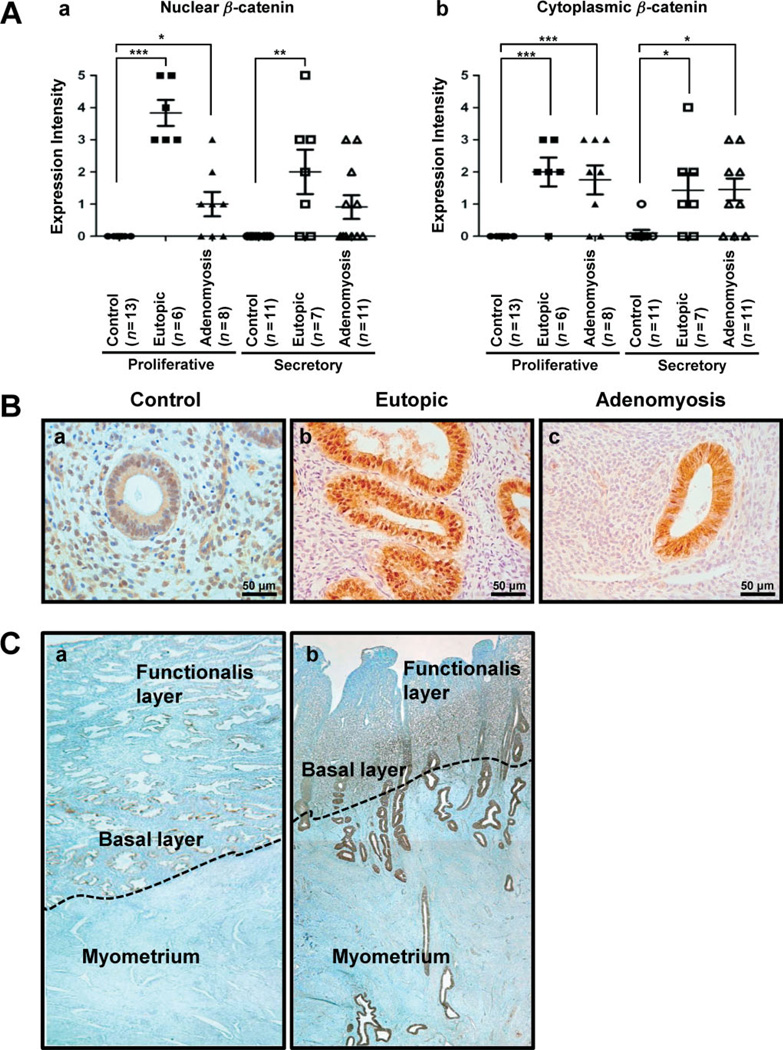
To determine whether β-catenin signalling is dysregulated in adenomyosis, its expression was examined by immunohistochemistry in endometrium from patients with and without adenomyosis (Figure 1A, B). The protein level of nuclear β-catenin was significantly higher in epithelial cells of eutopic endometrium (n = 6) and adenomyosis lesions (n = 8) compared to control endometrium without adenomyosis (n = 13) in proliferative phase samples (Figure 1Aa). In secretory phase samples, the level of nuclear β-catenin was also significantly higher in epithelial nuclei of eutopic endometrium compared with control endometrium (Figure 1B). A higher level of nuclear β-catenin was observed in adenomyosis lesions at the secretory phase, although there was no significant difference. The expression of cytoplasmic β-catenin was weak in control endometrium (n = 13), but the level of cytoplasmic β-catenin in eutopic (n = 6) and adenomyosis lesions (n = 8) was significantly higher compared to control endometrium in the proliferative phase (Figure 1Ab). In the epithelial cells of eutopic (n = 7) and adenomyosis (n = 11) lesions at secretory phase, cytoplasmic β-catenin intensity was also significantly higher than in control endometrium (n = 11) (Figure 1B). However, the expression of nuclear and cytoplasmic β-catenin was not different between proliferative and secretory endometrium in controls and adenomyosis. In addition, the level of membranous β-catenin expression was not different, depending on menstrual cycles and adenomyosis disease (see supplementary material, Figure S1).
Figure 1.
Activation of β-catenin in endometrial tissue from women with adenomyosis. (A) Nuclear and cytoplasmic β-catenin was scored by measuring expression intensity of endometrial epithelial cells from control women (n = 24), eutopic endometrium (n = 13) and adenomyosis lesion (n = 19). (B) Photomicrographs represent immunostaining for β-catenin in human endometrium, with and without adenomyosis. β-Catenin was confined to the cell cytoplasm. Nuclear accumulation of β-catenin was prominent in eutopic endometrium (Bb, Bc). (C) The expression of β-catenin was observed in full-thickness endometrium, without (a) and with adenomyosis (b). ***p < 0.001; **p < 0.01; *p < 0.05; one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple range test
To better understand the integration of β-catenin in adenomyosis, we examined full-thickness endometrium of patients with and without adenomyosis (Figure 1C). Weak expression of membranous β-catenin was observed in full-thickness endometrium of control patients. However, myometrium of the control did not have any β-catenin staining (Figure 1Ca). The endometrial expression of β-catenin was gradually increased at the basal layer and adenomyosis lesions of the myometrium compared with the zona functionalis layer in adenomyosis (Figure 1Cb). These results suggest that activation of β-catenin may play an important role in the pathogenesis of adenomyosis.
Myometrial defects in mice with uterine specific stabilization of β-catenin
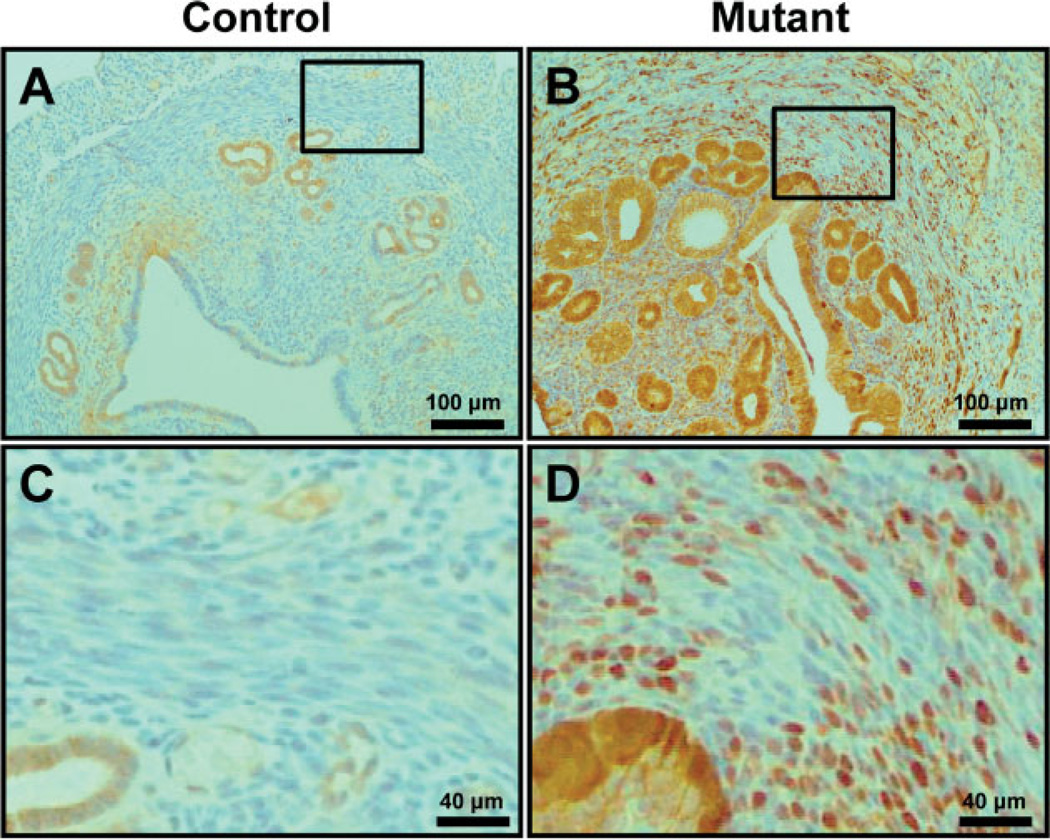
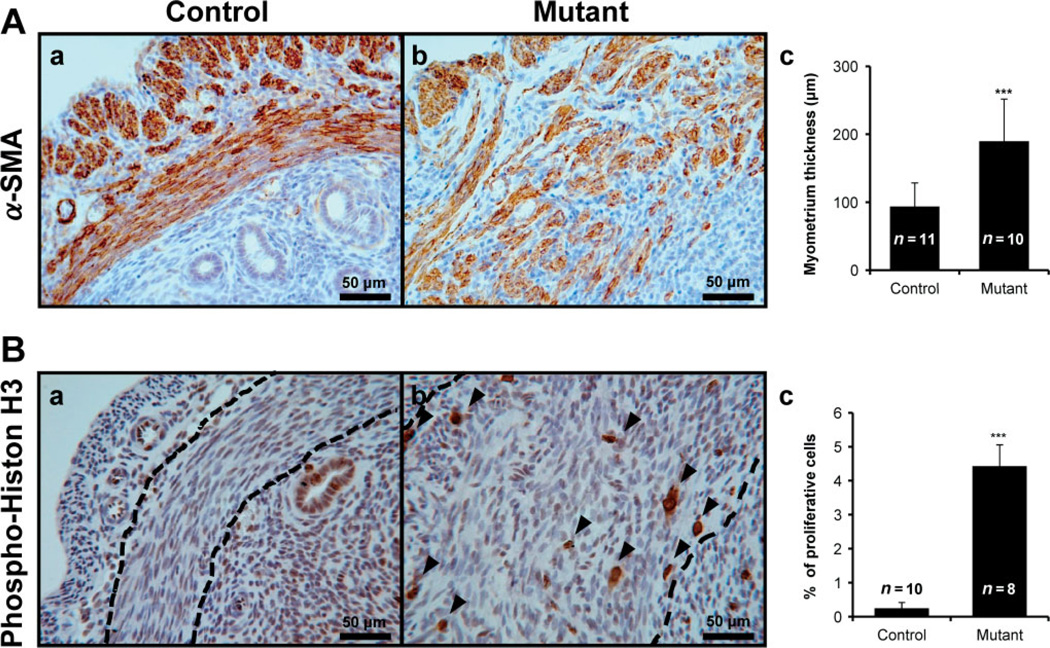
To determine whether abnormal activation of β-catenin in the murine uterus results in adenomyosis, Ctnnb1f(ex3)/+ mice were crossed with PRcre mice (PRcre/+ Ctnnbf(ex3)/+) [35]. Our previous data showed that mutant mice (PRcre/+ Ctnnb1f(ex3)/+) are infertile, hormone-insensitive and have endometrial glandular hyperplasia [35]. In addition, we observed an increase of β-catenin levels and nuclear localization in the myometrium and epithelium in the uterus of mutant mice compared to control mice (PRcre/+ and Ctnnbf(ex3)/+) (Figure 2). Histological assessment of uteri from 2 month-old mutant mice revealed severe defects in the myometrium. To investigate these defects in more detail, smooth muscle actin (α-SMA) immunohistochemistry was employed to specifically mark the myometrium. Control mice showed regular concentric layers of smooth muscle cells (Figure 3Aa); however, mutant mice exhibited an abnormal, irregular structure which exhibited an interwoven pattern and scattering of α-SMA-positive cells in the myometrium (Figure 3Ab). The thickness of the myometrium area is significantly increased in mutant mice (189.85 + 62.09 µm; n = 11) compared with control mice (93.88 + 34.6 µm; n = 10) (Figure 3Ac). In order to determine the extent of cell proliferation in the myometrium of mutant mice, we performed immunohistochemical staining for phosphohistone H3, a mitotic marker, in control and mutant mice at 2 months of age. Immunohistochemical staining of phospho-histone H3 demonstrated that proliferation was significantly increased in the myometrium of mutant mice (4.43 + 0.63%; n = 8) compared to control mice (0.25 + 0.17%; n = 10) (Figure 3B). These results suggest that activation of β-catenin in the uterus exhibited abnormal irregular structure and increased cell proliferation at the myometrium.
Figure 2.
Analysis of conditionally dominant stabilized β-catenin in the murine uterus. Six week-old control mice and mutant mice were sacrificed. Immunohistochemical analysis for β-catenin-demonstrated cytoplasmic β-catenin was observed in control mice (A, C) but nuclear accumulation of β-catenin in the epithelium and myometrium of mutant mouse uteri (B, D); (C, D) high-magnification pictures of the boxed areas in (A, B)
Figure 3.
Abnormal irregular structure and highly active proliferation in myometrium of uteri of mutant mice. (A) Immunohistochemical localization for smooth muscle actin (α-SMA), a smooth muscle cell marker, was examined in uterus of control (a) and mutant (b). The myometrial area of mutant mice were significantly increased (c) and revealed abnormal irregular structure. (B) Phospho-histone H3 was used for identification of proliferation in control (a) and mutant mice (b). Proliferative ability was increased in the myometrium of mutant mice compared with control mice (c); arrowheads, positive phospho-histone H3 cells; dashed lines, inner circular layer of myometrium. ***p < 0.001; one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple range test
Development of adenomyosis in mice with uterine-specific stabilization of β-catenin
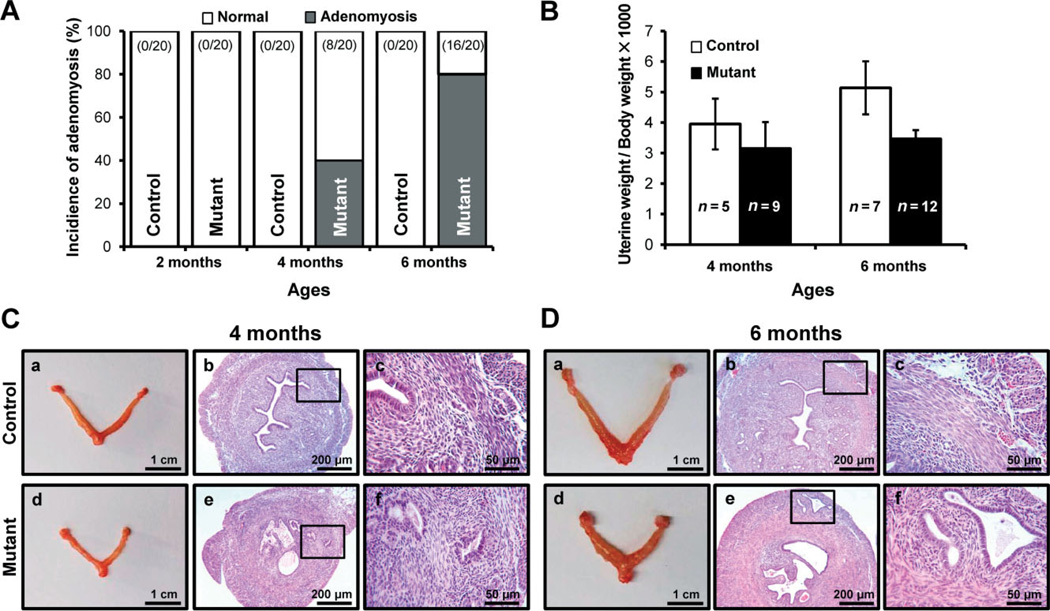
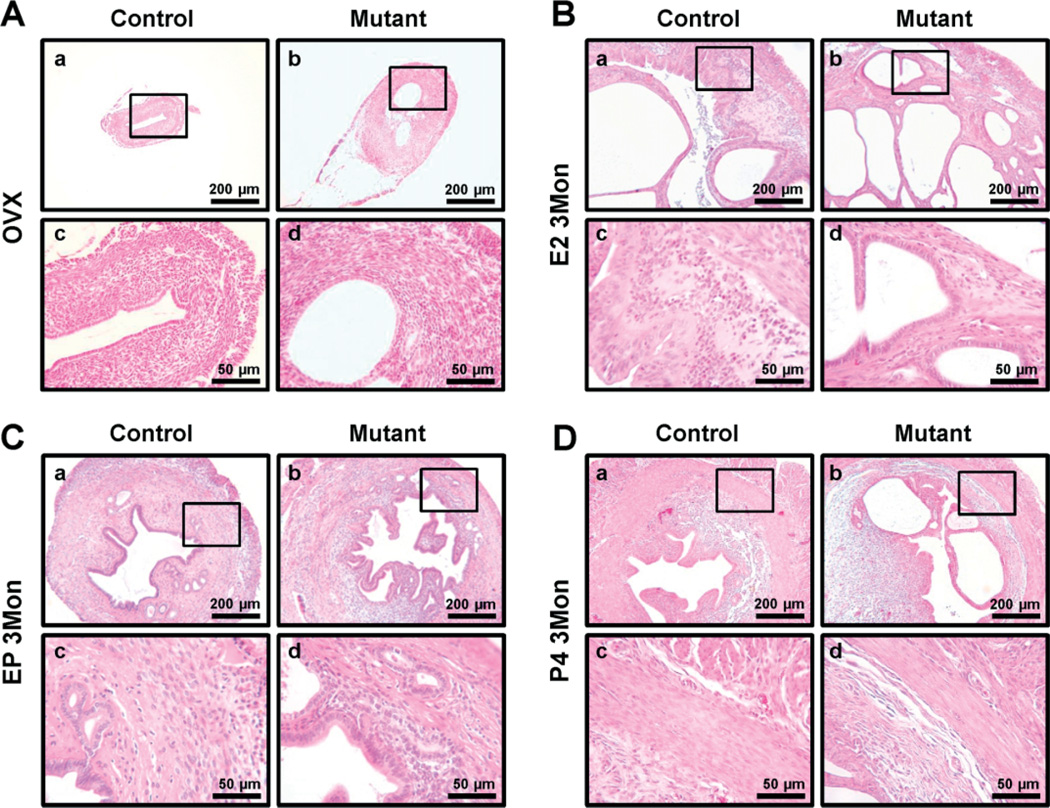
In addition to the abnormal myometrial structure, mutant mice showed glands and stromal cells in the myometrium (Figure 4). To identify the progression of adenomyosis in mutant mice, we sacrificed control and mutant mice at 2, 4 and 6 months of age (n = 20/age/genotype) and investigated their histology. In 4 month-old mutant mice the incidence of adenomyosis was 40%, and endometrial glandular hyperplasia was observed in all mutant mice (Figure 4A, C). At 6 months of age, 80% of mutant mice had adenomyosis in the myometrium (Figure 4A, D). In our previous study, uterus weight of the mutant mice at 4 weeks of age was significantly decreased compared to controls, and this persisted until 8 weeks of age [35]. Interestingly, the uterus weight of mutant mice was similar to control mice at 4 months (3.95 + 0.83% and 3.15 + 0.23%, respectively) and 6 months (5.14 + 0.87% and 3.47 + 0.28%, respectively) of age (Figure 4B). The uterine diameter was larger in mutant mice than in control mice, while the uterine length in mutant mice was smaller than in control mice (Figure 4C, D). The thickness of the myometrium area was significantly increased in mutant mice compared with control mice. In adenomyosis, the human uterus is larger than the normal uterus [13]. Therefore, these results suggest that highly active proliferation in the myometrium of mutant mice may cause the enlarged uterus of adenomyosis.
Figure 4.
Development of adenomyosis in mice with uterine-specific stabilization of β-catenin. (A) Incidence of adenomyosis development was scored in control and mutant mice; control and mutant mice at 2, 4 and 6 months of age were sacrificed. (B) Uterine weight was determined for control and mutant mice at 4 and 6 months of age. The uterine weight was not different between control and mutant mice. (C, D) Gross anatomy and histology of control and mutant mice uteri at 4 (C) and 6 (D) months of age. Uterine length of mutant mice was smaller than control mice, but the same thickness as in control mice. In histology analysis, adenomyosis phenotype was observed in mutant mice
Epithelial–mesenchymal transition in adenomyosis development
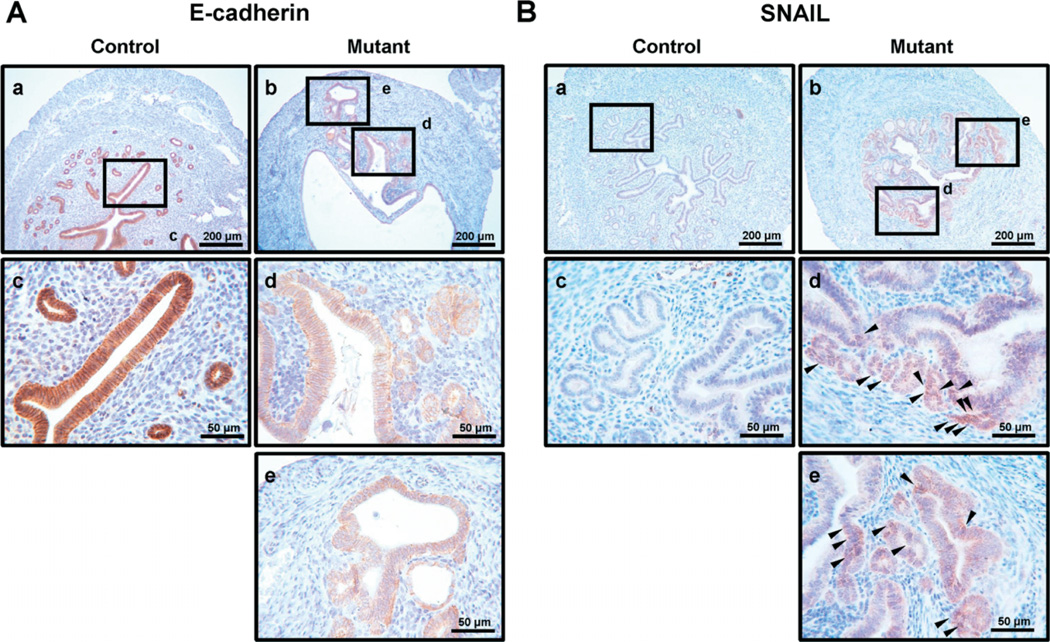
In order to determine whether adenomyosis lesions have endometrial epithelial cells in mutant mice, we performed immunohistochemistry for E-cadherin, an epithelial cell marker [38,39]. We detected E-cadherinpositive epithelial cells in the adenomyosis lesions of mutant mice but not in the myometrium of control mice at 4 months (Figure 5A). Interestingly, the expression of E-cadherin was lower in epithelial cells of eutopic (Figure 5Ad) and adenomyosis lesions (Figure 5Ae) of mutant mice compared to control mice (Fig 5Aa, c), consistent with EMT. EMT plays an important role in embryogenesis, fibrosis and tumour metastasis [40]. EMT is initiated by a number of transcription factors, including Snail, Slug, Twist, ZEB1 and SIP1, via the repression of E-cadherin expression [41]. Due to the invasive behaviour and cytoskeletal rearrangement of endometrial epithelial cells during ectopic implantation, we reasoned that EMT might be involved in the adenomyosis development observed in mutant mice. In order to determine whether adenomyosis development is related to EMT in mutant mice, we evaluated the levels of EMT markers in control and mutant mice. Snail, a member of the Snail family of zinc finger transcription factors, is a mediator of EMT and induces EMT by directly repressing epithelial markers such as E-cadherin and by up-regulating mesenchymal markers [42]. We performed immunohistochemistry to examine the expression of Snail between control and mutant mice (Figure 5B). Snail-positive cells were not detected in control uteri (Figure 5Ba, c). However, Snail was expressed in some epithelial cells of mutant uteri but not stromal cells (Figure 5Bb, d, e). ZEB1 is also an important repressor of E-cadherin, contributing to EMT.
Figure 5.
Expression of E-cadherin and Snail in the uterus of control and mutant mice. (A) Immunohistochemical analysis of E-cadherin demonstrated suppression of E-cadherin in mutant mouse epithelial cells (b, d, e) compared with control mice (a, c) at 4 months. (B) Immunohistochemical analysis of Snail demonstrated induction of Snail in epithelial cells in mutant (b, d, e) but not in control (a, c) mice at 4 months
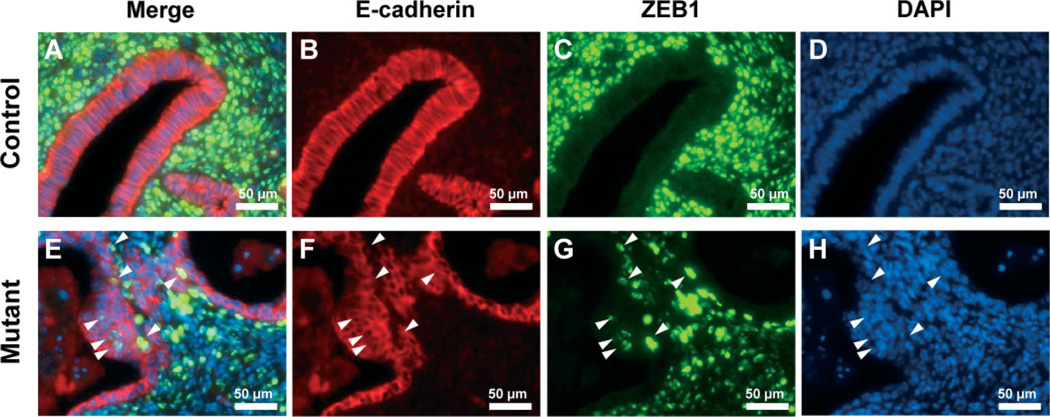
We examined the expression of ZEB1 and E-cadherin in control and mutant mice using double immunofluorescence staining. Interestingly, although the number of ZEB1-positive epithelial cells was sparse, the nuclear expression of ZEB1 was observed in some epithelial cells of the uterus in mutant mice (Figure 6E, G). In control mice, positive ZEB1 expression was limited in the majority of the stroma and myometrium (Figure 6A, C). These results support that abnormal activation of β-catenin in PR-positive cells induces expression of Snail and ZEB1 in some epithelial cells.
Figure 6.
The expression of ZEB1 in uterus of control and mutant mice. Immunofluorescence analysis of E-cadherin (red; B, F) and ZEB1 (green; C, G) was performed in control (A–D) and mutant (E–H) mice. The expression of ZEB1 was observed in epithelial cells of the uterus in mutant but not control mice. Merged images (A, E) and DAPI images (D, F); arrowheads, ZEB1-positive cells
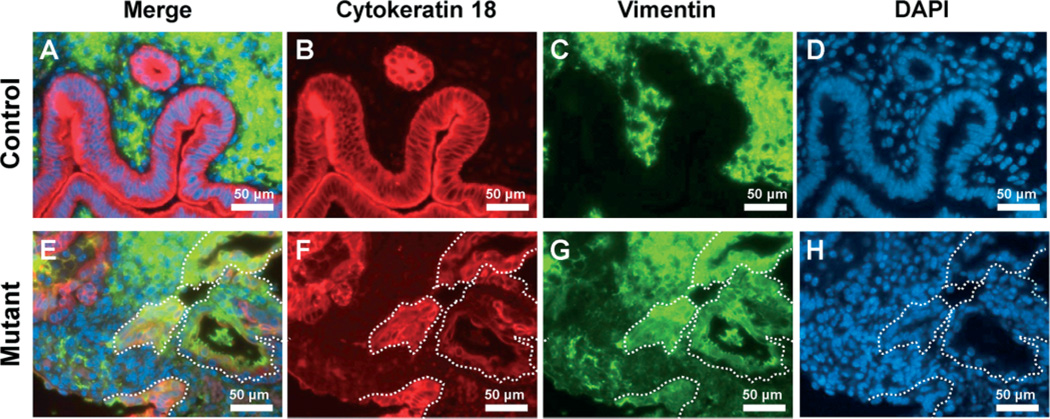
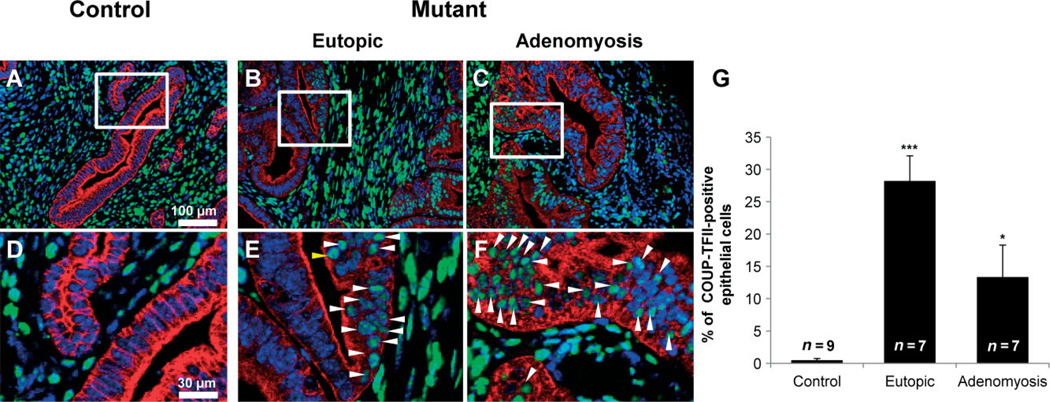
The expression of mesenchymal markers is observed after EMT [43]. Vimentin is used widely as a marker of EMT, which takes place during embryogenesis and metastasis [44,45]. The expression of vimentin was limited in stromal cells of control mice. However, vimentin was expressed in stromal cells as well as some epithelial cells of mutant mice (Figure 7). Chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) is highly expressed in mesenchymal cells and plays critical roles during mouse development [39]. In the female uterus, COUP-TFII is expressed in uterine stromal cells and the myometrium but not in epithelial cells [38]. The expression of COUP-TFII was observed in 29.50 + 2.24% of eutopic epithelial cells and 13.36 + 4.95% of adenomyotic epithelial cells of mutant mice (n = 7), while its expression was limited in endometrial stromal cells and the myometrium of control mice (0.50 + 0.26%, n = 9) (Figure 8). These results suggest that abnormal activation of β-catenin contributes to adenomyosis development through induction of EMT.
Figure 7.
The expression of vimentin in uteri of control and mutant mice. Immunofluorescence analysis of cytokeratin 18 (red; B, F) and vimentin (green; C, G) was performed in control (A–D) and mutant (E–H) mice. The expression of vimentin was observed in epithelial cells of the uterus in mutant but not control mice; white dotted lines, vimentin-positive epithelial cells
Figure 8.
The expression of COUP-TFII in uteri of control and mutant mice. Immunofluorescence analysis of COUP-TFII (green) and cytokeratin 18 (red) was performed in uteri of control (A, D) and mutant (B, C, E, F) mice. COUP-TFII was observed in epithelial cells of mutant mice (B, C, E, F), while its expression was limited to endometrial stromal cells and the myometrium of control mice (A, D). (D–F) High-magnification pictures of the boxed areas in (A–C); arrowheads, COUP-TFII-positive cells. (G) COUP-TFII-positive cells were significantly increased in mutant mice. *p < 0.05; ***p < 0.001, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple range test
Ovarian steroid hormone dependency of adenomyosis development
It has been suggested that adenomyosis is an oestrogen-dependent disorder in which oestrogen causes growth and progression of adenomyosis [46]. We examined the role of ovarian steroid hormones in the development of the adenomyosis phenotype in the mutant mice. Mutant and control mice were ovariectomized and treated with vehicle, oestrogen (E2), oestrogen plus progesterone (E2 + P4) or P4 for 3 months and sacrificed at 6 months of age. Ovariectomized mutant mice did not develop adenomyosis, as observed in intact mutant mice (Figure 9A). This demonstrates that the adenomyosis phenotype of mutant mice is dependent on ovarian hormone stimulation. Although control mice showed endometrial hyperplasia as expected from chronic E2 treatment, they did not show the adenomyosis phenotype. All of the mutant mice treated with E2 developed adenomyosis (Figure 9B). This result supports that adenomyosis is an oestrogen-dependent disease. Ovariectomized mutant mice treated with E2 + P4 for 3 months developed adenomyosis, as did intact mutant mice (Figure 9C). However, ovariectomized mutant mice treated with P4 for 3 months did not develop adenomyosis (Figure 9D). Therefore, these results demonstrate that β-catenin activation and oestrogen is important to the aetiology of adenomyosis.
Figure 9.
Ovarian steroid hormone dependent development of adenomyosis in mutant mice. (A) Uteri of 6 month-old ovariectomized control and mutant mice treated with vehicle. Histological analysis showed that mutant mice did not develop the adenomyosis phenotype. (B) Formation of adenomyosis in ovariectomized mutant mice treated with E2; adenomyosis induced in the uteri of ovariectomized mutant mice treated with E2 for 3 months; histological analysis showed that mutant mice developed the adenomyosis phenotype. (C) Uteri of 6 month-old ovariectomized control and mutant mice treated with E2 + P4 for 3 months; histological analysis showing adenomyosis in the ovariectomized mutant mice treated with E2 + P4 for 3 months. (D) Uteri of 6 month-old ovariectomized control and mutant mice treated with P4; histological analysis showed that mutant mice did not develop the adenomyosis phenotype
Epithelial–mesenchymal transition in human adenomyosis
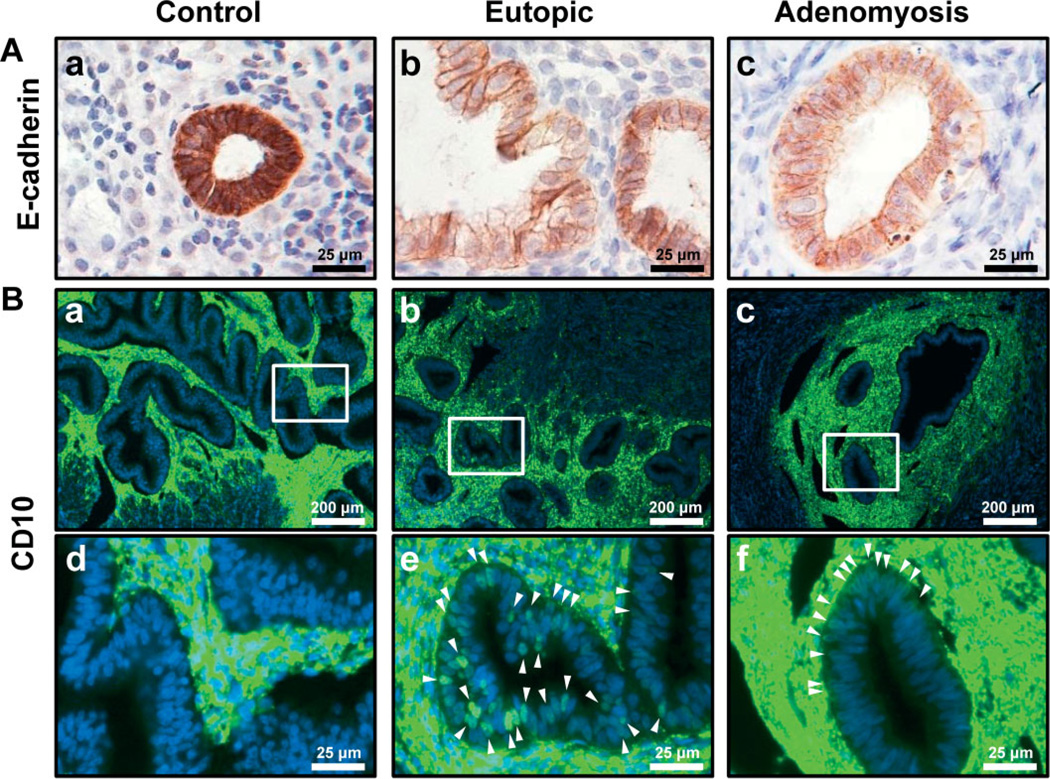
To elucidate the impact of EMT in human adenomyosis, immunohistochemistry for E-cadherin and CD10 was performed with control endometrium (proliferative phase, n = 13; secretory phase, n = 11) and eutopic endometrium (proliferative phase, n = 6; secretory phase, n = 7) and adenomyosis lesion (proliferative phase, n = 8; secretory phase, n = 11) from patients with adenomyosis (Figure 10). As shown in the mutant mice, eutopic endometrium and adenomyosis lesion from patients with adenomyosis showed decreased E-cadherin level in epithelial cells compared to control endometrium (Figure 10A). The expression of CD10 was observed in some epithelial cells of eutopic endometrium and adenomyosis lesions, while its expression was limited in endometrial stromal cells in control endometrium (Figure 10B). To confirm that CD10-positive cells are not present in infiltrating lymphocytes rather than epithelial cells, we examined the expression of CD3, CD4 and CD11b in CD10-positive cells. CD3 is expressed on T helper and T cytotoxic lymphocytes [47,48] and CD4 is expressed on T helper lymphocytes [49,50]. CD10-positive epithelial cells were neither CD3- nor CD4-positive in human endometrium with adenomyosis (see supplementary material, Figures S2 and S3). CD11b was found on monocytes, macrophages, granulocytes, some B cells, dendritic cells and natural killer cells [51]. CD10-positive epithelial cells were also CD11b-negative cells (see supplementary material, Figure S4). Lymph node was used as positive control of CD3, CD4 and CD11b immunostaining. These results confirmed that CD10-positive cells are epithelial cells but not infiltrating lymphocytes. Therefore, these results support that EMT contributes to adenomyosis development in humans.
Figure 10.
Repression of E-cadherin and induction of CD10 expression in epithelial cells of human adenomyosis. (A) Immunohistochemical analysis of E-cadherin showed suppression of E-cadherin expression in epithelial cells of a eutopic endometrium (b) and an adenomyosis lesion (c) compared to normal endometrium (a). (B) The expression of CD10 was detected in some epithelial cells of eutopic endometrium (b, e) and adenomyosis lesion (c, f) but not control endometrium (a, d)
Discussion
Adenomyosis is a common benign gynaecological disorder. The majority of symptomatic women who are non-responsive to pharmacological therapy require surgical intervention [5]. With the aid of transvaginal ultrasonography and MRI, this disease is now frequently diagnosed in infertility patients, where it can interfere with implantation and cause subfertility and miscarriage [9–11]. Given the prevalence of adenomyosis and associated symptoms, there is, unfortunately, no good evidence-based treatment to date except hysterectomy. Hysterectomy for adenomyosis treatment results in costly health care and may not be an option for those women wishing to maintain future fertility. The aetiology and pathophysiology of adenomyosis is still unknown. Our results suggest that activation of β-catenin plays an important role in the pathogenesis of adenomyosis (Figure 1).
Adenomyosis has been studied in many different animal models, including mice, rats, rabbits, dogs, cats and non-human primates [52]. To address the mechanism of disease development, several studies in the mouse model or hormonal treatment have shown an increased incidence of adenomyosis [53–56]. However, the development of appropriate animal models is still required to understand the molecular mechanism of adenomyosis. In order to study the pathophysiological role of β-catenin activation in adenomyosis, we used mice in which β-catenin was stabilized in the reproductive tract, using previously generated mice with modified β-catenin alleles and the PRcre mouse [35,57,58]. In these animals the uterus exhibited an abnormal irregular structure in the myometrium (Figure 3A), and proliferation was significantly increased in the myometrium of mutant mice compared to control mice (Figure 3B). We have provided evidence that mice with conditional uterine activation of β-catenin develop adenomyosis (Figure 4) and provide a novel model system to investigate the genetic and molecular events involved in the transition from normal to adenomyosis. Constitutive activation of β-catenin in the uterine mesenchyme (Amhr2cre/+ Ctnnbf(ex3)/+) also shows myometrial hyperplasia, develops mesenchymal tumours and causes occasional adenomyosis [59]. WNT–β-catenin signalling molecules are important and should be tightly regulated for uterine function [35,60–63]. These findings suggest that tight regulation of β-catenin in uterine mesenchymal cells and epithelial cells is important in physiological uterine function and that dysregulation of β-catenin causes several pathological conditions, including endometrial hyperplasia, uterine tumours and adenomyosis.
β-catenin interacts with E-cadherin in cell–cell adherens junctions. EMT of tumour cells is associated with nuclear accumulation of β-catenin. β-Catenin was shown to induce EMT during gastrulation in the sea urchin [64] and also in human cell culture systems [65,66]. Moreover, increased activity of β-catenin leads directly to a loss of epithelial cell differentiation [67,68]. E-cadherin is reduced in the uterine epithelial cells of women with endometriosis compared with endometriosis-free controls [69]. The most important hallmark of EMT is loss of E-cadherin. Its loss is a requirement for detachment, invasion, distribution and metastasis, which are linked directly to activated β-catenin signalling. We observed that E-cadherin expression was decreased in the uterus of mutant mice as well as in human adenomyosis (Figures 5A, 10A). EMT is initiated by a number of transcription factors, including Snail, Slug, Twist, ZEB1 and SIP1, via repression of E-cadherin expression [41]. Snail and ZEB1 expression has been associated with tumour cell migration, invasion and metastasis [41]. We observed induction of Snail and ZEB1 and repression of E-cadherin in some epithelial cells of the uterus in mutant mice (Figures 5, 6). Our results suggest that loss of E-cadherin and aberrant expression of Snail and ZEB1 are associated with EMT and induce the metastatic potential.
The expression of mesenchymal markers is observed after EMT in epithelial cells of mutant mice. We observed limited expression of vimentin in stromal cells of control mice. However, vimentin was expressed in stromal cells as well as some epithelial cells of mutant mice (Figure 7). COUP-TFII-positive cells are observed in some epithelial cells of mutant mice (Figure 8). COUP-TFII is strongly expressed in the stromal compartment and plays critical roles in development and tumour formation [39,70]. COUP-TFII induced the extracellular matrix-degrading proteinases matrix metalloproteinase 2 (MMP2) and urokinase-type plasminogen activator (uPA), which are known to play critical roles in angiogenesis and metastasis [71]. CD10 is also a useful molecule in tumour invasion. CD10 is a cell-surface, zinc-dependent metalloproteinase and CD10 expression has been detected within the invasive area of various cancers, such as prostate, breast, colorectal and skin carcinomas [72–75]. In our results, CD10 was expressed in some epithelial cells of human adenomyosis, whereas the control epithelial cells were negative (Figure 10B). These results suggest that EMT is essential for adenomyosis development.
In the development of adenomyosis, two theories are now generally accepted. One is endomyometrial invagination of the endometrium, and the other is the de novo development of adenomyosis from Müllerian rests [2]. It has been proposed that adenomyosis is an ovarian steroid hormone-dependent disorder, resulting from high oestrogen levels. It correlates positively with endometrial hyperplasia, endometrial cancer and endometriosis, which are also associated with unopposed oestrogen [17,76]. Chen et al. have suggested that oestrogen-induced epithelial–mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis [77]. Changes of EMT markers, including E-cadherin and vimentin, were linked to oestrogen levels. Mutant mice treated with E2 and E2 + P4 still develop adenomyosis. However, mutant mice treated with vehicle or P4 showed abnormal luminal epithelial structure but did not develop adenomyosis (Figure 9). These results suggest that β-catenin is a critical factor associated with unopposed oestrogen for adenomyosis development.
In conclusion, our results demonstrate a role of β-catenin activation in adenomyosis development, using the PRcre/+ Ctnnbf(ex3)/+ mouse model and human adenomyosis. We identified that β-catenin activation caused induction of Snail and ZEB1 expression and repression of E-cadherin expression and mesenchymal cell marker expression in some epithelial cells during the pathogenesis of adenomyosis. These findings provide great insights into our understanding of the aetiological and pathophysiological role of β-catenin signalling and into the development of new therapeutic approaches in adenomyosis.
Supplementary Material
Acknowledgements
We would like to thank the Human Female Reproductive Tract Biorepository and the Spectrum Health Medical Group, Department of Obstetrics, Gynaecology and Reproductive Biology and Women’s Health (Elizabeth Leary MD, Calvin Leazenby MD and Diana Bitner MD) and Susan D Ferguson for their help in obtaining the samples. We would also like to thank Dr Dong S Darling for ZEB1 antibody, Dr Jennifer Richer and Francesco J DeMayo for helpful discussions, Sung-Nam Cho for technical assistance, and Sharra Poncil and Thuy Tran for their help in manuscript preparation. This study was supported by the National Institutes of Health (Grant No. R01HD057873), the American Cancer Society (Research Scholar Grant No. RSG-12-084-01-TBG) and the World Class University programme (Grant No. R31-10056) through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (to JWJ), the National Institutes of Health (Grant No. R01HD067721, to BAL), the NICHD (Cooperative Agreement No. U54HD40093, as part of the Specialized Cooperative Centres Programme in Reproduction and Infertility Research, to ATF) and the Ministry of Education, Science and Technology, Republic of Korea (Research Grant No. 20120006091, to JML).
Footnotes
No conflicts of interest were declared.
Author contributions
SJO carried out experiments, analysed data and wrote the paper; JHS and HSL conceived experiments and analysed data; THK and JYY carried out experiments; BAL carried out experiments and analysed data; MMT and JPL provided transgenic mice; JYA, RRB, REL, ATF and JML conceived experiments and analysed data; and JWJ conceived experiments, analysed data and wrote the paper. All authors contributed to the final manuscript version.
SUPPORTING INFORMATION ON THE INTERNET
The following supporting information may be found in the online version of this article.
Figure S1. The expression of membranous β-catenin in endometrial tissue from women with and without adenomyosis
Figure S2. The expression of CD10 and CD3 in human endometrium with and without adenomyosis
Figure S3. The expression of CD10 and CD4 in human endometrium with and without adenomyosis
Figure S4. The expression of CD10 and CD11b in human endometrium with and without adenomyosis
References
- 1.Tamai K, Togashi K, Ito T, et al. MR imaging findings of adenomyosis: correlation with histopathologic features and diagnostic pitfalls. Radiographics. 2005;25:21–40. doi: 10.1148/rg.251045060. [DOI] [PubMed] [Google Scholar]
- 2.Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312–322. doi: 10.1093/humupd/4.4.312. [DOI] [PubMed] [Google Scholar]
- 3.Wang PH, Su WH, Sheu BC, et al. Adenomyosis and its variance: adenomyoma and female fertility. Taiwan J Obstet Gynecol. 2009;48:232–238. doi: 10.1016/S1028-4559(09)60295-3. [DOI] [PubMed] [Google Scholar]
- 4.Uduwela AS, Perera MA, Aiqing L, et al. Endometrial–myometrial interface: relationship to adenomyosis and changes in pregnancy. Obstet Gynecol Surv. 2000;55:390–400. doi: 10.1097/00006254-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Vercellini P, Vigano P, Somigliana E, et al. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006;20:465–477. doi: 10.1016/j.bpobgyn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Koike N, Tsunemi T, Uekuri C, et al. Pathogenesis and malignant transformation of adenomyosis [review] Oncol Rep. 2012;29:861–867. doi: 10.3892/or.2012.2184. [DOI] [PubMed] [Google Scholar]
- 7.Brosens I, Pijnenborg R, Benagiano G. Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis. Placenta. 2013;34:100–105. doi: 10.1016/j.placenta.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Kunz G, Beil D, Huppert P, Adenomyosis in endometriosis—prevalence, impact on fertility, et al. Evidence from magnetic resonance imaging. Hum Reprod. 2005;20:2309–2316. doi: 10.1093/humrep/dei021. [DOI] [PubMed] [Google Scholar]
- 9.de Souza NM, Brosens JJ, Schwieso JE, et al. The potential value of magnetic resonance imaging in infertility. Clin Radiol. 1995;50:75–79. doi: 10.1016/s0009-9260(05)82983-6. [DOI] [PubMed] [Google Scholar]
- 10.Campo S, Campo V, Benagiano G. Infertility and adenomyosis. Obstet Gynecol Int. 2012;2012:7806132. doi: 10.1155/2012/786132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis LS, Saso S, Chatterjee J, et al. Adenomyosis and infertility. Reprod Biomed Online. 2012;24:586. doi: 10.1016/j.rbmo.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261–264. doi: 10.1097/01.gco.0000169103.85128.c0. [DOI] [PubMed] [Google Scholar]
- 13.Devlieger R, D’Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139–147. doi: 10.1093/humupd/dmg010. [DOI] [PubMed] [Google Scholar]
- 14.Salim R, Riris S, Saab W, et al. Adenomyosis reduces pregnancy rates in infertile women undergoing IVF. Reprod Biomed Online. 2012;25:273–277. doi: 10.1016/j.rbmo.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688–691. doi: 10.1016/s0029-7844(99)00659-6. [DOI] [PubMed] [Google Scholar]
- 16.Sammour A, Pirwany I, Usubutun A, et al. Correlations between extent and spread of adenomyosis and clinical symptoms. Gynecol Obstet Invest. 2002;54:213–216. doi: 10.1159/000068385. [DOI] [PubMed] [Google Scholar]
- 17.Parazzini F, Vercellini P, Panazza S, et al. Risk factors for adenomyosis. Hum Reprod. 1997;12:1275–1279. doi: 10.1093/humrep/12.6.1275. [DOI] [PubMed] [Google Scholar]
- 18.Schindler AE. Progestogen deficiency and endometrial cancer risk. Maturitas. 2009;62:334–337. doi: 10.1016/j.maturitas.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Schindler AE. Hormonal contraceptives and endometriosis/adenomyosis. Gynecol Endocrinol. 2010;26:851–854. doi: 10.3109/09513590.2010.534612. [DOI] [PubMed] [Google Scholar]
- 20.Kazandi M, Zeybek B, Terek MC, et al. Grade 2 endometrioid adenocarcinoma arising from adenomyosis of the uterus: report of a case. Eur J Gynaecol Oncol. 2010;31:719–721. [PubMed] [Google Scholar]
- 21.Saegusa M, Hashimura M, Yoshida T, et al. β-Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer. 2001;84:209–217. doi: 10.1054/bjoc.2000.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saegusa M, Okayasu I. Frequent nuclear β-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001;194:59–67. doi: 10.1002/path.856. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Sagae S, Nishioka Y, et al. Mutations of the β-catenin gene in endometrial carcinomas. Jpn J Cancer Res. 1999;90:55–59. doi: 10.1111/j.1349-7006.1999.tb00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeda T, Yoshinaga K, Semba S, et al. Mutational analysis of the CTNNB1 (β-catenin) gene in human endometrial cancer: frequent mutations at codon 34 that cause nuclear accumulation. Oncol Rep. 2000;7:323–326. doi: 10.3892/or.7.2.323. [DOI] [PubMed] [Google Scholar]
- 25.Fukuchi T, Sakamoto M, Tsuda H, et al. β-Catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998;58:3526–3528. [PubMed] [Google Scholar]
- 26.Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 27.Aberle H, Bauer A, Stappert J, et al. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacios J, Gamallo C. Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998;58:1344–1347. [PubMed] [Google Scholar]
- 29.Cloke B, Huhtinen K, Fusi L, et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462–4474. doi: 10.1210/en.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno-Bueno G, Hardisson D, Sanchez C, et al. Abnormalities of the APC/β-catenin pathway in endometrial cancer. Oncogene. 2002;21:7981–7990. doi: 10.1038/sj.onc.1205924. [DOI] [PubMed] [Google Scholar]
- 31.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, β-catenin, and ZEB1 in malignant progression of cancer. Cancer Metast Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 32.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 33.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Tillo E, Siles L, de Barrios O, et al. Expanding roles of ZEB factors in tumorigenesis and tumor progression. Am J Cancer Res. 1:897–912. [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong JW, Lee HS, Franco HL, et al. β-Catenin mediates glandular formation and dysregulation of β-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 37.Franco HL, Dai D, Lee KY, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurihara I, Lee DK, Petit FG, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee DK, Kurihara I, Jeong JW, et al. Suppression of ERα activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24:930–940. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Acloque H, Adams MS, Fishwick K, et al. Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 42.Fan F, Samuel S, Evans KW, et al. Overexpression of Snail induces epithelial–mesenchymal transition and a cancer stem celllike phenotype in human colorectal cancer cells. Cancer Med. 2012;1:5–16. doi: 10.1002/cam4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, Yan Q, Long X, et al. Vimentin affects the mobility and invasiveness of prostate cancer cells. Cell Biochem Funct. 2008;26:571–577. doi: 10.1002/cbf.1478. [DOI] [PubMed] [Google Scholar]
- 44.Gilles C, Polette M, Mestdagt M, et al. Transactivation of vimentin by β-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–2664. [PubMed] [Google Scholar]
- 45.Upton MP, Hirohashi S, Tome Y, et al. Expression of vimentin in surgically resected adenocarcinomas and large cell carcinomas of lung. Am J Surg Pathol. 1986;10:560–567. doi: 10.1097/00000478-198608000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Zhou S, Yi T, Liu R, et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteom. 2012;11 doi: 10.1074/mcp.M112.017988. M112.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jedryka M, Chrobak A, Chelmonska-Soyta A, et al. Matrix metalloproteinase (MMP)-2 and MMP-9 expression in tumor infiltrating CD3 lymphocytes from women with endometrial cancer. Int J Gynecol Cancer. 2012;22:1303–1309. doi: 10.1097/IGC.0b013e318269e27b. [DOI] [PubMed] [Google Scholar]
- 48.Chuang SS, Lin CN, Li CY, et al. Uterine leiomyoma with massive lymphocytic infiltration simulating malignant lymphoma. A case report with immunohistochemical study showing that the infiltrating lymphocytes are cytotoxic T cells. Pathol Res Pract. 2001;197:135–138. doi: 10.1078/0344-0338-5710024. [DOI] [PubMed] [Google Scholar]
- 49.Yamagami W, Susumu N, Tanaka H, et al. Immunofluorescence-detected infiltration of CD4+ FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. Int J Gynecol Cancer. 2011;21:1628–1634. doi: 10.1097/IGC.0b013e31822c271f. [DOI] [PubMed] [Google Scholar]
- 50.Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–116. doi: 10.1111/j.1600-065X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 51.Khanh do T, Mekata E, Mukaisho K, et al. Prognostic role of CD10+ myeloid cells in association with tumor budding at the invasion front of colorectal cancer. Cancer Sci. 2011;102:1724–1733. doi: 10.1111/j.1349-7006.2011.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greaves P, White IN. Experimental adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20:503–510. doi: 10.1016/j.bpobgyn.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Mori T, Nagasawa H. Mechanisms of development of prolactin-induced adenomyosis in mice. Acta Anat (Basel) 1983;116:46–54. doi: 10.1159/000145724. [DOI] [PubMed] [Google Scholar]
- 54.Guttner J. Adenomyosis in mice. Z Versuchstierkd. 1980;22:249–251. [PubMed] [Google Scholar]
- 55.Ostrander PL, Mills KT, Bern HA. Long-term responses of the mouse uterus to neonatal diethylstilbestrol treatment and to later sex hormone exposure. J Natl Cancer Inst. 1985;74:121–135. [PubMed] [Google Scholar]
- 56.Parrott E, Butterworth M, Green A, et al. Adenomyosis—a result of disordered stromal differentiation. Am J Pathol. 2001;159:623–630. doi: 10.1016/S0002-9440(10)61733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harada N, Tamai Y, Ishikawa T, et al. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soyal SM, Mukherjee A, Lee KY, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 59.Tanwar PS, Lee HJ, Zhang L, et al. Constitutive activation of β-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81:545–552. doi: 10.1095/biolreprod.108.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arango NA, Szotek PP, Manganaro TF, et al. Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Zhang JV, Cao YJ, et al. Inhibition of the β-catenin signaling pathway in blastocyst and uterus during the window of implantation in mice. Biol Reprod. 2005;72:700–706. doi: 10.1095/biolreprod.104.033837. [DOI] [PubMed] [Google Scholar]
- 62.Mohamed OA, Jonnaert M, Labelle-Dumais C, et al. Uterine Wnt/β-catenin signaling is required for implantation. Proc Natl Acad Sci USA. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rider V, Isuzugawa K, Twarog M, et al. Progesterone initiates Wnt-β-catenin signaling but estradiol is required for nuclear activation and synchronous proliferation of rat uterine stromal cells. J Endocrinol. 2006;191:537–548. doi: 10.1677/joe.1.07030. [DOI] [PubMed] [Google Scholar]
- 64.Logan CY, Miller JR, Ferkowicz MJ, et al. Nuclear β-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development. 1999;126:345–357. doi: 10.1242/dev.126.2.345. [DOI] [PubMed] [Google Scholar]
- 65.Morali OG, Delmas V, Moore R, et al. IGF-II induces rapid β-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene. 2001;20:4942–4950. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 66.Eger A, Stockinger A, Schaffhauser B, et al. Epithelial–mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of β-catenin and upregulation of β-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J Cell Biol. 2000;148:173–188. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mariadason JM, Bordonaro M, Aslam F, et al. Down-regulation of β-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res. 2001;61:3465–3471. [PubMed] [Google Scholar]
- 68.Naishiro Y, Yamada T, Takaoka AS, et al. Restoration of epithelial cell polarity in a colorectal cancer cell line by suppression of β-catenin/T-cell factor 4-mediated gene transactivation. Cancer Res. 2001;61:2751–2758. [PubMed] [Google Scholar]
- 69.Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N -cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159:1839–1852. doi: 10.1016/S0002-9440(10)63030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin J, Chen X, Yu-Lee LY, et al. Nuclear receptor COUP-TFII controls pancreatic islet tumor angiogenesis by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor-2 signaling. Cancer Res. 2010;70:8812–8821. doi: 10.1158/0008-5472.CAN-10-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Navab R, Gonzalez-Santos JM, Johnston MR, et al. Expression of chicken ovalbumin upstream promoter-transcription factor II enhances invasiveness of human lung carcinoma cells. Cancer Res. 2004;64:5097–5105. doi: 10.1158/0008-5472.CAN-03-1185. [DOI] [PubMed] [Google Scholar]
- 72.Albrecht M, Gillen S, Wilhelm B, et al. Expression, localization and activity of neutral endopeptidase in cultured cells of benign prostatic hyperplasia and prostate cancer. J Urol. 2002;168:336–342. [PubMed] [Google Scholar]
- 73.Kesse-Adu R, Shousha S. Myoepithelial markers are expressed in at least 29% of oestrogen receptor negative invasive breast carcinoma. Mod Pathol. 2004;17:646–652. doi: 10.1038/modpathol.3800103. [DOI] [PubMed] [Google Scholar]
- 74.Ogawa H, Iwaya K, Izumi M, et al. Expression of CD10 by stromal cells during colorectal tumor development. Hum Pathol. 2002;33:806–811. doi: 10.1053/hupa.2002.125773. [DOI] [PubMed] [Google Scholar]
- 75.Takahara M, Chen S, Kido M, et al. Stromal CD10 expression, as well as increased dermal macrophages and decreased Langerhans cells, are associated with malignant transformation of keratinocytes. J Cutan Pathol. 2009;36:668–674. doi: 10.1111/j.1600-0560.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 76.Bergholt T, Eriksen L, Berendt N, et al. Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod. 2001;16:2418–2421. doi: 10.1093/humrep/16.11.2418. [DOI] [PubMed] [Google Scholar]
- 77.Chen YJ, Li HY, Huang CH, et al. Oestrogen-induced epithelial–mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol. 2010;222:261–270. doi: 10.1002/path.2761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.