Abstract
Background
Venous thromboembolism (VTE) is a frequent complication in melanoma patients (pts) with brain metastases (BM). The management of these pts is challenging due to the high risk of intracranial hemorrhage (ICH), and limited data on the safety of anticoagulation in this scenario. We reviewed the treatments and outcomes among melanoma pts with BM and VTE at our institution to determine the safety of anticoagulation in these patients.
Methods
A retrospective chart review was performed to identify melanoma pts with BM who were diagnosed with VTE. The clinical characteristics of the BM and the VTE, treatments given for VTE, subsequent intracranial hemorrhage (ICH) and overall survival (OS) were collected. Characteristics and outcomes were compared between pts who received systemic anticoagulation and those who did not.
Results
A total of 74 evaluable melanoma pts with BM and VTE were identified. Fifty seven (77%) pts received systemic anticoagulation. There was no significant difference in the number (p=0.40) or maximum diameter (p=0.55) of brain metastasis between the patients who were or were not anticoagulated. Two (4%) pts who received anticoagulation developed ICH, which was not statistically different from pts who were not anticoagulated (0%, p=1.00). There was a trend towards longer OS from VTE among patients who received systemic anticoagulation (median OS 4.2 vs 1.2 months, p=0.06).
Conclusion
Anticoagulation for VTE did not significantly increase the risk of ICH or decrease OS in pts with melanoma BM. This data supports the safety of systemic anticoagulation for VTE in these patients.
Keywords: Anticoagulation, brain metastases, intracranial hemorrhage, melanoma, venous thromboembolism
INTRODUCTION
The incidence of melanoma is on the rise. In 2011 an estimated 70,230 patients were diagnosed with melanoma, and 8,790 patients died from this disease, in the United States [1]. Melanoma has a high risk of metastasizing to the central nervous system (CNS). Melanoma is the third most common source of brain metastases, exceeded only by lung and breast cancer. As breast and lung cancer are much more common, this data reflects the high proclivity of melanoma to metastasize to the brain [2]. Up to 75% of patients with stage IV melanoma develop brain involvement, and such patients have a median survival of ~ 4 months [3].
Venous thromboembolism (VTE) is a common phenomenon in cancer patients. It is estimated that 4% – 20% of cancer patients will develop VTE, and is an important cause of cancer-related mortality[4, 5]. The risk of VTE is particularly high in patients with brain tumors [6]. Mechanical treatments for VTE, such as IVC filters, are prone to complications, particularly in patients with hypercoaguable conditions such as cancer. Thus, systemic anticoagulation, with either low-molecular weight heparin (LMWH) or Vitamin-K antagonists, is generally recommended for patients with VTE [7]. However, this therapy does incur a risk of hemorrhage, which can be particularly devastating when it occurs intracranially. Thus, the management of VTE in patients with brain metastases represents a common and challenging clinical scenario.
The management of VTE in patients with brain metastases from melanoma is particularly challenging due to the significant risk of hemorrhage in brain metastases from this disease [8, 9]. Little data is available regarding the relative risk of ICH in melanoma patients with brain metastases receiving anticoagulation. In order to develop rational management approaches for these patients we reviewed the clinical characteristics and outcomes of metastatic melanoma patients with brain metastases who were diagnosed with VTE to determine the relative risk and benefits of systemic anticoagulation in these patients.
METHODS
Patients
Under an IRB-approved protocol, we reviewed the records of patients treated in the Department of Melanoma Medical Oncology at the University of Texas MD Anderson Cancer Center from June 1, 1996, to June 30, 2006. Patients were selected who had radiographic evidence of parenchymal brain involvement at the time of diagnosis of a VTE, including deep venous thrombosis (DVT) or pulmonary embolism (PE). For each patient data was collected regarding pre existing characteristics (medical co-morbidities, presence of extra-cranial metastases); the number, maximal size, and hemorrhage of intraparenchymal brain lesions; the type of VTE (DVT, PE, both) and clinical presentation (clinically suspected, incidental finding or symptomatic); treatments given for VTE, including IVC filter placement and type and dose of systemic anticoagulation; and the occurrence of hemorrhagic events (intracranial, extracranial) and overall survival after the diagnosis of VTE. For patients who received anticoagulant therapy for PE, subsequent radiographic imaging was reviewed for VTE resolution.
Statistical Analysis
Fisher ‘s exact test was used to assess the association between categorical variables and the use of systemic anticoagulation in patients with VTE, and between the on-study outcomes of intra-cranial (ICH) and extracranial hemorrhage. The method of Kaplan and Meier was used to estimate the distribution of overall survival from the date of the VTE event, and distributions were compared between groups with the log-rank test. All statistical tests were performed two-sided, and no adjustment was made for the multiplicity of testing.
RESULTS
Patient Characteristics and Treatments for VTE
Eighty one patients with melanoma brain metastases who were diagnosed with VTE were identified. Seven of these patients had surgical resection of their brain metastases within a week of the VTE event, and thus were considered inevaluable for the subsequent risk of ICH. The clinical characteristics at the time of diagnosis of the VTE of the seventy four evaluable patients are shown in Table 1. Seventy (95%) of the patients had concurrent extracranial disease, and forty six (62%) had at least one medical co-morbidity. Six patients (8%) were receiving prophylactic anticoagulation at the time of diagnosis of VTE for atrial fibrillation (n=3), post-surgical care (n=1), cellulitis in the LE (n=1) and chronic PE (n=1).
Table 1.
Patient characteristics and use of anticoagulation in patients with melanoma brain metastasis (BM) and venous thromboembolic event (VTE)
| Patient Characteristics at the VTE Event |
Level | No Systemic Anticoagulation for VTE (n=17) N (%) |
Systemic Anticoagulation for VTE (n=57) N (%) |
P – Value1 |
|---|---|---|---|---|
| Co-morbidity* | Yes | 10 (59%) | 36 (63%) | 0.78 |
| No | 7 (41%) | 21 (37%) | ||
| Extra-cranial metastases | Yes | 16 (94%) | 54 (95%) | 1.00 |
| No | 1 (6%) | 3 (5%) | ||
| Already receiving systemic anticoagulation** | Yes | 1 (6%) | 5 (9%) | 1.00 |
| No | 16 (94%) | 52 (91%) | ||
| Hemorrhagic BM | Yes | 7 (41%) | 14 (25%) | 0.22 |
| No | 10 (59%) | 43 (75%) | ||
| Number of BM | >4 | 8 (47%) | 20 (35%) | 0.40 |
| 1–4 | 9 (53%) | 37 (65%) | ||
| Diameter for the largest BM | >1cm | 10 (71%) | 31 (61%) | 0.55 |
| <1cm | 4 (29%) | 20 (39%) | ||
| ND | 3 | 6 | ||
| Location of the largest BM | Frontal | 7 (41%) | 16 (28%) | 0.94 |
| Frontoparietal | 1 (6%) | 3 (5%) | ||
| Parietal | 3 (18%) | 14 (25%) | ||
| Temporal | 2 (12%) | 10 (18%) | ||
| Occipital | 2 (12%) | 6 (10%) | ||
| Cerebellum | 2 (12%) | 8 (14%) |
Fisher's exact test.
Cardiovascular, renal, pulmonary or autoimmune diseases, intercurrent infections and secondary malignancies.
Indicates surgery within 2 weeks of the VTE event.
BM= brain metastasis; ND= Not determined
Fifty seven (77%) of the evaluable patients received systemic anticoagulation for VTE. There was no significant difference between those patients who were and those who were not anticoagulated in the prevalence of medical co-morbidities (p=0.78), extra-cranial disease (p=1.00), or the antecedent use of prophylactic anticoagulation (p=1.00) (Table 1). Among the patients who received anticoagulation, thirty seven (65%) had one to four brain metastases, while twenty (35%) had more than four; this distribution was not significantly different from the patients who did not receive anticoagulation (p=0.40) (Table 1). Thirty one (61%) of the patients who were systemically anticoagulated had a maximum intracranial tumor diameter of greater than 1 cm, which was similar to the patients who were not anticoagulated (71%, p=0.55). There was also no significant difference in the location of the largest intracranial lesion between the two groups of patients (p=0.94). There was evidence of intratumoral hemorrhage at the time of diagnosis of VTE in twenty one (28%) of the seventy four patients overall; fourteen of these patients were subsequently treated with systemic anticoagulation, seven were not (p=0.22). Nineteen of the patients were diagnosed with a PE only, twenty five with DVT only, and 30 with both PE and DVT (Table 2). The type (p=0.57) and clinical presentation (p=0.66 for DVT, p=0.97 for DVT) of VTE also showed no significant differences between patients who did and did not receive systemic anticoagulation.
Table 2.
VTE event-characteristics and use of anticoagulation.
| Characteristics for VTE |
Level | Received No Anticoagulation N (%) |
Received Anticoagulation for VTE N (%) |
P – Value1 |
|---|---|---|---|---|
| Type of event | PE | 5 (30%) | 14 (24%) | 0.57 |
| DVT | 7 (40%) | 18 (32%) | ||
| both | 5 (30%) | 25 (44%) | ||
| Clinical presentation for PE | symptomatic | 3 (33%) | 20 (51%) | 0.66 |
| clinically suspected | 1 (1%) | 2 (5%) | ||
| incidental | 6 (66%) | 17(44%) | ||
| not-evaluated | 1 | 6 | ||
| Clinical presentation for DVT | symptomatic | 6 (60%) | 22 (51%) | 0.97 |
| clinically suspected | 4 (40%) | 15 (35%) | ||
| incidental | 2 (20%) | 6 (14%) | ||
| not-evaluated | 2 | 5 |
Fisher's exact test.
VTE= venous thromboembolism. PE= pulmonary embolism. DVT= deep venous thrombosis.
Among the fifty seven patients who received anticoagulation, twenty six were treated with systemic anticoagulation alone, while thirty one also underwent IVC filter placement (Table 3). Among the patients who were not anticoagulated, thirteen had an IVC filter, and four were given only supportive measures due to extensive disease and decline in status. The most common anticoagulant used was enoxaparin, which was used at full dose (1 mg/kg SC every 12 hours or 1.5 mg/kg SC every 24 hours) in six patients, and at a reduced dose (0.5 mg /Kg SC every 12 hours or 1 mg/kg once daily) in forty one. Fifteen of the patient treated with reduced-dose enoxaparin had radiographically diagnosed pulmonary emboli that were re-evaluated after approximately six weeks of anticoagulation; all fifteen had radiographic resolution of their thrombi.
Table 3.
Treatments given for VTE.
| Treatment | N (%) | |
|---|---|---|
| Any Treatment for VTE | IVC filter alone | 13 (18%) |
| Systemic anticoagulation alone | 26 (35%) | |
| IVC filter + systemic coagulation | 31 (42%) | |
| No treatment | 4 (5%) | |
| Systemic anticoagulation given | LMWH | 44 (59%) |
| Heparin/vitamin K inhibitor | 13 (18%) | |
| None | 17 (23%) |
IVC= inferior vena cava; LMWH= low molecular weight heparin.
Bleeding Events
The median follow-up for the evaluable patients from the diagnosis of VTE was 3.4 months (range 0.3 to 30.3). Among the seventy four patients who were diagnosed with VTE with BM from melanoma, ten patients died within one month without interval CNS imaging. Three of these patients received anticoagulation, seven did not. The cause of death for all three patients who received systemic anticoagulation was extensive progressive disease; there were no clinical findings to suggest intracranial or extracranial bleeding events at the time of death. All of the remaining patients (n=64) had subsequent CNS imaging performed after the diagnosis of VTE, and were considered evaluable for the analysis of the risk of bleeding events. For the patients with intracranial hemorrhage at the time of VTE diagnosis, images were reviewed for evidence of new or worsening hemorrhage.
Six patients developed a hemorrhagic event subsequent to VTE diagnosis (Table 4). Two of these events were intracranial, while four were extracranial. Both episodes of ICH (2/64 patients with CNS imaging) occurred in patients who received systemic anticoagulation and did not have evidence of ICH when VTE was diagnosed. The overall incidence of ICH in the patients who received anticoagulation (4%) did not differ significantly from those who did not (0%, p=1.0). One patient with two brain metastases (maximum diameter 1.4 cm) developed asymptomatic ICH after 3 weeks of low dose of LMWH, and survived over 12 months after the ICH. The second patient who developed ICH had numerous intracranial lesions (at least 10; maximum diameter 6.1 cm), had been diagnosed at an outside facility with DVT and was treated with coumadin for one week prior to transferring to our center for a partial seizure, ICH and prolonged INR. The patient had altered mental status with clinical evidence of significant disease progression and died a few days later. Extracranial bleeding events occurred in two (4%) patients receiving anticoagulation and two (12%) who did not, which also was not significantly different (p=0.22). Three of the extracranial bleeding events occurred in patients who had evidence of intratumoral hemorrhage in their brain metastases at the time of VTE diagnosis. Two of these patients received anticoagulation (enoxaparin); none of the three patients had worsening or new intracranial hemorrhage.
Table 4.
Hemorrhagic events after the diagnosis of VTE.
| Variable | Event | No Anticoagulation | Coagulation | P-value1 |
|---|---|---|---|---|
| ICH | No | 17 (100%) | 55 (96%) | 1.00 |
| Yes | 0 (0%) | 2 (4%) | ||
| Other Post-Coagulation Hemorrhage | No | 15 (88%) | 55 (96%) | 0.22 |
| Yes | 2 (12%) | 2 (4%) |
Fisher exact test.
ICH = intracranial hemorrhage.
Survival
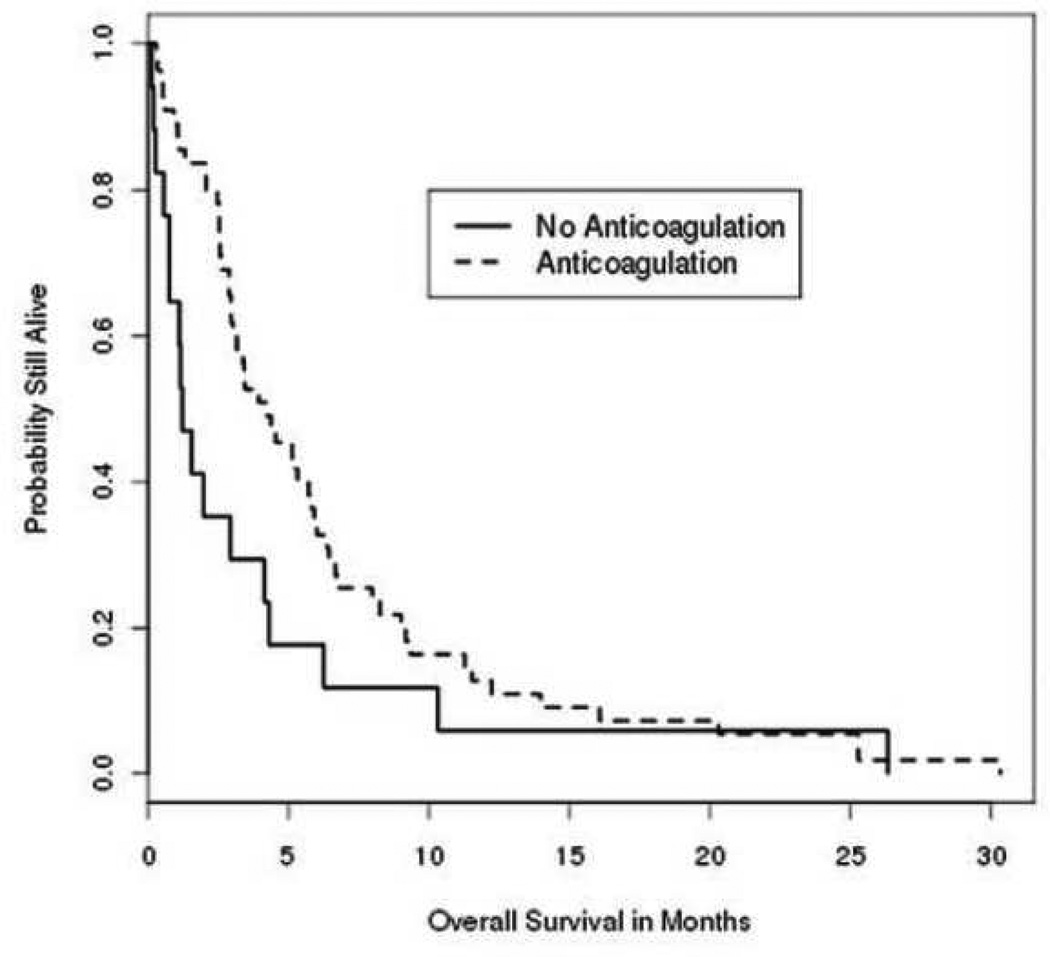
All seventy four patients with VTE and BM were included in the analysis of survival from VTE diagnosis (median OS=3.4 months). The patients who received anticoagulation had a median survival of 4.2 months from the diagnosis of VTE. The patients who were not anticoagulated had a median survival of 1.2 months (p=0.06 versus anticoagulated) (Figure 1). Consistent with previous studies, patients with more than four brain metastases (median OS 2.6 months) had significantly shorter survival than patients with four or less brain metastases (5.4 months, p=<0.0001). The number of brain metastases also correlated with survival from VTE among the patients receiving systemic anticoagulation (2.6 months for 1–4 BMs vs. 5.9 months for >4 BMs, p=<0.0001) (Table 5). Neither the size of the largest BM (p=0.80) nor the existence of hemorrhage (p=0.23) at the time of VTE correlated significantly with survival. There was a significant (p=0.002) association of survival with the location of the largest BM, with the worst outcomes observed with temporal lobe BMs (median 1.1 months), and longer survival with frontal (5.3 months) and parietal lobe (5.1 months) BMs.
Figure 1.
Overall survival after the diagnosis of VTE in patients with melanoma brain metastasis(es). Solid line, No systemic anticoagulation; Dashed line, Received systemic anticoagulation.
Table 5.
Survival from the diagnosis of VTE in patients with melanoma brain metastasis
| Patient Characteristics at the VTE Event |
Level | # Patients |
Median Survival (months) |
Hazard Ratio |
95% CI | P – Value1 |
|---|---|---|---|---|---|---|
| Co-morbidity* | Yes | 46 | 3.7 | 0.95 | 0.59 – 1.53 | 0.83 |
| No | 28 | 2.9 | ---- | ---- | ||
| Extra-cranial disease | Yes | 70 | 3.0 | 2.21 | 0.79 – 6.16 | 0.12 |
| No | 4 | 9.6 | ---- | ---- | ||
| Already receiving systemic anticoagulation** | Yes | 6 | 7.2 | 0.59 | 0.34 – 1.02 | 0.06 |
| No | 68 | 3.0 | ---- | ---- | ||
| Hemorrhagic BM | Yes | 21 | 3.4 | 1.37 | 0.82 – 2.30 | 0.23 |
| No | 53 | 3.4 | ---- | ---- | ||
| Number of BM | >4 | 28 | 2.5 | 2.72 | 1.62 – 4.55 | <0.0001 |
| 1–4 | 46 | 5.5 | ---- | ---- | ||
| Diameter for the largest BM | >1cm | 41 | 3.4 | 1.07 | 0.64 – 1.78 | 0.80 |
| <1cm | 24 | 4.4 | ---- | ---- | ||
| ND | 9 | 2.5 | **** | *** | ||
| Location for the largest BM | Frontal | 23 | 5.3 | 0.50 | 0.23 – 1.07 | 0.002 |
| Frontoparietal | 4 | 3.8 | 0.74 | 0.23 – 2.38 | ||
| Parietal | 17 | 5.1 | 0.54 | 0.24 – 1.22 | ||
| Temporal | 12 | 2.5 | 1.11 | 0.48 – 2.58 | ||
| Occipital | 8 | 1.3 | 2.69 | 1.02 – 7.06 | ||
| Cerebellum | 10 | 2.7 | ---- | ---- |
Fisher's exact test.
Cardiovascular, renal, pulmonary or autoimmune diseases, intercurrent infections and secondary malignancies.
Indicates surgery within 2 weeks of the VTE event.
BM= brain metastasis. ND= no data.
Not included in model analysis.
DISCUSSION
Epidemiologic data demonstrates that the incidence and mortality of melanoma have increased dramatically over the last 50 years [10]. Similarly, there has been a dramatic rise in the annual incidence of brain metastasis across all cancers, possibly reflecting the increased efficacy of therapies to control extra-cranial metastases [11].However, such therapies often fail to prevent the development of brain metastases, and the mortality due to these tumors. The management of VTE in melanoma patients with brain metastases is a challenging and increasingly common clinical scenario. In this single-center retrospective study we observed a 4% incidence of ICH with the use of systemic anticoagulation in melanoma patients with known brain metastases. This rate is relatively low, and did not differ significantly from the rate of ICH in VTE patients who did not receive systemic anticoagulation. In addition, there was no evidence that VTE patients with melanoma brain metastases who were anticoagulated systemically had shorter survival from the diagnosis of VTE than patients who were not.
To our knowledge, this represents the first analysis of the risk of ICH among patients with melanoma brain metastases treated with systemic anticoagulation. The results of this study are similar to previous reports regarding the use of anticoagulation in patients with primary brain tumors. Among a large cohort of patients with high-grade gliomas, ICH developed in 2 of 103 (1.9%) patients who were diagnosed with VTE and treated with UFH followed by warfarin, which was nearly identical to the ICH rate (2.2%) among the gliomas patients without VTE and not receiving anticoagulation[12]. The majority of bleeding events in the UFH cohort in that study occurred in the setting of supra-therapeutic levels of anticoagulation[13–15]. The safety of warfarin for long-term use was evaluated in 22 patients with malignant gliomas, none of whom developed ICH[16]. However, the use of long-acting vitamin-K antagonist requires very close monitoring with regular follow-ups, and a well-informed patient who can maintain a relatively stable diet. Drug interactions with medications that are commonly used in patients with brain metastases (i.e. anticonvulsants, certain antibiotics, cimetidine) can lead to fluctuations in the intensity of anticoagulation and requires careful assessment. As with unfractionated heparin, hemorrhagic complications associated with the use of warfarin frequently occur in the context of over-coagulation [17]. Over the last decade clinical trials have demonstrated superior efficacy, and equivalent safety, for LWMH in the prevention of recurrent VTE events in cancer patients [18, 19]. Supported by this data, the majority of the melanoma patients in our cohort were treated with enoxaparin, a LMWH that is effective and approved for use in patients with DVT and PE. The recommended dosing of enoxaparin for treatment of VTE is 1 mg/kg subcutaneously twice a day or alternatively 1.5 mg/kg once a day. Our patients were treated with either of these dosages based on the treating-physician’s discretion, but this did not affect the outcomes.
The similar rate of ICH observed in our cohort of patients with BM from melanoma with that previously reported in patients with gliomas is reassuring, but not necessarily expected. While there are a number of studies that support that the vasculature of tumors is different from that of normal tissue, there is also evidence that the vasculature of brain metastases may be quite different from primary brain tumors. The vasculature of brain metastases appears to be different from primary brain tumors in their expression of specific proteins (i.e. P-glycoprotein), and some studies have reported that histologically the vessels of brain metastases retain features of the vasculature of the primary tumors from which they originate [20, 21]. In addition, mechanical disruption could result from the penetration of metastatic tumor cells through the vascular endothelium of the CNS, which is required for the establishment of BMs, and thus could increase the risk of ICH. Functional differences between the vasculature of primary and metastatic brain tumors are also suggested by demonstrated differences in the penetration of paclitaxel in the periphery of tumors and surrounding normal tissues in patients who were administered this agent immediately prior to craniotomy [22].
We acknowledge that the retrospective nature of this study results in several limitations in the analysis performed. While we did not identify significant differences in the number, maximal size, or presence of hemorrhage in the intracranial metastases between the patients who were and who were not anticoagulated, the short median survival after VTE diagnosis among the patients who were not anticoagulated suggests that other factors (i.e. hospice referral, poor performance status) could have contributed to, or correlated with, their poor outcomes. Thus, we cannot be sure that the groups of patients who were and were not anticoagulated were fully equivalent/balanced. However, even without this comparator group, the relatively low incidence (4%) of ICH among the anticoagulated patients is reassuring. This in fact is much lower than the prevalence of ICH hemorrhage that was detected at the time of VTE diagnosis in this cohort of patients (28%), which is similar to the prevalence of hemorrhage (34.6%) reported in a cohort of 355 melanoma patients with brain metastases at the Memorial Sloan Kettering Cancer Center [23]. The confirmation of a lack of ICH, or a lack of worsening of existing ICH, by CNS imaging in fifty five of the fifty seven patients supports the likelihood of this low risk with anticoagulation; in turn, we acknowledge that the risk of ICH could be underestimated in the patients who were not anticoagulated in this cohort due to the rapid demise of seven of those patients without CNS imaging. We also acknowledge that conclusions about the safety of systemic anticoagulation in melanoma patients with brain metastases are limited by the non-uniformity of the regimens used in this retrospective cohort. However, the safety data from the 41 patients treated with enoxaparin at a total dose of 1 mg /Kg per day, which was effective for resolving pulmonary emboli in 15 of 15 evaluated patients, supports that this is a reasonable regimen to consider for such patients. Definitive conclusions about safety would require a randomized trial. Finally, while we observed no difference in the risk of bleeding between patients with a maximum BM diameter of <1 (n=24) versus >1 cm (n=41), we note that very few patients in this cohort had large BMs (3 pts with 1–2 cm; 12 pts, 2–3 cm; 3 pts, > 3 cm). Thus, the relative risk of systemic anticoagulation in melanoma patients with large (>3 cm) BMs remains to be defined.
In summary, the emergence of more effective therapies in melanoma, and the growing availability of clinical trials for patients with CNS involvement, supports the need for an improved understanding of the risk factors for ICH in these patients. Such information will be important not only to clinical management, but also to the interpretation of clinical events noted in trials. Our data supports that systemic anticoagulation can be used safely in melanoma patient with VTE with small (< 3 cm) brain metastases, based on the prevalence of ICH and survival observed in this retrospective cohort of patients. However, additional data from other sites with large populations of melanoma patients and/or randomized trials of systemic anticoagulation will be necessary for definitive conclusions.
Acknowledgements
Gregory W. Gladish, MD, Department of Daignostic Radiology, University of Texas MD Anderson Cancer Center
Source of funding: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflicts of interest: None
REFERENCES
- 1.Siegel R, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Sawaya R, et al. In: Metastatic Brain Tumors, in Brain Tumors. Kaye AH, Laws ER, editors. Philadelphia, PA: Churchill Livingstone; 2001. pp. 999–1026. [Google Scholar]
- 3.Davies MA, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. doi: 10.1002/cncr.25634. [DOI] [PubMed] [Google Scholar]
- 4.Khorana AA, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 6.Levitan N, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78(5):285–291. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lyman GH, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25(34):5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 8.Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983;1(4):313–317. doi: 10.1007/BF00165714. [DOI] [PubMed] [Google Scholar]
- 9.Wronski M, Arbit E. Surgical treatment of brain metastases from melanoma: a retrospective study of 91 patients. J Neurosurg. 2000;93(1):9–18. doi: 10.3171/jns.2000.93.1.0009. [DOI] [PubMed] [Google Scholar]
- 10.Beddingfield FC., 3rd The melanoma epidemic: res ipsa loquitur. Oncologist. 2003;8(5):459–465. doi: 10.1634/theoncologist.8-5-459. [DOI] [PubMed] [Google Scholar]
- 11.Barnholtz-Sloan JS, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 12.Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol. 1983;13(3):334–336. doi: 10.1002/ana.410130320. [DOI] [PubMed] [Google Scholar]
- 13.Levine MN, et al. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):287S–310S. doi: 10.1378/chest.126.3_suppl.287S. [DOI] [PubMed] [Google Scholar]
- 14.Hirsh J, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1 Suppl):64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 15.Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73(2):493–498. doi: 10.1002/1097-0142(19940115)73:2<493::aid-cncr2820730240>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Choucair AK, Silver P, Levin VA. Risk of intracranial hemorrhage in glioma patients receiving anticoagulant therapy for venous thromboembolism. J Neurosurg. 1987;66(3):357–358. doi: 10.3171/jns.1987.66.3.0357. [DOI] [PubMed] [Google Scholar]
- 17.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lee AY. Anti-thrombotic therapy in cancer patients. Expert Opin Pharmacother. 2003;4(12):2213–2220. doi: 10.1517/14656566.4.12.2213. [DOI] [PubMed] [Google Scholar]
- 19.Meyer G, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162(15):1729–1735. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 20.Gerstner ER, Fine RL. Increased Permeability of the Blood-Brain Barrier to Chemotherapy in Metastatic Brain Tumors: Establishing a Treatment Paradigm. J Clin Oncol. 2007;25(16):2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 21.Hirano A, Zimmerman HM. Fenestrated blood vessels in a metastatic renal carcinoma to the brain. Lab Invest. 1972;26:465–468. [PubMed] [Google Scholar]
- 22.Fine RL, et al. Randomized Study of Paclitaxel and Tamoxifen Deposition into Human Brain Tumors: Implications for the Treatment of Metastatic Brain Tumors. Clinical Cancer Research. 2006;12(19):5770–5776. doi: 10.1158/1078-0432.CCR-05-2356. [DOI] [PubMed] [Google Scholar]
- 23.Raizer JJ, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199–207. doi: 10.1215/15228517-2007-058. [DOI] [PMC free article] [PubMed] [Google Scholar]