Abstract
Purpose
The dosimetric impact of dose-probability based PTV margins for liver cancer patients receiving SBRT was compared to standard PTV based on the internal target volume (ITV). Plan robustness was evaluated by accumulating the treatment dose to ensure delivery of the intended plan.
Methods and Materials
Twenty patients planned on exhale CT for 27–50 Gy in 6 fractions using an ITV-based PTV and treated free-breathing were retrospectively evaluated. Iso-toxic, dose-escalated plans were created on mid-position CT, representing the mean breathing position, using a dose-probability PTV. The delivered doses were accumulated using biomechanical deformable registration of the daily cone-beam CT based on liver targeting at the exhale or mean breathing position, for the exhale and mid-position CT plans respectively.
Results
The dose-probability PTVs were on average 38% smaller than the ITV-based PTV enabling an average±standard deviation increase in the planned dose to 95% of the PTV of 4.0±2.8 Gy (9±5%) on the mid-position CT (p<0.01). For both plans, the delivered minimum GTV doses were greater than the planned nominal prescribed dose in all 20 patients and greater than the planned dose to 95% of the PTV in 18 (90%) patients. Nine patients (45%) had one or more GTVs with a delivered minimum dose more than 5 Gy higher with the mid-position CT plan using dose-probability PTV, compared to the delivered dose with the exhale CT plan using ITV-based PTV.
Conclusions
For iso-toxic liver SBRT planned and delivered at the mean respiratory, reduced dose-probability PTV enables a mean escalation of 4 Gy (9%) in 6 fractions over ITV-based PTV. This may potentially improve local control without increasing the risk of tumor under-dosing.
INTRODUCTION
Evidence of a dose-response relationship for local tumor control is emerging for liver cancer patients treated with stereotactic body radiotherapy (SBRT)[1–4]. Low rates of serious toxicities follow SBRT, providing a rationale to investigate iso-toxic dose-escalation techniques. Individualized dose-allocation schemes modeling liver normal tissue complication probabilities (NTCP) have been implemented clinically, however normal tissues still limit dose in the majority of patients[5].
Reducing the component of the planning target volume (PTV) accounting for breathing motion can spare normal liver tissue. Ten Haken et al. [6] modeled a 7% increase in tumor control probability after eliminating the breathing margin. This may be possible using active breath hold SBRT delivery, however approximately 40% of patients are unsuitable[7]. Abdominal compression passively reduces liver motion by only 2.3 mm on average[8]. Gating allows for reduced PTVs and an iso-NTCP median dose-escalation of 21%[9]. However, gating requires continuous real-time monitoring[10] and larger online workload versus other strategies.
For free-breathing radiotherapy, a simple and widely used PTV design creates an internal target volume (ITV), a union of the tumor’s positions on respiratory-correlated (4D) CT. A further linear expansion for setup uncertainties defines the PTV. This strategy aims for 100% tumor dose coverage over the entire breathing cycle, but effectively overcompensates as the tumor cannot be simultaneously at all phases. Margin ‘recipes’ have also been derived under simplified conditions, ensuring specific dosage and confidence levels (e.g. 90% of patients receive 95% dose)[11]. These dose-probability PTVs combine patient-specific breathing motion with population treatment uncertainties. They are 34% smaller on average than ITV-based PTV in liver SBRT[12]. Their dosimetric impact and robustness to liver SBRT uncertainties requires evaluation as there may be a reluctance to reduce margins clinically.
Delivered doses were previously accumulated with deformable registration of the treatment cone-beam CTs, for free-breathing liver SBRT plans using ITV-based PTVs[13]. Residual targeting errors exceeded the setup margin of 3 mm in 30% of patients, however the delivered tumor doses were lower than prescribed in only 1 patient (3.3%) with substantial inter-fraction liver deformation. The large ITV component of the PTV, intended to only compensate for breathing, may have nullified the dosimetric impact of these setup errors.
The aims of this study were to investigate liver SBRT planning at the mean breathing position with dose-probability PTVs enabling normal tissue sparing and dose-escalation. SBRT delivery was simulated with a guidance strategy based on 4D cone-beam CT and rigid registration. Deformable registration was used to reconstruct the delivered doses to evaluate the robustness of the plans. Iso-toxic dose-escalation via margin reduction may be safely explored to improve local control provided the risk of target under-dosing does not increase.
METHODS
Patient data
Twenty patients with primary or metastatic liver cancer previously treated on dose-escalation trials of liver SBRT for 27–49.8 Gy in 6 fractions were retrospectively investigated. The median total gross tumor volume (GTV) was 174 cm3 (range: 26–2402 cm3) over a median of 2 GTVs per patient (range: 1–3). Patients were treated free-breathing or with abdominal compression. The median GTV breathing amplitude was 8 mm (range: 1–21 mm).
Clinical planning was done on the exhale 4DCT (Pinnacle3 v9.2; Philips Medical Systems, Madison WI). The GTV contour was based on fused contrast-enhanced voluntary breath-hold CT and magnetic resonance images (MRI). The PTV (clinical PTV) was applied asymmetrically around each GTV at exhale, encompassing the patient-specific breathing motion plus a 5 mm expansion to account for other uncertainties (e.g. setup errors). In practice, the patient-specific motion was assessed as 90–100% of the motion measured on 4DCT, fluoroscopy and cine-MRI. The lack of liver-GTV contrast on 4DCT prevented direct GTV contouring on all 4D phases and the creation of a traditional ITV.
Daily image-guidance involved rigidly registering the planning exhale 4DCT liver contour to a free-breathing kilovoltage 3D CBCT, biased towards the superior part of the blurred liver diaphragm (i.e. the exhale position). Uncertainty in targeting the GTV results from using non-4D and non-contrast CBCT, the liver as a surrogate and residual liver deformation[13]. The residual population systematic (random) GTV errors were quantified with retrospective DIR of 4D CBCT to be 1.8 (1.7) mm left-right (LR), 2.2 (1.9) mm anterior-posterior (AP) and 2.6 (2.4) mm superior-inferior (SI)[13].
4D CBCT was not available clinically at the time these patients were treated, so the CBCTs were retrospectively 4D-sorted for this study[14].
SBRT planning
For this study, three plans were created with the same intensity-modulated radiotherapy (IMRT) technique. Individual prescribed doses were maximized based on a previously implemented liver iso-NTCP scheme after minimizing the normal liver dose[15]. The patient’s risk level assigned clinically (5–20%) was maintained across the three plans. The primary IMRT objectives were to maximize the dose to 95% of the PTV (PTVD95) while constraining the maximum luminal gastrointestinal organ doses to 31–36 Gy to 0.5 cm3 (details in [3]). If the last criteria could not be achieved, a lower dose than permitted by the iso-NTCP schema was prescribed, and the plan was re-optimized until all criteria were met. Plans were normalized so the prescribed dose covered at least 95% of the PTV.
PTV strategies and planning datasets
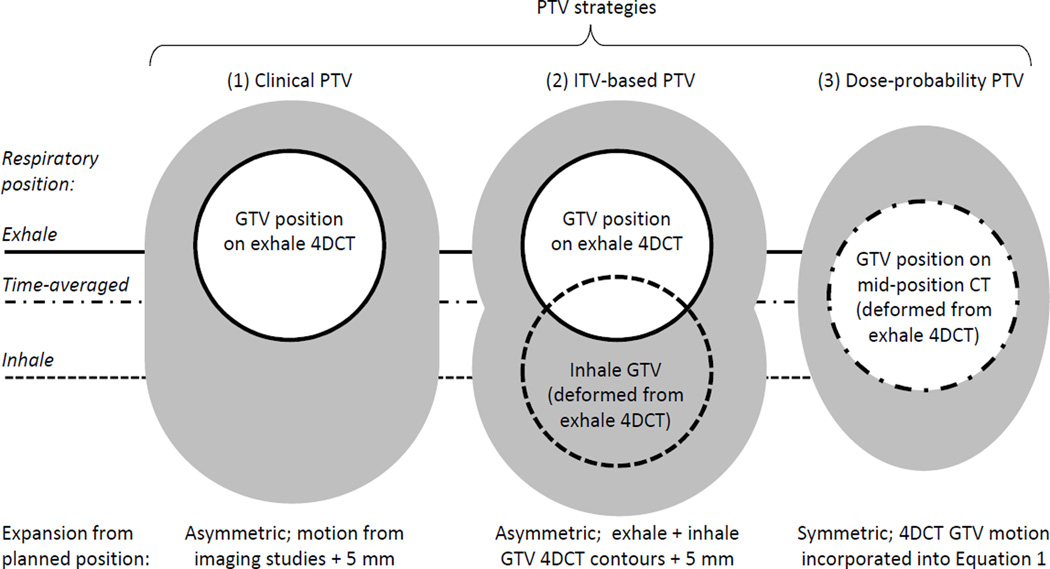
For this study, three plans were compared overall, two on the exhale 4DCT and one on the midposition CT (Fig. 1).
Fig. 1.
Schema of the different PTV strategies. Clinically, the GTV was delineated on exhale 4DCT. DIR of 4DCT allowed for propagation of the exhale contours into the inhale (2) or time-averaged position (3). Figure adapted from [24].
The first exhale 4DCT plan used the clinical PTV described above, termed the ‘clinical PTV plan’. Because this encompassed 90–100% (based on physician preference) of the breathing motion plus a setup margin it is similar in concept to ITV approaches.
The second exhale 4DCT plan’s PTV was standardized across all patients by using an ITV based on the exhale and inhale GTV contours as follows, termed the ‘ITV-based PTV plan’. Each exhale 4DCT was deformed to the inhale 4DCT using Morfeus, a biomechanical model-based deformable image registration (DIR) algorithm. This DIR establishes correspondence between images using guided-surface projections between contours of the liver, spleen and body on each image. These serve as boundary conditions for the model while tissues without secondary contoured surfaces (e.g. GTV) deform via the assigned biomechanical properties and are solved with finite element analysis. The mean and standard deviation of the absolute errors in the DIR-predicted positions of vessel bifurcations within the liver are ≤1.7 mm in any direction[16]. The exhale and deformed-inhale GTV contours were combined into an ITV and symmetrically expanded by 5 mm to create the ITV-based PTV, ensuring consistently constructed ITVs between all patients.
The third plan was created on a mid-position CT representing the patient anatomy in its time-averaged respiratory position allowing for dose-probability PTVs, termed the ‘dose-probability PTV plan’. Intermediate 4DCT phases were not archived for these patients therefore the mid-position CT was retrospectively reconstructed using DIR of each patient’s exhale and inhale 4DCT[12]. The exhale 4DCT and planning contours were deformed along the exhale-to-inhale linear trajectory into the mid-position CT geometry, with an accuracy of <2 mm versus the true time-averaged position[12].
This mid-position CT plan used the van Herk[11] PTV derivation to ensure 90% of patients receive the nominal dose prescribed to the 90% isodose (typical for liver SBRT):
| (1) |
where Σ and σ are the population standard deviations (SD) of all systematic and random errors respectively, and σpenumbra is the SD of the penumbra in water (3.2 mm[11]). The Σ and σ are the quadratic addition of the published uncertainties in GTV position due to DIR inaccuracy [16,17], intra-fraction errors specific to free-breathing liver SBRT[18], and residual inter-fraction GTV errors. It was assumed that after rigid liver alignment with 4D CBCT, deformation would cause residual errors in GTV position, therefore the inter-fraction errors were calculated as the centre-of-mass difference between the GTV and liver from previously studied patients[13]. The SD of the patient-specific 4DCT breathing motion was added quadratically to the population random errors (σ). Because the SD can be approximated as 1/3 the amplitude, this margin is smaller than the ITV-based PTV encompassing the full amplitude[19]. Rit et al. showed dose-probability PTVs are valid even with significant breath-to-breath variability[20]. The dose-probability PTV was applied symmetrically around the GTV on the mid-position CT. The baseline margin for all patients prior to the quadratic addition of the patient-specific breathing amplitude into σ was 3.1 mm LR, 3.9 mm AP and 4.7 mm SI.
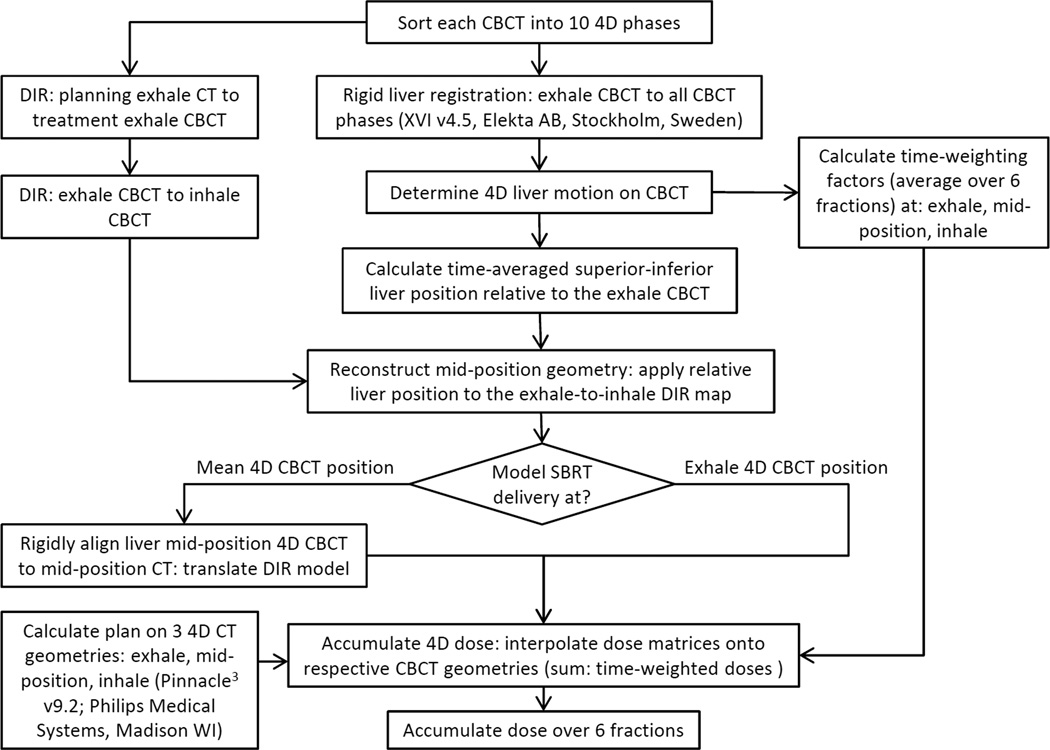
Delivered dose reconstruction to evaluate PTV robustness
The delivered dose was modeled as previously described and is summarized in Fig. 2[13]. DIR of all six 4D CBCTs allowed for residual inter-fraction targeting errors, deformations, and daily breathing motion to be accumulated into the delivered dose distributions for each plan. For the two exhale 4DCT plans, the residual position from the clinical treatment was used, including any post-CBCT corrections. For the mid-position CT plan, the liver was rigidly corrected at mean breathing position on 4D CBCT retrospectively, prior to dose reconstruction. This correction simulates the current scenario where online deformable registration is not available for direct GTV targeting.
Fig. 2.
Workflows to model the delivered dose following CBCT-guidance based on the liver’s exhale or mean breathing position.
Changes >1 Gy between the delivered and planned doses are assumed to be potentially clinically significant. Maximum and minimum doses are reported to 0.5 cm3. To evaluate DIR reliability, 4D CBCT contours were repeated for 10 patients (1 fraction each). This intra-observer variability resulted in an average (standard deviation) variation in the minimum delivered GTV dose of 0.0 (0.4) Gy.
RESULTS
Planning and dose-escalation
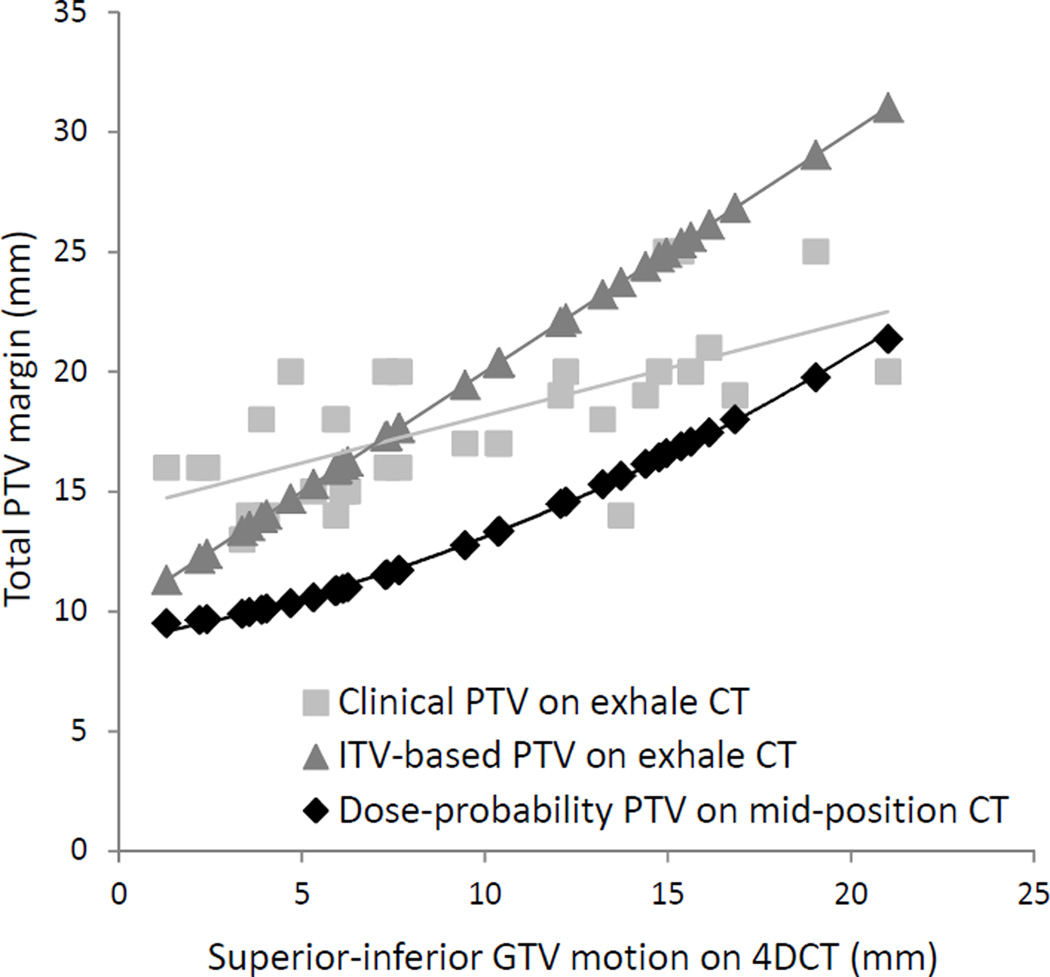
All PTVs are compared to breathing motion in Fig. 3. Clinical PTVs were on average 5% smaller in volume than ITV-based PTVs (p=0.08).
Fig. 3.
PTV size (total SI margin) versus the breathing amplitude. Above 6 mm of motion the difference in total SI expansion exceeds 5 mm between the dose-probability and ITV-based PTVs.
The average prescription isodose for the dose-probability PTV plans after optimization was 90% (range: 85–98%), equal to the isodose the margin was tailored for.. Dosimetry for the dose-probability PTV plans compared to the clinical and ITV-based PTV plans is shown in Table 1. All plans met the normal tissue dose constraints with similar PTV coverage. For all plans, the planned minimum GTV doses were 2–4% higher on average than the PTVD95, owing to the large dose heterogeneity allowed for SBRT distributions.
Table 1.
Planning dosimetry for the dose-probability PTV plans, and its change from the clinical and ITV-based PTV plans.
| Dose-probability PTV plans on mid- position CT, AVG±SD |
Δ in dose-probability PTV plan parameters on mid- position CT compared to: |
||
|---|---|---|---|
| Clinical PTV plan on exhale CT, Δ AVG±SD |
ITV-based PTV plan on exhale CT, Δ AVG±SD |
||
| Target dosimetry | |||
| PTV–GTV volume | 128±69 cm3 | −70±45 cm3 or −35±9 %* | −79±41 cm3 or −38±3 %* |
| Nominal prescription | 40.3±7.6 Gy | 3.9±2.8 Gy or 9±5%* | 3.7±2.4 Gy, or 9±6%* |
| PTVD95 | 42.6±8.4 Gy | 4.5±3.0 Gy or 10±6%* | 4.0±2.8 Gy or 9±5%* |
| Normal liver dosimetry | |||
| Mean dose (excluding GTV) | 16.1±3.2 Gy | −0.4±1.5 Gy or −3±9% | −0.5±1.7 Gy or −3±11 % |
| Effective volume‖, | 0.39±0.12 | −0.05±0.04* | −0.05±0.06* |
| Liver NTCP¶, | 2.4±4.0 % | −0.5±1.5% | −0.5±1.4% |
| PTV coverage quality | |||
| Volume receiving ≥ prescribed dose | 99±1% | 1±1%* | 0±1% |
| RTOG conformality index† | 1.29±0.15 | 0.0±0.22 | −0.06±0.14 |
| RTOG coverage quality index†† | 0.98±0.05 | 0.05±0.12 | 0.03±0.11 |
| RTOG homogeneity index# | 1.17±0.06 | −0.04±0.05* | −0.03±0.05 |
Abbreviations: AVG=average; CT=computed tomography; GTV=gross tumor volume; ITV=internal target volume; NTCP=normal tissue complication probability; PTV=planning target volume; PTVD95=dose to 95% of the PTV; RTOG=Radiation Therapy Oncology Group; SD=standard deviation.
Notes:
p<0.01 two-tailed paired Student’s T-test;
calculated as per Kutcher et al.(19);
calculated with Lyman-linear quadratic model corrected for the dose/fraction (α/β=2.5 Gy)(14);
ratio of nominal prescription isodose volume/PTV;
ratio of min PTV dose to 0.5cm3/nominal prescription dose;
ratio b of max PTV dose to 0.5cm3/nominal prescription dose.
Compared to the clinical PTV plans, the PTVD95 for the dose-probability PTV plans were more than 5 Gy higher in 55% of patients for least one GTV (53% of all GTVs). Compared to ITV-based PTV plans, the PTVD95 for the dose-probability PTV plans were more than 5 Gy higher in 50% of patients for at least one GTV (50% of all GTVs). In 15% of patients at least one GTV’s PTVD95 for the dose-probability PTV plans was within ±1 Gy (the minimum ‘clinical relevance criteria’) of the clinical or ITV-based PTV plans’ PTVD95, but none were more than 1 Gy lower.
Factors affecting the dose-escalation between dose-probability PTV and ITV-based PTV plans were investigated. Patients with GTV breathing motion <10 mm had an average±SD dose-escalation using the dose-probability PTV plans of 5.2±3.8 Gy versus 3.8±1.7 Gy for >10 mm (p=0.28). Smaller GTV size (p=0.01) and PTVs that did not overlap normal luminal gastrointestinal tissues (p=0.04) had a higher magnitude of dose-escalation.
Residual GTV treatment uncertainties
Following rigid alignment of each 4D CBCT’s mean liver position to the mid-position CT, the residual systematic (random) GTV errors, defined about the centre-of-mass, were estimated with DIR to be 0.9 (1.0) mm LR, 1.4 (1.1) mm AP and 1.0 (0.9) mm SI. These are reduced by 1.6 (1.5) mm SI versus errors reported following exhale CT to 3D CBCT liver alignment[13].
Mean changes in GTV breathing amplitude (4D CBCT – 4D CT) were ≤1.5 mm. In 2 patients the amplitude increased by 5 and 8 mm, and these variations were present for all three PTV strategies.
Delivered versus planned doses
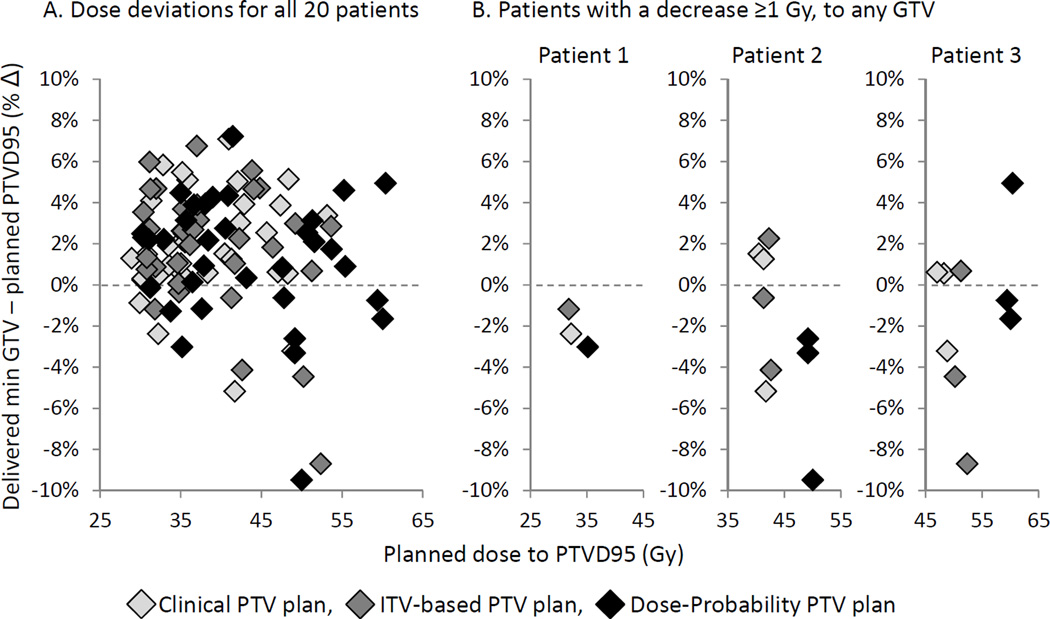
The delivered min GTV doses were first compared to their initial planned nominal prescribed doses. For all three strategies the delivered doses were on average 2.3–3.1 Gy (6–8%) higher and not more than 1 Gy lower for any patient. Using stricter criteria, the delivered min GTV doses were next compared to their planned PTVD95 (Fig. 4). For all three strategies the delivered doses were on average 0.5–0.9 Gy (1–3%) higher than the PTVD95, but lower by at least 1 Gy in 10% of patients. The average dose deviation (delivered min GTV–planned PTVD95) was narrower for the dose-probability PTV plan (0.5 Gy, or 1%) versus the ITV-based PTV plan (0.9 Gy, or 3%), p=0.046.
Fig. 4.
Delivered min GTV dose deviations relative to the planning PTVD95 for (A) all patients combined, and (B) separately for three patients each with a decrease ≥1 Gy to any GTV (No. 2 and 3 each have three GTVs).
In all three strategies, 2 different patients (10%) had at least one GTV with a delivered min dose more than 1 Gy lower than its PTVD95 (Fig. 4B). The largest delivered min GTV dose decrease was −2.2 Gy (−5%), −4.6 (−10%) and −4.7 Gy (−10%) for the clinical, ITV-based, and dose-probability PTV plans respectively. In the worst example, a patient with 3 GTVs (patient 3 on Fig. 4B) had liver deformation causing residual mean 3D GTV displacements of 5–7 mm, and a mean 8 mm larger breathing motion on 4D CBCT versus 4D CT. For this patient, all three plans had a delivered min GTV dose decrease of at least 1 Gy relative to their PTVD95. The delivered min doses however with the dose-probability PTV plan were >5 Gy higher than delivered with the clinical or ITV-based PTV plans, due to the planned dose-escalation that was possible with the dose-probability PTV.
For all three strategies the delivered normal luminal gastrointestinal tissues doses did not exceed planning constraints by more than 1 Gy with only one exception. The delivered max dose for one patient’s esophagus increased by 1.9 Gy (6%) relative to the planned dose only for the clinical PTV plan, and exceeded the planning constraint by 1.4 Gy.
Delivered doses between all strategies
The actual delivered min GTV doses are compared amongst the three strategies in Table 2. The delivered min GTV doses with the dose-probability PTV plans were not lower than either clinical or ITV-based PTV plans by more than 1 Gy in any case.
Table 2.
Increase in the actual delivered GTV doses with the dose-probability PTV plan compared to the delivered doses with the clinical or ITV-based PTV plans.
| Delivered minimum GTV dose to 0.5cm3 |
Δ delivered dose for the dose-probability PTV plan compared to: | |
|---|---|---|
| Delivered dose for the clinical PTV plan |
Delivered dose for the ITV-based PTV plan |
|
| AVG±SD (max) | 4.1±3.1 Gy* (12.8 Gy) or 9±6 % (21 %) | 3.5±3.0 Gy* (11.4 Gy) or 8±6 % (19 %) |
| No. (%) GTVs with an increase ≥5 Gy | 15 GTVs (44%) | 14 GTVs (41%) |
| No. (%) patients with an increase ≥5 Gy to any GTV | 8 patients (40%) | 9 patients (45%) |
Abbreviations: as per Table 1. Notes:
p<0.01 two-tailed paired Student’s T-test.
DISCUSSION
To the authors’ knowledge this is the first investigation of dose-probability PTV for liver SBRT including the quantification of dose-escalation. Dose-probability PTVs allowed for iso-toxic plans 4.0 Gy (9%) higher on average than ITV-based PTV plans. Of greater concern with margin reduction is whether planned dose distributions conforming to reduced PTVs are equally robust to treatment uncertainties. To evaluate this, the delivered doses were modeled with deformable dose accumulation, assuming image-guidance was based on rigid CBCT liver alignment as per current clinical protocol. An equivalent rate of delivered tumor coverage was observed between PTV strategies, including the significantly smaller dose-probability PTVs. In summary, a complete liver SBRT planning and delivery strategy based on the mean breathing position was evaluated which can be implemented clinically with respiratory-correlated imaging, and simple rigid liver registration.
Linearly adding breathing motion to other margin expansions resulted in similar clinical and ITV-based PTVs. Dose-probability PTVs however treat breathing motion as a random error only (≈1/3 the amplitude), assuming planning on the mid-position CT systematically accounts for the motion. The 8 mm median amplitude in this study is lower than the overall population because larger breathers received SBRT under active breath-hold. Dose-probability PTVs were also tailored to the 90% iso-dose, although iso-doses of 65% have been used for liver SBRT[21] requiring even smaller margins with the potential for further dose-escalation.
The dose-probability PTV was designed for free-breathing liver SBRT, and the exact margin may invalid for other techniques (e.g. active breath hold) or institutions. The original margin recipe included GTV delineation uncertainties[11]. These were not explicitly incorporated into any margin evaluated in this study, but are under investigation using DIR-enabled correlation of in vivo imaging to ex vivo histopathology[22]. In clinical practice these potential systematic errors are thought to be minimized using fused contrast-enhanced MRI, ‘over-contouring’ at ambiguous GTV borders, and contour peer-review. Additional margin validation is warranted using an independent cohort or modeling studies (e.g. Monte Carlo[23]), due to the limited number of patients investigated.
Dose-probability PTV adoption has been slow versus ITV methods, even for the more commonly treated lung which similarly benefits [24]. The barrier to widespread implementation is likely the presumed-difficultly in establishing the mean breathing position. For liver SBRT planning, two groups recently validated the mid-position CT accuracy using DIR or simple rigid registration[12,25]. For delivery, this study modeled rigid liver localization at the mean 4D CBCT position to account for baseline shifts, which is a commercially-available capability. The population residual GTV errors following this strategy (caused by residual liver deformation) were likely reduced compared with exhale 4DCT to blurred 3D CBCT alignment, because the former strategy aligns analogous respiratory positions. PTVs were evaluated with DIR-based dose accumulation however image-guidance using rigid liver registration was simulated. Hugo et al. [26] also demonstrated the mean lung tumor position can be accurately localized by registering an average (i.e. blurred) planning 4DCT to free-breathing 3D CBCTs. This requires further investigation as liver tumors are poorly visualized on both 3D and 4D CBCT.
Leinders et al. [27] recently piloted daily adaptive IMRT re-optimization for liver SBRT. In 2 of 12 GTVs the intervention permitted better PTV dose coverage, but dose-escalation beyond the initial prescription was not investigated. A similar impediment to other strategies (e.g. gating), the authors acknowledge the current online workloads are prohibitive to clinical implementation. In comparison, the current study’s results suggest dose-escalation is feasible with PTV reduction, CBCT guidance and rigid liver registration.
Each PTV strategy had two patients (10%) where the delivered min GTV dose to any GTV was 5–10% lower than the PTVD95. These resulted from liver deformations, thus even margins fully encompassing the breathing motion (i.e. clinical and ITV-based PTV plans) also experienced tumor under-dosage. Despite the dose-escalation, the delivered normal tissue doses did not exceed the planning dose constraints for the dose-probability PTV plans. Dosimetric validation is progressing[28], but implementation of deformable dose reconstruction for SBRT quality assurance may allow for identification of delivered dose deviations and inform whether re-planning is warranted.
CONCLUSION
Iso-toxic liver SBRT planning at the mean breathing position coupled with dose-probability PTVs allows for an average planned dose-escalation of 4 Gy (9%) in 6 fractions compared to ITV-based margins. When the delivered dose was evaluated with deformable dose reconstruction, the risk of under-dosing the tumor did not increase despite the reduced margins. This strategy has the potential to improve local control and can be readily implemented with respiratory-correlated imaging at planning and SBRT delivery and widely-available rigid registration.
SUMMARY.
Iso-toxic, 6 fraction liver SBRT plans were compared between exhale 4DCT with internal target volume-based margins, and mid-position CT representing the mean breathing position with dose-probability margins. The smaller dose-probability margins enabled an average planned dose escalation of 4 Gy. The delivered dose was also reconstructed using deformable registration of the treatment cone-beam CT. The nominal prescribed doses were delivered to all of the tumors despite the smaller margins.
Acknowledgements
The authors thank Graham Wilson for his technical support. This research is supported by a Canadian Institutes for Health Research (CIHR) Fellowship, and a U.S. National Institutes of Health grant 5RO1CA124714-02. Patient data was acquired during clinical trials supported by the National Cancer Institute of Canada grant 18207 and the Canadian Institutes for Health Research (CIHR) grant 202477.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification: KK Brock, JL Moseley, and LA Dawson have financial interest in the technology reported in this manuscript through a licensing agreement with RaySearch Laboratories.
REFERNCES
- 1.Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K, Flentje M. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45:838–847. doi: 10.1080/02841860600904821. [DOI] [PubMed] [Google Scholar]
- 2.Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, Kim J, Dinniwell R, Brierley J, Kavanagh BD, Dawson LA, Schefter TE. Stereotactic body radiotherapy for colorectal liver metastases: A pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 3.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA. Sequential phase i and ii trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 4.McCammon R, Schefter TE, Gaspar LE, Zaemisch R, Gravdahl D, Kavanagh B. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:112–118. doi: 10.1016/j.ijrobp.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 5.Eccles CL, Bissonnette JP, Craig T, Taremi M, Wu X, Dawson LA. Treatment planning study to determine potential benefit of intensity-modulated radiotherapy versus conformal radiotherapy for unresectable hepatic malignancies. Int J Radiat Oncol Biol Phys. 2008;72:582–588. doi: 10.1016/j.ijrobp.2008.06.1496. [DOI] [PubMed] [Google Scholar]
- 6.Ten Haken RK, Balter JM, Marsh LH, Robertson JM, Lawrence TS. Potential benefits of eliminating planning target volume expansions for patient breathing in the treatment of liver tumors. Int J Radiat Oncol Biol Phys. 1997;38:613–617. doi: 10.1016/s0360-3016(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 7.Dawson LA, Eccles C, Bissonnette JP, Brock KK. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005;62:1247–1252. doi: 10.1016/j.ijrobp.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 8.Eccles CL, Patel R, Simeonov AK, Lockwood G, Haider M, Dawson LA. Comparison of liver tumor motion with and without abdominal compression using cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2011;79:602–608. doi: 10.1016/j.ijrobp.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Wagman R, Yorke E, Ford E, Giraud P, Mageras G, Minsky B, Rosenzweig K. Respiratory gating for liver tumors: Use in dose escalation. Int J Radiat Oncol Biol Phys. 2003;55:659–668. doi: 10.1016/s0360-3016(02)03941-x. [DOI] [PubMed] [Google Scholar]
- 10.Briere TM, Beddar S, Balter P, Murthy R, Gupta S, Nelson C, Starkschall G, Gillin MT, Krishnan S. Respiratory gating with epid-based verification: The mdacc experience. Phys Med Biol. 2009;54:3379–3391. doi: 10.1088/0031-9155/54/11/007. [DOI] [PubMed] [Google Scholar]
- 11.van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 12. XXX. [Google Scholar]
- 13. XXX. [Google Scholar]
- 14.Sonke JJ, Zijp L, Remeijer P, van Herk M. Respiratory correlated cone beam ct. Med Phys. 2005;32:1176–1186. doi: 10.1118/1.1869074. [DOI] [PubMed] [Google Scholar]
- 15.Dawson LA, Eccles C, Craig T. Individualized image guided iso-ntcp based liver cancer sbrt. Acta Oncol. 2006;45:856–864. doi: 10.1080/02841860600936369. [DOI] [PubMed] [Google Scholar]
- 16. XXX. [Google Scholar]
- 17. XXX. [Google Scholar]
- 18.Case RB, Sonke JJ, Moseley DJ, Kim J, Brock KK, Dawson LA. Inter- and intrafraction variability in liver position in non-breath-hold stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:302–308. doi: 10.1016/j.ijrobp.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 19.Lujan AE, Larsen EW, Balter JM, Ten Haken RK. A method for incorporating organ motion due to breathing into 3d dose calculations. Med Phys. 1999;26:715–720. doi: 10.1118/1.598577. [DOI] [PubMed] [Google Scholar]
- 20.Rit S, van Herk M, Zijp L, Sonke JJ. Quantification of the variability of diaphragm motion and implications for treatment margin construction. Int J Radiat Oncol Biol Phys. 2012;82:e399–e407. doi: 10.1016/j.ijrobp.2011.06.1986. [DOI] [PubMed] [Google Scholar]
- 21.Mendez Romero A, Zinkstok RT, Wunderink W, van Os RM, Joosten H, Seppenwoolde Y, Nowak PJ, Brandwijk RP, Verhoef C, JN IJ, Levendag PC, Heijmen BJ. Stereotactic body radiation therapy for liver tumors: Impact of daily setup corrections and day-to-day anatomic variations on dose in target and organs at risk. Int J Radiat Oncol Biol Phys. 2009;75:1201–1208. doi: 10.1016/j.ijrobp.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Milot L, Guindi M, Gallinger S, Moulton CA, Brock KK, Dawson LA, Haider MA. Mr imaging correlates of intratumoral tissue types within colorectal liver metastases: A high-spatial-resolution fresh ex vivo radiologic-pathologic correlation study. Radiology. 2010;254:747–754. doi: 10.1148/radiol.09090508. [DOI] [PubMed] [Google Scholar]
- 23.Sonke JJ, Rossi M, Wolthaus J, van Herk M, Damen E, Belderbos J. Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam ct guidance. Int J Radiat Oncol Biol Phys. 2009;74:567–574. doi: 10.1016/j.ijrobp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Wolthaus JW, Sonke JJ, van Herk M, Belderbos JS, Rossi MM, Lebesque JV, Damen EM. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys. 2008;70:1229–1238. doi: 10.1016/j.ijrobp.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Kruis MF, van de Kamer JB, Sonke JJ, Jansen EP, van Herk M. Registration accuracy and image quality of time averaged mid-position ct scans for liver sbrt. Radiother Oncol. 2013;109:404–408. doi: 10.1016/j.radonc.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 26.Hugo GD, Liang J, Campbell J, Yan D. On-line target position localization in the presence of respiration: A comparison of two methods. Int J Radiat Oncol Biol Phys. 2007;69:1634–1641. doi: 10.1016/j.ijrobp.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leinders SM, Breedveld S, Mendez Romero A, Schaart D, Seppenwoolde Y, Heijmen BJ. Adaptive liver stereotactic body radiation therapy: Automated daily plan reoptimization prevents dose delivery degradation caused by anatomy deformations. Int J Radiat Oncol Biol Phys. 2013;87:1016–1021. doi: 10.1016/j.ijrobp.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 28. XXX. [Google Scholar]