Abstract
Background
Various factors are related to the occurrence of postoperative pancreatic fistula (POPF) following pancreatoduodenectomy (PD). Some of the strongest are identified intra- or postoperatively, which limits their utility in predicting this complication. The preoperative prediction of POPF permits an individualized approach to patient consent and selection, and may influence postoperative management. This study sought to develop and test a score to predict POPF.
Methods
A post hoc analysis of a prospectively maintained database was conducted. Consecutive patients were randomly selected to modelling and validation sets at a ratio of 2 : 1, respectively. Patient data, preoperative blood tests and physical characteristics of the gland (assessed from preoperative computed tomography images) were subjected to univariate and multivariate analysis in the modelling set of patients. A score predictive of POPF was designed and tested in the validation set.
Results
Postoperative pancreatic fistula occurred in 77 of 325 (23.7%) patients. The occurrence of POPF was associated with 12 factors. On multivariate analysis, body mass index and pancreatic duct width were independently associated with POPF. A risk score to predict POPF was designed (area under the receiver operating characteristic curve: 0.832, 95% confidence interval 0.768–0.897; P < 0.001) and successfully tested upon the validation set.
Conclusions
Preoperative assessment of a patient's risk for POPF is possible using simple measurements. The present risk score is a valid tool with which to predict POPF in patients undergoing PD.
Introduction
The centralization of pancreatoduodenectomy (PD) procedures into high-volume centres has reduced perioperative mortality.1–4 Morbidity, however, remains high and affects 16–41% of patients, even in specialist units.3,5–7 The occurrence of a postoperative pancreatic fistula (POPF) is a major contributor to both morbidity and mortality. The importance of POPF has been recognized and, in order to facilitate the auditing of outcomes, comparisons between centres performing PD and studies reviewing PD, the International Study Group for Pancreatic Fistula (ISGPF) has defined three grades of POPF.8 Various interventions (such as techniques of visceral reconstruction and abdominal drainage) and therapeutic drugs (such as somatostatin analogues) aimed at decreasing rates of POPF have been subject to review.9–13 These meta-analyses do not support a protective role against the development of clinically significant POPF.
In the absence of effective strategies to decrease the incidence of POPF, it is important to identify risk factors for its occurrence. Patient characteristics associated with POPF include increased body mass index (BMI), advanced age, male sex and comorbidity.14–18 Further factors that may predict POPF are the findings of preoperative biochemical tests, but these have been less thoroughly investigated. A study of 2894 patients found elevated urea and low albumin to be predictive of postoperative complications.19 However, this study did not define these complications and predated the ISGPF definition of POPF. A more recent study identified high levels of both haemoglobin and sodium to be associated with POPF as defined by the ISGPF.20 A further variable attracting increasing attention is pancreatic steatosis, a significant risk factor for POPF.14,17,18 However, this can only be identified at pathological assessment and thus has no role in preoperative risk analysis. Other factors that relate to POPF and can be identified at preoperative imaging include the pancreas gland width, duct width, and visceral and superficial fat thickness.16,20–23 Providing an individualized assessment of POPF risk in the preoperative phase would facilitate the provision of accurate patient counselling and obtaining of consent, and might alter aspects of clinical management such as patient selection for PD, the timing of drain removal and the instigation of early enteral feeding.
The aim of this study was to review patient factors that may relate to POPF in order to design a preoperative predictive risk score for POPF in patients undergoing PD.
Materials and methods
Consecutive patients undergoing PD at a single centre [University Hospitals Birmingham National Health Service (NHS) Trust, Birmingham, UK] between February 2007 and February 2012 were identified from a prospectively maintained database. Patients for whom preoperative computed tomography (CT) imaging was unavailable were excluded from the study.
All patients underwent PD performed or supervised by a consultant hepatopancreatobiliary surgeon. Whether a pancreatojejunostomy (PJ) or pancreatogastrostomy (PG) was performed depended upon which surgeon performed the procedure. This decision was not made intraoperatively and reflects each surgeon's individual preferred technique of pancreatic anastomosis. Pancreatic duct stenting was not performed. A single tube drain was placed posterior to the hepaticojejunostomy and adjacent to the PJ or PG anastomosis via the right flank in each patient. Patients received 100 μg of a subcutaneous somatostatin analogue, octreotide, three times daily for the first 5 days postoperatively. A feeding nasojejunal tube was placed in each patient under a standard protocol to administer feed from the first postoperative day. Drain fluid amylase levels were assessed on postoperative day 5 and, if the patient was clinically well without biochemical evidence of POPF, the drain was removed and enteral feeding instigated.
Data gathered for each patient referred to patient variables including: gender; age; smoking history; comorbidity; BMI, and pathological diagnosis (pancreatic adenocarcinoma, cholangiocarcinoma, ampullary carcinoma, duodenal carcinoma, neuroendocrine tumour, intraductal papillary mucinous neoplasm, pancreatitis, other).
Data on preoperative biochemical results included: white cell count (WCC); haemoglobin; platelet count; international normalized ratio (INR); aspartate transaminase (AST); alanine transaminase (ALT); alkaline phosphatase (ALP); sodium; potassium; urea; creatinine; bilirubin; albumin, and neutrophil to lymphocyte ratio (NLR). Values immediately preceding the PD were recorded. The values were considered as continuous data.
Data on the surgical technique used included details of whether a pylorus-preserving PD or a classic Whipple procedure was conducted, and whether a PJ or a PG was performed.
Preoperative CT imaging was assessed. The sequence of slices 1 mm in thickness was selected for review. Data collected referred to the anteroposterior thickness of the pancreas gland and the width of the pancreatic duct (both obtained at the level of the confluence of the superior mesenteric and portal veins), and to the anteroposterior thickness of the superficial fat adjacent to the umbilicus and the anteroposterior thickness of the perirenal fat at the level of the left umbilical vein. Each measurement was recorded in triplicate and the values were averaged. Measurements were obtained by a single researcher (KJR) blinded to the patient's clinical outcome. A second researcher (a hepatobiliary radiologist, HM) reviewed the images for a random selection of 50 patients and recorded the same measurements. The comparison of the two sets of measurements provided an assessment of intraclass correlation.
Postoperative pancreatic fistula was defined and classified according to the ISGPF definition into Grades A, B and C.8
Statistical analysis
Initially, the patient group was randomly divided into two sets at a ratio of 2 : 1 by an independent statistician. The larger of the two sets, the ‘modelling set’, was used to produce a risk scoring model, which was then applied to the smaller ‘validation set’ in order to validate its predictive accuracy.
To produce the risk score, each of the potential predictors were first considered univariably for the data in the modelling set. For the categorical variables, Fisher's exact test was used to compare rates of pancreatic leak across the different levels of the factor. Received operating characteristic (ROC) curves were produced for the continuous variables to ascertain whether they were significant predictors of POPF. The factors found to be significant at this stage of the analysis were then entered simultaneously into a backwards stepwise binary logistic regression. The resulting model was then converted into a risk score.
The new risk score was then applied to the data in the validation set. An ROC curve was produced to test whether the score remained a significant predictor of POPF in this second set of data. The modelling and validation sets were then divided into deciles and quintiles, respectively, based on the risk score, and the actual rates of POPF in each category calculated. This allowed the relationship between the risk score and actual patient outcomes to be compared graphically.
Data describing the cohorts were expressed as the median (interquartile range). The Mann–Whitney U-test and chi-squared test were used to explore cohort characteristics.
Statistical tests were two-tailed. A P-value of <0.05 was accepted as indicative of statistical significance. All analyses were performed using IBM spss Version 19.0 (IBM Corp., Armonk, NY, USA). A medical statistician (JH) provided advice during the study design, and performed the analyses and modelling.
Results
A total of 325 patients were included in the study; 217 patients were randomly selected into the modelling set and the remaining 108 were selected into the validation set. Table 1 describes the whole cohort and provides a comparison between the validation and test sets. There was no significant difference in any characteristic between the two sets.
Table 1.
Characteristics of the whole group and patients in the modelling and validation sets. There was no significant difference in any characteristic between the modelling and validation sets
| Whole group (n = 325) | Modelling set (n = 217) | Validation set (n = 108) | P-value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 67 (61–73) | 67 (60–73) | 68 (61–72) | 0.955 |
| Gender, male, n (%) | 182 (56.3%) | 120 (55.8%) | 62 (57.4%) | 0.812 |
| BMI, kg/m2, median (IQR) | 24.9 (22.5–27.8) | 25.0 (22.5–27.8) | 24.6 (22.6–27.8) | 0.952 |
| Smoker, n (%) | 53 (16.3%) | 33 (15.2%) | 20 (18.5%) | 0.651 |
| Preoperative diabetes, n (%) | 46 (14.2%) | 31 (14.3%) | 15 (13.9%) | 1.000 |
| Diagnosis, n (%) | ||||
| Pancreatic adenocarcinoma | 128 (39.4%) | 88 (40.6%) | 40 (37.0%) | 0.169 |
| Cholangiocarcinoma | 47 (14.5%) | 33 (15.2%) | 14 (13.0%) | |
| Ampullary carcinoma | 68 (20.9%) | 40 (18.4%) | 28 (25.9%) | |
| Duodenal carcinoma | 20 (6.2%) | 11 (5.1%) | 9 (8.3%) | |
| Neuroendocrine tumour | 15 (4.6%) | 10 (4.6%) | 5 (4.6%) | |
| Benign | 22 (6.8%) | 16 (7.4%) | 6 (5.6%) | |
| Other | 25 (7.7%) | 19 (8.8%) | 6 (5.6%) | |
| PPPD/Whipple procedure, n | 294/31 | 193/24 | 101/7 | 0.231 |
| PJ/PG, n | 176/149 | 117/100 | 59/49 | 0.907 |
| Pancreatic fistula, n (%) (ISGPF Grade A/B/C) | 77 (23.7%) (29/28/20) | 48 (22.1%) (21/19/8) | 29 (26.9%) (8/9/12) | 0.420 |
IQR, interquartile range; BMI, body mass index; PPPD, pylorus-preserving pancreaticoduodenectomy; PJ, pancreaticojejunostomy; PG, pancreaticogastrostomy; ISGPF, International Study Group on Pancreatic Fistula.
Risk factors for POPF
The results of the univariable analysis on the modelling set are reproduced in Tables 2 and 3 and the results of the multivariable analysis in Table 4. The resulting model contained BMI (P = 0.011) and pancreatic duct width (P < 0.001). A risk score derived from this model to predict the likelihood of POPF is calculated thus:
Table 2.
Univariate analysis of continuous factors in the modelling set (n = 217)
| Factor | n | Pancreatic fistula | a | P-value | ||
|---|---|---|---|---|---|---|
| No | Yes | AUROC (SE) | ||||
| Patient factor | ||||||
| Age, years, median (IQR) | 217 | 66.7 (59.8–73.4) | 66.9 (60.7–73.3) | 0.502 (0.047) | + | 0.961 |
| BMI, kg/m2, median (IQR) | 211 | 24.7 (22.0–27.0) | 26.8 (24.0–32.4) | 0.677 (0.045) | + | < 0.001 |
| Biochemical result, median (IQR) | ||||||
| White cell count, ×109/l | 216 | 7.70 (6.60–9.80) | 7.90 (6.63–9.48) | 0.508 (0.046) | − | 0.865 |
| Platelet count, ×109/l | 216 | 306 (244–395) | 297 (237–342) | 0.552 (0.046) | − | 0.271 |
| Haemoglobin, g/dl | 216 | 12.6 (11.7–13.5) | 12.6 (11.0–13.7) | 0.506 (0.051) | − | 0.901 |
| International normalized ratio | 212 | 1.00 (1.00–1.10) | 1.00 (0.90–1.10) | 0.516 (0.049) | − | 0.743 |
| Aspartate transaminase, IU/l | 209 | 52.0 (28.0–84.5) | 32.0 (21.3–69.5) | 0.598 (0.048) | − | 0.039 |
| Alanine transaminase, IU/l | 48 | 50.0 (30.3–140.3) | 40.0 (15.8–118.8) | 0.559 (0.115) | − | 0.568 |
| Alkaline phosphatase, IU/l | 216 | 558 (280–955) | 293 (193–672) | 0.630 (0.047) | − | 0.006 |
| Sodium, mmol/l | 216 | 139 (136–142) | 140 (138–142) | 0.548 (0.045) | + | 0.308 |
| Potassium, mmol/l | 213 | 4.30 (3.90–4.60) | 4.20 (3.90–4.47) | 0.529 (0.046) | − | 0.534 |
| Urea, mmol/l | 216 | 5.20 (4.30–6.68) | 5.70 (4.63–7.10) | 0.558 (0.047) | + | 0.220 |
| Creatinine, μmol/l | 216 | 75.0 (64.0–92.8) | 78.5 (66.8–98.5) | 0.567 (0.048) | + | 0.157 |
| Bilirubin, μmol/l | 216 | 39.5 (14.0–130.0) | 14.0 (6.0–50.0) | 0.659 (0.047) | − | 0.001 |
| Albumin, g/l | 216 | 41.0 (38.0–44.0) | 41.0 (38.0–43.0) | 0.502 (0.046) | − | 0.969 |
| Neutrophil count, ×109/l | 216 | 5.00 (3.80–6.60) | 5.30 (4.25–6.35) | 0.523 (0.045) | + | 0.632 |
| Lymphocyte count, ×109/l | 216 | 1.90 (1.40–2.40) | 1.85 (1.30–2.45) | 0.519 (0.047) | − | 0.687 |
| Neutrophil : lymphocyte ratio | 216 | 2.74 (1.91–4.00) | 3.29 (1.66–4.85) | 0.518 (0.049) | + | 0.711 |
| Data from CT imaging, median (IQR) | ||||||
| Pancreas width, mm | 217 | 14.9 (12.7–17.8) | 17.2 (14.3–20.0) | 0.644 (0.044) | + | 0.002 |
| Pancreas duct diameter, mm | 217 | 4.90 (1.75–7.15) | 0.00 (0.00–1.47) | 0.801 (0.033) | − | < 0.001 |
| Renal fat thickness, mm | 217 | 10.0 (4.5–16.1) | 11.5 (6.3–18.8) | 0.581 (0.046) | + | 0.086 |
| Superficial fat thickness, mm | 217 | 13.0 (9.0–19.3) | 14.2 (9.2–24.0) | 0.552 (0.048) | + | 0.276 |
+ or − indicates a positive or negative association with pancreatic fistula, respectively.
AUROC, area under the receiver operating characteristic curve; SE, standard error; IQR, interquartile range; BMI, body mass index; CT, computed tomography.
Table 3.
Univariate analysis of categorical factors in the modelling set (n = 217)
| Factor | n | Rate of leaks | P-valuea |
|---|---|---|---|
| Sex | 1 | ||
| Male | 120 | 21.7% | |
| Female | 95 | 22.1% | |
| Pathological diagnosisb | < 0.001 | ||
| Pancreatic carcinoma/cholangiocarcinoma/ampullary carcinoma | 167 | 17.4% | |
| Duodenal carcinoma | 16 | 62.5% | |
| Benign disease/neuroendocrine tumour/other disease | 34 | 26.5% | |
| Operation | 0.434 | ||
| Pylorus-preserving pancreaticoduodenectomy | 193 | 21.2% | |
| Whipple procedure | 24 | 29.2% | |
| Pancreatic reconstruction | 0.140 | ||
| Pancreaticojejunostomy | 117 | 17.9% | |
| Pancreaticogastrostomy | 100 | 27.0% | |
| Smoker | 0.042 | ||
| Non-smoker | 108 | 24.1% | |
| Ex-smoker | 66 | 25.8% | |
| Current smoker | 33 | 6.1% | |
| Preoperative diabetes | 0.842 | ||
| No | 180 | 21.7% | |
| Type 1 | 5 | 20.0% | |
| Type 2 | 26 | 26.9% | |
Fisher's exact test.
Because of the number of different pathological diagnoses, some were grouped together. Patients with pancreatic adenocarcinoma, ampullary carcinoma and cholangiocarcinoma had similar rates of pancreatic fistula (PF) as did those with other diseases, neuroendocrine tumours and benign disease.
Table 4.
Multivariate backwards stepwise logistic regression model on the data in the modelling set (n = 217)
| Factor | Coefficient | Odds ratio (95% CI) | P-value |
|---|---|---|---|
| Constant | −3.026 | 0.049 | 0.01 |
| Body mass index | 0.107 | 1.113 (1.025–1.209) | 0.011 |
| Pancreatic duct width | −0.404 | 0.668 (0.568–0.786) | <0.001 |
95% CI, 95% confidence interval.
Confidence intervals (95% CIs) can be calculated as follows: lower CI = (risk score*0.814) − 2, and upper CI = (risk score*1.110) + 5.7. An ROC curve was produced to test the accuracy of this risk score in predicting POPF (Fig. 1). The area under the ROC curve was found to be 0.832 (95% CI 0.768–0.897; P < 0.001).
Figure 1.
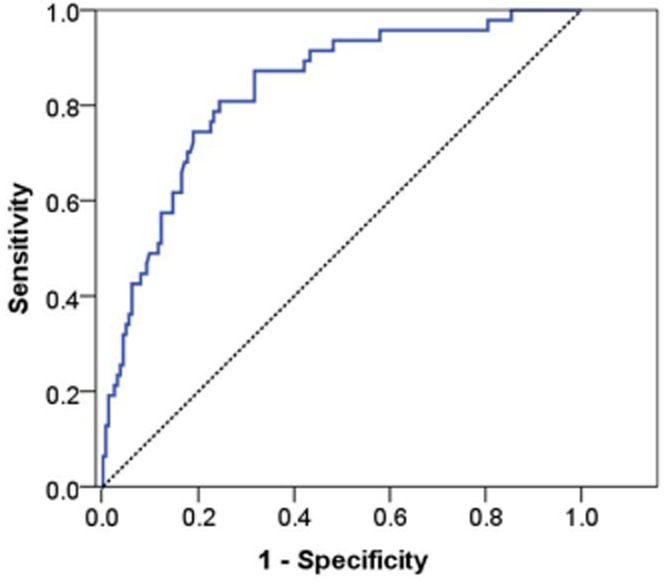
Receiver operating characteristic curve of risk score in the modelling set
In order to validate the risk score, it was applied to the validation set. The area under the ROC curve for the validation set was found to be 0.751 (95% CI 0.649–0.852), which did not differ significantly from the area under the ROC curve for the modelling set (P = 0.241).
Figure 2 further evaluates the performance of the risk score. Patients in the modelling set were divided into deciles based on their risk scores. Patients in the validation set were then divided into quintiles because there were fewer patients in this set. The observed rate of pancreatic leaks was then calculated for each of the percentiles and plotted at the mean value within each interval. A reference line was added to indicate where the points would lie if the risk score had performed perfectly. As Fig. 2 shows, the plotted points are close to this line, a further indication of the accuracy of the risk score. Figure 3 demonstrates graphically the risk for POPF relative to BMI and pancreatic duct width as predicted by the score. Thus, obese patients (BMI: 30–35 kg/m2) with a narrow duct (< 3 mm in diameter) have a 30–55% risk for developing POPF, whereas patients with a pancreatic duct diameter of >10 mm, regardless of BMI, have a risk of <5%.
Figure 2.
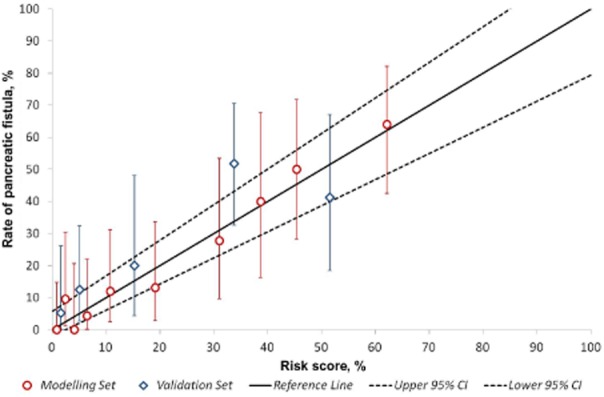
Comparison between the scoring system and observed rates of postoperative pancreatic fistula (POPF). The performance of the risk score in both the modelling and validation cohorts is demonstrated with the actual observed rate of POPF; bars represent 95% confidence intervals (CIs). There were twice as many patients within the modelling group (divided into deciles) as the validation group (divided into quintiles). The solid line indicates the perfect performance of the score; the dashed lines indicate the 95% CI
Figure 3.
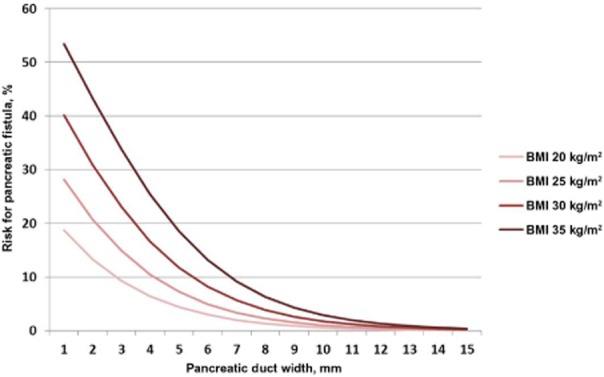
Graphical representation of risk for postoperative pancreatic fistula (POPF) as predicted by the risk score. Pancreatic duct width was measured from preoperative computed tomography imaging. BMI, body mass index
A web-based calculator for this risk score is available at http://www.uhb.nhs.uk/preoperative-prediction-of-pancreatic-fistula-calculator.htm.
Associations between severity of POPF and independent variables
The associations between ISGPF grade, pancreatic duct width and BMI were explored in the whole cohort. There was no significant difference in pancreatic duct width or BMI between patients with POPF of ISGPF Grade A and those with Grade B or C POPF (Table 5).
Table 5.
Associations of International Study Group on Pancreatic Fistula grades of postoperative pancreatic fistula (POPF), pancreatic duct width and body mass index, showing the lack of significant difference between associations with POPF of Grade A, and POPF of Grades B and C
| Postoperative pancreatic fistula | ||||
|---|---|---|---|---|
| None (n = 246) | Grade A (n = 28) | Grades B + C (n = 48) | P-value | |
| Pancreatic duct width | 5 (1.7–7.2) | 0 (0–0) | 0 (0–2.7) | 0.097 |
| Body mass index | 24.5 (22.1–27.0) | 26.4 (24.0–30.6) | 26.3 (23.7–30.3) | 0.936 |
Interobserver variation in data derived from CT measurements
There was generally strong correlation between reviewers’ observations of preoperative CT imaging. Assessments of pancreatic duct width by the surgeon and hepatobiliary radiologist produced an intraclass correlation of 0.831 and a mean ± [2 × standard errors of the mean (SEM)] difference of 0.48 ± 0.51 mm.
Discussion
This study reviewed various factors associated with the occurrence of POPF following PD with the aim of developing a preoperative score predictive of POPF. Variables identified intra- or postoperatively were purposefully excluded from analysis. The main findings were that BMI and pancreatic duct width independently correlated (positively and negatively, respectively) with POPF. These results were used to construct a statistical model to predict risk for POPF. The utility of this score was validated successfully in a separate cohort of patients.
This is not the first study to develop a predictive score for POPF. Gaujoux et al. identified that BMI, pancreatic steatosis and an absence of pancreatic fibrosis were independently associated with POPF.14 The index derived in that study was based upon the presence or absence of these categorical variables and gave a score of 0–3. Callery et al. reported a well-conducted study that used four variables to construct a score with four categorical risks for POPF.24 The variables were an assessment of pancreatic texture (firm or soft), pancreatic duct width, pathology and intraoperative blood loss. Yamamoto et al. developed a risk score based upon preoperative variables and included five factors yielding a score of 0–7.25 This latter score and the present score share a clear advantage as they can be applied in the preoperative phase, but only the present study uses continuous data and presents a patient's risk as a continuous value. Considering the pancreatic duct width as a binary outcome greatly limits the ability of this variable to predict POPF risk. Furthermore, recording a patient's BMI and pancreatic duct width is easy and reproducible. The score reported by Yamamoto et al.25 requires four measurements from CT imaging. The strong correlation between the interpretation of data obtained from CT imaging by the surgeon and hepatobiliary radiologist in the present study demonstrates that this risk assessment can be carried out by the surgeon in the outpatient setting.
A preoperative score predictive of POPF clearly cannot include characteristics of the gland that are identified intra- or postoperatively, some of which are strongly associated with POPF. These include the histological assessment of pancreatic steatosis14,17,18 and fibrosis,14,18 a soft or hard gland at palpation16,24,26–28 and pancreatic duct width.22–24 Increasing evidence, however, indicates that an indirect assessment of the gland is possible. Fatty pancreas, first described in 1933,29 is associated with obesity14,17 and a local inflammatory state, so-called non-alcoholic pancreatic disease.30 The association between obesity and pancreatic disease has only recently been appreciated and an increased BMI associated with POPF following PD14,31 and distal pancreatectomy.32 Hepatic steatosis, related to increased BMI, is reliably assessed by measuring hepatic density in non-contrast CT imaging.33,34 A recent study assessed the utility of CT imaging to indirectly assess pancreatic density as a surrogate for steatosis and the relationship with POPF; a significant association was identified.20 In the present study, preoperative CT scans for over 75% of patients did not include a non-contrast CT sequence. It was therefore not possible to assess pancreatic density as determined by CT analysis in this study.
Pancreatic steatosis can be assessed using other radiological modalities, including magnetic resonance imaging (MRI),35–37 magnetic resonance spectroscopy38,39 and endoscopic ultrasound.40 The clinical application of these techniques is limited by their infrequent use; for example, fewer than 10% of patients undergoing PD are assessed by MRI.41
Quantifying a patient's risk for POPF prior to PD is essential if the clinician is to provide individualized care to a patient considered for PD because POPF is responsible for the majority of morbidity and mortality following this procedure. The data presented in this study demonstrate that an individual's risk for POPF can vary from <1% to >50% based upon considerations of BMI and pancreatic duct width alone. Elucidating a patient's risk for POPF has several advantages. Preoperative consent and counselling can be tailored to each patient. The selection of patients and operative technique can be optimized as it is in patients with borderline fitness and those with lesions of uncertain pathological behaviour. For example, the decision to undertake partial or total pancreatic resection in a patient with an intraductal papillary mucinous neoplasm may be influenced by the risk for POPF, and a patient with a cystic lesion of the head of the pancreas that is deemed to be benign but is associated with a risk for malignant transformation in the future is likely to be influenced into pursuing conservative or surgical treatment depending upon his or her risk for POPF. Patients with proven malignant disease are unlikely to decline PD if they are deemed to be at high risk for POPF, but their clinical management may vary. Patients at low risk for POPF may benefit from the early removal of abdominal drains and/or the instigation of enteral feeding. Those at high risk may benefit from the placement of a feeding jejunostomy at the time of PD as the incidence of infective complications is lower than with parenteral nutrition, and complications with tube obstruction or dislodgement are lower than with nasojejunal feeding.42 Finally, trials of operative or therapeutic interventions aimed at decreasing rates of POPF have largely failed to demonstrate clinical benefit. Studies may now be designed to target those patients with the highest degree of risk for POPF.
It can be argued that ISGPF Grade A fistulae are not clinically relevant and therefore should not be considered as representing an important complication. There was no significant difference in either pancreatic duct width or BMI between patients with Grade A POPF and those with Grade B or C POPF. Both of these factors independently predispose to POPF as defined by an amylase-rich drain effluent, but whether other unidentified factors influence which patients will develop clinically relevant POPF is unclear and warrants further investigation. All factors analysed in this study were used to discriminate between patients who developed Grade A POPF and those who developed Grade B or C POPF; however, no significant factor was identified (data not shown). There are theoretical advantages to measuring visceral fat rather than BMI. Visceral fat can be regarded as a metabolic organ in its own right, in comparison with superficial fat, and is associated with metabolic syndrome,43 which, in turn, has been associated with fatty pancreas.38,44,45 Tranchart et al. reviewed the potential role of the visceral fat area (VFA) in predicting POPF.46 In non-contrast CT images, the VFA emerged as an independent predictor of Grade B and C POPF in comparison with no POPF and Grade A POPF.46 However, Tranchart et al. observed that the BMI of patients with Grade B or C POPF was significantly different from that of patients without POPF or with Grade A POPF, presumably in keeping with the VFA findings.46 This lack of correlation between BMI and the occurrence of Grade A POPF differs from the findings of the present study; furthermore, assessing the VFA is more complex than the technique used in the present study and requires additional software and training.46 In addition, the area under the ROC curve generated from the predictive score in the present study (0.832) is higher than that obtained by Tranchart et al.46 using VFA alone (0.77), which indicates the former's greater sensitivity and specificity in the prediction of POPF. In another study, retro-renal fat thickness was associated with POPF,21 but this was not observed in the present cohort or by Tranchart et al.46 Although there is some conflict among the data regarding which assessment of body fat is most useful in predicting POPF, there is overwhelming evidence for the association of obesity with POPF. Whether obesity and its relation to risk for POPF are most optimally assessed by physical characteristics such as BMI, radiological characteristics such as VFA or possibly pancreatic density or the pathological assessment of the resected gland remains to be elucidated. The advantage of using BMI is that it is the easiest characteristic to measure and is available in the preoperative period.
The other component of the predictive score is pancreatic duct width. Its negative association with POPF has been observed previously.16,22,23 Others have considered pancreatic duct width as a binary variable and refer to it as either normal or wide. Considering duct width as a continuous variable makes it possible to identify risk for POPF with much greater predictive accuracy.
In conclusion, this study has developed a score predictive of POPF based solely upon preoperative information, which is simple and quick to use and has been validated within a separate cohort of patients. An individualized assessment of risk for POPF enhances preoperative counselling and patient selection for PD. Furthermore, the stratification of risk may permit a change to established clinical practice and facilitate research into risk factors for and strategies to decrease POPF amongst those patients at highest risk.
Acknowledgments
The authors would like to acknowledge Chris Coldham, who is responsible for prospective data collection within the Hepatobiliary and Pancreatic Surgery Unit, University Hospitals Birmingham, and Professor J. A. C. Buckels and Mr D. Mayer, consultant surgeons within the same unit. Thanks to Gordon McKenzie (medical student, University of Birmingham) for help with data collection.
Conflicts of interest
None declared.
References
- 1.Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008;95:357–362. doi: 10.1002/bjs.5982. [DOI] [PubMed] [Google Scholar]
- 2.de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404–410. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, Nakeeb A, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010;145:634–640. doi: 10.1001/archsurg.2010.118. [DOI] [PubMed] [Google Scholar]
- 4.van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aroori S, Puneet P, Bramhall SR, Muiesan P, Mayer AD, Mirza DF, et al. Outcomes comparing a pancreaticogastrostomy (PG) and a pancreaticojejunostomy (PJ) after a pancreaticoduodenectomy (PD) HPB. 2011;13:723–731. doi: 10.1111/j.1477-2574.2011.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Connor S, Alexakis N, Garden OJ, Leandros E, Bramis J, Wigmore SJ. Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg. 2005;92:1059–1067. doi: 10.1002/bjs.5107. [DOI] [PubMed] [Google Scholar]
- 10.Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Knaebel HP, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2011;(5) doi: 10.1002/14651858.CD006053.pub4. CD006053. [DOI] [PubMed] [Google Scholar]
- 11.Diener MK, Tadjalli-Mehr K, Wente MN, Kieser M, Buchler MW, Seiler CM. Risk–benefit assessment of closed intra-abdominal drains after pancreatic surgery: a systematic review and meta-analysis assessing the current state of evidence. Langenbecks Arch Surg. 2011;396:41–52. doi: 10.1007/s00423-010-0716-0. [DOI] [PubMed] [Google Scholar]
- 12.Koti RS, Gurusamy KS, Fusai G, Davidson BR. Meta-analysis of randomized controlled trials on the effectiveness of somatostatin analogues for pancreatic surgery: a Cochrane review. HPB. 2010;12:155–165. doi: 10.1111/j.1477-2574.2010.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wente MN, Shrikhande SV, Muller MW, Diener MK, Seiler CM, Friess H, et al. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg. 2007;193:171–183. doi: 10.1016/j.amjsurg.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15–23. doi: 10.1016/j.surg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Lermite E, Pessaux P, Brehant O, Teyssedou C, Pelletier I, Etienne S, et al. Risk factors of pancreatic fistula and delayed gastric emptying after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg. 2007;204:588–596. doi: 10.1016/j.jamcollsurg.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–959. doi: 10.1016/j.gassur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 17.Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058–1064. doi: 10.1097/SLA.0b013e31814a6906. [DOI] [PubMed] [Google Scholar]
- 18.Rosso E, Casnedi S, Pessaux P, Oussoultzoglou E, Panaro F, Mahfud M, et al. The role of ‘fatty pancreas’ and of BMI in the occurrence of pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2009;13:1845–1851. doi: 10.1007/s11605-009-0974-8. [DOI] [PubMed] [Google Scholar]
- 19.Winter JM, Cameron JL, Yeo CJ, Alao B, Lillemoe KD, Campbell KA, et al. Biochemical markers predict morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2007;204:1029–1036. doi: 10.1016/j.jamcollsurg.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Roberts KJ, Storey R, Hodson J, Morris-Stiff G, Smith AM. Can pancreatic fistula following resection be predicted by the use of preoperative CT imaging? Pancreatology. 2013;13:423–428. doi: 10.1016/j.pan.2013.04.322. [DOI] [PubMed] [Google Scholar]
- 21.House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–278. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 22.Pratt WB, Callery MP, Vollmer CM., Jr Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–428. doi: 10.1007/s00268-007-9388-5. [DOI] [PubMed] [Google Scholar]
- 23.Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, Huang YT. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. doi: 10.3748/wjg.v11.i16.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM., Jr A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto Y, Sakamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2011;35:2747–2755. doi: 10.1007/s00268-011-1253-x. [DOI] [PubMed] [Google Scholar]
- 26.DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuks D, Piessen G, Huet E, Tavernier M, Zerbib P, Michot F, et al. Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg. 2009;197:702–709. doi: 10.1016/j.amjsurg.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Kim WS, Choi DW, Choi SH, Heo JS, Kim MJ, Song SC, et al. Clinical validation of the ISGPF classification and the risk factors of pancreatic fistula formation following duct-to-mucosa pancreaticojejunostomy by one surgeon at a single centre. J Gastrointest Surg. 2011;15:2187–2192. doi: 10.1007/s11605-011-1726-0. [DOI] [PubMed] [Google Scholar]
- 29.Ogilvie R. The islands of the Langerhans in 19 cases of obesity. J Pathol. 1933;37:473–481. [Google Scholar]
- 30.Mathur A, Marine M, Lu D, Swartz-Basile DA, Saxena R, Zyromski NJ, et al. Non-alcoholic fatty pancreas disease. HPB. 2007;9:312–318. doi: 10.1080/13651820701504157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimoda M, Katoh M, Yukihiro I, Kita J, Sawada T, Kubota K. Body mass index is a risk factor of pancreatic fistula after pancreaticoduodenectomy. Am Surg. 2012;78:190–194. [PubMed] [Google Scholar]
- 32.Sledzianowski JF, Duffas JP, Muscari F, Suc B, Fourtanier F. Risk factors for mortality and intra-abdominal morbidity after distal pancreatectomy. Surgery. 2005;137:180–185. doi: 10.1016/j.surg.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 33.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 34.Speliotes EK, Massaro JM, Hoffmann U, Foster MC, Sahani DV, Hirschhorn JN, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–899. doi: 10.1111/j.1440-1746.2008.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat–water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring) 2010;18:841–847. doi: 10.1038/oby.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Z, Kim MJ, Kim JH, Jin SY, Kim YB, Seo D, et al. Prediction of postoperative pancreatic fistula in pancreaticoduodenectomy patients using preoperative MRI: a pilot study. HPB. 2009;11:215–221. doi: 10.1111/j.1477-2574.2009.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH, Kim MA, et al. Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg. 2010;251:932–936. doi: 10.1097/SLA.0b013e3181d65483. [DOI] [PubMed] [Google Scholar]
- 38.Lingvay I, Esser V, Legendre JL, Price AL, Wertz KM, Adams-Huet B, et al. Non-invasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009;94:4070–4076. doi: 10.1210/jc.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zyromski NJ, Mathur A, Gowda GA, Murphy C, Swartz-Basile DA, Wade TE, et al. Nuclear magnetic resonance spectroscopy-based metabolomics of the fatty pancreas: implicating fat in pancreatic pathology. Pancreatology. 2009;9:410–419. doi: 10.1159/000199436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Haddad M, Khashab M, Zyromski N, Pungpapong S, Wallace MB, Scolapio J, et al. Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case–control study. Pancreas. 2009;38:672–675. doi: 10.1097/MPA.0b013e3181a9d5af. [DOI] [PubMed] [Google Scholar]
- 41.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerritsen A, Besselink MG, Cieslak KP, Vriens MR, Steenhagen E, van Hillegersberg R, et al. Efficacy and complications of nasojejunal, jejunostomy and parenteral feeding after pancreaticoduodenectomy. J Gastrointest Surg. 2012;16:1144–1151. doi: 10.1007/s11605-012-1887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 44.Lee JS, Kim SH, Jun DW, Han JH, Jang EC, Park JY, et al. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol. 2009;15:1869–1875. doi: 10.3748/wjg.15.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8:169–177. doi: 10.1038/nrgastro.2011.4. [DOI] [PubMed] [Google Scholar]
- 46.Tranchart H, Gaujoux S, Rebours V, Vullierme MP, Dokmak S, Levy P, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg. 2012;256:139–145. doi: 10.1097/SLA.0b013e318256c32c. [DOI] [PubMed] [Google Scholar]


