Abstract
Objective
Routine extrahepatic bile duct (EBD) resection in non-jaundiced patients with gallbladder cancer (GBC) is controversial. The aim of this study was to retrospectively analyse patterns of recurrence in patients who underwent resection of GBC without routine EBD resection.
Methods
This analysis referred to 58 patients who had undergone explorative laparotomy for GBC during 2000–2012 at a single, tertiary referral centre. Overall survival, time to recurrence, and patterns of recurrence were assessed in patients who underwent conventional negative-margin (R0) resection without routine EBD resection.
Results
Of 58 patients submitted to explorative laparotomy for GBC, 26 (45%) patients underwent R0 resection without EBD resection (tumour stage T1b in five patients, T2 in 17, T3 in three, and T4 in one). The 3-year survival rate among these patients was 78% at a median follow-up of 33 months (range: 13–127 months). Seven patients developed recurrent disease at a median of 9 months (range: 2–25 months) after resection. No patients developed isolated recurrent disease at the EBD.
Conclusions
Of 26 patients resected for GBC, none developed isolated recurrent disease at the EBD after conventional resection of GBC without EBD resection. This finding suggests that routine EBD resection is of no additional value.
Introduction
The surgical treatment of gallbladder cancer (GBC) is predominantly guided by the extent of tumour invasion.1,2 A potentially curative resection requires tumour-free margins and regional lymphadenectomy in patients with disease of stage T1b or higher.3,4 The aim of regional lymphadenectomy is to improve the curative potential of resection5,6 and to assess the stage of the tumour for prognostication.7,8 Resection of the extrahepatic bile duct (EBD), however, is controversial in GBC patients, whether or not they show involvement of the EBD. For patients with EBD involvement, there is disagreement on the benefit of surgical resection.9,10 The present paper discusses the value of routine EBD resection in patients who have no obstructive jaundice, no macroscopic involvement of the EBD, and a negative cystic duct margin.
Routine EBD resection has been performed for GBC with varying frequency in many centres.1,10–14 The rationale for this approach is that cancer cells may have spread not only to the large lymph vessels in the subserosal layer around the EBD, which are resected during conventional lymphadenectomy, but also to small lymph vessels in the submucosal layer of the EBD.10,13,15 However, no studies have been able to show improved survival with routine EBD resection.12,16 In addition, it has been demonstrated that EBD resection does not increase the completeness of lymphadenectomy of the hepatoduodenal ligament.1 Hence, although EBD resection is associated with increased morbidity and mortality, there is no evidence that routine EBD resection is of any oncological benefit.1,17
In the Academic Medical Centre (AMC) in Amsterdam, GBC resection without EBD resection has been the standard approach in patients who have no signs of obstructive jaundice, no macroscopic involvement of the EBD and a negative cystic duct margin. It is uncertain whether this policy's potential leaving of cancer cells in the submucosal layers of the EBD negatively impacts survival. As most patients develop distant disease as the initial pattern of recurrence after GBC resection,18 the present study was based on the hypothesis that GBC patients are unlikely to develop an isolated regional recurrence at the EBD after GBC resection without routine EBD resection.
The aim of this study was to assess patterns of recurrence in patients who underwent conventional resection of GBC without routine EBD resection.
Materials and methods
Patients
Consecutive patients who underwent explorative laparotomy for GBC at the study institution tertiary care referral centre between January 2000 and April 2012 were identified by reviewing pathology registries and medical billing records. Inclusion criteria required patients to have undergone negative-margin (R0) resection of GBC (i.e. adenocarcinoma of hepatobiliary origin or papillary malignancy, originating at the gallbladder or at the cystic duct) and laparotomy at the AMC Amsterdam, either because of a primary suspicion of GBC or subsequent to a previous non-curative cholecystectomy at a referring centre (i.e. incidental GBC found at pathology review). All patients with a positive-margin resection (R1 or R2) or no resection were excluded, as were patients with an indication for EBD resection. Indications for EBD resection were: preoperative obstructive jaundice; macroscopic involvement of the EBD, and a positive cystic duct margin. Clinical and pathological data were retrospectively collected from the medical records.
Surgical resection
Resection of the tumour in patients who presented with a primary suspicion of GBC consisted of a locoregional lymphadenectomy and en bloc cholecystectomy with a hepatic resection. Definitive resections were performed in patients who had undergone a previous non-curative cholecystectomy at a referring centre, consisting of locoregional lymphadenectomy for GBC of stage pT1b or higher, and additional hepatic resection for disease of stage pT2 or higher. Hepatic resections consisted of the excision of a wide wedge of segments IVb and V, or a right hemi-hepatectomy extended to segment IVb depending on the liver involvement of the tumour. Indications for right hemi-hepatectomy were: hepatic invasion not radically resected with segment IVb/V wedge resection, and tumour involving the right posterior portal pedicle. Infiltration into other adjacent organs required the resection of involved structures, most commonly parts of the colon or duodenum. The EBD was preserved in all patients in this study cohort. Standard lymphadenectomy included the harvesting of all locoregional lymph nodes [i.e. the N1 lymph nodes according to the seventh edition of the tumour–node–metastasis (TNM) classification],4 and excision of all lymphatic tissue in the hepatoduodenal ligament, resulting in the complete skeletonization of the portal vein up to its bifurcation and the proper hepatic artery, including the left and right hepatic arteries. Lymph nodes beyond the hepatoduodenal ligament (i.e. along the common hepatic artery) were not included in the standard lymphadenectomy.
Follow-up
Overall survival, disease-free survival and patterns of recurrence were analysed. Patterns of recurrence were determined from computed tomography (CT) imaging studies conducted 3 months postoperatively and when indicated by clinical signs of recurrence (e.g. recurrent jaundice, abdominal complaints or fatigue) at further clinical follow-up. For patients who had no clinical follow-up until 1 April 2012, disease status was assessed by contacting the primary care physicians who had monitored clinical signs of recurrence and had referred patients for CT imaging if indicated. If applicable, details on tumour recurrence were obtained from the regional hospitals at which tumour recurrence had been diagnosed. Tumour markers were not monitored in a standard way. The diagnosis of recurrent disease was confirmed by histological analysis if possible. Since 2010, patients with recurrent disease have been considered for systemic treatment with cisplatin and gemcitabine.19 ‘Patterns of recurrence’ were classified as local (liver resection margin, EBD or liver hilum), or distant (intrahepatic but away from the resection margin, retroperitoneal lymph nodes, peritoneum and abdominal wall).18 ‘Time to recurrence’ was defined as the time between definitive resection and the first suspicious radiological finding of recurrent disease. ‘Overall survival’ was defined as the time between definitive treatment and the date of death, which was retrieved from the Dutch municipal population register.
Statistical analysis
Continuous data are presented as the median (range). Independent variables were dichotomized. Associations with the dependent variable for tumour recurrence were analysed using Fisher's exact test. Kaplan–Meier estimates were used in analyses of overall survival and disease-free survival, and differences in overall survival were analysed using the log-rank (Mantel–Cox) test. P-values of < 0.05 were considered to indicate statistical significance.
Results
A total of 58 patients who had undergone explorative laparotomy with curative intent for GBC were identified at the AMC Amsterdam. Twenty patients did not undergo resection at explorative laparotomy because of extensive disease or distant metastases. Thirty-eight patients underwent a resection (resection rate: 66%). Patients who required an EBD resection (for macroscopic EBD involvement in three patients and for a positive cystic duct margin in three patients), and patients in whom an R1 or R2 resection was achieved (n = 6) were excluded. An R0 resection without EBD resection was achieved in 26 patients; these patients represent the study group (Fig. 1). Demographics, treatment and pathology results are presented in Table 1.
Figure 1.
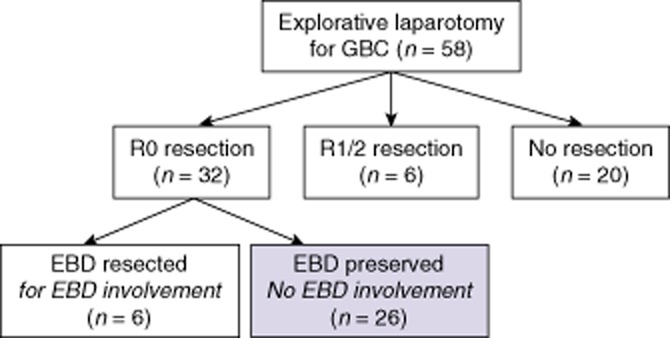
Flowchart showing surgical outcomes in patients undergoing explorative laparotomy for gallbladder cancer (GBC). In 26 patients, a negative-margin (R0) resection was achieved with preservation of the extrahepatic bile duct (EBD)
Table 1.
Demographics, treatment and pathology of patients undergoing negative-margin (R0) resection of gallbladder cancer without resection of the extrahepatic bile duct (n = 26)
| Characteristics | Value |
|---|---|
| Sex, female, n | 19 |
| Age, years, median (range) | 64 (48–82) |
| Preoperative jaundice, n | 0 |
| Previous cholecystectomy, n | 12 |
| Tumour stage, n | |
| T1b | 5 |
| T2 | 17 |
| T3 | 3 |
| T4 | 1 |
| Node stage, n | |
| N0 | 18 |
| N1 | 6 |
| Nx | 2 |
| Hepatic resection, n | |
| Cholecystectomy | 5 |
| Segment IV/V wedge | 19 |
| Right hemi-hepatectomy | 2 |
| Additional resections, n | |
| Partial duodenum | 1 |
| Regional lymphadenectomy, n | 26 |
| Lymph nodes harvested, median (range) | 3 (0–11) |
Nx, lymph node status not available in pathology report.
A previous laparoscopic cholecystectomy (i.e. an incidental finding of GBC) had been performed in 12 patients, who had been referred for definitive surgery. In these 12 patients, the depth of tumour infiltration was pT1b in three patients, in whom only lymphadenectomy was undertaken at explorative laparotomy. The remaining nine of the 12 patients had pT2 disease and underwent segment IV/V resection in combination with lymphadenectomy at explorative laparotomy. The median time between cholecystectomy and definitive surgery was 8 weeks (range: 3–17 weeks).
Survival
Ten of the 26 study patients had died at last follow-up; of these 10 patients, three showed no signs of GBC recurrence at the time of death. The median length of follow-up of the 16 patients who remained alive at the last follow-up was 33 months (range: 13–127 months). The rate of 3-year overall survival was 78%; median survival was not reached (Fig. 2). Lymph node status was significantly associated with overall survival (P = 0.04), whereas depth of tumour invasion was not.
Figure 2.
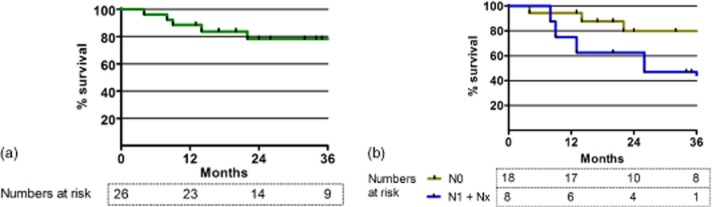
Survival curves for patients who underwent negative-margin (R0) resection of gallbladder cancer with preservation of the extrahepatic bile duct. No patients were lost to follow-up. (a) Overall survival curve. The median survival was not reached. The estimated 3-year survival rate was 78%. (b) Survival curves according to lymph node status. Patients with N1 (n = 6) and Nx (n = 2) disease were pooled. Patients with N0 disease had significantly better survival than patients with N1/Nx disease (P = 0.04). No significant association emerged between survival and depth of tumour invasion. Nx, lymph node status not available in pathology report
Initial disease recurrence
Univariate analysis found no statistically significant associations between the depth of tumour invasion or lymph node status, and tumour recurrence (Table 2). The anatomical locations of initial tumour recurrences are presented in Table 3. Four patients developed recurrence at a local site, involving the liver resection margin in two patients, the liver hilum in one patient, and the EBD in one patient. Local recurrences in these four patients were confirmed histologically. The two patients with local tumour recurrence at the liver hilum or the EBD had synchronous recurrence at distant sites: one patient had synchronous peritoneal metastases, and one patient had synchronous abdominal wall and greater omentum metastases, which may have resulted from tumour spill at a previous laparoscopic cholecystectomy. The median time to initial recurrence was 9 months (range: 2–25 months). Nineteen patients had no evidence of tumour recurrence at the time of death or at the most recent follow-up.
Table 2.
Univariate analysis of factors associated with recurrence
| Factor | No recurrence (n = 19), n | Recurrence (n = 7), n | P-value |
|---|---|---|---|
| Depth of tumour invasion | 0.28a | ||
| T1b | 5 | 0 | |
| T2 | 12 | 5 | |
| T3 | 2 | 1 | |
| T4 | 0 | 1 | |
| Lymph node status | 0.15a | ||
| N0 | 15 | 3 | |
| N1 | 4 | 2 | |
| Nx | 0 | 2 | |
Analyses (Fisher's exact tests) were based on comparisons between patients with stage T1b versus T2/3/4 disease and N0 versus N1/Nx disease, respectively.
Nx, lymph node status not available in pathology report.
Table 3.
Characteristics, survival and sites of initial disease recurrence in patients who developed recurrence after negative-margin (R0) resection of gallbladder cancer without extrahepatic bile duct (EBD) resectiona
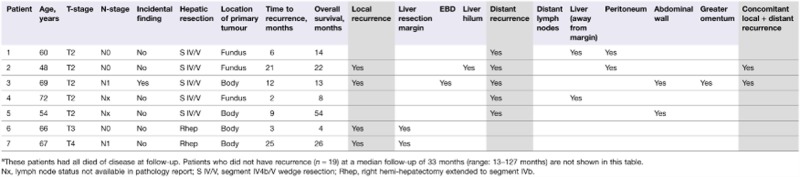 |
Discussion
In this cohort of 58 patients undergoing explorative laparotomy for GBC, no patients underwent routine resection of the EBD. Of 26 patients who underwent R0 resection of GBC without EBD resection, four patients developed local recurrence. Two patients had local recurrence in the region of the EBD (i.e. at the liver hilum or in the EBD), but both of these patients also presented with synchronous distant metastases. No patient developed an isolated local recurrence in the region of the EBD.
A retrospective nationwide study conducted in Japan compared EBD preservation and EBD resection in 399 and 194 patients, respectively.12 This study included only patients without macroscopic involvement of the EBD. No difference in overall survival was found for any subgroup of patients grouped by T-stage or N-stage. Two Western studies have also compared patients in whom the EBD had been preserved or resected, and revealed no differences in overall survival. However, in these studies > 50% of EBD resections were mandated by clinical involvement of the EBD representing more extensive disease.1,20 The present study shows that of 26 GBC patients who underwent an R0 resection without routine EBD resection, not a single patient presented with an isolated recurrence in the region of the EBD. These results support the omission of routine EBD resection in the treatment of patients with GBC.
By contrast with these results, several studies have investigated histological tumour spread to the EBD in the resection specimen of non-jaundiced patients. These studies found tumour cells in the EBD in 19 of 44 patients with T2/3 GBC and in 16 of 42 patients with T3/4 GBC.10,13 A third study found tumour cells in the submucosal lymph vessels of the EBD in three of 15 patients after resection of T2 GBC.15 Another advantage of routine EBD resection is that it may enhance locoregional lymphadenectomy, but no differences in lymph node count were found between patients who had undergone EBD resection and those who had not.1 To summarize, omitting EBD resection potentially leaves behind micro-metastases in submucosal lymphatic tissue in the EBD; however, it is unlikely that routine EBD resection will improve overall survival in patients undergoing R0 resection of GBC without macroscopic involvement of the EBD.
Although the benefit of routine EBD resection is uncertain, it is associated with increased morbidity and mortality in both Western and Asian series. In a series of 104 patients who had undergone resection for GBC, 33% of patients in whom the EBD had been resected developed a complication that required a re-intervention, whereas only 13% of patients in whom the EBD had been preserved did so.1 Another recent study showed that the rate of postoperative bile leakage increased from 3% to 14% when liver resections for various indications were combined with EBD resection.17
The observation in this study that most patients who present with recurrence after radical resection of GBC are diagnosed with distant metastases is consistent with the findings of previous studies.18 This finding reflects the aggressive biology of GBC and the associated poor survival, especially in patients with positive lymph nodes. Micro-metastases remain undetectable by preoperative imaging and during surgery, suggesting a role for adjuvant systemic treatment. However, a recent meta-analysis based on six retrospective studies involving 4450 patients with GBC found no significant difference in 5-year survival between patients who did and did not receive adjuvant treatment.21 Systemic treatment with cisplatin plus gemcitabine is currently established as palliative treatment,19 but benefits established in a palliative setting do not always translate to an adjuvant setting. Randomized trials are needed to further evaluate adjuvant therapies in GBC.
This study is limited by its retrospective study design and the lack of a control group of patients submitted to routine EBD resection. Some patients in the study group had undergone a previous laparoscopic cholecystectomy. This difference in treatment introduces heterogeneity into the relatively small group of patients. In addition, the median time between laparoscopic cholecystectomy and definitive surgery may have exposed patients to occult tumour progression, although only one of 12 patients with a previous laparoscopic cholecystectomy developed tumour recurrence during the study period. Furthermore, the study is limited by its small sample size and short follow-up. As well as imposing limitations on the general conclusion, the small sample size of patients with this rare disease hampered the detection of factors prognostic of tumour recurrence other than lymph node status. However, this study is the first to explore the value of routine EBD resection by analysing initial patterns of recurrence.
In conclusion, this study shows that none of 26 GBC patients who underwent an R0 resection without routine EBD resection developed isolated recurrence in the region of the EBD. This finding suggests there is no additional value to be derived by performing routine resection of the EBD in patients who have no involvement of the EBD and have a negative cystic duct margin.
Conflicts of interest
None declared.
References
- 1.D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806–816. doi: 10.1245/s10434-008-0189-3. [DOI] [PubMed] [Google Scholar]
- 2.Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, et al. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14:833–840. doi: 10.1245/s10434-006-9097-6. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. 2013. NCCN Clinical Practice Guidelines™ in OncologyHepatobiliary Cancers. Gallbladder Cancer 2012. 27-2-2013.
- 4.Edge SB American Joint Committee on Cancer. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 7th edn. New York, NY: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 5.Hari DM, Howard JH, Leung AM, Chui CG, Sim MS, Bilchik AJ. A 21-year analysis of stage I gallbladder carcinoma: is cholecystectomy alone adequate? HPB. 2013;15:40–48. doi: 10.1111/j.1477-2574.2012.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen EH, Abraham A, Jarosek S, Habermann EB, Al-Refaie WB, Vickers SA, et al. Lymph node evaluation is associated with improved survival after surgery for early-stage gallbladder cancer. Surgery. 2009;146:706–711. doi: 10.1016/j.surg.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 7.Goetze TO, Paolucci V. The prognostic impact of positive lymph nodes in stages T1 to T3 incidental gallbladder carcinoma: results of the German Registry. Surg Endosc. 2012;26:1382–1389. doi: 10.1007/s00464-011-2044-z. [DOI] [PubMed] [Google Scholar]
- 8.Downing SR, Cadogan KA, Ortega G, Oyetunji TA, Siram SM, Chang DC, et al. Early-stage gallbladder cancer in the Surveillance, Epidemiology, and End Results database: effect of extended surgical resection. Arch Surg. 2011;146:734–738. doi: 10.1001/archsurg.2011.128. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310–315. doi: 10.1245/aso.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Nishio H, Ebata T, Yokoyama Y, Igami T, Sugawara G, Nagino M. Gallbladder cancer involving the extrahepatic bile duct is worthy of resection. Ann Surg. 2011;253:953–960. doi: 10.1097/SLA.0b013e318216f5f3. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto Y, Kosuge T, Shimada K, Sano T, Hibi T, Yamamoto J, et al. Clinical significance of extrahepatic bile duct resection for advanced gallbladder cancer. J Surg Oncol. 2006;94:298–306. doi: 10.1002/jso.20585. [DOI] [PubMed] [Google Scholar]
- 12.Araida T, Higuchi R, Hamano M, Kodera Y, Takeshita N, Ota T, et al. Should the extrahepatic bile duct be resected or preserved in R0 radical surgery for advanced gallbladder carcinoma? Results of a Japanese Society of Biliary Surgery Survey: a multicentre study. Surg Today. 2009;39:770–779. doi: 10.1007/s00595-009-3960-6. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, et al. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136:1012–1017. doi: 10.1016/j.surg.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Kohya N, Miyazaki K. Hepatectomy of segment IVa and V combined with extrahepatic bile duct resection for T2 and T3 gallbladder carcinoma. J Surg Oncol. 2008;97:498–502. doi: 10.1002/jso.20982. [DOI] [PubMed] [Google Scholar]
- 15.Chikamoto A, Tsuji T, Nakahara O, Sakamoto Y, Ikuta Y, Tanaka H, et al. Cancer cells spread through lymph vessels in the submucosal layer of the common bile duct in gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:557–561. doi: 10.1007/s00534-009-0101-y. [DOI] [PubMed] [Google Scholar]
- 16.Yokomizo H, Yamane T, Hirata T, Hifumi M, Kawaguchi T, Fukuda S. Surgical treatment of pT2 gallbladder carcinoma: a re-evaluation of the therapeutic effect of hepatectomy and extrahepatic bile duct resection based on the longterm outcome. Ann Surg Oncol. 2007;14:1366–1373. doi: 10.1245/s10434-006-9219-1. [DOI] [PubMed] [Google Scholar]
- 17.Hoekstra LT, van Gulik TM, Gouma DJ, Busch OR. Post-hepatectomy bile leakage: how to manage. Dig Surg. 2012;29:48–53. doi: 10.1159/000335734. [DOI] [PubMed] [Google Scholar]
- 18.Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 19.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 20.Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478–1486. doi: 10.1007/s11605-007-0309-6. [DOI] [PubMed] [Google Scholar]
- 21.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]


